Recent advances in enhancing reactive oxygen species based chemodynamic therapy
Xinchao Li, Rui Luo, Xiuqi Liang, Qinjie Wu, Changyang Gong
State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu 610041, China
Keywords:Chemdynamic therapy Fenton or fenton-like reactions Reactive oxygen species Increasing hydrogen peroxide levels Reducing glutathione levels External energy intervention Combination therapy
ABSTRACT Chemodynamic therapy (CDT), defined as an in situ oxidative stress response catalyzed by the Fenton or Fenton-like reactions to generate cytotoxic hydroxyl radicals (·OH) at tumor sites, exhibits conspicuous inhibition of tumor growth.It has attracted extensive attention for its outstanding edge in effectiveness,lower systemic toxicity and side effects, sustainability, low cost and convenience.However, the inconformity of harsh Fenton reaction conditions and tumor microenvironment hamper its further development,based on which, numerous researchers have made efforts in further improving the efficiency of CDT.In this review, we expounded antitumor capacity of CDT in mechanism, together with its limitation, and then summarized and came up with several strategies to enhance CDT involved tumor therapy strategies by 1) improving catalytic efficiency; 2) increasing hydrogen peroxide levels at tumor sites; 3) reducing glutathione levels at tumor sites; 4) applying external energy intervention; 5) amplifying the distribution of hydroxyl radicals at tumor sites; and 6) combination therapy.Eventually, the perspectives and challenges of CDT are further discussed to encourage more in-depth studies and rational reflections.
1.Introduction
Chemodynamic therapy (CDT), an emerging therapeutic strategy recently proposed by Bu’s group [1], is defined asin situoxidative stress through Fenton reaction or Fenton-like reaction to generate hydroxyl radicals (·OH) at tumor site [2].Catalyzed by transition metal or specific enzyme, CDTcan convert endogenous hydrogen peroxide (H2O2), which is abundant in tumor, to more lethal·OH, trigger apoptosis, necrosis or ferroptosis and inhibit tumor progress.In other words, CDT is relying on the conversion of reactive oxygen species (ROS).Reactive oxygen species, along with cell metabolism, are byproduct of molecular oxygen, also described as oxygen atoms-containing chemically reactive species [3,4].With single or more unpaired electrons, ROS are extremely unstable and short-lived molecules, for example, superoxide anion radicals(O2-·), H2O2and·OH.Different types of ROS show undeniably distinct diffusion capabilities and reactivities, which is the reason why chemodymic therapy can inhibit tumor progress but still exist some limitations impede its further development.For example,the reactivity or oxidizability, weakens from·OH, O2-·, to H2O2; In contrast, the diffusion capability enhances in order.Hydroxyl radicals are extremely reactive and almost do not diffuse after its formation, while O2-·is highly active and has limited diffusion capacity, especially through certain channels.H2O2readily diffuses through membranes making it even an ideal candidate for intracellular signaling [5].
Benefited from the amazing effectiveness, lower systemic toxicity and side effects, sustainability, low cost and convenience, CDT has drawn increasing attention.Concretely speaking, profiting from the unique killing mechanism that it can convert abundant H2O2in situto more lethal·OH, CDT displays incredible therapeutic effects.Additionally, taking H2O2as the reaction substrate, Fenton reaction is triggered by tumor acidic microenvironment logically and selectively, which means it is relatively safe in normal tissues for the neutral or alkaline conditions and low level of H2O2.Of note,transition metal ions, ferrous ions for example, can consume H2O2,and thus produce·OH and iron ions continuously, as a recycling catalyst.Subsequently, iron ions react with H2O2to regain ferrous ions.Therefore, sustainability is achieved.What increases the likelihood of clinical transformation is that CDT has no requirement of sophisticated therapeutic instruments, reducing economic cost of tumor treatment prominently.Eventually, without external field stimulation makes it such a convenient and potential therapy in clinical application, which of course causes it an adjuvant therapy with ease when other separate treatment hits a bottleneck.Moreover, CDT avoids lots of limitations compared with photodynamic therapy (PDT) that generates cytotoxic singlet oxygen (1O2) when stimulating photosensitizer (PS) with a specific wavelength light and oxygen.For example, (1) the best absorption wavelength of PS are ranging from 700 nm to 850 nm [6], which impedes penetration depth of light, 3 mm approximately underneath the skin;(2) high oxygen consumption is another considerable problem for hypoxic tumor; (3) side effect from PS cytotoxicity in the dark and the undesirable white-light activation; and (4) accurate delivery PS to sub-cellular organelles for exerting powerful toxicity remains an issue, CDT ignores the limitation of light, oxygen and dark toxicity in mechanism.Like PDT, sonodynamic therapy (SDT) is faced with similar problems, although with deeper penetration.
With shining advantages, there are also some imperfections limiting the efficiency of CDT when standing on the shoes of harsh Fenton reaction conditions.For example, low catalytic efficiency,insufficient H2O2as reactants, and continuous loss of·OH as products, which all restrict its ideal effect.Specifically, inefficient catalytic activity, limited endogenous H2O2in tumor and the resistance of antioxidant systemin vivoall impede the accumulation of products,·OH.Besides, accelerating the accumulation of·OH is not the final aim, but tumor cells death.Hence, the killing process of·OH to tumor cells also should be taken into consideration.The extremely active state of·OH limits their spread, which means the powerful killing ability of·OH can only work locally and thus limit their damage to tumor.
Herein, we summarize and come up with some strategies about how to solve those problems.We take how to improve CDT involved tumor treatment as how to increase the performance of Fenton reaction and intensify tumor cell damage, and we thus putting forward the following points to realize our idea, (1) improving catalytic efficiency; (2) increasing hydrogen peroxide levels at tumor site; (3) reducing glutathione levels at tumor site; (4) applying external energy intervention; (5) amplifying the distribution of hydroxyl radicals at tumor site; and (6) combination therapy.
2.Improving catalytic efficiency
Zhanget al.reported the facile synthesis of amorphous iron nanoparticles (AFeNPs), which could response the mild acidity and the overproduced H2O2in tumor microenvironment (TME) and generate substantial·OH rapidly, when compared with their crystalline counterpart, iron nanocrystals (FeNCs).It is the first time to enhance the efficiency by increasing the release of ferrous ion [1].Although several iron-based nanomaterials has been reported, such as ferric oxide [7], Fe3O4[8], [FeO(OH)n] [7,9], M(Sn, Mn)Fe2O4[10,11], and ferric pyrophosphate [12], there are always some problems caused by strict reaction conditions when referring to ironbased nanocarriers.The optimal pH of iron-based Fenton reaction is ranging from 2 to 4, which limits catalytic efficiency because the pH of TME predominantly ranges from 6.5 to 7, the pH of endosomes in tumor cells is approximately 5.0 and that of lysosomes is approximately 4.5 [13].Therefore, some efforts have been made to improve catalytic efficiency in the following description.
2.1.Ameliorating the conditions of iron-based Fenton reaction
Due to the lower optimal pH, it is obvious to take measures to reduce the pH of tumor.Liuet al.[14] designed a unique amorphous iron oxide (AIO) RNAi NP platform with small size, which has a perfect penetration in tumor (Fig.1a).Notably, the nanoparticle can result in MCT4 gene silencing, which blocks intracellular lactate efflux and causes cancer cell acidosis.Moreover, the accumulation of hydrogen ion can exacerbate Fenton reaction after AIO is taken by tumor cell.
Rate-limiting step of Fenton reaction is the reduction of iron ion to ferrous ion.Hence, it is a wise choice to add some reducing agents to lower the redox barrier.As shown in Fig.1b, Zhang’s group synthesized an ATP-responsive autocatalytic Fenton nanosystem GOx@ZIF@MPN for tumor ablation based on the overexpressed ATP in tumor [15].On arriving at tumor site, GOx@ZIF@MPN nanosystem split up into GOx, Fe3+, tannic acid (TA).The released TA can turn Fe3+into Fe2+as a reductant, which was a reactant of Fenton reaction, and thus enhancing the generation rate of·OH.CuS has also been descriped to have a similar effect [16].
Accelerating electron transfer is another perspective to achieve the same goal.In this strategy, electron shuttle between Fe2+and Fe3+, an accelerator, provides a possible way for accelerating their circulation and improving the efficiency of CDT.Liet al.skillfully took advantages of the Lewis acidity of aluminum and ligandfield effects of N-graphene, and thus designed a Fe, Al, N coincorporated graphitic nanozyme (Fe/Al-GNE) (Fig.1c), in which Al could attract electrons and drive their transfer to Fe3+and graphitic sheet further accelerated electrons transfer due to its perfect electrical conductivity [17].Unsurprisingly, Fe/Al-GNE showed satisfactory viability suppression bothin vitroandin vivo.Gong et al.constructed Hafnium-based nanoscale metal-organic frameworks (Hf-nMOFs) and functionalized them with Fe3+to get Hf-BPY-Fe.Upon irradiation, Hf4+in Hf-BPY-Fe generated a mass of high-energy electrons, which could not only convert H2O to·OH partly, but also accelerate the reduction from Fe3+to Fe2+.Ideal results ofin vitroandin vivoprovided a proof of concept for multistep or full-process radiosensitization based on ferric ion functionalized Hf-nMOFs [18].
As mentioned above, pH is the key bottleneck problem of CDT.Nevertheless, the pH of some areas on the surface of solid tumor is neutral for their rich vascular distribution and adequate oxygen supply, which leads to an inefficient result even accompanied by recurrence and metastasis.In order to crack acidity addiction and raise CDT efficiency, Bu’s group prepared a complex of ferrouscysteine-phosphotungstate nanoparticles (FcPWNPs) on account of the chelation of PWO and Fe3+, which prevented iron ion from rapidly precipitating out as inert ferric oxyhydroxides [Fe(OH)x]at a neutral pH (Fig.1d).Meanwhile, the CDT efficiency was increased by the electron shuttling properties of Phosphotungstate(PWO), which expedited the transfer process from ferric to ferrous ions [19].Their strategy achieved a comprehensive treatment from surface to interior of tumor, which provided a novel way to treat tumor.Transmission electron microscopy (TEM) images show the diameter of FcPWNPs is approximately 120–130 nm.The effect of FcPWNPs was also verified by inhibiting 4T1 growthin vitroandin vivo.
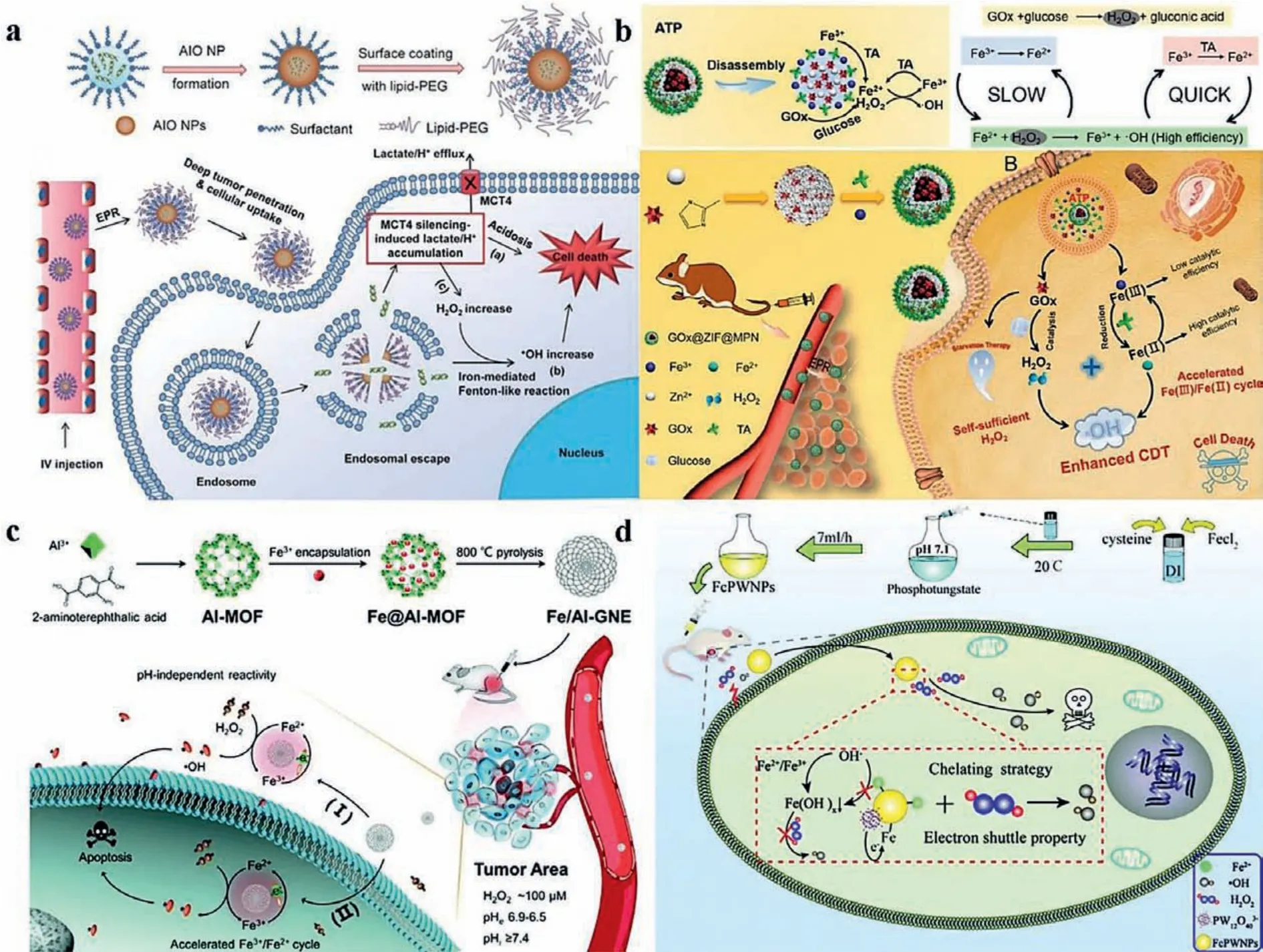
Fig.1.Schematic illustration of ameliorating the condition of iron-based Fenton reaction.(a) Amorphous iron oxide (AIO) RNAi NP platform was used to acidize TME.Copied with permission [14].Copyright 2018, Wiley Publishing Group.(b) GOx@ZIF@MPN nanosystem was designed for reducing redox barrier between Fe3+ and Fe2+.Reproduced with permission [15].Copyright 2018, American Chemical Society.(c) Fe, Al, N co-incorporated graphitic nanozyme (Fe/Al-GNE) was demonstrated to accelerate electron transfer between Fe3+ and Fe2+.Copied with permission [17].Copyright 2020, Royal Society of Chemistry.(d) Ferrous-cysteine–phosphotungstate nanoparticles (FcPWNPs)were reported to prevent precipitation of Fe3+ at a neutral pH and accelerate electron transfer simultaneously.Copied with permission [19].Copyright 2019, Royal Society of Chemistry.
2.2.Changing catalyst types
Caused by the limitation of ferrous ion, even in ideal pH, the Fenton reaction rate catalyzed by Fe2+is low, about 63 L mol-1s-1, which leads to slow generation of ROS [20].Compared with Fe2+, cuprous ion (Cu+) has a higher reaction rate with looser conditions rather than strictly acidic surrounding.Its highest reaction rate (1 × 104L mol-1s-1) can almost reach a ~160-fold increase over that of Fe2+[21,22].Of note, copper is a cofactor of many enzymes that have biological activity, can conjugate protein and many other ligands, which may induce severe systemic toxicity if free copper is excess [23–25].So, it is of significance to deliver copper to tumor site accurately.Recently, self-assembled copper-amino acid mercaptide nanoparticles (Cu-Cys-NPs), displayed in Fig.2a, have been designed forin situglutathioneactivated and H2O2-reinforced CDT because copper ions have a high coordination ability with sulfhydryl-group-containing ligands[26].They detected the cytotoxicity of Cu-Cys-NPs, which showed Cu-Cys-NPs possessed relatively higher cytotoxicity toward cancer cells than toward normal cells.Then, NOD-SCID immune-deficient mice bearing MCF-7 orthotopic xenografts were established to verify whether Cu-Cys-NPs had antitumor abilityin vivo.Satisfactorily, Cu-Cys-NPs revealed obviously inhibition compared with doxorubicin (DOX) to drug-resistant breast cancer.Many other articles[16,27-29] also reported the advantages of Fenton-like reaction catalyzed by cuprous ion.
There are still some other elements that can catalyze Fenton-like reactions, Mn2+, Mo5+for example.Bicarbonate(HCO3-)/carbon dioxide (CO2), as one of the most important physiological buffers, is indispensable for Mn2+-mediated Fenton-like reaction (Fig.2b) [30,31].Liuet al.designed a Mo2C-derived POM as PTT/CDT dual-agent, which relied on massive generation of noxious1O2during the oxidation of Mo5+to Mo6+presumablyviathe Russell mechanism [31].What is more, Ag [32], Au [33], and W [34] were also reported to have Fenton-like reaction.
To combat microbial invasion and inflammation, neutrophils and monocytes, taking H2O2and halide as reactants, can generate hypochlorous ion (ClO-) with the help of myeloperoxidase (MPO)[35].ClO-can further react with H2O2, and generates1O2.As a result, nanosystems have been developed as carriers of MPO for production of ClO-and1O2[36].However, its application was limited by the instable chemical property, which results in the appearance of chloroperoxidase (CPO), a more stable MPO-like enzyme [37].There are still some problems, the safety and immunogenicity of this exogenous enzyme need further explore.Hence, Zhao’s group reported a nanocarrier with the cargo of NaClO, loaded into zeolitic imidazolate framework (ZIF-8) with poloxamer 188 (F68) coating outside (Fig.2c) [38].This1O2-based chemodyanmic therapy that was realized by intravenous (i.v.) delivery of hybrid nanocarriers(ClO@MOF/F68) and concurrent intraperitoneal (i.p.) administration of Ascorbate (Asc) [39], which can provide H2O2at the pharmacological concentration only in the extracellular fluids of solid tumors.The anti-tumor effects were both evaluatedin vitroandin vivo(Fig.2d).This work, to some extent, expanded the concept of CDT medicated by Fenton reaction and gated the avenues of selectively ROS-based antitumor therapy.
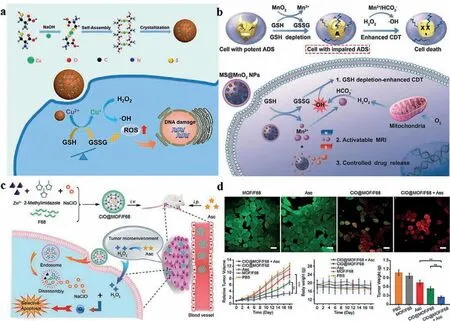
Fig.2.Non-iron catalyst applied to chemodynamic therapy (CDT).(a) Schematic depiction of Cuprous ion based self-assembled copper-amino acid mercaptide nanoparticles(Cu-Cys-NPs).Copied with permission [26].Copyright 2018, American Chemical Society.(b) Schematic depiction of manganese ion doped MS@MnO2 NPs.Copied with permission [31].Copyright 2018, Wiley Publishing Group.(c) Schematic depiction of NaClO loaded hybrid nanocarriers (ClO@MOF/F68) work together with ascorbate for generalized CDT.Copied with permission [38].Copyright 2019, Wiley Publishing Group.(d) Anti-tumor effect of ClO@MOF/F68 evaluated both in vitro and in vivo.Reproduced with permission [38].Copyright 2019, Wiley Publishing Group.
Many other catalysts were consolidated in Table 1 [15,17,18,32,33,40-64].
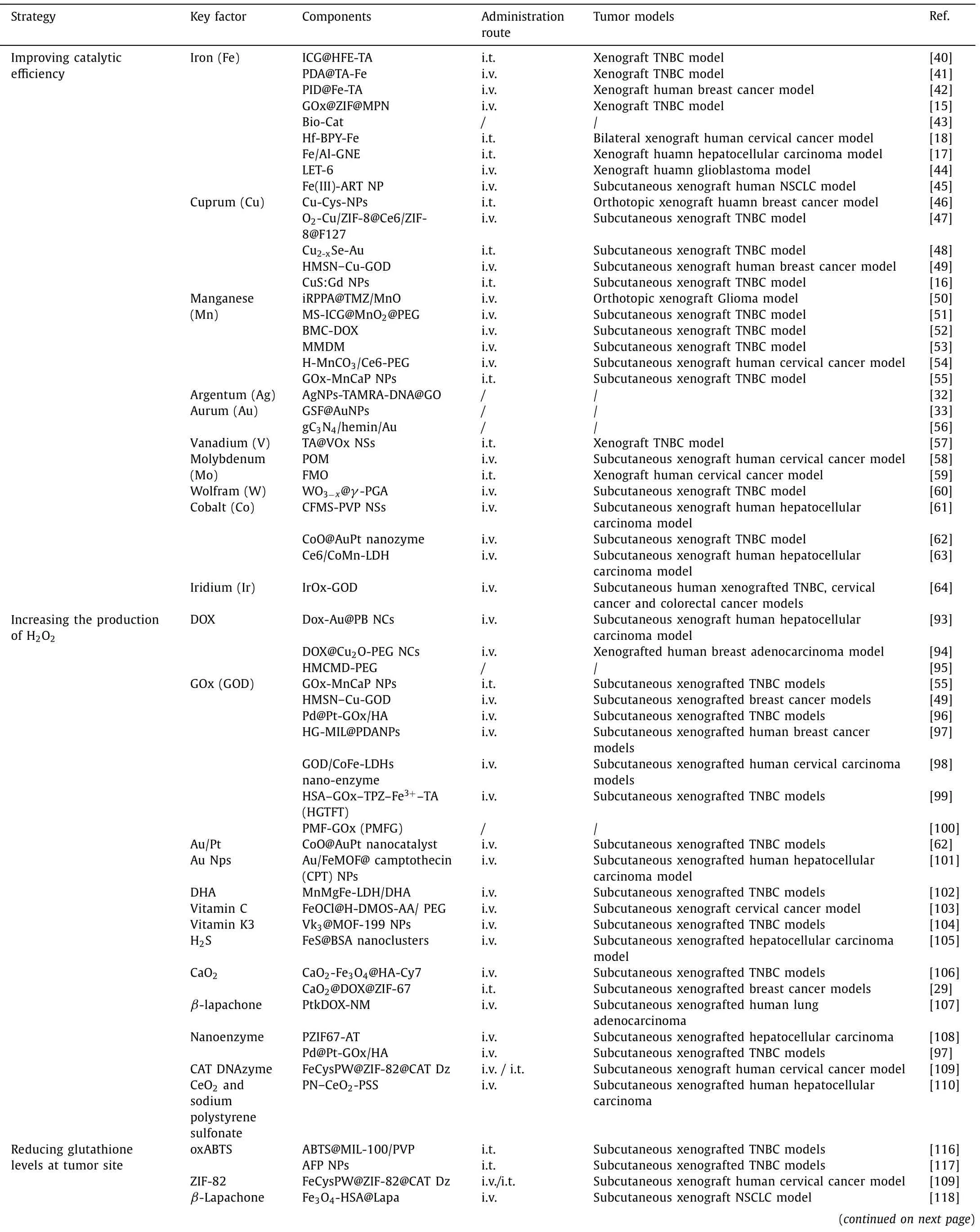
Table 1 Various strategies reported for enhancing ROS based CDT.
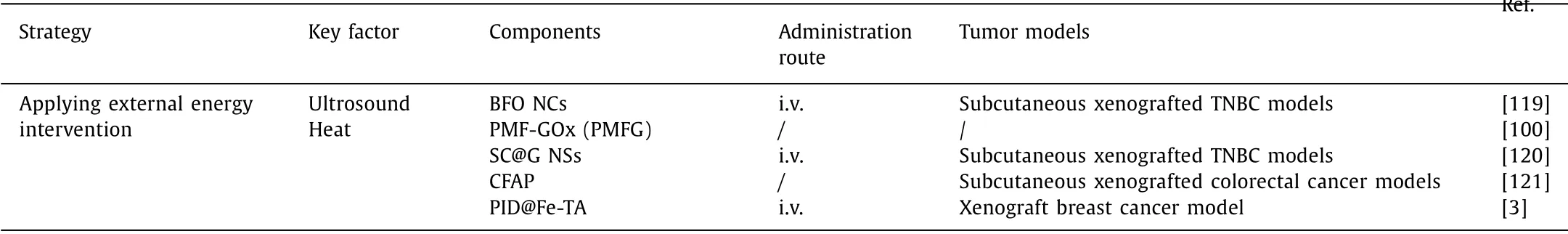
Table 1 (continued)
3.Increasing hydrogen peroxide levels at tumor site
As the source of·OH, it is of significance to accumulate H2O2at tumor site for improving the efficiency of CDT.On the one hand,methods of how to enhance the generation of endogenous H2O2are a good choice.Generated by incomplete oxidative phosphorylation in the mitochondria and non-mitochondrial membrane-bound nicotinamide adenine dinucleotide phosphate oxidase (NOX), O2-·can be catalyzed by superoxide dismutase (SOD) into H2O2.Besides, H2O2can be generated from NOX4 and DUOX1/2, which are family members of NOX.Xanthine oxidase (XO), which can oxidize hypoxanthine to xanthine with the production of H2O2, is another source of H2O2.On the other hand, the endogenous H2O2might be finite, and as a result, delivering exogenous H2O2is another better strategy.In view of those ideas, increasing work has paid attention to raising the level of H2O2.
3.1.Enzymes delivered to tumor site
The generation and degradation of H2O2are controlled by different enzymes, so the level of H2O2in tumor can be regulated by several enzymes.
Glucose oxidase (GOx) is widely described as a potential catalyst, by which glucose can be catalyzed to produce gluconic acid and H2O2with involvement of oxygen.On one hand, H2O2provides abundant source for Fenton reaction.In the meantime, gluconic acid leads to increased acidity in TME, which provides beneficial condition for Fenton reaction.On the other hand, the consumption of glucose causes a lack of energy at tumor site, which is called limotherapy clinically.Designated as GOx-Fe3O4@DMSNs nanocatalysts, Shi’s group successively integrated GOx and synthetic ultrasmall Fe3O4NPs into the large mesopores of dendritic MSN and formed a biodegradable and sequentially functioning nanocatalysts within the tumor tissue, which showed efficient tumor suppressionviaTME-responsive sequential catalytic reactions with high tumor specificity and therapeutic efficacy (Fig.3a) [8].Ge’s group also demonstrated a polymersome nanoreactor, which can be specifically initiated by tumor acidic microenvironment cascade reactions through ultrasensitive pH-responsive membranes permeability and transportation of small molecules [65].GOx catalyzed glucose into gluconic acid and H2O2, which promoted the Fenton reaction of Fe3O4and released a mass of·OH.Whereafter, hydroxyl radicals triggered prodrugs CPT release by cleavage of thioketal linkers.It is an orchestrated cooperative cancer treatment, including starving therapy, CDT, and chemotherapy.The nanoreactor designed with tumor activated specifically cascade reactions represented an insightful paradigm for precise cancer treatment.Many other work [15,66,67] also have focused their attention on GOx, of note, the presence of hypoxic zones in tumor tissue may limit the activation of therapeutic nanoreactors, indicating it is promising to solve the supply of oxygen.
SOD, another important source of H2O2, has been studied recently.Leiet al.synthesized composite PBFS nanoparticles consisting of prussian blue (PB) nanocube core (Fig.3b), which is excellent photothermal agent for the photothermal therapy (PTT) approved by FDA, and fibrous mesoporous silica shell (FS).PBFS is a nanocarrier of Cu, Zn-SOD and GSH-activated ferrocene-based prodrug of disulfanediylbis(ethane-2,1-diyl) bis(ferrocenylcarbamate)(DiFe) [68].PBFS-PSS/DiFe/SOD nanoparticles could not only exert the function of CDT, but also PTT when stimulated with near infrared laser.
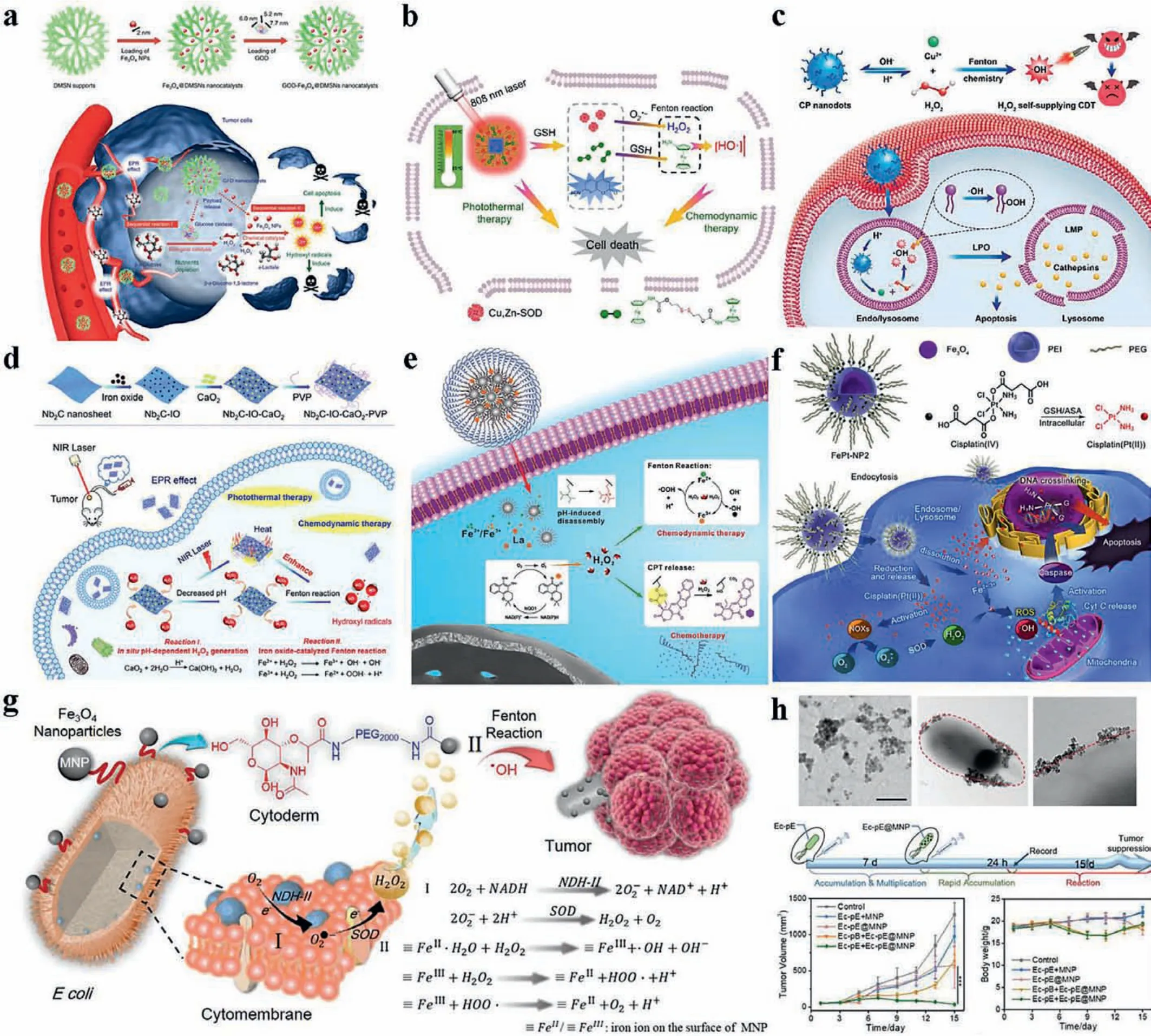
Fig.3.Strategies of raising hydrogen peroxide levels in tumor.Enzymes catalyzing the produce of H2O2 were delivered to tumor.Such as (a) GOx; (Copied with permission[8].Copyright 2017, Nature) and (b) SOD (Copied with permission [68].Copyright 2019, Springer-Verlag).Peroxide delivered to tumor can also achieve the same goal.(c) copper peroxide (Copied with permission [70].Copyright 2019, American Chemical Society) and (d) calcium peroxide (Copied with permission [71].Copyright 2019,Royal Society of Chemistry) were attempted to improve the effect of CDT.ROS-produced-drugs, such as (e) lapachone (Copied with permission [76].Copyright 2019, Wiley Publishing Group) and (f) camptothecin (Reproduced with permission [80].Copyright 2017, American Chemical Society), were also reported to accumulate H2O2 in tumor.(g)Bioengineered bacterium E.coli modified with Fe3O4 nanoparticles (MNP) were reported to generate H2O2 in situ continuously.Reproduced with permission [90].Copyright 2019, Wiley Publishing Group.Micro morphologies and anti-tumor effect were shown in (h).Reproduced with permission [90].Copyright 2019, Wiley Publishing Group.
3.2.Peroxide delivered to tumor site
Although the effectiveness of endogenous H2O2accumulation in tumor can be catalyzed by catalyst like SOD, GOx, and NOX, relying on endogenous substances alone still restricts its development.Encouragingly, peroxide containing a structural peroxy bond (–O–O–)can react with H2O under acidic conditions, liberating abundant amounts of H2O2[69].
Chen’s group first fabricated copper peroxide (CP) nanodot,which depended on poly(vinylpyrrolidone) (PVP) as stabilizer and deprotonation of hydroxyl ion.It was prepared by the reaction of copper(II) chloride (CuCl2), H2O2, and sodium hydroxide (NaOH)in an aqueous solution containing PVP at room temperature for 30 min [70].As shown in Fig.3c, the CP nanodot could simultaneous release Cu2+and H2O2at the acidic conditions of lysosomes once internalized into tumor cells, which induced generation of abundant·OH.The resulting·OH further triggered permeabilization of lysosomal membrane through lipid peroxidation,which caused cell death subsequentlyviaa lysosome-associated pathway.This study provided the first example of Fenton-like reactions triggered by metal peroxide nanoparticle and highlighted the preparation of CP nanodots for self-supplying H2O2, which provided a novel clue for CDT.
Shi’s group [71] synthesized a 2D Nb2C MXene nanosheets based on a simple chemical exfoliation methodology, then in combination with iron oxide (IO) and calcium peroxide (CaO2)in turn, modified with PVP finally (Fig.3d).The Nb2C–IO–CaO2nanoreactors acted as a H2O2self-supplying and PTT enhanced CDT agent, liberated abundant·OH leading to cell apoptosis.At the same year, Wang’s group designed and prepared cisplatin and ICG-encapsulated Fe3O4-embedded SP-PLGA lipopolymersomes (Pt/Fe3O4@SP-PLGA LPs)viaa double-emulsion process [72].The therapeutic element,·OH, was generated by the engineered reaction of succinic acid peroxide (SP) with IO in a near infrared irradiation/tumor acidic pH environment, which provided a general approach to support potent ROS-mediated cancer therapy.
3.3.ROS-produced-drugs delivered to tumor site
As mentioned above, chemotherapy is one of the most common therapies for ROS accumulation within several mechanisms increasing ROS levels.Some chemotherapeutic drugs such asβlapachone (Lap), DOX, CPT and platinum have been reported as ROS promoting drugs.Lap, which can generate H2O2through the catalysis of the nicotinamide adenine dinucleotide (phosphate) quinone oxidoreductase 1 (NQO1), shows great potential for tumor-specific H2O2level amplification [73].Its surprising effectiveness depends on the overexpression of NQO1 in tumor,making it selectively elevate ROS levels and redox cycles in tumor site [74,75].On the basis of the advantages of Lap, several groups focused on designing nanocarriers to deliver it accompanied by other treatments.Chen’s group [76] constructed a pH and H2O2dual-responsive nanomedicine (LaCIONPs), which was encapsulated with iron oxide nanoparticles (IONPs) and Lap, and could accumulate in tumor tissue specifically through the enhanced permeability and retention (EPR) effect (Fig.3e).Once internalized into cancer cell, the nanoparticles would respond the acidic surroundings and H2O2of tumor cells, and release Lap and Fe3+, resulting in an enhanced CDT.The next year, they developed another nanocarrier, in which Lap and ferric ions were loaded, consisting of pH-responsive polymer and an ROS-responsive poly-prodrug [77].After cleavage of the thioketal linker, CDT was initiated, meanwhile, DOX was released as chemotherapeutic drugs.Bothin vitroandin vivoexperiments demonstrated the potent antitumor activity and low systemic toxicity of the nanomedicine.
Cisplatin, with the ability to activate NOX, can efficiently convert O2to O2-·.Subsequently, O2-·is catalyzed by SOD enzyme to form H2O2, causing a striking upsurge of cellular H2O2[78,79].Hence, platinum may be an enhanced agent of CDT and numerous researchers have devoted themselves to the combination of platinum and Fenton reaction agents.Maet al.showed a programmed strategy of delivering a ROS-inducing anticancer drug cisplatin in the form of its cisplatin(IV) prodrugviaIO nanocarriers (Fig.3f) [80].Guided by magnetic-field and monitored by MRI,the nanocarriers could precisely locate at tumor site and preferentially increase the accumulation of Pt and Fe3+.Cisplatin then combined and damaged DNA as a chemotherapy drug, and activated NOX to generate O2-·simultaneously, which was then catalyzed to H2O2.The elevated level of H2O2assisted Fe3+in enhancing Fenton reactions.Yanget al.also reported an activatable semiconducting theranostic nanoparticle platform, containing cisplatin prodrug and ferric ion catalyst for ROS generation [81].Meanwhile, a nanoprobe consisting of inert semiconducting perylenediimide (PDI) and ROS activatable near-infrared dye (IR790s) was used in ratiometric photoacoustic imaging of ROS during tumor treatment.
DOX, a clinically approved anticancer drug that not only blocks topoisomerase II but also generates O2-·to further amplify the performance of CDT through the catalysis of NOX [82,83].Much work [84,85] was finished due to its arrestive mechanisms.CPT is selected as a model mitochondrial drug, which can act as a cellular respiration inhibitor to stimulate endogenous mtROS production and hyperpolarization of mitochondria [86–88], apart from the general inhibition of DNA topoisomerase I for cancer therapy [89].
3.4.Others
Zhang’s group bred bioreactors of Fenton reactions based on Nonpathogenic bacteriumEscherichia coliMG1655 (Ec), which was engineered with respiratory chain enzyme II (NDH-2) overexpression (Ec-pE) and chemically modified with magnetic Fe3O4nanoparticles (MNP) on the surface (Fig.3g) [90].During bacterial respiration, NDH-2 accepted electrons from Nicotinamide adenine dinucleotide (NADH), and then transferred them to oxygen to produce H2O2[91,92].Ec-pE@MNP was obtained for Fenton-like reaction catalysis, which could continuously generate H2O2, and plenty of·OH were produced to induce tumor cell death.The validity of their design for tumor growth inhibition is provedin vivo.(Fig.3h)
Some similar studies were also displayed in Table 1 [29,49,55,62,93-110].
4.Reducing glutathione levels at tumor site
Owing to the higher ROS levels in tumor, elevated reducing equivalents are developed to neutralize and eliminate ROS avoid their damage.In addition to glutathione (GSH), a set of enhanced‘antioxidant’ systems are also expressed in various subcellular compartments for the same function [111].Enzyme is main force in degradation of ROS, such as SOD, peroxidases.SOD has the ability of catalyzing O2-·(approximately 10–320 nanoseconds of halflife) into H2O2, which is less detrimental, but present longer (1 millisecond of half-life) before it undergoes further reduction.Peroxidases are another critical enzyme that catalyzes the degradation and detoxification of ROS, which include peroxiredoxins, catalase, and glutathione/glutathione peroxidase/glutathione reductase system.Peroxiredoxins (Prxs) [112,113], considered to be the forefront and main force for detoxification, is responsible for reducing cellular peroxides by approximately 90%, such as H2O2, organic hydroperoxides (ROOH) and peroxynitrite.Then thioredoxins(Trxs) reduce oxidized peroxiredoxins.As a substitute, thioredoxin reductases, which use NADPH as the source of reducing equivalents, reduce oxidized Trxs [114].Catalase can catalyze the reduction of H2O2to water.The same as the mode of peroxiredoxins,glutathione/glutathione peroxidase/glutathione reductase system is also a cycle reaction.The reaction between reduced GSH and ROS under the catalytic of glutathione peroxidase generates oxidized glutathione (GSSG), which is catalyzed by glutathione reductase into GSH, using NADPH as the source of reducing equivalents.In general, the reduction of ROS relies on NADPH, which comes from pentose phosphate pathway, folate metabolism or isocitrate dehydrogenase 2 (IDH2) and the transhydrogenase system.Therefore,there is no doubt that antioxidant system of tumor impairs the therapeutic effect of CDT due to reduced ROS levels.
Considering the powerful antioxidant capacity of antioxidant system at tumor site compared with normal tissue, in this review,we classify antagonist of antioxidant system as a separate category for more attention.Conceivably, on behalf of antioxidant system,the overexpressed GSH in tumor cells exhibits a direct and potent scavenging effect on the extremely reactive·OH, thereby greatly diminishing the efficacy of CDT and increasing the resistance of tumor cells to oxidative stress, which may be one of the most challenging obstacles for CDT.Hence, Linet al.showed the effectiveness of manganese dioxide (MnO2), which could not only eliminate GSH, but also triggered Fenton-like reactions mediated by Mn2+in the presence of physiological HCO3-, provided a paradigm for the design of metal-based CDT nanoagents with the ability to deplete intracellular GSH [31].Dinget al.reported a MnO2-coated Upconversion nanoparticles (UCNP), on which further anchored by cisplatinum prodrug and poly(ethylene glycol) (PEG), named UCMnPt.MnO2and cisplatinum prodrug (IV) can be reduced by GSH[115].The depletion of GSH, the enhanced generation of H2O2and DNA damage caused by cisplatinum all together presented excellent therapeutic effect.
In addition, Cu2+[27], Fe3+[12] and other materials were also described as oxidants that convert GSH into GSSG, which facilitates the efficiency of CDT (Table 1) [109,116-118].
5.Applying external energy intervention
The involvement of external energy is also a non-ignorable factor that can improve the efficiency of CDT for its facilitating effect on the accumulation of·OH from the perspective of Fenton reactions.Up to the present, light, heat and ultrasonic wave have been widely reported to affect the performance of CDT (Table 1)[3,100,119-121].
5.1.Light enhances CDT
As is reported, irradiating Fe2+and H2O2solution with ultraviolet (UV) or solar light can enhance the overall·OH productionviaphotoreduction of Fe3+to Fe2+and photolysis of H2O2atλ <260 nm, which is called “photo-Fenton” [122].Therefore, loads of·OH are produced in the process of photo-Fenton to enhance CDT Eqs.1 and 2.

Even so, tissue damage, limited penetration, and rapid attenuation in tissues are all impediment of UV light.In direct contrast to UV, near-infrared (NIR) light, NIR II laser (1064 nm) especially, is gradually getting more attentions for its ability of deeper penetration and lower tissue lesion.Thus, UCNPs based on anti-Stokes shift have become the focus of research, which provides an alternative way to transform longer wavelength NIR laser into shorter wavelength light (UV or visible light) [123].Lin’s group [11] fabricated a multifunctional nanoplatform (UCNPsplatinum(IV) (Pt(IV))–ZnFe2O4, denoted as UCPZ), which would show size-switchable character once the nanoplatform cleaved in response to GSH, because of the broken of Pt(IV) prodrugs linking UCNPs and ZnFe2O4.After exposure to near-infrared light (NIR),UCPZ in Fig.4a, served as the UV–vis source, triggered the PDT effect and endowed the energy for ZnFe2O4to produce·OH.They also constructed a nanoplatform (denoted as Y-UCSZ) consisting ofUCNPs, silica shell, and mesoporous ZnFe2O4shell through a facile hydrothermal method.The NIR-triggered and Yb3+/Tm3+codoped UCNPs emitted UV light, which sequently activated ZnFe2O4for the production of1O2and promoted Fenton reactions for the generation of·OH.Moreover, DOX loaded in Y-UCSZ released for chemical therapy at last for better curative effect.In vitroandin vivoassays authenticated the prominent anticancer effectiveness of the DOX loaded PEG/Y-UCSZ, indicating its potential application in the cancer treatment field [124].Huet al.designed a ternary complex (UCSRF) as photochemotherapy agent displayed in Fig.4b, consisting of (1) UCNP cores based on lanthanide-doped nanocrystals for converting NIR light to UV or visible photons to catalyze photo-Fenton reaction; (2) mesoporous silica shell coated on the UCNP core for Fenton reagent (Fe2+) loading and delivery;(3) Ru2+complex co-conjugated on the surface of mesoporous silica shell to bind mitochondrial DNA [125].Therefore, photo-Fenton promoted the generation of·OH and led to the mitochondrial DNAbinding tumor cell apoptosis by minimizing the distance between ROS and mitochondrial DNA molecules, which established a novel photochemotherapy strategy for efficient tumor therapy.
5.2.Heat enhances CDT
Apart from exposing to UV/visible light, raising temperature at tumor site is another feasible alternative to promote Fenton reactions, which might be attributed to the increase of both the collision frequency of molecules on the surface of the catalyst and the fraction of molecules that possesses energy in excess of the activation [126,127].In spite of the efficiency promotion, the disintegration of H2O2under relatively high temperature have to be taken into consider.NIR, known as the stimulus for the photothermal conversion, is mainly in the NIR I window (750–1000 nm),where the absorbance by biological tissues, blood and water is low[128,129].Bu’s group represented a novel TME-mediated nanoplatform employing antiferromanetic pyrite nanocubes (Fig.4c), exploiting the intratumoral, overproduced peroxide for self-enhanced magnetic resonance imaging (MRI) and PTT/CDT [130].However,compared to the low maximum permissible exposure (MPE, for example, 0.33 W/cm2for 808 nm) of NIR I window, the NIR II window (1000–1350 nm) has drawn widespread attention recently due to the deeper penetration of the tissue and higher MPE to the laser(1 W/cm2).Chen’s group [131] synthesized ultrasamll (≤5 nm) PEGylated Cu2-xS nanodots, which could be a novel Fenton agent for nanocatalytic tumor therapy (Fig.4d).Taking unique advantages of copper’s prominent photothermal conversion efficiency at NIR-II biowindow (1000–1350 nm), Cu2-xS nanodots, showing ideal properties of Fenton reagent, could achieve self-enhancement in the production of ROS based on the mechanism of heat enhancing CDT efficiency.Meanwhile, both systematicin vitroandin vivoexperiments have demonstrated the high therapeutic efficacy of Cu2-xSenabled synergistic PTT and CDT.
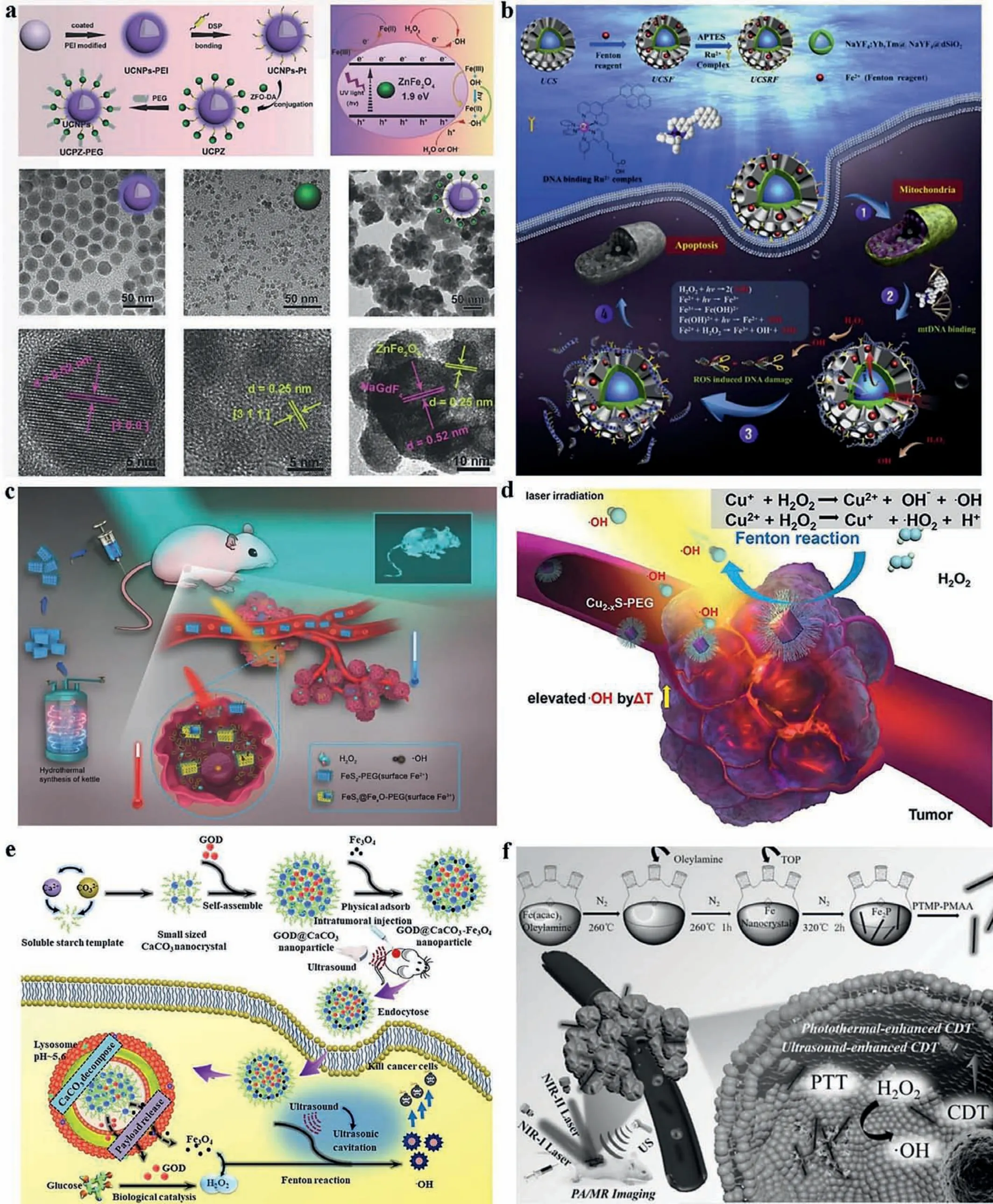
Fig.4.External energy applied to enhance CDT.Light was described to induce photo-Fenton reaction, which was utilized by (a) a multifunctional nanoplatform, upconversion nanoparticle-platinum(IV)–ZnFe2O4 (UCPZ) (Reproduced with permission [11].Copyright 2018, Wiley Publishing Group) and (b) a ternary complex (UCSRF) (Copied with permission [125].Copyright 2017, Elseiver) for production of hydroxyl radical.Heat was reported to enhance CDT as well.Heat facilitated the production of ·OH via photothermal conversion capacity of materials in (c) NIR I window (Copied with permission [130].Copyright 2017, Wiley Publishing Group) or (d) NIR II window (Reproduced with permission [131].Copyright 2019, Elseiver).US was also demonstrated to enhance CDT via sono-Fenton reaction.The production of ·OH induced by (e) GOx@CaCO3-Fe3O4 (Reproduced with permission [136].Copyright 2019, Elseiver) and (f) ferrous phosphide nanorods (FP NRs) (Reproduced with permission [137].Copyright 2019, Wiley Publishing Group) could be enhanced by US.
5.3.Ultrasound enhances CDT
Approved for therapeutic purposes in clinic, ultrasound (US) has become a significant adjuvant therapy for its safety, noninvasiveness, real-time visualization ability.US can promote·OH and H2O2generation Eqs.3-5 through ultrasonic cavitation and ultrasonic dissocation of water and molecular oxygen, which can enhance the efficiency of CDT in a way and thus called “sono-Fenton” [132].Optimizing the ability of “sono-Fenton” reaction to promote the production of reactive oxide species, SDT has been noticed gradually.As an emerging and representative US-based therapeutic modality for cancer noninvasive treatment, SDT utilizes sonosensitizer to produce ROS and features high tissue-penetrating capability, nonionizing property, high controllability and low cost [133].After working, SDT can induce effective tumor cell deathviapathways of apoptosis and/or necrosis [134,135].With high spatial accuracy,SDT can stimulate sonosensitizers and induce selective damage only at the site of the lesion rather than the tissues ultrasound passed.

Chenet al.[136] synthesized mesocrystalline CaCO3particlesviaself-assembly in a mild condition, loaded with GOx, which was delivered into tumor cells to catalyze glucose into H2O2(Fig.4e).Then, stirring them with prepared Fe3O4nanoparticles,they got pH-dependent GOx@CaCO3-Fe3O4particles.After endocytosed by tumor cells, the particles disintegrated and released GOx and Fe3O4in response of acidic environment.Subsequently,GOx catalyzed superoxide anion into H2O2, which provided reactant for iron mediated Fenton reactions leading to the generation of cytotoxic·OH.Meanwhile, assisted by cavitation effect of SDT,loads of·OH were produced to enhance the CDT efficiency.Liuet al.also reported an US-enhanced Fenton agent for CDT.They obtained one-dimensional (1D) ferrous phosphide nanorods (FP NRs)by a high-temperature method, and modified them by trithiolterminated poly(methacrylic acid) (PTMP-PMAA) through a ligand exchange route to improve their hydrophilicity and biocompatibility (Fig.4f) [137].The FP NRs exhibited excellently intensive efficiency of the Fenton reaction, owing to cavitation effect of SDT and high photothermal conversion efficiency, resulted by strong absorption in the NIR II window and high molar extinction coefficient under irradiation with 1064 nm laser.Bothin vitroandin vivoexperiments authenticated its outstanding inhibition of tumor, indicating the FP NRs, based on metallic phosphide, had overcome the low maximum permissible exposure and tissue penetration depth, which initiated a novel path for designing multifunctional integrated nanocarrier.
6.Amplifying the distribution of hydroxyl radicals at tumor site
Limited by low-permeability of nanomedicine at tumor site, it is not enough to cause tumor ablation even with great Fenton reaction outcome [138].Tumor is a complex filled with tumor cells,tumor blood vessels, extracellular matrix, and metabolic waste, all of which contribute to the physical barriers of tumor, lead to high solid and liquid pressures, and poor mobility [139].Therefore, Fenton reactions should be existed extensively in tumor and increasing permeability is of great significance.
Liet al.constructed an ultrasmall gold nanoparticles-loaded,Cu2+-tannic acid complexes deposited hollow mesoporous organosilica nanoparticle, on which 2-(1-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) was hybridized, and then coated with PVP to form HMON-Au-Col@Cu-TA-PVP nanoreactors(Fig.5a) [140].In this system, ultrasmall gold nanoparticles catalyzed glucose into gluconic acid and oxygen like GOx and assisted with the generation of·OH and depletion of GSH of Cu2+-TA complex.Moreover, the generated oxygen provided reactant for HPPH,a photosensitizer, to produce1O2.In addition, collagenase enhanced the penetration of HMONs and O2infiltration for extensive distribution of toxic ROS and effective tumor ablation.Bothin vitro3D tumor spheroids andin vivotumor accumulation showed the necessity of collagenase for deep penetration (Fig.5b).Antitumor experiment showed excellent tumor inhibition of HMON-Au-Col@Cu-TA-PVP compared with HMON-Au@Cu-TA-PVP.As shown in Fig.5c, Zhanget al.reported a novel hypoxia-responsive copper metal-organic framework nanoparticles (Cu-MOF NPs) for CDT and SDT [141].After accumulating at tumor site, large Cu-MOF NPs responded with hypoxia, degraded and released Cu2+and Ce6 for deeper penetration.In this strategy, intratumoral penetration was significantly reinforced andin vivoantitumor assay showed prominent tumor suppression (Fig.5d).
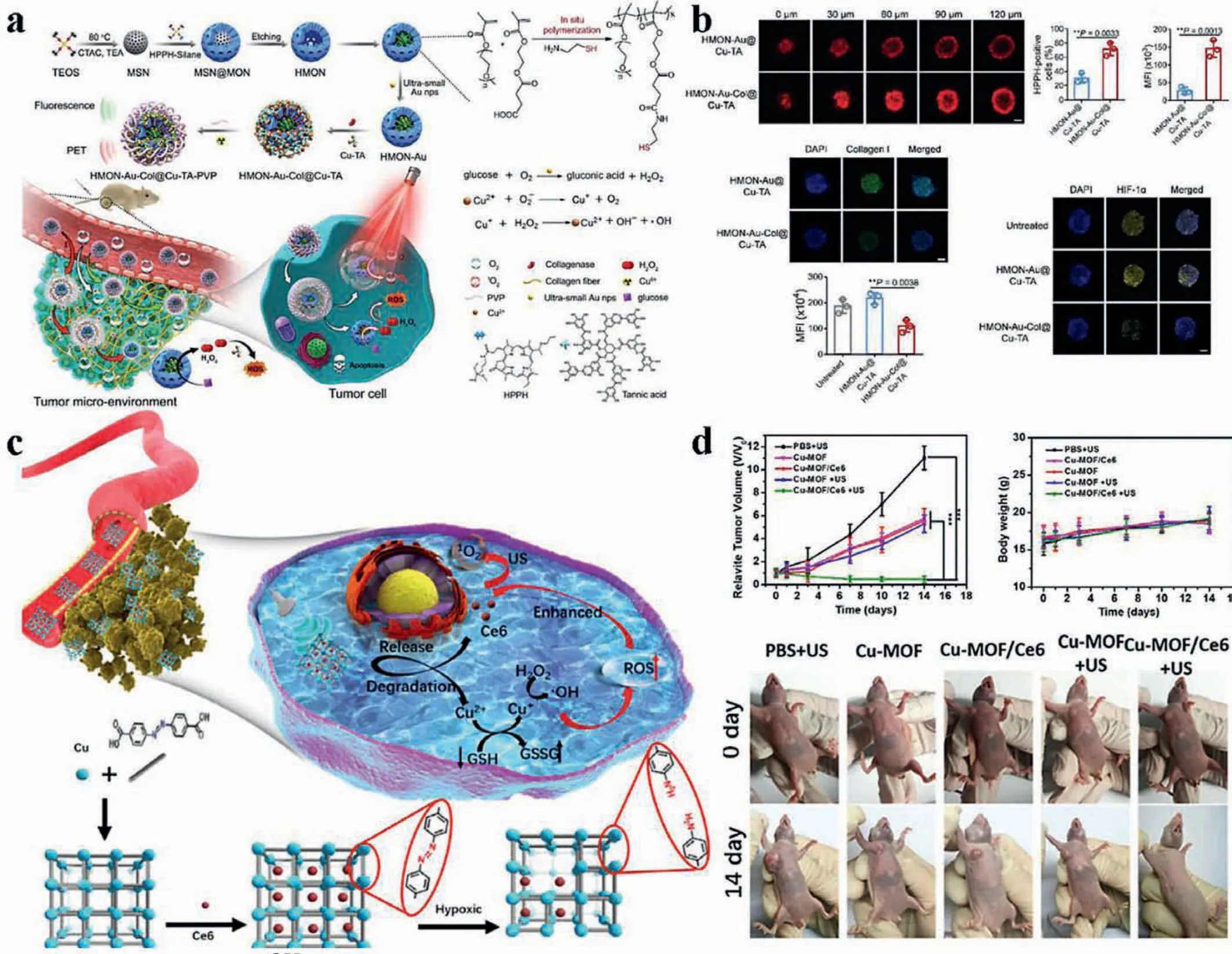
Fig.5.Uniform distribution of ·OH in tumor.(a) HMON-Au-Col@Cu-TA-PVP nanoreactors penetrated deeply in tumor with collagenase for extensive distribution of toxic ROS.Copied with permission [140].Copyright 2020, Wiley Publishing Group.(b) Penetration of the nanoreactors with or without collagenase both in vitro and in vivo.Reproduced with permission [140].Copyright 2020, Wiley Publishing Group.(c) Cu-MOF NPs achieved deep penetration due to its degradation into Cu2+.Copied with permission [141].Copyright 2020, Elsevier.(d) Anti-tumor assay showed the excellent effectiveness of Cu-MOF.Reproduced with permission [141].Copyright 2020, Elsevier.
7.Combination therapy
Owing to a single killing mechanism, monotherapy is gradually unable to achieve ideal effectiveness, because tumor has evolved a series of escape mechanisms and/or resistance mechanisms that lead to the shrinkage of the tumor rather than disappearance or disappearance but then recurrence even metastasis.To date,based on multi-killing mechanisms, combination therapy with diverse strengths has been valued gradually owing to its coordination of multiple therapeutic approaches.CDT always induces apoptosis that can hardly redeploy immune response to cure tumor completely.Therefore, it is of urgent need to combine CDT with other therapeutic approaches such as chemotherapy, PDT, PTT.
7.1.Chemotherapy
Up to now, chemotherapy has become one of the three main clinical approaches to treat tumor, since it was used successfully to treat solid tumors in the 1950s [142,143].Chemotherapeutic drugs can be divided into five categories on the basis of their mechanisms, such as:interfering nucleic acid biosynthesis; affecting the structure and function of DNA directly; interfering transcription and preventing RNA synthesis; interfering protein synthesis and its function and affecting hormone balance.However,with the extensive utilization of chemotherapeutic drugs, there occurs multidrug resistance during chemotherapy, and thus resulting in desensitization of patients who take the same type of chemotherapeutic drugs persistently.The reason why drug resistance events occurs frequently contains that drug efflux, drug activation and inactivation, alterations in drug targets, DNA damage repair, deregulation of apoptosis, autophagy and activation of prosurvival signaling, oncogenic bypass and pathway redundancyetc.[144].On the other hand, severe side-effects are non-ignorable for chemotherapy.Long-term use of chemotherapeutic drugs is associated with significant negative side-effects including nausea, vomiting, hair loss, loss of appetite, fatigue, peripheral neuropathy and anemia [144], which reduced the quality of life for patients.Hence,combining chemotherapy and other therapeutic approaches is a promising trend to improve the effectiveness of cancer treatment.CDT, as a continuous source of cytotoxic ROS, has been widely reported to combine chemotherapy and leads to excellent effect compared with monotherapy.
Lianget al.engineered monodispersed SPIONs, co-loaded with GOx and Mitomycin C (MMC, a hypoxia-activated drug), which were reported as theranostic nanomedicine (SMG), for tumorspecific and MRI-guided synergistic therapy after triggered by a unique sequential reaction in TME (Fig.6a) [145].When delivered to TME, SMG responded to acidic and hypoxic conditions,and released GO, together with MMC, which cooperated with SPIONs for enhanced-CDT and chemotherapy.The elaborately designed synergistic chemotherapy and CDT nanomedicine exhibited a marvelous tumor-inhibition effects towards 4T1 breast tumor xenografts (77.96%), simultaneously with high biocompatibility and therapeutic biosafety.Penget al.fabricated Cu(DDC)2nanoparticlesviaa coordination strategy between Cu(II) and carboxylic groups in poly(ethylene glycol)-b-poly(ester-carbonate) (PEC), which was generated by thein situreaction of disulfiram (DSF) and Cu(II)(Fig.6b) [146].The strategy solved the rapid metabolism of DSF in blood, at the same time, the nanoparticles released Cu(II) that could be reduced by GSH and subsequently induce CDT and disulfiram that could exert chemotherapy together inhibited tumor growth notably, after internalized into cancer cells.Xueet al.prepared a metal-organic framework (MOF) MIL-100viaa microwaveassisted synthesis, which loaded DOX in it and was modified by hyaluronic acid (HA) on the surface [85].It is also a combination of chemotherapy and CDT.
7.2.Photodynamic therapy
Although with similar tumor-killing mechanisms [147], it is a common combination of CDT and PDT, which might because the process to add transition metal ions into nanocarriers of photosensitizer is easy to achieve, and the result has a synergistic effect in treatment.As shown Fig.6c, Liuet al.reported biodegradable cancer cell membrane-coated mesoporous copper/manganese silicate nanospheres (mCMSNs), exhibiting excellent CDT/PDT synergistic therapeutic effects [148].mCMSNs targeted cancer cells owing to the homotypic targeting ability of cancer cell membrane,subsequently, catalyzed endogenous H2O2into oxygen which was then converted into1O2with a 635 nm laser irradiation accompanied by GSH-activated Fenton agents Cu2+and Mn2+, and thus achieving exceptional cancer targeting and therapeutic effects bothin vitroandin vivo.Combining CDT with PDT, Leiet al.[149] tactfully designed a porphyrin-ferrocene theranostic agent based on the reaction of acyl halide and amino group, which could release porphyrin as a photosensitizer and ferrocene as a Fenton agent when responding to acidic environment of TME (Fig.6d).Besides,Ce6 [150,151], TCPP [152] have also been reported to combine with CDT as photosensitizers.Xuet al.developed copper/manganese silicate nanosphere (CMSNs)-coated lanthanide-doped nanoparticles(LDNPs@CMSNs) with ultrabright upconversion (UC) and downconversion (DC) NIR-II emission for trimodal imaging guided PDT/CDT[153].Irradiated with NIR-II laser, CMSNs were activated by UC photons and produced1O2as photosensitizers.Subsequently, Cu+and Mn2+were released after responded to GSH and achieved systematic therapy of PDT and CDT.
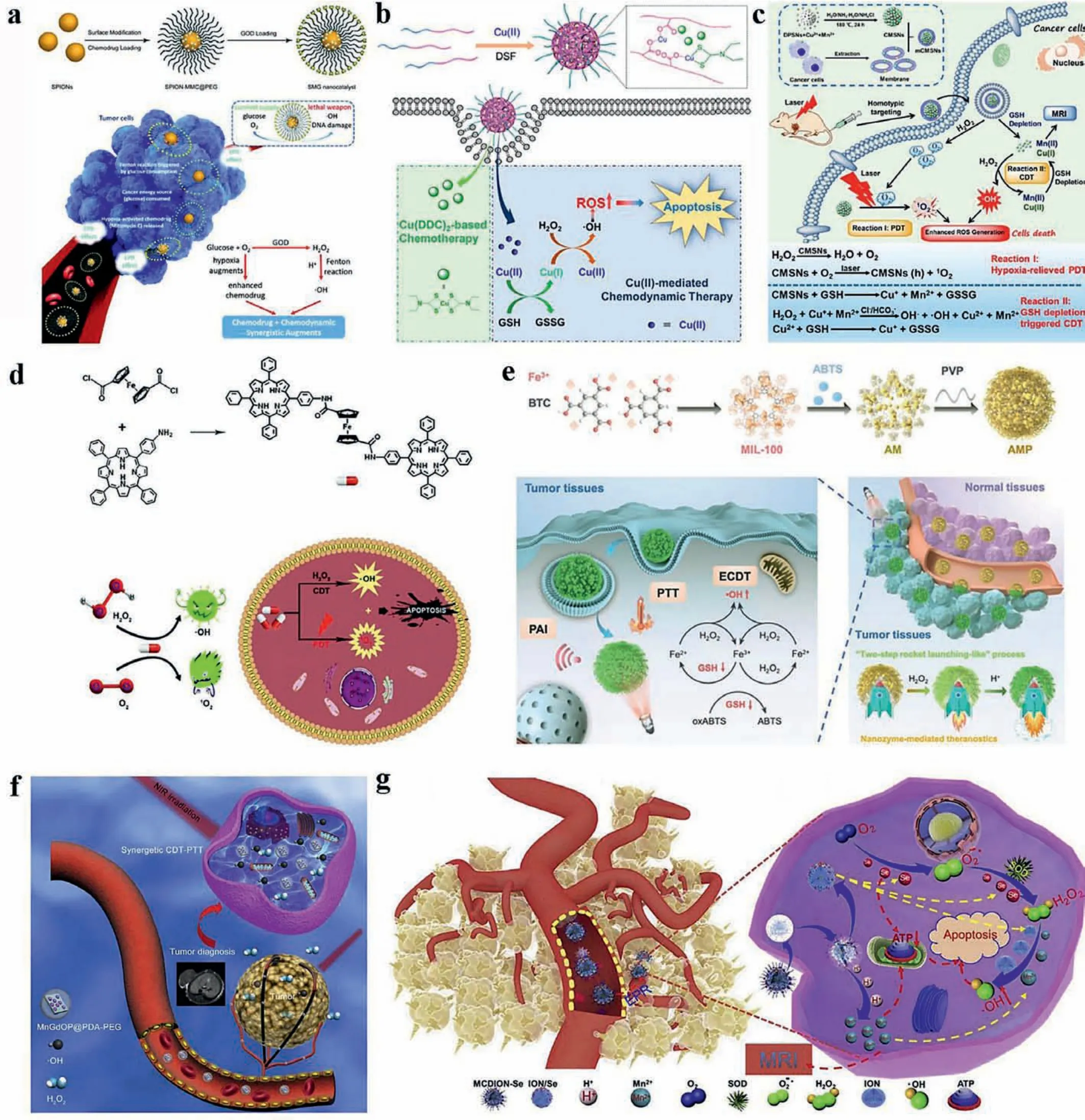
Fig.6.Combination therapy of CDT and chemotherapy, PDT, PTT or starvation therapy.Chemotherapeutic drugs, such as (a) Mitomycin C (Reproduced with permission [145].Copyright 2019, Royal Society of Chemistry) and (b) disulfiram (Reproduced with permission [146].Copyright 2019, American Chemical Society), were combined with Fenton or Fenton-like agents for better efficacy.PDT was also combined with CDT by irradiating the complex of photosensitizers and Fenton agents as shown in (c) (Reproduced with permission [148].Copyright 2019, American Chemical Society) and (d) (Reproduced with permission [149].Copyright 2018, Royal Society of Chemistry).Photothermal agents together with Fenton agents were exposed to laser for combination therapy, including (e) (Reproduced with permission [159].Copyright 2019, Wiley Publishing Group)and (f) (Copied with permission [160].Copyright 2019, Elsevier).(g) A nano-selenium-coated manganese carbonate-deposited iron oxide nanoparticles (MCDION-Se) were designed for ATP inhibition and ·OH production.Reproduced with permission [171].Copyright 2019, Elsevier.
7.3.Photothermal therapy
Exhibiting many advantages such as high specificity, minimal invasiveness and precise spatial-temporal selectivity, photothermal therapy, which can generate heat to burn cancer cells relying on photothermal agents triggered by NIR, have become a burgeoning therapeutic strategy currently [154–157].However, there exist some restrictions that hamper its further development.Limited depth of light penetration induced by tissue absorption and light scattering, is the fundamental flaw of PTT, which supports the treatment of superficial solid tumor rather than deep tumor[158].Additionally, the accurate delivery of photothermal agents towards tumor site is difficult, which usually results in nonignorable side-effects or heat damage to normal tissues around tumor.Of note, overexpression of heat shock protein (Hsp) in tumor cells contributes to their resistance to PTT.Therefore, measures should be taken to further improve its efficacy.CDT, with different tumor-killing mechanisms, is accepted to synergize PTT.Chen’s group reported a 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) loaded ABTS@MIL-100/poly(vinylpyrrolidine)(AMP) nanoreactors (NRs) theranostic nanoreactor (Fig.6e), which could be activated by TME to turn on its PTT function, simultaneously produced·OH in response to the high H2O2level and disrupted intracellular GSH that endowed the AMP NRs with enhanced chemodynamic therapy [159].The abundant features endowed AMP NRs potential therapeutic value with a combination of CDT and PTT.Zhaoet al.[160] engineered a nanoplate(MnGdOP@PDA-PEG) consisting of polydopamine (PDA) coat shell,which showed high photothermal conversion efficiency, and Mn doped Gd2O3, which exhibited effective generation of·OH for heat enhanced CDT (Fig.6f).Their works both provide novel ideas for constructing integrated nanocarriers with the function of CDT and PDT.Zhanget al.reported a novel 2D nanosheet based on facile liquid-exfoliated FePS3, which exhibited striking photothermal conversion efficiency of up to 43.3% [161].In addition, large specific surface area of Fenton agents allowed it remarkably catalytic efficiency.Powerful efficiency of operations eradicated HeLa tumor without relapsein vivo.Several other work [162–164] also focused on the combination of CDT and PTT, which may be a potential strategy to tackle tumor growth.
7.4.Starvation therapy
Starvation therapy, an emerging tumor therapeutic approach,can inhibit tumor growth, retard tumor progression and elongate the life span of patientsviareducing blood supply to the tumor site, depriving or consuming its nutrients and/or oxygen, all of which are of great importance during the progression of tumor because the faster transportation and metabolism rely on nutrients for tumor growth and metastasis [165–169].Nevertheless, agents inducing starvation therapy, such as angiogenesis inhibiting agents,vascular disrupting agents and transarterial chemoembolization,are limited by several disadvantages like low targeting efficiency,elevated tumor hypoxia, induced drug resistance and increased tumor metastasis risk, which all are in the way of further application of starvation therapy agents in clinic [170].As a result, it is urgent and significant to combine starvation therapy with other therapeutic approaches.CDT, a potential treatment strategy, is considered to synergize starvation therapy by the consumption of nutriment and the accumulation of cytotoxic ROS simultaneously.
Accompanied by the consumption of glucose and oxygen, GOx,can generate H2O2that is the reactant of Fenton agents and gluconic acid that can promote Fenton reaction rate due to the enhanced acidity.Plenty of work based on GOx has been described in section II.Xiaoet al.designed a nano-selenium-coated manganese carbonate-deposited iron oxide nanoparticles (MCDION-Se)to achieve the combination of CDT and starvation therapy (Fig.6g)[171].They tactfully took advantages of nano-selenium (nano-Se),an inhibitor of ATP, which could convert oxygen into O2-·, activate SOD to catalyze accumulated O2-·into H2O2in vivo, and thus providing source of the generation of·OH mediated by Mn2+ions,meanwhile, inhibit the generation of adenosine triphosphate (ATP)and thus starving cancer cells.What is more, the released iron oxide nanoparticles (IONs) could also mediate the improvement of·OHviaFenton reaction.Overall, this work provided a nanoplatform that combined CDT with starvation therapy for cancer therapy.
7.5.Sonodynamic therapy
SDT, based on US, a non-invasive, deep penetrating mechanical wave, and sonosensitizers, which can generate ROS under the stimulation of US and the formation cavitation, is suitable for deep-seated tumors.However, potent sonosensitizers with high sensitivity, safety and penetration still remains a huge challenge.Hence, Linet al.designed US and GSH dual responsive vesicles of Janus Au-MnO nanoparticles (JNPs) coated with PEG and a ROS-sensitive polymer (Fig.7a) [172].The JNPs disintegrated into smaller Au-MnO nanoparticles under the stimulation of US, which improved the penetration of nanoparticles.Subsequently, in response of abundant GSH at tumor site, MnO was transferred into Mn2+, which can generate ROSviaFenton reaction.Otherwise, SDT could also directly enhance the generation of ROS due to the stimulation of sonosensitizers and increased cavitation.This strategy exhibits hopeful application in enhancing tumor-specific synergistic therapyviacombining CDT with SDT.Baiet al.synthesized Fe-doped, PEGylated TiO2nanodots (Fe-TiO2-PEG NDs) with good physiological stability and biocompatibility (Fig.7b) [173].The nanodots showed enhanced production capacity of1O2because of the reducing band gap of TiO2for the existence of Fe.Furthermore,·OH induced by iron further enabled synergy of CDT and SDT.Upon i.v., the nanodots showed efficient tumor accumulation and excellent antitumor therapeutic effectviathe synergistic effect of SDT and CDT.
7.6.Gas therapy
Nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S), known as three endogenous gaseous transmitters with versatility, shows bimodal pharmacological character in cancer, in that not only the inhibition of their biosynthesis but also elevation of their concentration beyond a certain threshold can exert anticancer effects [174].NO mediated cell death involves the inhibition of mitochondrial activity, DNA damage and activation of downstream pathways such as the p53 and caspaseactivation pathways, culminating in tumor cell lysis [175,176].In spite of quantities of research have paid attention to delivering NO donor into tumor site, there are still some limitations in precise targeting and location-controlled release of NO.Huet al.[177] designed a nanoscale coordination polymer, which was formed by a NO donor (1,5-bis[(L-proline-1-yl)diazen-1-ium-1,2-diol-O2-yl]-2,4-dinitrobenzene, BPDB) sensitive to GSH coordinating with iron ions (Fig.7c).Upon responding to GSH, the obtained Fe(II)-BNCP could release NO and Fe2+rapidly, both of which worked together for cell death induced by NO,·OH and peroxynitrite anion (ONOO-).Meanwhile, the nanosystem showed desirable solubility, circulation stability and biocompatibility.Sunet al.demonstrated Cu(II)-flavone coordination polymer (NCu-FleCP)prodrug mediated CO and CDT (Fig.7d) [178].After ingested by tumor cells, it could release Fle and Cu+accompanied with GSH depletion.Subsequently, Fle released CO upon NIR light illumination and Cu+produced toxic·OH for tumor elimination.Utilizing bovine serum albumin (BSA) as a template, Heet al.synthesized metastableγ-phase MnS for pH-responsive T1-weighted MRI guided integration of H2S and CDT (Fig.7e) [179].In acidic TME,MnS@BSA could be disintegrated into Mn2+and H2S gas.The former could exert its role as Fenton agents and contrast agent for T1-weighted MRI, and the latter could be used as gas therapy.
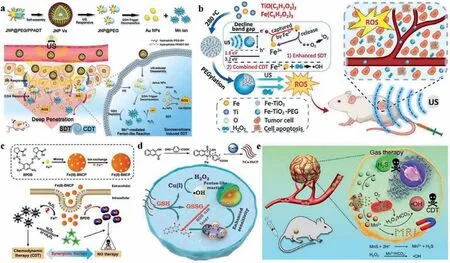
Fig.7.Combination therapy of CDT and SDT or gas therapy.Sonosensitizers, including (a) Au-MnO NPs (Reproduced with permission [172].Copyright 2019, Wiley Publishing Group) and (b) TiO2 (Copied with permission [173].Copyright 2020, American Chemical Society), were used to induce SDT for enhancing CDT.Gas therapy containing (c) NO(Reproduced with permission [177].Copyright 2019, American Chemical Society), (d) CO (Reproduced with permission [178].Copyright 2020, Wiley Publishing Group) and(e) H2S (Copied with permission [179].Copyright 2020, Ivyspring) was combined with CDT.
7.7.Multimodality therapy
Single treatment can’t kill tumor cells thoroughly, which can only inhibit tumor growth in a way, and often leads to recurrence with time.On the contrary, referring to different mechanisms, multimodality therapy is a novel trend in tumor therapeutic approach recently due to rapid and various gene mutability of tumor during infinite proliferation, damage resistance and repair mechanisms,and metastasis.
Wanet al.[180] reported a nano-MOF (NMOF) constructed by Fe-TCPP with a CaCO3mineralized coating, which could trigger collapse of nanoplatform specifically at tumor site dependent on the weakly acidic microenvironment and high concentration of GSH, release dihydroartemisinin (DHA) and thus activating TCPP(Fig.8a).Briefly, upon arriving in tumor, CaCO3coating of the prepared nanoplatform was dissolved in weakly acidic microenvironment and generated Ca2+.Subsequently, NMOF with DHA cargo was exposed and internalized by tumor cells.Responding to GSH,NMOF dissociated into Fe3+and activated TCPP.Then, Fe3+was reduced into Fe2+for CDT which also cooperated with DHA for Fe2+-activated, DHA-mediated apoptosis.Additionally, Ca2+could assist the anticancer effect of DHA because DHA could influence the cell membrane as well the Ca2+pump ATPase, and led to an increased cytosolic Ca2+level, which activated calpains, especially calpain-2, to induce oncosis-like cell death [181–184].Meanwhile,TCPP could mediate PDT as a photosensitizer.All in all, this work displayed triply synergistic effect (CDT, OT and PDT) and exhibited joyous surprise in therapeutic efficiency for ablating tumor completely bothin vitroandin vivo.
Considering the limitation of PDT like oxygen reliance, inherent flaws of traditional photosensitizers and harsh Fenton reaction conditions of CDT, it is of great need to design a novel nanosystem with powerful therapeutic effect on the basis of ROS.Herein,Liuet al.reported biocompatible copper ferrite nanospheres (CFNs)with enhanced ROS production under irradiation with a 650 nm laser through direct electron transfer and photo-enhanced Fenton reaction and high photothermal conversion efficiency upon exposure to 808 nm laser, exhibiting a considerable improved synergistic treatment effect (Fig.8b) [185].Taking BSA as surfactant,they synthesized CFNs by one-step hydrothermal method, which contained two redox pairs (Fe2+/Fe3+and Cu+/Cu2+) with abilities of converting H2O2into·OH and GSH depletion.Simultaneously,CFNs could also catalyze the generation of oxygen through H2O2,which improved the requirement of PDT in a way.What is worth mentioning is that the high photothermal conversion efficiency of CFNs under irradiation of 808 nm laser, it provided CFNs excellent PTT effect.In a word, such an “all-in-one” nanosphere combining CDT, PDT and PTT developed a new idea for effective tumor eradication.As shown in Fig.8c, Zhaoet al.took different measures to improve the efficiency of CDT and PDT, they tactfully fabricated ROS-activatable liposomes (RALP) that integrated hexadecyloxaliplatin carboxylic acid (HOC) and Fe3O4, which create a positive feedback loop among the key players of PDT/CDT to simultaneously address their therapeutic challenges [186].Once delivered to tumor site, RALP@HOC@ Fe3O4would be cleaved because of the thioketal bond, which was ROS-liable to shed PEG corona.HOC, a prodrug, could be reduced into oxaliplatinviathe depletion of GSH and exert cytotoxic effects.Fe3O4was regarded as Fenton agent with enhanced efficiency for reduced GSH.
Metastasis is the uppermost reason for death of patients.However, there are very few studies put their efforts in metastasis when referring to CDT alone.Fortunately, Lin‘s group constructed a multifunctional cascade bioreactor on the basis of hollow mesoporous Cu2MoS4(CMS) with GOx cargo (Fig.8d).This cascade bioreactor exhibited multiple interrelated functions, for example, Fenton-like, glutathione peroxidase-like and catalase-like activity.When internalized into tumor cells, CMS worked in different mechanisms.CMS promoted·OH due to Cu+and Mo4+,which could be enhanced by the generation of gluconic acid and H2O2through GOx and the depletion of GSH mediated by Cu2+and Mo6+.Besides, CMS catalyzed the catabolism of H2O2to produce oxygen, which acted as a positive feedback amplifier for the function of GOx and replenished the oxygen required by PDT.Under irradiation of 1064 nm laser, CMS showed remarkable tumorkilling ability induced by PTT because of its excellent photothermal conversion efficiency.Combined with checkpoint blockade therapy(anti-CTLA-4),in vivoexperiments authenticated CMS@anti-CTLA-4 elicited robust immune responses by ablating primary tumors effectively and inhibiting cancer metastasis.There is no doubt that it was the first time to construct such a multifunctional cascade bioreactor based on hollow mesoporous CMS loaded with GOx for synergetic cancer therapy by CDT, PTT, starvation therapyand immunotherapy [187].
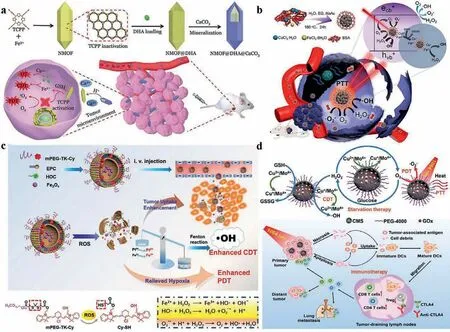
Fig.8.Schematic illustration of multimodality therapy.(a) CDT was combined with PDT and oncosis therapy.Copied with permission [180].Copyright 2019, Wiley Publishing Group.(b) CDT was combined with PDT and PTT.Copied with permission [185].Copyright 2018, American Chemical Society.(c) CDT was combined with PDT and chemotherapy.Reproduced with permission [186].Copyright 2019, Wiley Publishing Group.(d) CDT was combined with PTT, starvation therapy and immunotherapy.Reproduced with permission [187].Copyright 2019, Wiley Publishing Group.
Great efforts have been made to combine CDT with other therapeutic approaches [19,66,188-190].We of course believe, because of the combination therapy, we can take an important step forward in the treatment of cancer.
8.Conclusion and overlook
Recently, an updated report has showed that the death rate from cancer in the United States continued to decline, with an overall reduction of 31% during 1991 to 2018, which meant more than 3.2 million patients escaped from the clutches of death [191].The past decade (2008–2018) has witnessed overall cancer death rates fall by an average of 1.5% a year contributed by great advances of therapeutic approaches like targeted therapy and immunotherapy.However, there are still some defects in the mainstream clinical treatment.For example, aggressive surgery can only resect primary tumor in a way, but it shrivels when referring to metastasis.Radiotherapy, taking effect on metastasis due to the defined “abscopal effect”, is also limited by the relatively poor accuracy external radiation treatment and the robust, non-ignorable inflammatory response around the niduses.The utilization efficiency of cytotoxic drugs, a deficiency of chemotherapy owing to drug resistance and lack of targeting, often results in tumor recurrence and severe side effects.Furthermore, thermal therapy needs to take the expression of heat shock protein in cancer cells into consideration for the evocable thermal tolerance.PDT must take measures to solving the problem of its oxygen reliance and hypoxic nature of solid tumors.Immunotherapy, showing great potential to treat cancer, is related to tumor type and physical conditions of individuals.Thus, enormous challenges have to be overcome in exploring novel,more effective therapeutic approaches for cancer.CDT, an emerging treatment, which takes advantages of the properties of tumor microenvironment, exhibits satisfactory selectivity and specificity,possesses powerful cytotoxic effects on tumor cells, and saves cost without unique apparatus or method of administration.Therefore,CDT has a promising light as an adjuvant therapy.
Nevertheless, strict reaction conditions of classic Fenton reaction limit its development.Of note, CDT is different from Fenton reaction to some extent, because its ultimate goal is causing the death of tumor cellsin vivorather than just increasing outcome of Fenton reactions.In other words, we should not only consider how to improve the outcome of Fenton reactions, but also allow for the lethality of·OH to tumor cells.In addition, harsh Fenton reaction conditions are difficult to completely achieved in living body for the sake of security.To maximize the performance of Fenton reactions without exacerbating security issues, this review has summarized several feasible strategies for more ideal therapeutic effect,such as (1) improving catalytic efficiency; (2) increasing hydrogen peroxide levels at tumor site; (3) reducing glutathione levels at tumor site; (4) applying external energy intervention; (5) amplifying the distribution of hydroxyl radicals at tumor site; and (6) combination therapy.All these methods have been discussed deeply in this review based on the improvement of CDT, behind which we further focused on the mechanisms that might be of guiding significance to future research.It may be an advisable choice to combine the above strategies flexibly for their own unique advantages and disadvantages.For example, strategy 1 focused on the catalytic effi-ciency of catalyst, which can only accelerate the production of·OH but cannot determine its ultimate concentration.However, strategy 2 and 3, with the vision of broaden sources of income and reduce expenditure, are committed to improving the accumulation of aggregate rather than production rate of·OH, which may be detrimental to anti-tumor effect for the imbalance of limited killing rate and rapid multiplication.Strategy 4 integrates the focus of previous strategies, which can both accelerate and accumulate the production of·OH, but with unattractive efficacy in both ways.Strategy 5 shifts the emphasis to the Achilles’heel of·OH, which is extremely reactive and untransferable.Therefore, amplifying the distribution of hydroxyl radicals at tumor site is dominant.Monotherapy often leads to drug tolerance of tumor cells due to the intrinsic heterogeneity.Strategy 6 can get rid of those troubles by combining CDT with other treatments.To sum up, integrate the above strategies may improve the efficacy of CDT significantly.Although tremendous efforts have been made to improve the efficiency of CDT, there are still some key points waiting to be focused on in the following section.
Firstly, targeting to tumor site accurately is an essential problem, because Fenton agents can also cause damage to normal cells due to ROS generation including H2O2during cellular respiration in spite of harsh conditions.Hence, designing nanosystems with specific and accurate targeting to tumor site must be drawn urgent attention.Of note, integration system of imaging and treatment may provide dynamic trace display of CDT inducer and guide rational design and medication.In addition, penetration is another significant problem.The Fenton or Fenton-like reaction based CDT relies on the generation of·OH, which are extremely reactive and almost non-diffusing after its formation.Therefore, nanocarriers modified with penetrating peptides or owning TME responsive size reduction function for deep penetration can be fabricated to widely deliver Fenton agents, or·OH, to tumor site.Furthermore, transmembrane ability of nanoplatform with Fenton agents also affects the effect of CDT.It is because both the higher concentration of intracellular H2O2and more acidic environments can accelerate Fenton reaction.In lieu of trans-membrane, it may be a wonderful choice to induce tumor cell lysis by other treatments and thus resulting in the release of intracellular H2O2and hydrion for locally cascade amplified CDT, although the generation of·OH in cytoplasm leads to more powerful cytotoxicity than in TME.At last, risk must be taken into consideration when looking for ways to improve the efficacy of CDT.For example, the dosage of transition metal ions-based Fenton agents should be taken into account cautiously.As is reported, the intake of ferrous ion in cancer cells is proved to be increased dramatically compared to normal cells, which is crucial to many fundamental cellular processes, including DNA synthesis, proliferation, cell cycle regulation and the function of proteins containing iron-sulfur clusters [192].One real concern, however, is whether high dosage intake of dietary iron has an auxo-action on tumorigenesis of normal tissues because high intake of dietary iron is associated with an increased risk for some cancers.Potential damage of heavy metal ions also should be taken into consideration.In broad terms, concepts of CDT should be broadened to encourage other ROS based non-metal ions agents.
Although tumor growth is suppressed, no treatment basically can cure it for the poor outcomes of monotherapy.Similarly, CDT alone is difficult to cure a tumor completely because of the high tolerance and insensitivity of cancer stem cells to ROS, which has been widely improved to be linked to drug resistance, recurrence and metastasis [193].Hence, this review has also summarized combination therapy of CDT and other treatments up to now.It may be an excellent choice to combine CDT with immunotherapy aided by immunogenic death-induced therapeutic approaches,which is worth exploring further to prevent tumor metastasis and recurrence.
In summary, as a flourishing research frontier, there is still plenty of room for improvement worthy of exploring.The future tasks can pay attention to improving Fenton reaction efficiency, simultaneously combining with other therapies to produce a synergistic effect.For clinical use, theranostics nanoplatform should be designed for tracing the moving trajectory of Fenton agentsin vivoand medication guide.In addition, pharmacokinetics and adverse reaction of transition metal ionsin vivoalso needs to be understood clearly.All in all, as a promising and booming new therapeutic strategy, difficulties are together with opportunities, while hope and challenges are coexistent.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.81822025) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University(No.ZYYC08002).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
- Encapsulation strategies on 2D materials for field effect transistors and photodetectors
