Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
Zengqiang Gao, Junjun Li, Zhicheng Zhang, Wenping Hu
Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science & Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin 300072, China
Keywords:CO2 reduction Electrocatalysis Carbon materials Chemicals and fuels Single-atom catalysts
ABSTRACT The increase of atmospheric CO2 concentration has caused many environmental issues.Electrochemical CO2 reduction reaction (CO2RR) has been considered as a promising strategy to mitigate these challenges.The electrocatalysts with a low overpotential, high Faradaic efficiency, and excellent selectivity are of great significance for the CO2RR.Carbon-based materials including metal-free carbon catalysts and metal-based carbon catalysts have shown great potential in the CO2RR, owing to the tailorable porous structures, abundant natural resources, resistance to acids and bases, high-temperature stability, and environmental friendliness.In this review, various carbon materials including graphene, carbon nanotubes,quantum dots, porous carbon, and MOF-derived catalysts, etc., for the CO2RR have been summarized.Particularly, recent progress in terms of the mechanism and pathway of CO2 conversion has been comprehensively reviewed.Finally, the opportunities and challenges of carbon-based electrocatalysts for the CO2RR are proposed.
1.Introduction
The fossil fuels supply about 75% of global energy demands, and the CO2emissions associated with excessive fossil fuel consumption resulted in global extremes, ocean acidification, and sea-level rise.Currently, the atmospheric CO2concentration has increased to 418.94 ppm in 2021 [1].Converting CO2into sustainable fuels and chemical feedstocks,viabiochemical, photocatalytic, and electrocatalytic reactions,etc., provides a promising approach to close the carbon cycle and alleviate energy and environmental problems.To date, electrocatalytic CO2reduction reaction (CO2RR) has been considered as an important route to address the above-mentioned issues, because of its moderate conditions, controllable process, minimized chemical consumption, and easy-to-scale-up applications[2–5].However, CO2has a stable molecular structure (high C=O dissociation energy of 803 kJ/mol), leading to a high kinetic barrier [6–8].During the electrochemical reduction of CO2, the multiproton-coupled electron transfer processes usually generate a significant number of intermediates, resulting in low product selectivity.Therefore, the rational design and controlled synthesis of advanced electrocatalysts with high activity, selectivity, and stability are highly desired.
Until now, various electrocatalysts including noble metals,metal alloys, and transition metal oxides have been developed, exhibiting outstanding electrocatalytic performance for the CO2RR.However, it remains a great challenge to fabricate the electrocatalyst with low cost, highly exposed active sites, and industrial-grade current densities.Up to now, carbon-based materials have been regarded as one of the most promising catalysts for the CO2RR owing to the inherent advantages, such as abundant natural resources, resistance to acids and bases, high-temperature stability,and environmental friendliness.The tunable electronic structures and excellent electrical conductivity of carbon-based materials are viable to reduce the energy barrier for C–C coupling [9].Despite the poor catalytic activity of carbon materials, their performance can be greatly improvedviadoping heteroatoms or constructing metal/carbon composites.More importantly, constructing carbonbased single-atom catalysts or tandem catalysts becomes a potential strategy to achieve a good catalytic activity, selectivity, as well as stability [10,11].In this review, we have comprehensively summarized the recent development of metal-free and metal-based carbon materials for the CO2RR, involving synthetic strategies, catalytic active sites, and reaction mechanisms.
2.The catalytic mechanisms for CO2 reduction
The complex processes of electrochemical CO2reduction yield various reduction products.According to the number of carbon atoms, the reduction products can be classified as C1products (e.g.,CO and HCOOH), C2products (e.g., C2H4, C2H6and C2H5OH) and C3products (e.g., C3H6and C3H7OH).The value-added multi-carbon(C2+) products are more favored over the C1products.The standard potentials for the conversion of CO2into different products are similar (Table 1) and the standard potential for H2within this range would decrease the selectivity for high-value products [12–14].The principle of the CO2RR contributes to investigate a favorable kinetic pathway to bypass the hurdles of the high overpotential and hinder the process of the HER.The reaction pathways of the CO2RR are shown in Fig.1.First, CO2is adsorbed on the surface of the catalysts, forming unstable*CO2δ- radicals which are readily protonated to generate bidentate intermediate (*OCHO) or adsorption intermediate (*COOH) [15,16].For example, Songet al.found that the formation of*COOH and*CO are more beneficial on pyridinic N and pyrrolic N than that on graphite N and pure carbon.The energy for the*CO-to-*CHO conversion on pyridinic N sites is 0.82 eV, whereas it is a slight exothermicity of -0.24 eV on pyrrolic N sites, revealing that the formation of*CHO is thermodynamically advantageous on pyrrolic N [17].Furthermore, the metal center with various d-electron structures on metal-based carbon materials exhibited unusual adsorption energies of CO2-related intermediatesviathe fine-tuning of crystal facets, and chemical states, which can adsorb CO2or the related intermediates [18].Then, the H+is adsorbed on*CO2δ-viaa proton-coupled electron transfer process or hydrogenation towards different products.Significantly,*CO intermediates are crucial intermediates to produce multi-carbon products [19].Since protons can be accepted by C and O, the reaction in this step is divided into two parts by the position of accepted protons:(1) O in*CO firstly accepts protons to generate*COH and combines with several protons to produce CH4by losing one water molecule [20].(2) The protonated*CO generates*CHO and finally produces methanol through several subsequent hydrogenation processes [21].
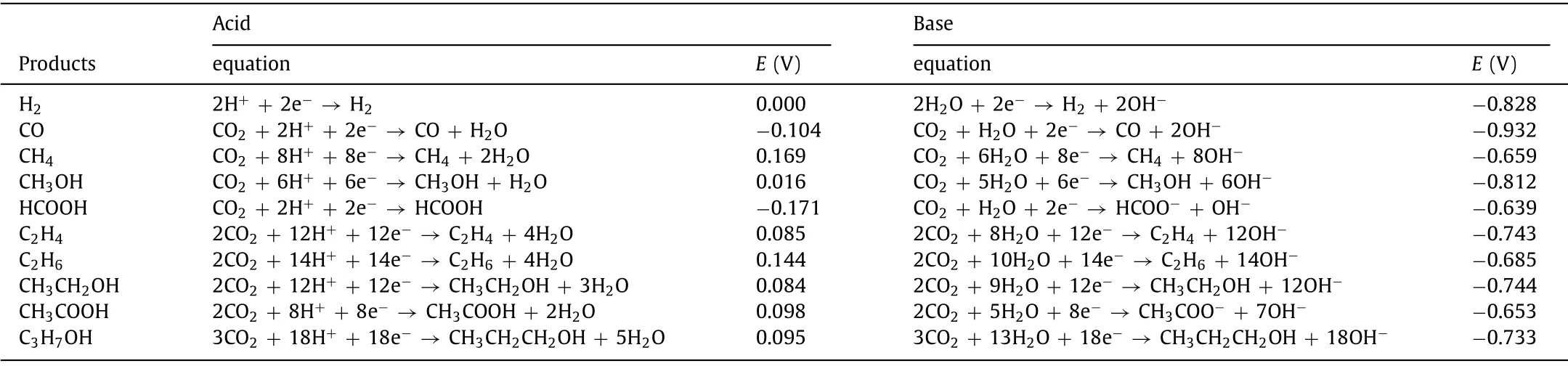
Table 1 The electrochemical thermodynamic reactions for CO2 reduction and their corresponding standard redox potentials.
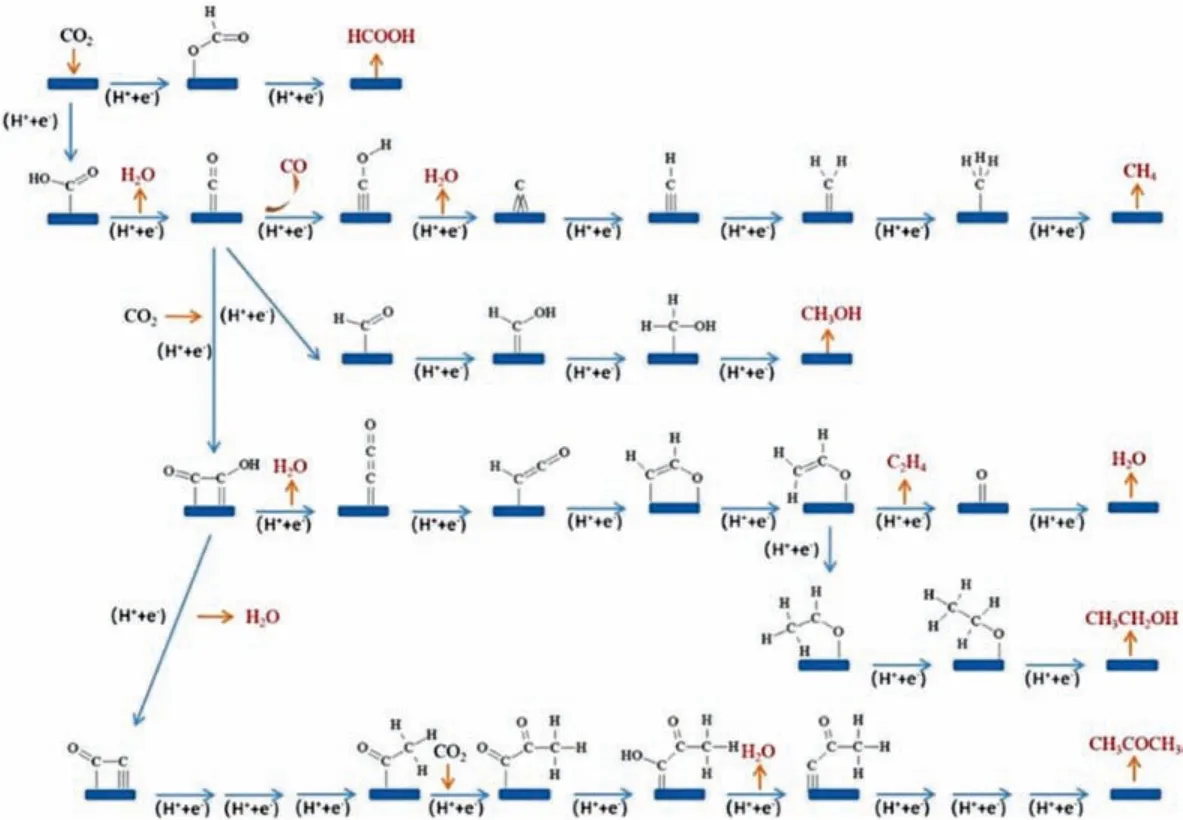
Fig.1.The reaction pathways for the CO2RR on the catalysts.Reproduced with permission [22].Copyright 2021, Wiley-VCH.
For the reaction path of the C2product, the protonated*CO attached to another*CO produces*OCCOH.*OCCOH is then further protonated to form*OCHCH2.The intermediate discards the O attached to the active site to generate C2H4or is repeatedly hydrogenated to generate CH3CH2OH [22,23].After*OCCOH discarding one water molecule, the two C atoms are attached to the active sites.Finally, CH3COCH3is formed by hydrogenation and protonation.For generating C2+products, the Cu, the only metal, can convert*CO to*CHO,*COH, or*OCCO, due to the suitable*CO adsorption energy, whereas Ag, Au, Fe, Ni and Pt own weaker or stronger*CO adsorption energy [24].By constructing metal-based carbon catalysts, the electronic structures of the active center can be regulated by adjusting coordination environments such as the species and numbers of coordination atoms and metal elements [25].For example, the Ni 3d orbital in N-doped graphene possessed an unpaired electron which could be transferred from Ni(I) to the C 2p orbital of CO2through the delocalization effect, forming the CO2δ˙-species to reduce the energy barrier for the CO2RR [26].
3.Carbon-based materials for CO2RR
3.1.Metal-free carbon materials
Carbon materials with high surface area and electronic conductivity are usually utilized as an effective matrix to load other components.Particularly, porous carbon materials can facilitate the adsorption and reduction of CO2, as well as the rapid penetration of electrolytes due to the well-developed porosity and rich specific surface chemistry.Therefore, the structural configurations for carbon frameworks have significant influences on catalytic performance [27–29].However, most of the metal-free carbon catalysts are electrochemically inert due to the negligible ability to activate CO2.Thus, various strategies for the preparation of nanoporous heteroatom-doped carbon materials (e.g., chemical doping and post-treatment) have been developed for the CO2RR.For example, the incorporation of heteroatom (e.g., B, N or S)can efficiently improve the electrochemical activity, because of the electronic properties effectively modulated by heteroatom doping.The tunable porous structure can increase the local CO2concentration through the nanoconfinement effect.The change of electronic properties arising from heteroatom doping is favorable to optimizing charge-carrier concentration, offering numerous catalytic active sites and promoting C–C coupling reactions.More importantly, the catalytic performance of carbon-based materials could be precisely tailored by the active sites within the carbon matrix.
Kumaret al.reported that the N-doping carbon nanofibers (NCNFs) showed about 13 times higher current density than Ag bulk for CO2-to-CO conversion by using ionic liquids (ILs, 1-ethyl-3-methylimidazolium tetrafluoroborate) as the electrolyte (Figs.2A and B) [30].The ILs enabled CO2to form an intermediate complex(EMIM-CO2) and thus promote the FECO, endowing NCNFs with good stability (Fig.2C).The unchanged N 1s X-ray photoelectron spectroscopy (XPS) spectrum before and after the CO2RR test indicated that positively charged carbon atoms were the active site rather than doping nitrogen.The redistribution of the charge and spin density of oxidized carbon atoms after doping nitrogen improved the catalytic activity of the NCNFs.
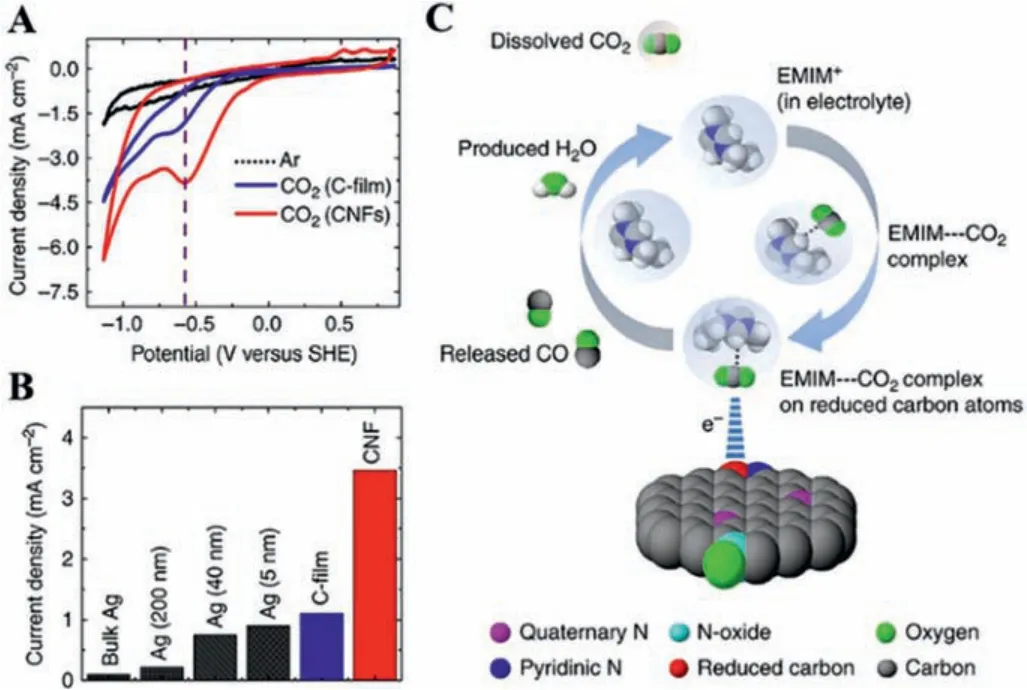
Fig.2.(A) Cyclic voltammetry (CV) of C-film and CNFs in Ar or CO2.(B) The current density of different materials.(C) Scheme of mechanism for CO2-to-CO conversion over NCNFs.Reproduced with permission [30].Copyright 2013, Nature.
Given their unique electronic and geometric features, nitrogendoped carbon nanotubes (NCNTs) were also employed for CO2-to-CO conversion, showing a low overpotential of -0.18 VRHEand selectivity of 80% [31].The catalytic activity of NCNTs synthesized by the liquid chemical vapor deposition method was mainly attributed to pyridinic N which had a lone pair of electrons to bind CO2, followed by pyrrolic and graphitic N (Figs.3A and B).The onset potential of the pyridinic N site was -0.30 VRHE, lower than that of Cu (-0.31 VRHE), Au (-0.63 VRHE) and Ag (-0.79 VRHE), which resulted in the current density of NCNTs four times that of CNTs.
The hierarchically structured porous N-doped carbon/CNT composite membrane (HNCM/CNT) was further developed and displayed high activity, selectivity, and stability for formate with a FE of 81% (Fig.3C) [32].The increased pyridinic N defects in the HNCM/CNT catalyst contributed to the improvement of catalytic performance towards CO2RR, because the basic carbon atom adjacent to the pyridinic N is prone to adsorb CO2.Xuet al.further tailored nitrogen contents by adjusting the ratio of nitrogen species and the optimized catalysts showed a good selectivity of 90% for CO2-to-CO conversion and stability of over 60 h [33].Density functional theory (DFT) calculations illustrated that the radical anion intermediate (CO2δ˙-) can be stabilized by N defects to lower the reduction barriers.
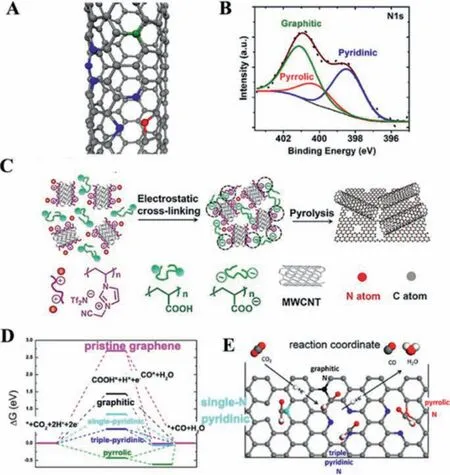
Fig.3.(A) Schematic illustration of N configuration.(B) XPS spectrum of N 1s for NCNTs.Reproduced with permission [31].Copyright 2015, American Chemical Society.(C) The fabrication process of HNCM/CNT [32].Copyright 2017, Wiley-VCH.(D)Free energy diagram of the CO2RR on NG.(E) Schematic illustration of N configuration to reduce CO2.Reproduced with permission [35].Copyright 2017, American Chemical Society.
Compared with CNTs, graphene without interference factors(e.g., curvature effects or the number of walls) can be used as a more suitable platform for an independent study of catalytic activity on different N-defect structures within the carbon network.The pyridinic N among the N-doping defects could endow adjacent carbon atoms with a positive charge.For example, N-doped graphene (NG) as a robust electrocatalyst could convert CO2into formate with a FE of 73% in aqueous electrolyte [34], while Ndoped 3D graphene foam (NGF) displayed a good electrocatalytic performance for CO2-to-CO conversion, rather than formate [35].Specifically, NGF showed comparable activity with a FECOof 85%at a lower overpotential of 0.47 V, compared to Au and Ag.DFT calculations confirmed that the free energy barrier could significantly decrease after adding N atoms in graphene owing to triplepyridinic N strongly binding intermediate COOH*(Figs.3D and E).
Considering the porous structure and chemical diversity on the surface, the nanoporous carbon (NPC) materials are also excellent electrocatalysts for the CO2RR.Yanget al.reported that the structure of carbon nanofiber membranes can be tuned by co-doping N and S and constructing hierarchical pores [36].The as-synthesized membranes with excellent flexible and free-standing properties exhibited a FECOof 94% at a current density of 103 mA/cm2.Besides,Se-doped carbon nanosheets (Se-CNs) with different sizes of the porous structure were also designed for the CO2RR [37].Impressively, Se-CNs with the optimized active sites, high surface area,and hierarchical pores, were able to promote penetration, transport, and the reaction of the reactants, and thus the partial current density of CO for Se-CNs was over 11 times that of CNs.Through edge-Se-C-N-configurations obtained by XPS and extended X-ray absorption fine structure (EXAFS) analysis, Se-CNs catalyst exhibited a smaller free energy barrier (0.32 eV) than that of CNs(0.63 eV), the proper adjustment of electronic structures made the electronic state of the active site more suitable for bonding CO2rather than adsorbing hydrogen.Accordingly, Se-CNs displayed better selectivity of CO (90%).
Fluorine-doped carbon (FC) by pyrolyzing commercial BP2000 with a fluorine source can also achieve a highly selective CO2-to-CO conversion with a FE of 89.6% and a small Tafel slope of 81 mV/dec [38].DFT calculations revealed that C4 and C9 located on the metal position of FC possessed the low Gibbs free energy barrier, conducive to COOH*adsorption (Figs.4A–C).Furthermore,Kuanget al.applied a second doping process to increase the density of pyridinic N and defects after pyrolysis of ZIF-8, achieving a good FECOof 92% [39].Subsequently, Panet al.achieving N, Pco-doping mesoporous carbon (N, P-mC) by annealing phytic-acidfunctionalized ZIF-8 in NH3and the obtained materials achieved selectivity close to 100% (Fig.4D) [40].
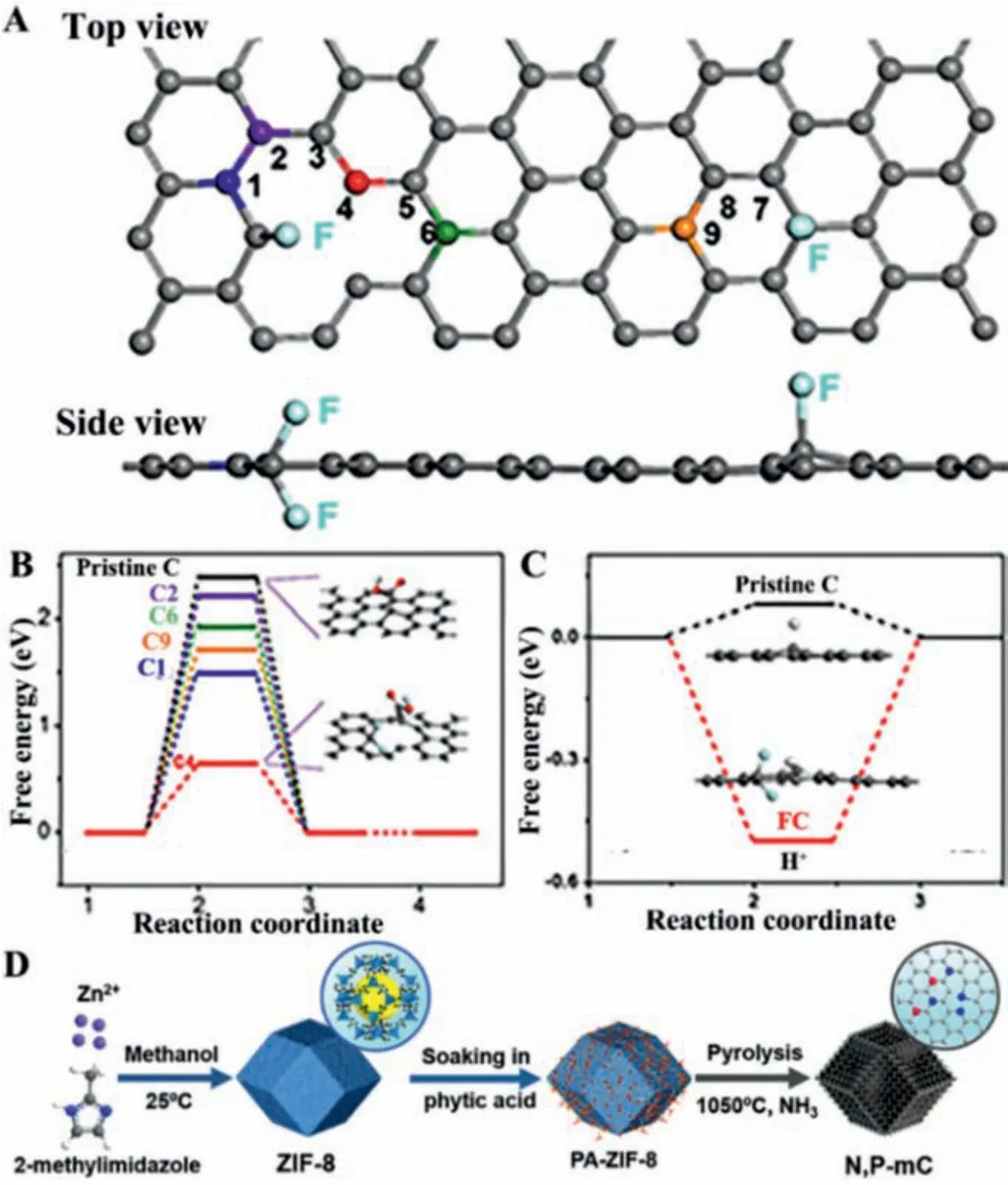
Fig.4.(A) The different view of the DFT model for FC.(B) Free energy diagram of the CO2RR to CO on FC and pristine C (with graphene model).(C) Schematic of the pathway on FC.Reproduced with permission [38].Copyright 2018, Wiley-VCH.(D)Fabrication scheme of N, P-mC.Reproduced with permission [39].Copyright 2021,Wiley-VCH.
N-Doped carbon can effectively enhance the activity of quantum dots towards CO2RR [41,42].For example, Ajayan and coworkers reported that N-doped graphene quantum dots (NGQDs)with an abundant density of N-doping defects could be prepared by exfoliating N-doped graphene oxide (GO) (Fig.5A) [43].Interestingly, different species could be regulated by potential on NGQDs,ranging from single-carbon to multi-carbon products.Local density of states and thermodynamic free energy of intermediate states further confirmed that N-doped GQD and CNT showed a much larger energy gain compared to NG, contributing to binding*COOH to achieve a high catalytic activity (Fig.5B).Owing to the easy,inexpensive and large quantity production and earth-abundance,graphene-like carbon nitride (g-C3N4) with abundant pyridinic nitrogen species is a promising candidate for heterogeneous catalysis[44].Luet al.constructed graphitic carbon nitride onto multiwall carbon nanotubes (g-C3N4/MWCNTs) by treating the precursor (dicyandiamide and MWCNT) in a H2SO4and HNO3mixture (3:1, v/v)and polycondensation, and the g-C3N4/MWCNTs with covalent attachment achieved a maximum FECOof 60% and prominent stability of 60 h [45].This work revealed that the electronic structures of g-C3N4can be effectively modified by metal-free carbon materials.Subsequently, Zhiet al.reported that g-C3N4/doped graphene materials could significantly boost CO2reductionviathe interfacial electron transfer [46].With the assistance of computational methods, the impact of heteroatom doping (X = B, N, O, P) was comprehensively explored.The observed variable adsorption energetics and electronic structures, among different doping cases, indicated that the interfacial electron transfer modulation can promote the catalytic performance for producing methane.
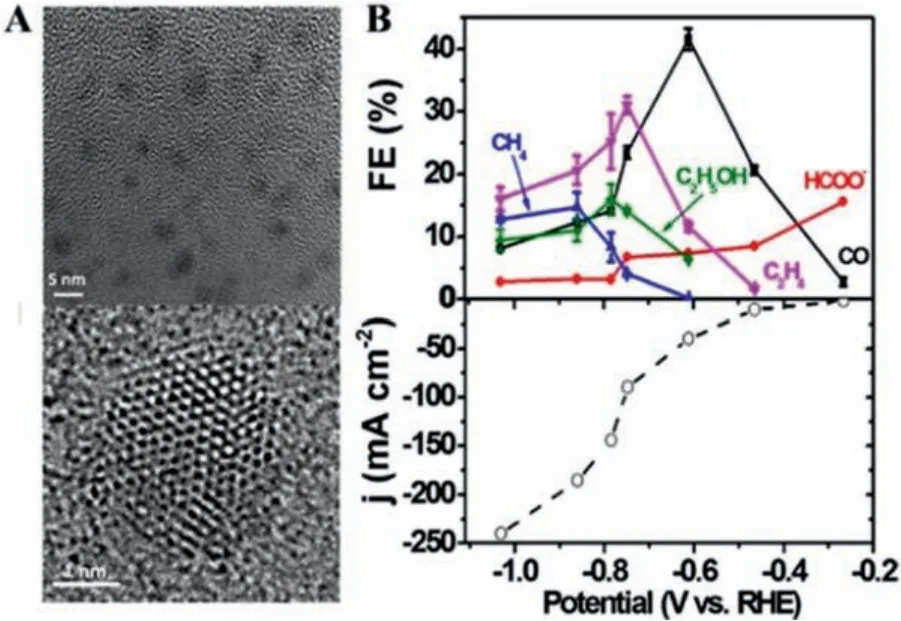
Fig.5.(A) TEM (TOP) and high-resolution TEM for N-doped carbon-based materials.(B) FE of different products and the current density of NGQDs.Reproduced with permission [43].Copyright 2017, American Chemical Society.
In addition to conventional CNT and graphene structures, diamond nanocrystals with unique tetrahedral bonding units can facilitate C–C coupling.Although the electrical conductivity of pristine diamonds is poor, it can be substantially promoted by doping.Through microwave plasma-enhanced chemical vapor deposition, Liuet al.deposited N-doped nanodiamond (NDD) on Si rod array substrates, achieving a high FE of 77.6% towards CH3COOH and improving the selectivity for C2+products (Figs.6A–C) [47].Afterward, the same group further applied a hot filament chemical vapor deposition method to achieve B and N element co-doped nanodiamond (BND) [48].The obtained materials can achieve a FE of 93.2% towards C2H5OH (Fig.6D), owing to the synergistic effect between B and N on BND.
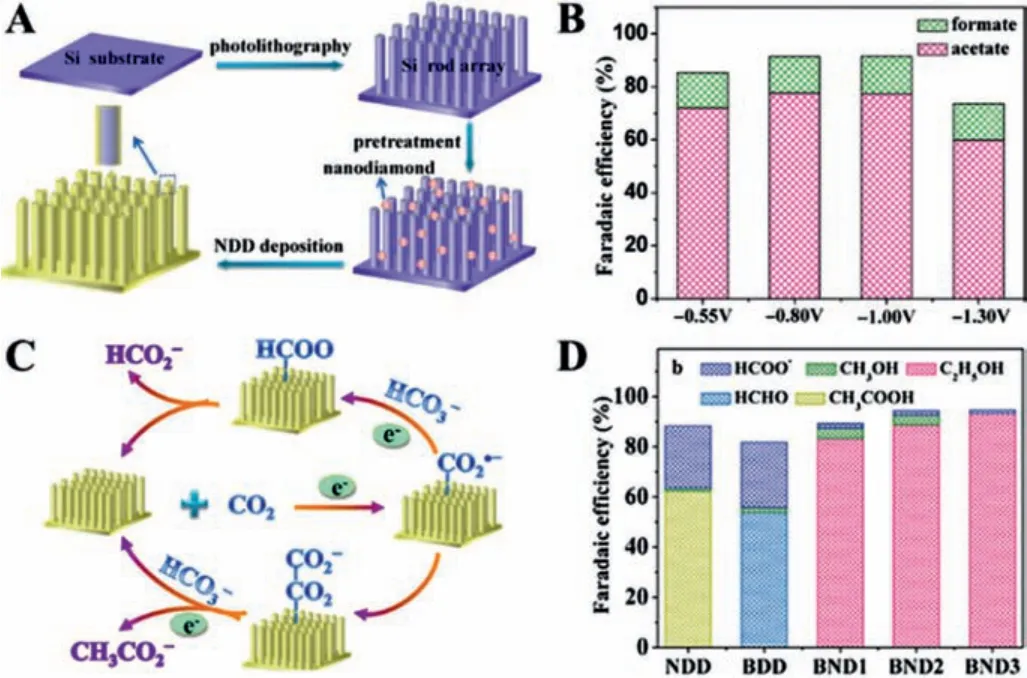
Fig.6.(A) Scheme of fabrication of NDD/Si rod array.(B) FE of different products.(C) The reaction pathways for NDD/Si rod array.Reproduced with permission [47].Copyright 2015, American Chemical Society.(D) FE for the different percentages of N in NDD materials.Reproduced with permission [48].Copyright 2017, Wiley-VCH.
3.2.Metal-based carbon materials
Anchoring metal on carbon composite is an ideal strategy for large-scale production.The synergistic effect between metals and carbon materials contributed to improving catalytic activity and selectivity towards specific products, where the metal elements(Au, Pd, Ag, Fe, Co, Ni, Cu, In,etc.) can efficiently adsorb and activate CO2to lower the energy barrier, and carbon materials with the high surface areas and tunable porous structure supply a large electrochemical active area to decrease the diffusion resistance [49–53].As known, Cu is regarded as the fundamental metal for electrocatalytically converting CO2into multi-carbon products.The strong defect sites within carbon-supported Cu catalysts can stabilize related intermediates and alter the selectivity of the catalyst for specific products.Generally, HOCCH*is a key intermediate that determines whether the product is ethylene or ethanol.Gangeriet al.investigated the conversion of CO2into various oxygenates by coating Fe or Pt onto CNT (Fe/CNT or Pt/CNT) [54].Although Fe/CNT exhibited better catalytic activity than Pt/CNT, the faster deactivation occurred on Fe/CNT due to the cross-over of the electrolyte, particularly K ions.It was found that FeOxcompounds were produced on the CNTs after reaction.Furthermore, Wanget al.investigated the confinement effect using amorphous N-doped carbon/Cu (N–C/Cu) to improve the selectivity for C2H5OH (52% ± 1%) [55].The capping layers (N–C) possessed a strong electron-donating ability, which can effectively promote C–C coupling and suppress the deoxygenation process to achieve an enhanced selectivity for ethanol.Zhonget al.also constructed Fe and N doped porous carbon nematosphere (FeNPCN) for the CO2-to-CO conversion, exhibiting a high FECOof 94% [56].3D hierarchical FeNPCN was prepared by carbonizing FeO-PDA formed by the polymerization of dopamine triggered by Fe species.The addition of the Fe atom can effectively promote the formation of the intermediates because Fe-N coordination is conducive to the formation of the COOH*intermediate.Moreover, the composition of the syngas,i.e., the ratio of CO and H2, could be efficiently adjusted.Similarly, Sunet al.integrated bismuth and bismuth oxide nanoparticles (NPs) on nitrogen-doped reduced graphene oxide nanosheets(Bi/Bi2O3/NrGO) by combining the hydrothermal method and calcination [57].The strong synergistic effect between Bi and Bi2O3together with the undercoordinated sites efficiently improved the catalytic performance, which achieved a FE of 85% for formate with a low Tafel slope of 166 mV/dec.Besides, Duet al.integrated CoO on N-doped mesoporous carbon and carbon nanotubes(MC–CNT/Co) for reducing CO2to CH3CHO and C2H5OH [58].The pyrrolic-N and pyridinic-N on MC–CNT can significantly promote the C–C coupling and stabilize*CO generated by CoO, which is favorable to forming C2H5OH.Superior to other N-containing carbon substrates, the g-C3N4with abundant pyridinic nitrogen atoms can efficiently contact with oxygen-bound intermediates (*OCHx,*O and*OH) thanks to high affinity, contributing to the deep reduction of CO2.For example, the g-C3N4as a molecular scaffold can appropriately modify the electronic structure of Cu, which could optimize some key reaction intermediates (Figs.7A and B) [59].Interestingly, Cu-C3N4with the strong adsorption of CO contributed to the lower free energy level for the intermediates (Figs.7C and D).Thus, the Cu-C3N4surface can greatly benefit the activation of CO2, leading to a more facile reaction to desirable products, compared to the Cu(111) surface and other Cu complexes on nitrogendoped carbons.Afterward, g-C3N4supported Au NPs was synthesized by Zhanget al.,achieving a FECOof over 90% in a wide range of potential from -0.45 VRHEto –0.85 VRHE[60].Impressively, the negatively charged Au surface induced by the interaction of two compounds could stabilize the key intermediate*COOH to enhance the CO2RR performance, further confirmed by the DFT.(Figs.7E-G).
To date, the engineering of defect, interface, strain, lateral size and thickness, and heteroatom doping have been considered as effective approaches to improve the catalytic activity of 2D materials [61,62].Interestingly, downsizing the metal NPs to single-atom level, single-atom catalysts (SACs) with unique electronic structure, low-coordinated metal atoms, strong metal-support interactions, and maximum atom utilization can integrate the advantages of both homogeneous and heterogeneous catalysts [63–66].SACs with isolated metal atoms dispersed on conductive support have shown excellent catalytic performance and thus become a research hot in the field of catalysis [67–69].Combining atomically dispersed metal sites and the coordinated atoms can greatly boost the catalytic performance towards CO2RR by facilitating the activation or the dissociation of the intermediates [70–73].More importantly, the fabrication of SACs contributes to systematically understanding the underlying relationship between material structure and properties at atomic level [74].Physical adsorption, polymerization, chemical bonding, or integration in frameworks endowed complexes with well-defined metal-Nx(M-Nx) sites.SACs with MN4sites as the efficient electrocatalysts for the CO2RR were confirmed by DFT calculations [75–80].To obtain heterogeneous catalysts with atomically dispersed metal sites, a common method is to pyrolyze metal precursors using appropriate nitrogen and carbon sources [81].In 2015, Strasseret al.prepared N-doped porous carbon black (N–C) containing different metals by repeated heat treatments and acid-leaching processes [82].They found that the N–C catalyst presented a similar selectivity to the N-doped carbon catalysts with M-N4moieties.More importantly, the metal sites could further reduce the adsorbed CO to hydrocarbons.Through a topo-chemical, catalyst with an exclusive Ni-N4-C structure can accelerate charge transfer and lower free energy to form*COOH[83].Atomic Fe dispersed on N-doped graphene catalysts with Fe-N4moieties showed a high FECOof over 90% in a flow cell reactor at low overpotential due to the high CO2adsorption and CO desorption of discrete Fe3+ions coordinated with pyrrolic N atoms[84].In 2020, Zhang and co-workers applied a feasible strategy to synthesize the In single-atom catalyst (In-SAs/NC) with exclusive isolated In-N4atomic interface sites for generating formate [85].By combining a wet-impregnation process and pyrolysis process,the main group of indium catalysts can be accurately regulated at the atomic scale.Therefore, catalyst displayed a large turnover frequency (TOF) of 12,500 h-1at 0.95 VRHE, and the FE for formate and current density could reach 96%, 8.87 mA/cm2, respectively.
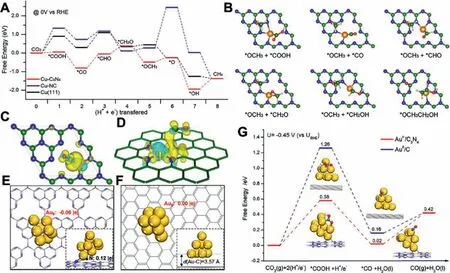
Fig.7.(A) The reaction pathways for Cu-C3N4.(B) The main reaction intermediates for producing CH3CH2OH on Cu-C3N4. The electron density for CO adsorbed on (C)Cu-C3N4 and (D) Cu-NC.Reproduced with permission [59].Copyright 2017, American Chemical Society.Au8 cluster adsorbed on C3N4 (E) at the hollow site and (F) at the graphene-carbon support.(G) The Gibbs free-energy diagram for Auδ˙-/C3N4.Reproduced with permission [60].Copyright 2018, American Chemical Society.
The coordinated N ligands act as a crucial role in improving the catalytic performance of SACs towards CO2RR.The surface-active sites can effectively suppress mass transfer and improve the atom economy.The supported Ni metal NPs can be transformed to thermal stable Ni single atoms that were mostly located on the surface of pyrolysis-treated ZIF-8 as the support, through thermal atomization (SE-Ni SAs@PNC) (Fig.8A) [86].When Ni nanoparticles contacted with N-doped carbon with abundant defects, this synthetic process not only transformed the Ni NPs into single atoms owing to the strong coordination and the continuous loss of atomic Ni species, but also created numerous pores to facilitate the contact of dissolved CO2and single Ni sites.Moreover, carbon substrate can stabilize the surface of atomization of Ni NPs.The contrast experiment that Ni NPs dispersed on the non-defective XC-72 matrix without any aggregation occurred the migration and aggregation of Ni atoms after a pyrolysis treatment revealed that the defective NC is a crucial factor for the formation of the SAs.Compared to pyridinic N-coordinated Ni (Ni-4N), pyrrolic N-coordinated Ni (Ni-3N)possessed lower free energy for*COOH intermediate.Pyrrolic Ncoordinated atomically dispersed metal ions also presented more outstanding activity and stability than pyridinic N-coordinated catalysts (Figs.8B and C).Besides, anchoring atomically dispersed Co-N5structure on polymer-derived hollow N-doped porous carbon spheres was also designed to implement a robust CO2RR [87].The X-ray absorption near-edge structure (XANES) and EXAFS revealed that a single Co atom possessed a positive charge and the valence of the Co element in the obtained catalyst was slightly higher than that of CoPc.Interestingly, the Co-N5sites were the active center and played a crucial role in the rapid formation of COOH*intermediate and the desorption of CO.
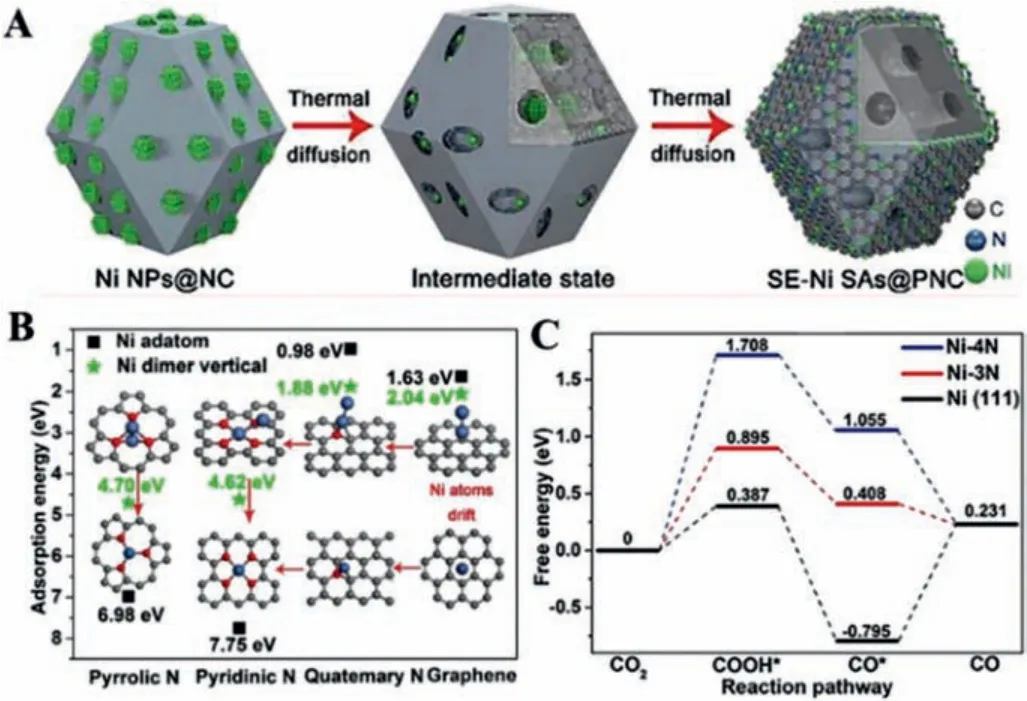
Fig.8.(A) The scheme of the transformation of Ni NPs into Ni SAs.(B) Adsorption energies of the Ni active site.(C) Free-energy diagram for the CO2-to-CO conversion.Reproduced with permission [86].Copyright 2018, Wiley-VCH.
Strasseret al.designed a series of M-N-Cviaan impregnation process and subsequent heat treatments (Fig.9A) [88].Specifically, the obtained Ni-N-C catalyst exhibited better catalytic performance, followed by Fe-N-C catalyst (Figs.9B and C).This result was further confirmed by Liet al.(Figs.9D and E) [78].The ultrathin graphene-like Ni-N-C catalystsviapace-confinementassisted molecular-level complexing approach achieved the highest FECOof 96% and the TOF of 1060 h-1at a moderate overpotential, compared to other M-N-C catalysts (M = Mn, Co, Fe, Cu,etc.).Besides, Panet al.confined low-valent Ni-based NPs within nitrogen-doped carbon (Ni-NC) catalyst for the CO2RR, exhibiting a maximum FECOof 98% [89].Interestingly, Ke and co-workers synthesized a highly active manganese N-heterocyclic carbene pincer complex ([MnCNCOMe]BF4) [90].The strong electron-donating ability of the -OMe group and electronic tuning by para substitutions are favorable to the conversion of CO2into CO.
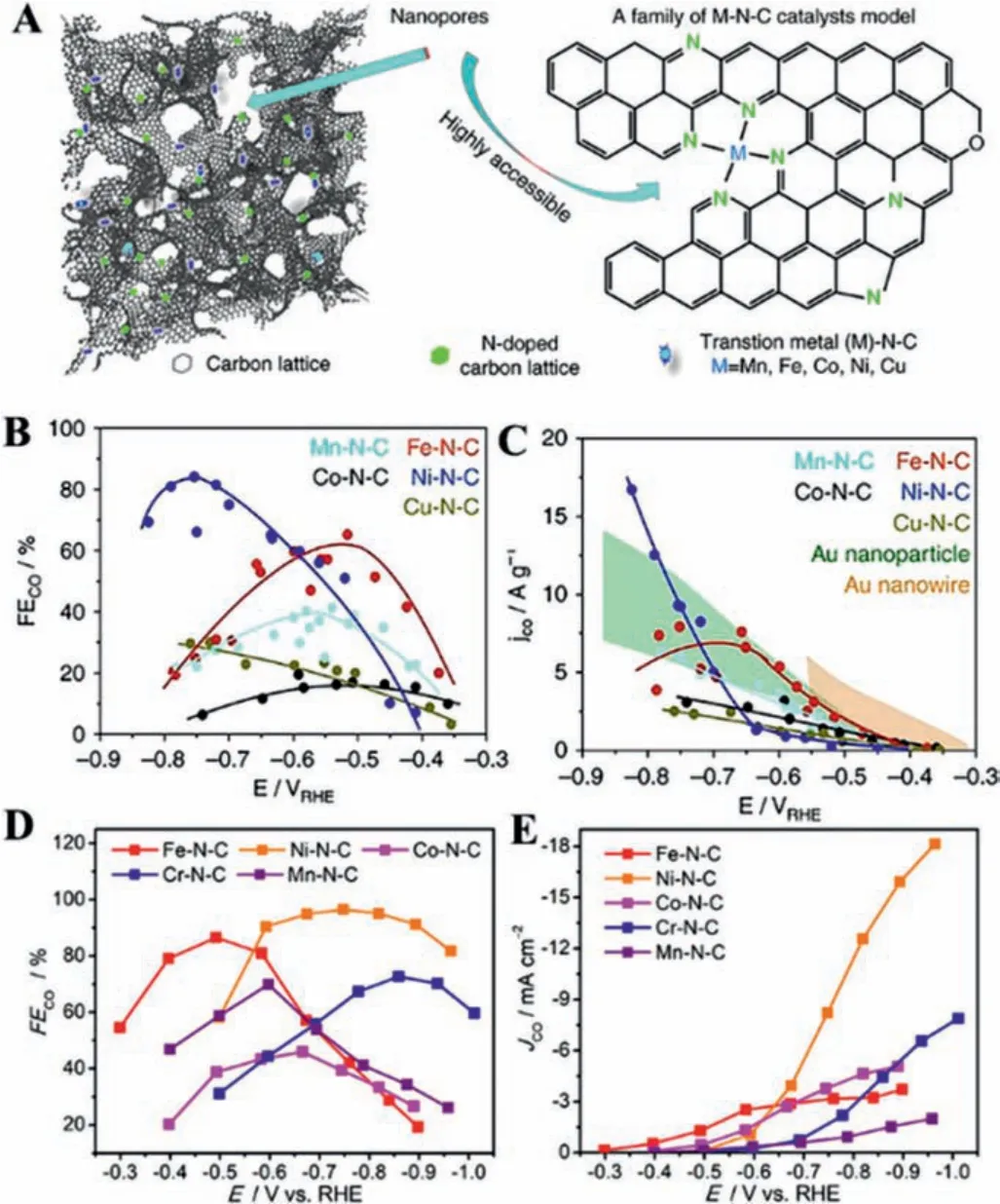
Fig.9.(A) Materials model and a schematic local structure.(B) FECO of different catalysts.(C) Catalyst mass-normalized CO partial currents of different catalysts.Reproduced with permission [87].Copyright 2017, Springer Nature.(D) FECO of different structures.(E) Catalyst mass-normalized CO partial currents of different structures.Reproduced with permission [78].Copyright 2018, Elsevier.
Metal-organic frameworks (MOFs) are porous hybrid materials consisting of metal nodes and organic ligands [91–94].The active sites within a particular metal-ligand periodic interval of MOFs are in a state of monoatomic dispersion.Pristine MOFs can be used as electrocatalyst precursors or templates to fabricate MOF-derived porous carbon nanomaterials with superior conductivity and stability.Inspired by electrocatalytic degradation of carbon tetrachloride by Co-porphyrinic MOF [95], Kubiak’s group applied electrophoretic deposition to construct a heterogeneous system by integrating redox-conductive Fe-porphyrins into thin MOF-525 films,which electrochemically covered catalytic sites (~1015sites/cm2)in conductive surface [96].The active Fe(0)-porphyrin species in Fe-based MOF films can produce 15.3 μmol/cm2of CO and 14.9 μmol/cm2of H2, whose current density can reach 2.3 mA/cm2within 0.5 h, and turnover number (TON) for CO in controlled potential electrolysis (CPE) was 272.With an acidic proton donor(trifluoroethanol, TFE), the TON of Fe(0)-porphyrin system reached 1520.Moreover, the current density and stability were up to 5.9 mA/cm2and 3.2 h, respectively, better than that without TFE.
Whereafter, Alboet al.further investigated the electrocatalytic reduction of CO2by using MOFs (HKUST-1, CuAdeAce) and metalorganic aerogels (CuDTA, CuZnDTA) [97].By cyclic voltammograms(CV), HKUST-1 showed the best current density after five scans among the above-mentioned four materials, indicating an excellent performance in transferring electrons.The HKUST-1 with a surface area of 1710 m2/g exhibited maximum yield rates of 5.62 × 10-6and 5.28 × 10-6mol m-2s-1for CH3OH and C2H5OH, respectively.Because the charge can be transported within MOF films by either linker or electron/hole redox hopping.3D porphyrin-based MOF-525 catalyst synthesized by dip-coating method admirably converted CO2into CO with low overpotential, achieving a maximum FECOof 91% and a TOF of 0.336 site-1s-1after 10 h electrolysis [98].Liuet al.applied epitaxially-grown molecular (ReL(CO)3Cl)to surface-grafted MOF catalyst, and monolithic coatings obtained current densities exceed 2 mA/cm2with a FECOof 93% [99].Due to the porosity and conformance of the inherited catalytic active sites after carbonization, MOF was a potential candidate in the field of non-noble metal catalysts.Zhaoet al.synthesized OD Cu/C catalysts by the pyrolysis of HKUST-1 to convert CO2into ethanol [100].The carbon matrix can protect Cu from deactivation and improve the efficient transfer of CO2and diffusion of the products to the solutions.The synergetic effect of the highly dispersed Cu and C matrix rendered the production rate for the methanol and ethanol up to 12.4 and 13.4 mg L-1h-1, respectively, with a total FE of 71.2%at -0.3 VRHE.In addition, the metal-metal interface plays an important role in improving the activity and selectivity of the catalysts.Three heterometallic HKUST-1 dopped ions (Zn, Ru, Pd) reported by Alboet al.were also applied to investigate the catalytic performance towards CO2RR [101].Ru(III)-electrocatalyst showed the highest yield of alcohol (47.2%).
Wu’s group prepared nitrogen-doped porous carbon by ZIFassisted strategy and ionic exchange, where Ni ions were utilized to replace Zn within the cavities of ZIF-8 (Ni SAs/N–C) (Fig.10A)[102].Due to the improved adsorption energy towards CO and electronic conductivity, the electron can rapidly shift from the electrode to the absorbed CO2to form a radical intermediate.Thus,the Ni SAs/N–C displayed a FECOof over 71.9% with a prominent TOF of 5273 h-1and the current density can reach 10.48 mA/cm2(Figs.10B and C).Based on the gas diffusion electrode (GDE), Li’s group applied Cu3(BTC)2to capture CO2for synthesizing hydrocarbons, in which capture capacity reached up to 1.8 mmol/g [103].Under relatively negative potentials, the FE of CH4for Cu3(BTC)2can double without the addition of Cu3(BTC)2.While for the competitive HER, the FE of H2was declined to 30%.
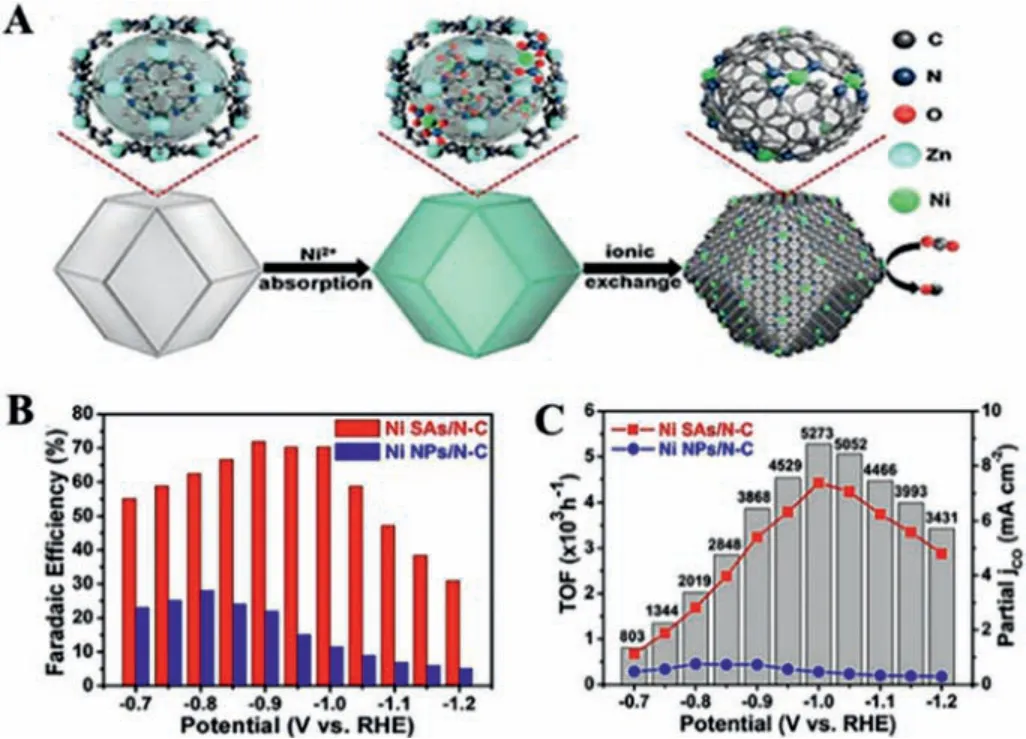
Fig.10.(A) Illustration of the synthesis process of Ni SAs/N–C.(B) FEs of CO.(C)TOF and partial CO current density.Reprinted with permission [102].Copyright 2017, American Chemical Society.
Yanget al.employed a multi-step pyrolysis approach to fabricate atomically dispersed transition metals (Ni, Co, NiCo, CoFe and NiPt) on N–CNTs (NiSA-N-CNTs) for the CO2-to-CO conversion [104].The obtained materials were subsequently characterized by aberration-corrected scanning transmission electron microscopy and X-ray absorption spectroscopy (XAS) to explore the coordination structures.While pyridinic N and graphitic N kept the same inex situXANES spectroscopy, Ni L-edge peak revealed higher photon energy, revealing that Ni-N species were the active centers for the CO2RR.The NiSA-N-CNTs can achieve a high FECOof 91.3% and a TOF of 11.7 s-1.It has been confirmed that the Ni-N4-C structure can exhibit excellent catalytic activity towards the CO2RR.Recently, low-coordination single-atom Ni electrocatalysts (Ni-N3-C)were obtainedviaa facile post-synthetic metal substitution (PSMS)strategy [105].After the pyrolysis of the Zn-MOF, acid treatment and decoration, abundant Ni-N3sites were formed (Fig.11A).Importantly, Ni-N3-C catalyst showed better catalytic performance than Ni-N4-C and N-C owing to the favorable kinetics.This result was also confirmed by XPS spectrum, synchrotron-based XAS measurement and Fourier transforms (FT) extended X-ray absorption fine structure.Ni-N3-C catalyst with a high TOF of 1425 h-1can be used in the Zn-CO2battery, showing a discharge potential of 0.41 V (Fig.11B).Moreover, integrating MOF with the function of support for electrochemical CO2-to-CO conversion is an intriguing strategy, which is capable of tailoring active molecular in the porous network to maximize active sites.The charge transport could be controlled by the morphology of MOF.Yaghiet al.incorporated the active molecule (TCPP-Co) into an Al-based MOF(Fig.11C) [106].They found that all of the active sites in homogeneous catalysts (porphyrin molecular) can contact the electrolyte,electrons and their transfer can be changed by adjusting the morphology and thickness of MOF.The selectivity for CO was over 76%at -0.7 VRHE, running for over 7 h.
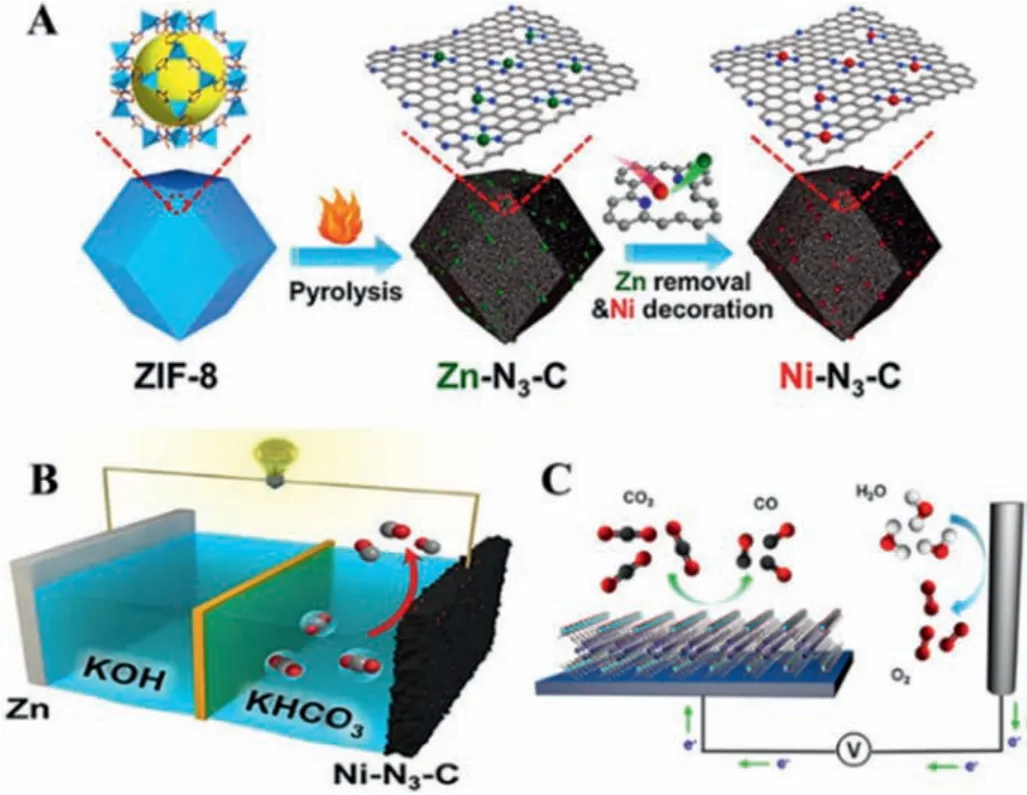
Fig.11.(A) Scheme of fabrication of Ni-N3–C catalyst.(B) The scheme of Zn-CO2 battery using Ni-N3–C as cathode catalyst.Reprinted with permission [101].Copyright 2021, Wiley-VCH.(C) The scheme of TCPP-Co with Al-based MOF for the CO2RR.Reprinted with permission [106].Copyright 2017, American Chemical Society.
Further, a NC/MOF hybrid comprising Ag NCs thin film (Ag@Al-PMOF) was constructed to improve the performance towards CO2RR and suppress HER [107].The change of the interface between the NPs and the MOFs can not only change the electronic structure of Ag but also contribute to suppressing HER and promoting the CO2RR, compared to the bare Ag NC counterparts.In 2019, Zhang and co-workers fabricated exclusive Bi-N4sites on porous N-doped carbon networks (Bi SAs/NC) through thermal decomposition of the Bi-MOF and dicyandiamide (DCD) [108].The synthesized complex achieved high activity for CO2-to-CO conversion with a highFECOof 97% and a TOF of 5535 h-1.Compared with the Bi clusters or nanoparticles, the Bi atoms achieved a higher FE of CO.It is worth noting that Bi is regarded as the material for generating formate, while BiN4/C with a low free energy barrier is conducive to the formation of CO.
Owing to the intrinsically electrically insulated property, pristine MOFs have been rarely studied in electrocatalysis.Recently,the electrical conductive MOFs have been constructedviathroughbond approach, extended conjugation approach, through-space approach, guest-promoted approach, and so on [109,110].The enhanced electrical conductivity can endow MOFs with excellent catalytic performance and provide a new pathway to boost the development of the CO2RR.For example, 2D Cu-based conductive MOF (copper tetrahydroxyquinone, Cu-THQ) was constructed for the CO2RR and achieved a FECOof 91% at a high current density of 173 mA/cm2, which are more active than other reported MOFs-based catalysts [111,112].The HRTEM images illustrated that Cu-THQ displayed a honeycomb arrangement with the AB stacking model (Fig.12A).The operando Cu K-edge X-ray absorption near-edge spectroscopy and DFT calculations revealed the existence of reduced Cu (Cu+) and Cu-THQ with the CO adsorption free energies was favorable to converting CO2into CO (Fig.12B).Meanwhile, 2D Cu-THQ with high atomic utilization and easy surface modification contributed to the improvement of performance [113].Sargentet al.systematically incorporated Ag NPs in face-centered cubic MOFs which was changed by modifying both the organic linker (1,4-benzene dicarboxylic acid, BDC, and 1,4-naphthalenedicarboxylic acid, NDC) and the metal node (Zr and Y),improving the FECOfrom 74% to 94% (Fig.12C) [114].The advanced technologies can contribute to the deep investigation of the mechanism reaction.In this work, XAS andin situRaman spectroscopy confirmed that the surface of Ag NPs in stable MOFs can effectively optimize the*CO binding mode by increasing local CO2concentration, achieving COatop/CObridgecontrol, rather than conventionally controlling the binding energy.
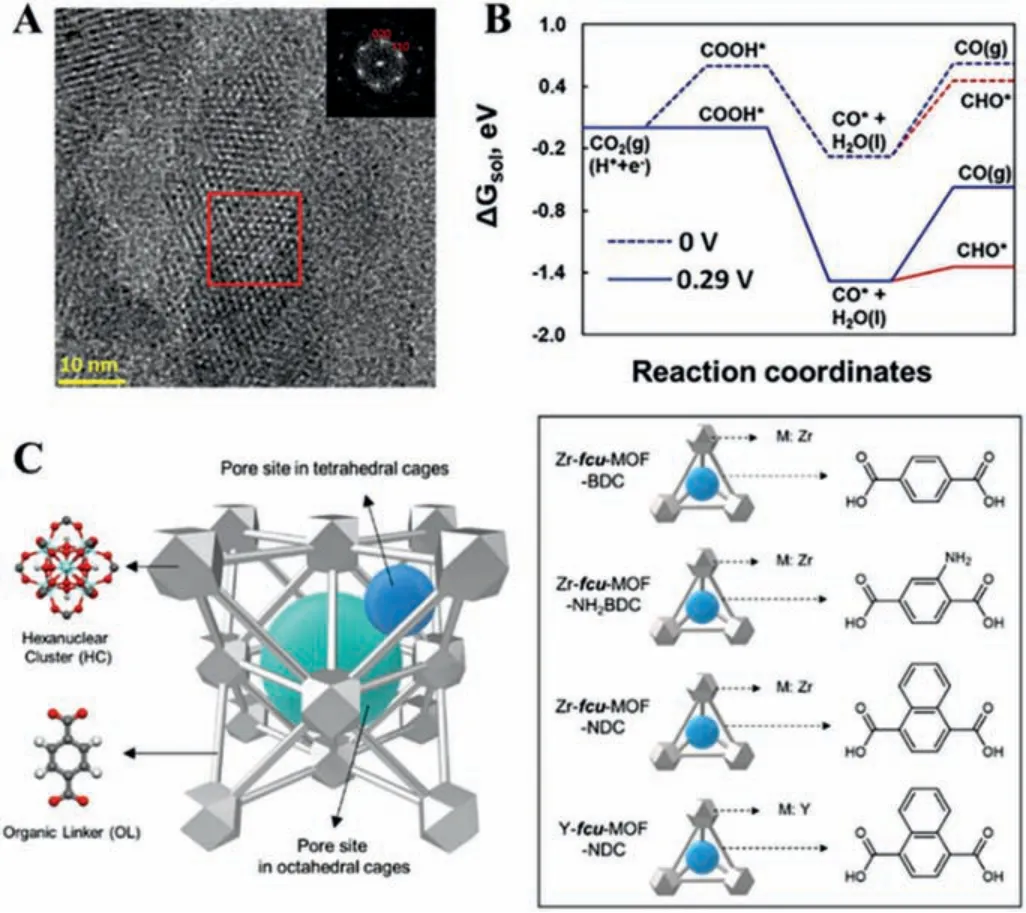
Fig.12.(A) HRTEM and Fourier transform of Cu-THQ.(B) The free energies of Cu-THQ.Reproduced with permission [111].Copyright 2021, Wiley-VCH.(C) The synthesized design strategies of MOF.Reproduced with permission [114].Copyright 2021,American Chemical Society.
To avoid the energy penalty, tandem catalysis has been considered as an efficient strategy to generate high-value products (C2+)[115].For example, Sargentet al.integrated FeTPP[Cl] (5,10,15,20-tetraphenyl-21H,23H-porphine iron(III) chloride) on a sputtered Cu electrode (FeTPP[Cl]/Cu) to investigate the molecule-metal interfaces [116].FeTPP[Cl] sites showed excellent catalytic activity for*CO, leading to the increased concentration of CO near the Cu surface, which can lower reaction energy for*CHCHOH to steer selectivity from C2H4to C2H6OH (41%).
Formate is an important chemical raw material, and many researchers focus on improving the performance of Bi-based, Inbased, and Sn-based catalysts for the conversion of CO2into formate.Bi-based catalysts are regarded as the most commercially available materials for producing formate [117].In 2020, Zhuet al.firstly obtained atomically thin bismuthene (Bi-ene) byin situelectrochemically transforming Bi-based metal-organic layers (Fig.13A)[118].Interestingly,in situattenuated total reflection-infrared spectroscopy (in situATR-IR) indicated that the adsorbed HCO3-groups participated in the first proton-couple electron transfer process during the CO2RR, which was consistent with the analysis of the Tafel test and DFT.The obtained Bi-ene in a flow cell reactor can achieve nearly full conversion for formate in a wider potential range and different electrolytes (Fig.13B).Furthermore, Bi(1,3,5-tris(4-carboxyphenyl)benzene) (Bi(btb)) as the precatalyst underwent a structural rearrangement upon electrocatalysis to produce Bi crystalline, achieving a FE of 95% towards formate [119].Similar results were also reported in previous literature [120–123].Relying on the Bi element, these reports achieved a high FE for the formate, but the structure change of Bi-based MOFs during the reaction process remains unclear.
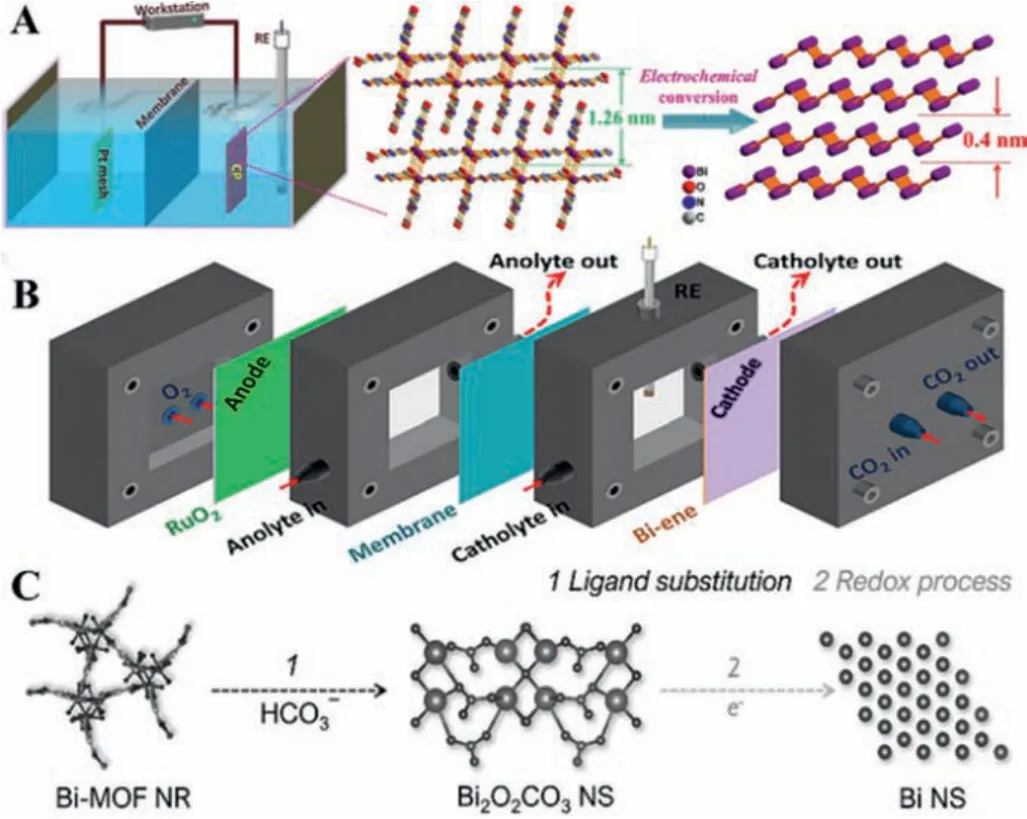
Fig.13.(A) The scheme of synthesis of Bi-ene.(B) Illustration of the self-designed flow cell.Reprinted with permission [118].Copyright 2020, Wiley-VCH.C) Diagram of reconstruction of Bi-MOF.Reprinted with permission [124].Copyright 2021,Wiley-VCH.
In 2021, Qiaoet al.monitored and controlled the reconstruction of a Bi-based MOF during the electrochemical CO2reduction(Fig.13C) [124].They found that Bi-based MOF was firstly converted into Bi2O2CO3in electrolyte and then Bi2O2CO3was mediated to Bi by applying potential.Bi catalysts after intentionally reconstructed exhibited high activity, excellent selectivity, and longterm durability for generating formate.This work indicates the significant impact of pre-catalyst reconstruction during the reaction and provides a pathway for the rational design of highly active and stable electrocatalysts.
4.Conclusions and perspective
The electrochemical reduction of CO2has been considered as one of the most promising approaches to close the carbon cycle, solve environmental issues and meet energy demands.Until now, various carbon-based catalysts including graphene, carbon nanotubes, quantum dots, porous carbon, and MOF-based catalysts have been fabricated, exhibiting good FEs and selectivity towards the CO2RR.Due to the tailorable porous structures, abundant resources, and excellent catalytic properties, carbon-based materials are efficient in the reduction of CO2to the value-added carbon products.The relevant experiments have been completed, as shown in Table 2.Concerning metal-based carbon catalysts, improving the utilization efficiency of metal atoms is of essential significance for reducing the catalyst cost and promoting sustainability.Through a combination of experiment and theory, precisely controlling the structure of metal-based carbon composite catalysts provides an enormous opportunity for investigating the relationship between structure and performance.
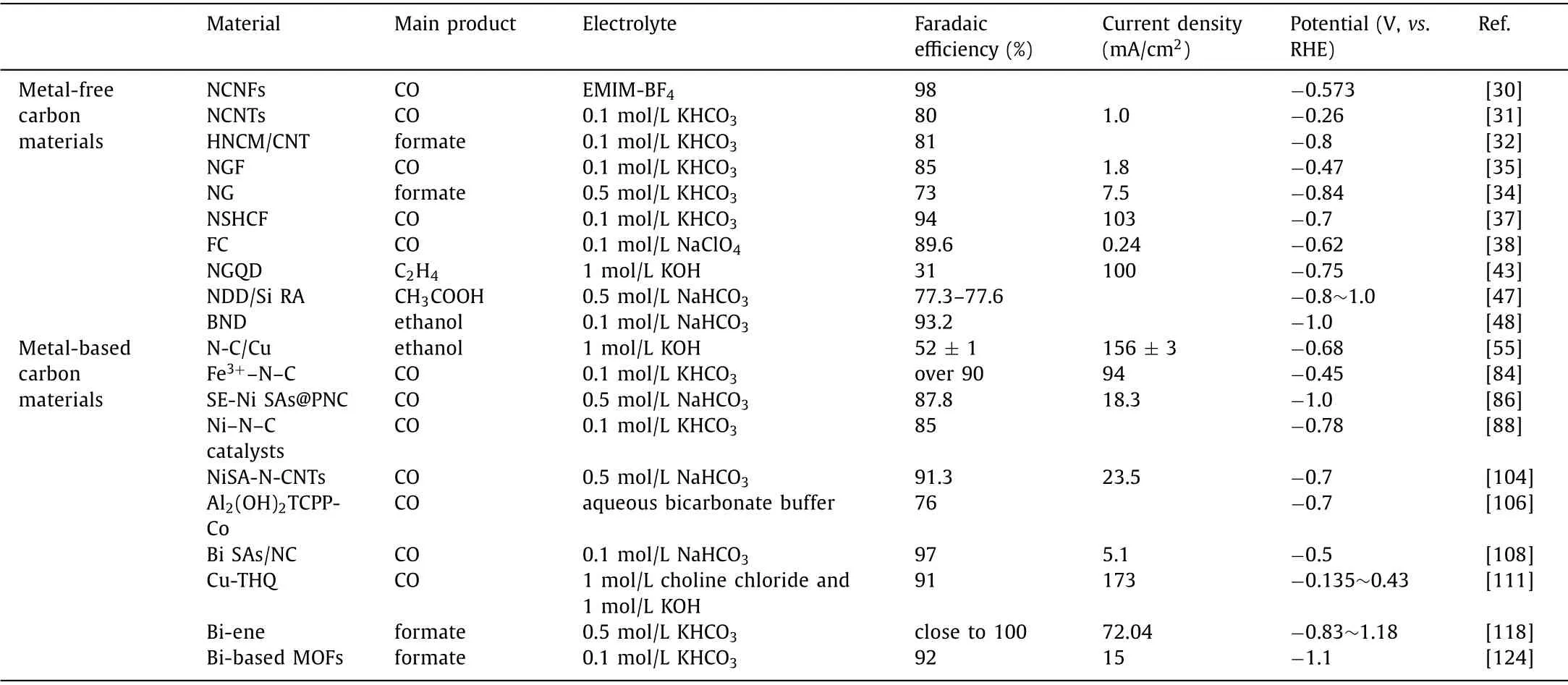
Table 2 The performance of different MOFs and their related products in the CO2RR.
Despite these promising achievements, the catalytic performance still needs to be improved especially in terms of current densities, FE, and selectivity.The catalytic performance of carbonbased catalysts depends on the surrounding environment, active sites, and physicochemical properties.Besides, the multiple electron pathways of the CO2RR (even more than 18-electron) would generate various related intermediates which can provide many arrangements for protonation, leading to the complexity of the product and uncontrollable process.Furthermore, more efforts are needed to further direct a deeper understanding of the catalytic process to find the optimal catalyst and reaction conditions.Therefore, for the in-depth understanding of possible pathways for the key intermediates and structure-activity relationships, various ad-vancedin situcharacterization technologies, such asoperandoXAS,in situRaman spectroscopy, electron paramagnetic resonance,in situsurface-enhanced infrared absorption spectroscopy,in situATRIR, andin situXPS should be further developed and adopted to detect electrochemical reaction or monitor the change of surface state and atomic structure.An electrocatalytic microdevice of the CO2RR like the chip should also be constructed to directly probe dynamic changes of the catalyst or electrochemical processes.
Identifying the specific role of each component involved in multiple carbon-based composites towards the CO2RR is a great challenge.In this regard, some new measures or approaches should be further taken to achieve this goal.For example, fabricating conductive MOFviadoping, ligands decoration, is a proper strategy for improving the performance of carbon-based catalysts.In addition, fabricating carbon-based tandem catalysts as models for target products, especially Cu-based carbon catalysts, deserves further exploration.Differently, the mass transfer of CO2also plays an important role in improving the current density.Some approaches can be utilized to overcome this problem in an aqueous solution:(1) performing this reaction in an organic solvent such as acetonitrile to promote the solubility of CO2; (2) adjusting reaction systems, such as increasing reaction pressure to promote the solubility of CO2; (3) designing new electrochemical cells, such as flow cell, overcoming the inherently low diffusion between CO2and the catalyst surface.
This review provides the guidance to explore promising strategies for the CO2RR, so as to achieve high energy efficiency,industry-compatible current densities, and admirable conversion efficiency in the future.Inspiringly, carbon-based materials are expected to achieve the high performance towards the CO2RR by combining the theory of computer science and quantum chemistry with experiments, which can significantly decrease the workload of researchers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.22071172), the Strategic Priority Research Program of the Chinese Academy of Sciences (No.XDB12030300), the Ministry of Science and Technology of China(Nos.2017YFA0204503 and 2018YFA0703200).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Encapsulation strategies on 2D materials for field effect transistors and photodetectors
