Recent developments of droplets-based microfluidics for bacterial analysis
Ruizhi Ning, Jinhi Fn, Ling Kong, Xue Jing, Yun Qin, To Du,Gungjin Zhng,*, Weiwei Wu
a Interdisciplinary Research Center of Smart Sensors, Academy of Advanced Interdisciplinary Research, Xidian University, Xi’an 710071, China
b The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China
c State Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases and Shaanxi Clinical Research Center for Oral Diseases,Department of Oral and Maxillofacial Surgery, School of Stomatology, Air Force Medical University, Xi’an 710032, China
d Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Clinical Research Center for Oral Diseases of Zhejiang Province, Key
Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Hangzhou 310006, China
Keywords:Droplet microfluidics Droplet generation Droplet manipulation High throughput Bacteria analysis
ABSTRACT Droplet-based microfluidics enables the generation of uniform microdroplets at picoliter or nanoliter scale with high frequency (~kHz) under precise control.The droplets can function as bioreactors for versatile chemical/biological study and analysis.Taking advantage of the discrete compartment with a confined volume, (1) isolation and manipulation of a single cell, (2) improvement of in-droplet effective concentrations, (3) elimination of heterogeneous population effects, (4) diminution of contamination risks can be achieved, making it a powerful tool for rapid, sensitive, and high-throughput detection and analysis of bacteria, even for rare or unculturable strains in conventional methods.This mini-review will focus on the generation and manipulation of micro-droplets and bacteria detection and analysis carried out by droplet-based microfluidics.Finally, applications with high potential of droplet-based bacteria analysis are briefly introduced.Due to the advantages of rapid, sensitive, high throughput, and compatibility with rare and unculturable bacteria in conventional methods, droplet-based microfluidics has tremendous potential of providing novel solutions for biological medicine, microbiological engineering, environmental ecology,etc.
1.Introduction
The biological world we live in is a symbiotic system that includes plants, animals, and microorganisms.All of them are indispensable in the circulating of substances and life.Broadly, in the ecological cycle, the plants act as the producer, animals the consumer, and microorganisms the decomposer.The group, microorganisms hardly seen by naked eyes, are widely involved in diverse fields.As a crucial member of microorganism, bacteria are widespread in soil and water or live in symbiosis with other organisms.Determination of the species, composition, the number of bacteria, and the chemical or biological molecules produced by bacteria are significantly crucial for the food industry, medicine,agriculture, and ecology.Due to their microscale dimension and compositional complexity in a native environment, bacteria investigation commonly requires primary culture, isolation, and pure culture for subsequent analysis [1].Conventional procedure from primary culture to analysis carried out on solid/liquid media involves prolonged time and sophisticated operation by laboratory technicians, and information loss of unculturable or heterogenous bacteria with a small population is inevitable [2,3].Therefore, a highly effective, sensitive, reliable, and easy handling bacteria detection and analysis platform is demanded.
Since the emergence of the initial microfluidic device in the 1980s [4], manipulation and analysis of chemical and biological molecules at microscale were achieved based on programmable channels and chambers with the lowest resolution in micrometer.Furthermore, dimensional consistency between cells (including bacteria cells) and microchambers makes microfluidic chips particularly suitable for cell research in a state of constant flow.Besides, microanalysis on the devices requires minor regent and analyte volume, leading to microfluidics a powerful tool in investigating cells and molecules at a low amount, even for a single cell.On the account of the above characters, this multidisciplinary technology exhibited tremendous potential and feasibility in biological and medical fields, such as microenvironment simulation [5], cellto-cell interaction [6], organ-on-a-chip [7], drug evaluation [8].
Droplet microfluidics is a subcategory of microfluidics, focusing on creating monodispersed droplets with precious dimensional control and improved frequency.Each generated compartment could function as an independent bioreactor.Encapsulation,isolation, incubation, and analysis of small groups of microorganisms, even for a single microbe, have been successfully carried out in droplets of picoliter or nanoliter with high throughout [9,10].Meanwhile, the confining volume and discrete feature of droplet microfluidics leads to superiorities in easy achieving of effective concentration to measurable levels of analytes and avoiding cross contamination between microreactors.Therefore, droplet microfluidics is significantly suitable for rapid [11], sensitive [12], heterogenous analysis of bacteria [13], pathogenic bacteria detection [14],and antibiotic susceptibility assay [15].Although several review articles have been addressed [16-18], a timely review of droplet microfluidics for bacteria analysis with the latest developments,which covers droplet generation, droplet and in-droplet particle manipulation, bacteria analysis, and applications, is still absent and needed.
This mini-review article briefly and timely reviews the developments of droplet microfluidics in bacteria analysis from several aspects:(1) droplet generation, including generation of monodispersed droplets, droplet arrays, and droplet components with interfaces constituted by diverse materials or in-droplet compartments.(2) Manipulation of droplets and in-droplet particles.(3)Bacteria analysis achieved by droplet-based microfluidics such as counting, detection, high throughput screening, and cell-to-cell interaction investigation.(4) Representative applications of bacteria analysis based on droplet microfluidics in microbiology, biological medicine, and environmental monitoring.Summaries, discussions,and critical thinking are finished from a bird eye’s sight, which might be valuable for new researchers in the field of microfluidics.
2.Droplet
The generation of monodisperse droplets under precious control plays a fundamental and critical role in the droplet-based analysis.Depending on characteristic differences of analysis methods or analytes, droplets generation needs to have high throughput, conduct in-site droplet formation/reaction, controllable droplet composition, or suitable droplet interfaces.This part will introduce methods for droplet generation under precious control of frequency, location, composition, and different interfaces.
2.1.Droplet generation
2.1.1.Based on nozzle architecture
Droplet generation based on microfluidic geometries is a passive, simple, easy-to-manipulate, and cost-effective approach to create droplets with high throughput.Different from microfluidic geometries of microwell/microchamber that form and trap droplets in-site, droplet production based on designed nozzle architectures,such as T-junction, flow focusing, coflowing andDropChopgeometry have the superiority in the generation of abundant homogenous droplets with high frequency for high throughput screening,utilizing the principle of hydrodynamics [19].
T-junction, flow focusing, and coflowing are widely studied and used in droplet microfluidics [20-22], since Thorsen originally adopted T-junction to generate droplets in 2001 [23].As shown in Fig.1a, T-junction geometry has two inlet channels joining perpendicularly, and the dispersed phase is sheared off into droplets by a flow of continuous phase at the junction.Flow focusing geometry consists of three inlet channels (one main channel and two symmetric side channels).The dispersed phase in the main channel is segmented by a continuous phase in the two symmetric side channels.Compared with T-junction, flow focusing geometry counteracts force influences other than the driving force along the main channel due to the symmetrical structures of side channels,therefore lead to more stable droplets; The coflowing geometry is composed of two co-axial channels, with dispersed phase flow in the inner channel and continuous phase in the outer channel.In this structure, droplets are pinched off from a continuous phase when surface tension force counteracts with drag force.However,the strong viscous shear force of the continuous phase easily leads to the problem of polydisperse droplet sizes [24].At present, flow focusing is the most common configuration for droplet generation with high throughput.Flow focusing nozzles can generate monodisperse droplets with picoliter and nanoliter level at a frequency of kHz, and frequency and droplet size can be regulated and controlled by altering the flow rate, channel size, and fluidic viscosity [25,26].Besides, multiple parallel droplet generation nozzles can be further integrated into one device to improve the frequency.For instance, Zeng developed a microfabricated emulsion generator array device containing multiple (up to 96) flow focusing channels, producing nanoliter-volume emulsion droplets up to 3.4 × 106h-1[27].
Despite the maturity and widespread adoption of T-junction and flow focusing geometries in droplet microfluidics, point-ofcare testing remains challenging, because these microfluidic devices need additional equipment to provide the driving force for fluidic flow, such as syringe pump.Posteket al.developed a microfluidic module,DropChop, that allows to passively generate monodisperse droplets smaller than 1 nL from larger droplets without additional equipment, by a novel geometry of microfluidic channels [28-30].As shown in Fig.1a, a large channel with a slope becomes shallower steadily and ends in a short slit.The slit then connects a large channel that has a dimension of the channel from upstream of the slope.When a disperse phase flows through the shallow channel and enters the broad and deep channel, its curvature gradually reduces due to the expansion in the large channel.Because the curvature of the neck of a droplet phase that is still in the shallow channel is limited from the bottom surface, at some time, the Laplace pressure in the neck cannot be matched by the decreasing capillary pressure in the growing balloon, then the dispersed phase splits and forms a separate droplet.Based on this novel design, Kaoet al.fabricated a gravity-driven step emulsification device and achieved the generation ofca.2000 monodisperse droplets at the volume of 2 nL within 10 min [28].
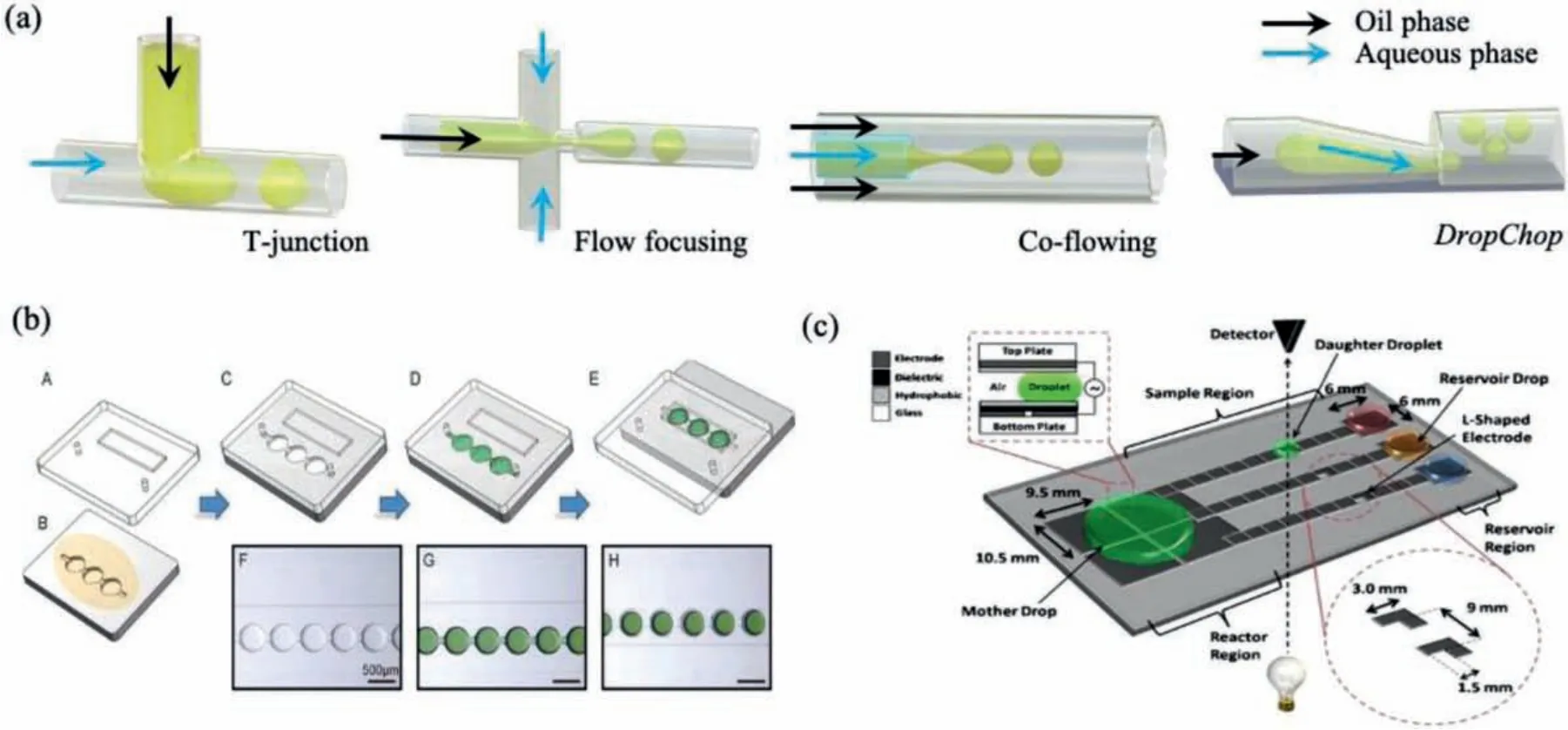
Fig.1.Schematics of droplets generation on microfluidic devices.(a) Droplets generated based on microfluidic geometries with different nozzle architectures.(b) Microwell arrays-assisted droplets formation using a SlipChip.Reproduced with permission [35].Copyright 2020, the Elsevier.(c) Digital droplets created on an EWOD device.Reproduced with permission [41].Copyright 2010, the Springer.
To sum up briefly, T-junction, flow focusing and co-flowing have the advantage of high frequency for droplet generation.Among them, flow focusing is the most applied approach in cell encapsulation, because droplets generation in the T-junction structure suffer from instability due to the asymmetry force, and the co-flowing easily leads to polydisperse droplets.As a newly emerged structure, theDropChophas the advantage in preparing monodisperse droplets without providing a driving force by additional equipment, but its frequency of droplet generation can hardly achieve that of flow focusing.
2.1.2.Based on microwell/microcage/microchamber
As another group of droplet generation method based on microfluidic geometries, microarrays of wells, cages and chambers can conduct in-site reaction and allow direct observation without the demand of droplet transfer or the risk of droplet merging and destabilization, because few or single bacteria is trapped in the designed position with a certain volume.The microstructures, such as microwell arrays, which can be fabricated on glass substrates using wet chemical etching [31], could provide trapping positions.These kinds of microfluidic devices are relatively suitable for studying the physiology, growth, and replication of individual bacterial cells over time.Here, we specify several typical methods that generate and separate droplets from bulk fluids using micro-wells, microcages, or micro-chambers.
SlipChip is a microfluidic device with two microfluidic plates with precisely designed microwells or fluidic ducts.By easy operation of slipping, relative movement of the imprinted micropatterns on the contacting surface of the two plates can partition the bulk liquid into droplets.They do not require auxiliary systems,e.g., pumps and pneumatic systems, for providing the driving force to manipulate and control the liquids, which have great potential for widespread applications [32-34].Despite the convenient operation, traditional ShipChips are limited by their requirements of precise alignment of one set of microwells over another, which is quite challenging in accurate placement under low resolution.Recently, Yuet al.achieved robust droplet formation by slip-induced flow without precise alignment of microfeature [35].As exhibited in Fig.1b, they designed an upper microfluidic plate with expansion channels and an underlayer plate with“chain-of-pearls” continuous fluidic channels.While the two plates are overlapped by simple manual slipping, surface tension drives the liquid to break at the “neck” between adjacent microwells and form independent droplets.Besides, other active procedures,such as centrifugal force [36] and magnetic force [37], can also be utilized to isolate target particles in droplets by particle trapping in microwells and subsequent removal of liquids in the main channel.
Unlike the above devices, the microfeatures for bacteria trapping are imprinted on top or bottom contacting surfaces to bulk fluid, devices with side microchannels branched to the main fluidic channel have advantages in easy fabrication and diminishing droplet detachment from the side microchannels by continuous fluid in the main channel.Hsieh successfully confined and cultivated single bacteria in side-branched pico-arrays and proved its feasibility as a new method for simple, fast, and precise counting of viable bacteria [38].Azizi performed a similar method that bacteria isolation and proliferation is carried out in a nanoliter-sized side microchamber-based microfluidic platform[39].They demonstrated the platform robust, easy-to-implement,short time-to-result for microbial antibiotic susceptibility testing(AST).
2.1.3.Other methods
Besides droplet generation based on microfluidic geometries,electrowetting on dielectric (EWOD), surface wettability, laser, and Inkjet technique also can be utilized in droplet generation.
EWOD-induced droplet generation is an active method that electrodes integrated into microdevices allow electrical control over droplet formation and motion based on a “wetting” force[40].Specifically, an electric field can change the interfacial energy between a fluid and its contacting surface.Since interfacial energy directly influences surface wettability, the hydrophilicity of the fluid on a hydrophobic surface can be temporarily triggered from “unwet” to “wet”.A typical two-planes structure of EWOD devices, including a ground electrode and a control electrode on the top and bottom plane, respectively, is revealed in Fig.1c.Both layers include an insulating hydrophobic layer to separate the droplets from the electrodes.When an electric potential is applied on the electrodes, the droplet begins to wet the surface and form a short liquid finger between the electrodes.Once the electrodes are switched off, the surface reverts to hydrophobic, which leads to segmentation of the liquid finger from the reservoir and form a droplet.The droplet size depends on the intensity and frequency of electric field, and the distance between the two planes.Using this technique, Auet al.constructed a digital microfluidics, in which daughter droplets are pulled out from mother drop and moved to transparent regions of the device to determine bacterial cell density by absorbance measurement (Fig.1c) [41].This EWOD-based digital device showed potential in automated culture and analysis of microorganisms.
Surface wettability can assist in droplet generation.Except in the manner of altering surface wettability in EWOD by electric field, hydrophilic micropillars can be used in preparing droplet arrays.Liuet al.developed an automated, fast, and flexible method based on surface wettability assisted fluid segmentation [42].Specifically, a continuous aqueous stream flowed out from a capillary probe was segmented and immobilized on hydrophilic micropillars of a microchip into massive oil-covered droplets with the probe rapidly scanning over the microchip.Through adjusting the micropillar top area, distance between adjacent micropillars, aqueous stream flow rate and microchip moving speed, they achieved droplet generation rate up to 50 droplets/s and tunable volume range from 0.25 nL to 350 nL.Besides, pulsed laser [43,44] and inkjet devices [45,46] provide additional options for droplet generation.For instance, Parket al.applied pulsed laser in dispersed phase to produce small voids, which extrudes the dispersed phase into a continuous phase and subsequently form droplets.Inkjet devices generate droplets through volume shrinkage of the ink chamber under piezoelectricity, which can perform high throughput and droplet patterning [45-47].
2.2.Generation of droplet arrays
Forming droplet arrays plays an important role in microfluidic droplet-based high throughput screening and analysis, because assays preformed in a continuous flow channel lack of effective means to conduct complex droplet manipulation and long-term bacteria cultivation.Depending on the process from droplet fabrication to droplet array formation, methods for droplet array generation can be roughly divided into two categories:one-step and two-step approach.
The one-step approach implies the generation of droplets and droplet arrays simultaneously,i.e., droplets are formed and immobilized in-site.Droplet generation based on microwell/microcage/microchamber, as well as surface wettability-induced droplet formation based on hydrophilic micropillars that we have introduced in previous sections, is essentially a one-step approach for droplet array generation.
In a two-step approach, droplets are first generated and then assembled into an array.A typical way for droplet assembly is based on droplet trapping in microwell/microcage arrays.Various measurements,e.g., SlipChip technique and mesh-grid, were developed to improve the filling rate and efficiency.Umet al.achieved easy trapping and consistent addition of droplets by applying a metal grid covered on poly (dimethylsiloxane) (PDMS) microwells separated by spacers (Fig.2a) [48].The introduced mesh grid acts as a microchannel that enhances the vertical velocity toward the wells.Thus, droplets will move directly into the wells and tend to stay in the wells.Moreover, on the mesh grid-assisted trapping array platform, it is possible to trap a second droplet and merge the two droplets by oil change, which provides open access for additional manipulation in a high-throughput manner.Xuet al.developed a rapid and straightforward method for large-scale droplet array formation by combining micro-cage array chip and slipping technique, as demonstrated in Fig.2b [49].Using this method,they prepared a monolayer droplet array containing approximately 1,000,000 droplets on a 5.5 cm × 5.5 cm micro-cage array chip within 90 s.Further on-chip cultivation and target droplet picking by capillary is possible, which was validated in bacteria screening.
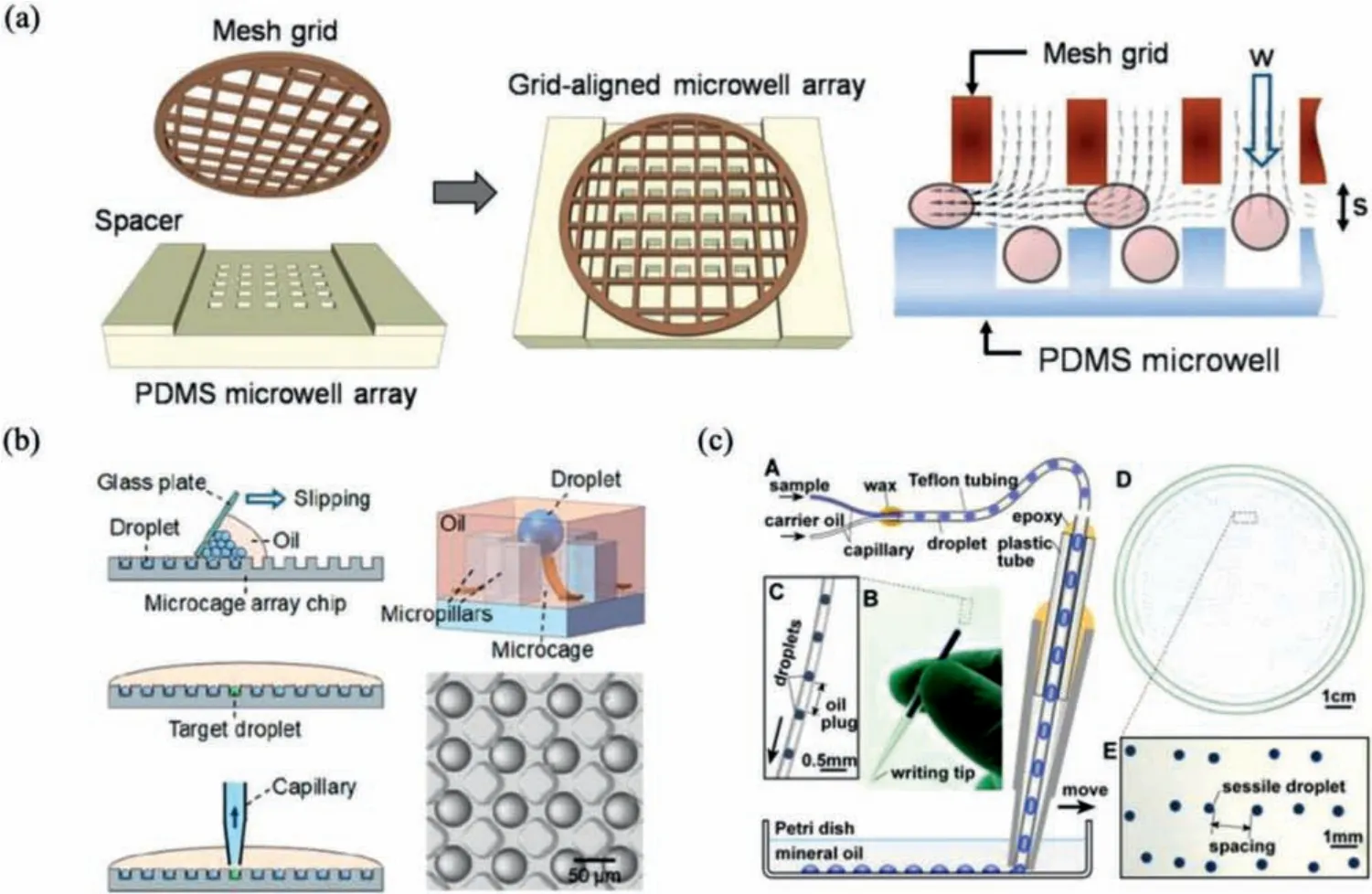
Fig.2.Methods of generating droplet arrays.(a) The formation of droplet arrays based a grid-aligned microwell array.Reproduced with permission [48].Copyright 2012,the Royal Society of Chemistry.(b) Rapid generation of droplet arrays based on a microcage chip by simple slipping.Reproduced with permission [49].Copyright 2019, the American Chemical Society.(c) Droplet arrays formed on an MSP.Reproduced with permission [51].Copyright 2016, the American Society for Microbiology.
2.3.Designable droplet structures and interfacial materials
Droplets as conventional bioreactor can be generated by droplet microfluidics in form of a water-in-oil (W/O) single emulsion containing a surfactant stabilized interface.Under requirements of specific conditions and applications, water-in-oil-in-water(W/O/W) double-emulsion, lipid vesicles, hydrogel microparticles,and multi-compartmental hydrogel particles are developed to prepare droplets with different interfaces and constitutions.
The emergence and employment of W/O/W double emulsion aims at overcoming the problems with W/O single emulsion, because the single emulsion droplets are incompatible with analysis in an aqueous phase.In addition, the inner droplet environment cannot easily communicate with the external phase.The preparation of double emulsion can be easily achieved by a double T-junction or flow focusing cross structure [52,53].In double emulsion, selectively permeable oil barrier can provide a discrete microenvironment, and aqueous core could be well defined and precisely modulated for bacterial cell cultivation and investigation of biological activities [53].Moreover, the coupling of highthroughput gene synthesis and double emulsion droplet screening system was demonstrated promising to produce and screen large amounts of synthetic genes efficiently [52].
Another way of settling the incompatibility of W/O emulsion droplets in an aqueous environment is to encapsulate cells using hydrogels.Two kinds of most-used gelling materials for cell encapsulation are agarose and alginate according to their biocompatibility.Agarose is a linear polysaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose.Agarose dissolves in near-boiling water and forms a gel after cooling down due to the hydrogen bonds and can be reversely disrupted by heating back to a liquid state.Agarose microencapsulation can be simply prepared by flow-focusing microfluidic devices, of which the dispersed phase is cells admixed with agarose.Eunet al.prepared agarose microencapsules with small volume of ~1–50 pL and carried out highthroughput isolation and analysis of bacterial cells in the microencapsules using fluorescence-activated cell sorting (FACS) and determined the minimum inhibitory concentration of rifampicin onEscherichia colicells [54].Alginic acid is a linear polysaccharide consisting of linkedβ-D-mannuronic acid (M) andα-L-glucuronic acid (G).Both M-blocks and G-blocks have hydroxyl and carboxyl in their chemical structures, which endows their salts, alginates,a hydrogel material for micro-encapsulation.When calcium ions are introduced in sodium alginate aqueous solution, counter-ion exchange of carboxylates occurs and calcium ions crosslink adjacent alginate chains and leads to gelation.Lianet al.[55] developed a pressure-driven flow focusing system to consistently create alginate micro-gels which includes a 3D flow focusing design to generate sodium alginate droplets and a downstream calcium introduction inlet to complete gelation.Wuet al.fabricated an integrated chip-capillary droplet generator in creating multicompartmental micro-gel particles.In this system, the upstream component of ingenious micro-fluidic chips is devoted for defining the internal microstructure of micro-gel, while the downstream component of capillary-based device is used as the flow focusing structure to prepare spherical hydrogel droplets.Ca-alginate is used as the dispersed phase, and acetic acid in the continuous oil phase diffused into droplets and triggered rapidin-situcrosslinking of Ca-alginate.This method is capable of creating well-divisional multi-compartmental particles, which is validated the application in monitoring the activity of independent cell line in a multicellular particle system [56].
Even though the gel/agarose microparticles allow a continuous exchange of the nutrients and reagents through diffusion, do not retain small molecules.Thus, employment of lipid vesicles as culture compartments was developed to address the limitations of micro-emulsion, double emulsion and hydrogel microparticles.Juskovaet al.reported an optimized water-in-oil transfer method to generate bacteria-encapsulating giant unilamellar vesicles.These lipid vesicles were demonstrated to be versatile culture compartments for bacteria cultivation combining advantages of both droplets and gel beads [57].
3.Manipulation
Generated droplets used as bioreactors for further bacteria detection and analysis usually involve subsequent manipulation of droplets or in-droplet particles, which aims to achieve functions such as reactant import, in-droplet aqueous homogenization,droplet concentration, purification (isolation), and screening.Several basic droplet manipulations, such as injection, splitting and merging, can be generally divided into passive and active methods,while in-droplet particle manipulation and sorting are commonly based on active paths.
3.1.Droplet manipulation
3.1.1.Injection, splitting, merging
As mentioned above, basic droplet manipulation of injection(fusion), splitting (fission) and merging (mixing) can be realized by passive and active approaches.Passive droplet manipulation does not require external power sources or devices to perform droplet manipulation.Instead, it relies on hydrodynamic forces created by channel design in a continuous flow, making it simple and suitable for high throughput analysis.Fig.3a exhibited a typical microchannel structure for essential droplet manipulation functions,including fission, fusion, and mixing [58].In passive fission, the shear force created by channel designs, such as T-junction, branching channels and channel obstructions, are used to split droplets at precise positions into controllable volumes.Liuet al.split generated plugs (droplets) into arrays of identical daughter plugs, where each plug contained clones of the original cell.They further analyzed each array by independent techniques, including cellulase assays, cultivation, cryo-preservation, gram staining, and fluorescencein-situhybridization [59].In passive fusion, channel design aims at controlling the location of droplet fusion and achieving frequency matching between two or more droplets under high flow conditions.In passive mixing, a zigzag channel design is always employed to enhance internal turbulent mixing of adjacent miscible fluids, with a shorter distance and shorter time than in laminar flow conditions.Hosokawa designed a zigzag channel for mixing multiple displacement amplification in cell lysate droplets, and the merged droplets were collected and carried out whole genome amplification for single bacteria cell sequencing and analysis [60].
Active droplet fission, fusion, and mixing could be achieved on EWOD-based digital microfluidic devices (Fig.3b) [61,62].As we elaborated in the droplet generation section, the EWOD technique has the property of electrical control over droplet formation and motion based on a “wetting” force, which leads to the generation of daughter microdroplets from the mother droplet by splitting.With the same principle that electric field can direct surface wettability and lead to droplet movement, droplet manipulation on EWOD-based microfluidic chips is feasible.Kalsiet al.constructed an EWOD digital microfluidic platform with 16,800 electrodes that can be independently controlled to conduct multiple and simultaneous droplet manipulation.By continuous mixing of droplets during DNA amplification, detection of the target DNA improved significantly by at least 100 times than a benchtop assay [62].Compared with the passive approach based on channel design, the active EWOD approach has superiority in performing complex manipulation in a small area within a short time under better control,unable to deal with massive droplets.
When he reached the first court of the castle he saw before him a flight of agate20 steps, and went up them, and passed through several splendidly furnished rooms
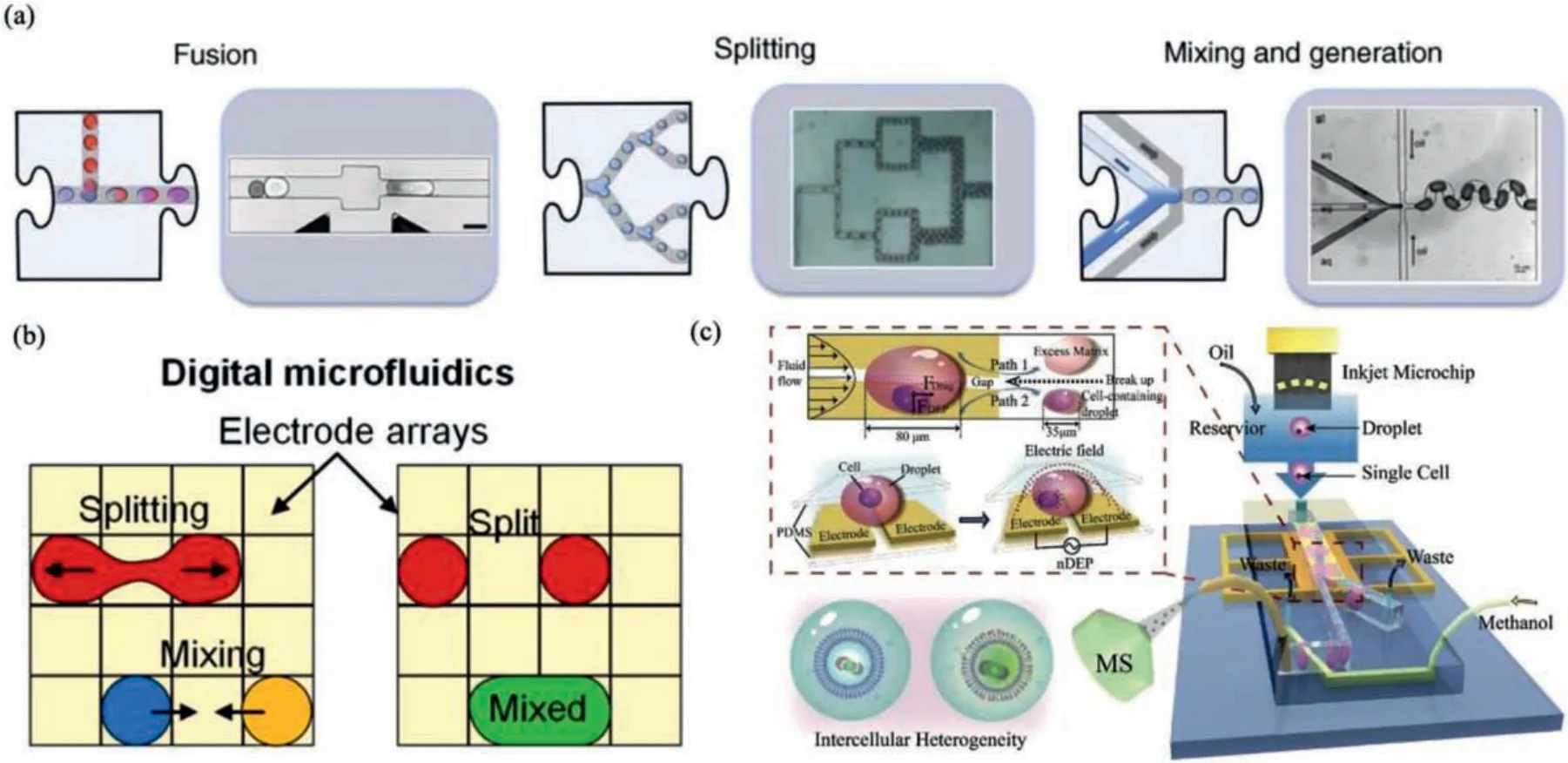
Fig.3.Manipulation of droplets and in-droplet particles.(a) Passive droplet manipulation relies on hydrodynamic forces created by microchannels.Reproduced with permission [58].Copyright 2010, the Elsevier.(b) Active droplet manipulation on EWOD-based digital microfluidic devices.Reproduced with permission [61].Copyright 2017,the AIP Publishing.(c) In-droplet cell manipulation and asymmetrical droplet splitting by a DEP microfluidic platform.Reproduced with permission [70].Copyright 2019, the Wiley.
3.1.2.Sorting
There are two ways of sorting:passive and active sorting, depending on action modes.Passive sorting takes full advantages of microfluidic chip structures and physical characters of target droplets to sort, without external fields or forces.For instance,size-based sorting using channel geometry or gravity.However, the passive sorting system lacks flexibility over sorting parameters because its bias and sorting parameter are coupled.Therefore, active sorting, which generally employs external fields (e.g., acoustic,electric, magnetic, and optical), has become the mainstream.In active sorting, recognition of target bacteria can be realized based on analysis of genetic molecules, metabolite, proteins or bacterial cells by fluorescence, light scattering,etc., and the detective fluorescence or light scattering can be transferred into an activating signal for digital sorting based on active droplet manipulation technique.Cell sorting based on acoustic, electric, magnetic, and optical forces are reviewed by Wyatt Shields IVet al.[63].We will mainly introduce the generally adopted active sorting method, dielectrophoresis (DEP), and a novel 3D particle sorting method under vacuum to isolate target cells [64].
DEP sorting is the most widely adopted sorting technique for droplet microfluidics due to its good compatibility with digital platforms for high throughput analysis.DEP refers to the movement of suspended cells in a non-uniform electric field due to their polarizability.Once an alternating current (AC) field is provided,the cell polarization is triggered, and cells migrate either toward or away from the region of strongest field intensity depending on the electrical permeability of the cell and the fluid.Cells with a higher permeability than the fluid are attracted toward the field maxima,which is known as positive DEP (pDEP), and the opposite is negative DEP (nDEP).The intensity of DEP force depends on the cell and fluidic electrical properties, on the cell size, and the voltage and frequency of the electric field applied.Consequently, DEP-based cell sorting has remarkable flexibility and selectivity.Wanget al.constructed vertical electrodes in the sidewall of microchannels as DEP switching for multiplexed switching of objects.They achieved switching cells and polystyrene microbeads to multiple outlets (up to five).High throughput digital sorting of bacteria-encapsulated droplets is the prevailing direction for droplet microfluidics-based bacteria analysis.On digital platforms, DEP-based sorting is the most employed sorting technique.Zureket al.performed cell lysate screening by DEP at ultrahigh throughput after clonal amplification in droplets.The assay sensitivity and DNA recovery improved by orders of magnitude [65].Hunget al.developed a microfluidic sorter capable of selecting fluorophores based on fluorescence lifetime and brightness at two excitation and emission colors using DEP, at a maximum droplet rate of 2.5 kHz [66].
Despite the extensive investigation and application of microfluidic-based sorting ranging from the active system (acoustic, electric, optical, or magnetic forces) to passive system (inertial forces, immobilization procedure), their dealing capacity is typically limited within 1 mL.Therefore, they are inapplicable to rapid diagnostic assays on low-abundance pathogens.Heddeet al.fabricated a 3D particle sorting device that scans a bulk sample at a rate excessing 400 μL/min by spinning a cylindrical, transparent sample vial.Behind the vial, a laser is focused on exciting and detecting target fluorescence [64].With a similar principle, Kang detected a single labelled bacterium in whole blood [67].Hedde further demonstrated that the principle is feasible in sorting target cells in real-time by a thin gauge needle or capillary dipping into the sample adjacent to the laser focus.Targets at concentrations of 1-100 colony-forming unites (CFUs)/mL in blood can be enriched to over 100-fold from 1 to 10 mL volume within minutes [64].
3.2.In-droplet particle manipulation
Even though diverse droplet manipulation techniques have been developed and utilized in different applications, further demand of in-droplet particle manipulation exists in specific areas, such as the necessity of in-droplet cell separation in the study of cellcell communication, drug screening and volume concentration of single-cell containing droplets.Compared with passive methods,active methods have the advantage of precise manipulation.Several active manipulation methods have been developed for cell manipulation inside nanoliter droplets using acoustophoresis and DEP[68,69].
Acoustophoresis is a contactless particle manipulation method based on the scattering of ultrasonic waves on particle surfaces.Acoustophoretic forces depend on the particle size and density difference to its surrounding medium.Also, acoustic forces are suitable for cell manipulation due to their rapid and precise spatial control without affecting cellular viability.Moreover, acoustophoresis is a label-free particle or cell manipulation method.Gerltet al.exploited an acoustofluidic chip to manipulate particles and cells in flowing droplets before the droplets split at a T-junction.They achieved a separation efficiency of 100% in more than 100 droplets at a frequency of 114 droplets/min [69].However, acoustophoresis can hardly separate two different populations of cells, because all cells suspended in a regular culture medium possess a positive acoustic contrast factor, leading all cells to move toward the same position in the droplet.
DEP, extensively used in droplet manipulation, can also be utilized in particle manipulation in droplets.As shown in Fig.3c,Zhanget al.achieved concentrating single cells in picoliter droplets on a microfluidic chip with a dielectrophoretic channel.Single cells in the dielectrophoretic channel were finally confined in a small area of the droplet, and a downstream Y-shaped structure was used to split the droplet into two asymmetrical daughter droplets.The droplet with a smaller volume containing the single cell was introduced to mass spectrometry for lipid analysis[70].Hanet al.developed an in-droplet label-free cell separation technology by utilizing different dielectrophoretic responses of two different cell types.Two pairs of angled planar electrodes were integrated into the microfluidic chip to generate positive or negative dielectrophoretic force acting on each cell type.A downstream Y-shaped structure split the droplet into two daughter droplets, each of which contains only one cell type[68].
4.Bacteria analysis
General expectations for bacteria analysis cover a wide range,including bacteria growth assay, determination of pathogenic bacteria, phenotype/genotype assay, bacteria chemotaxis in a gradient environment, cell-to-cell communication,etc.Several conventional detection and analysis methods,e.g., fluorescence-based observation and analysis, light scattering-based counting and metabolite detection, amplification-based molecular detection and bacterial typing, coculturing for cell-to-cell interaction,etc., have been well developed and widely used as golden standard in clinical diagnosis.Principles and technical processes of routine analytical methods have been comprehensively reviewed in previous publications.Here, we will briefly introduce what droplet microfluidics can do differently from the regular bacterial analysis, and how it is achieved by examples.
4.1.Bacteria growth
Simple, fast, and precise counting of viable bacteria has great significance for various microbiological applications, such as food safety, diagnosis of bacterial infection, AST.Conventional methods for bacteria growth assay using agar plating and microscopy need a prolonged time and sophisticated operation for bacteria isolation and cultivation.Meanwhile, information loss of unculturable or heterogenous bacteria with a small population is inevitable in the routine method.Droplet microfluidics provides an individual compartment for a single bacteria cell with a small volume, making bacteria growth rapid and adverse effects of multi-species population culture can be eliminated.Therefore, bacteria growth analysis performed in pico-liter or nanoliter droplets possess properties of simple, rapid, and precise, which brings a revolution in bacteria counting related fields, especially for AST.Fluorescence, light scattering, and surface-enhanced Raman scattering (SERS) are typically adopted detection methods in droplet-based bacteria growth analysis.
Detecting fluorescence light is the most general approach for bacteria detection [57,67], which can also be used in bacteria growth determination.e.g., Juskovaet al.fabricated a system to encapsulate bacteria inside giant unilamellar vesicles (GUVs) for realtime monitoring of microbial growth and production.They evaluated the bacteria growth in GUVs by monitoring sfGFP (a modified bacteria strain overexpressed the gene for green fluorescent protein) fluorescence, and the results proved the system a promising platform toward highly parallel microbial cultivation and monitoring as required in biotechnology, basic research, or drug discovery[57].Although fluorescent detection is widely used, bacteria do not inherently generate fluorescence signals.Additional modification of cells or their environment needs to be carried out to yield detective fluorescence.
The light scattering has superiority in detecting cell proliferation because it is inherently label-free and could work with any bacteria species with high throughput.Pacochaet al.achieved reliable detection of the proliferation of encapsulated bacteria with a high frequency of 1.2 kHz, using a sensitive and fast measurement of scattered light [71].The sensitivity of the light scattering method was verified by comparing with fluorescent signals simultaneously recorded.Meanwhile, the system’s applicability was confirmed with 12 different bacteria species, including Gram-positive and Gram-negative species.SERS can provide native and rich chemical information on bacteria compositions, including nucleic acids, proteins, lipids, and pigments, with features of labelfree, high-specific, and accurate sensing, making it promising for microbial characterization.SERS investigation of bacteria on conventional bacteria incubation platform usually suffers from background signal interference and low bacterial count.Bacteria encapsulated in a micro-droplet possess increased effective bacteria concentration, which suits employing SERS analysis in bacteria proliferation.Huanget al.developed a Microwell-SERS system to perform a rapid and high throughput AST [72].The microwells were directly attached to the SERS substrate to achieve minimal Raman signal interference.Finally, in-site SERS measurement of bacteriasecreted metabolite was used to evaluate the bacteria proliferation under antibiotic treatment.
4.2.Bacteria detection
Due to the increased effective bacteria concentration and high throughput feature of droplet microfluidics, their advantages in bacteria detection are mainly in two aspects:rapid separation and detection of rare or target bacteria species with specific phenotype/genotype, and amplification-free detection.
One chief objective of bacteria detection is the determination of bacteria phenotype/genotype.Droplet microfluidics is an ideal platform for conducting single-cell isolation, detection, and analysis with high throughput.When integrated with a sorting system, cells with specific targets (bacteria cells, nucleic acids, proteins, metabolites,etc.) can be isolated from a heterogeneous population.Micro-droplets have a higher effective concentration of targets than a bulk sample, leading to a reliable single copy detection, even for rare or unculturable species that can hardly be detected in routine manners.Nucleic acid cytometry is a general method for phenotype/genotype detection.Fig.4 shows the typical workflow of nucleic acid cytometry [73], which includes compartmentalization of samples in droplets, detection of the target sequence, sorting of positive droplets, and downstream analysis of the sorted droplets if needed.Aziziet al.prepared a sensitive biosensor for pathogenic bacteria (S.typhimurium) detection by combining droplet-based microfluidics with loop-mediated isothermal amplification (LAMP).The mathematical model proved that the biosensor using 20 μm droplets work up to 105× and 103× dilutions of original extracted RNA samples from pureS.typhimuriumand contaminated milk [39].Thibaultet al.combined transposoninsertion sequencing (Tn-Seq) with droplet microfluidics for identifying complex single-cell phenotypes.Droplet Tb-Seq enabled isolated growth of individual transposon mutants, free from the influence of the population.Consequently, 1%–3% of mutants inStreptococcus pneumoniaeexhibited different fitness in isolated growth[74].
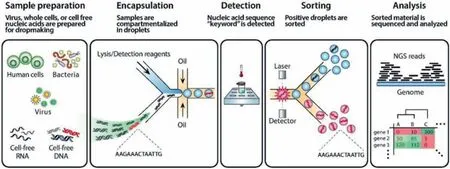
Fig.4.A typical workflow of nucleic acid cytometry.Reproduced with permission [73].Copyright 2017, the Royal Society of Chemistry.
Many high throughput bacteria analyses on droplet microfluidic platforms are amplification-based molecular detection [35,60,75].To perform amplification reactions, such as polymerase chain reaction (PCR) and LAMP, bacteria-encapsulated droplets commonly need to undergo an off-chip amplification process, and then reject subsequent microchip for amplicon detection [76], which increases the processing time and operational complexity.Therefore,amplification-free detection is desired.Raneet al.constructed a droplet microfluidic chip enabling amplification-free detection of single pathogenic cells.This fully integrated device contains functional units of cell isolation, lysis, probe-target hybridization, and fluorescent detection.The platform’s feasibility was demonstrated with a 16S rRNA mimic target and intact E.coli cells as the model pathogen [77].
In addition, image-based detection is a method for accurate identification of cell type and number, which requires accurate cell segmentation and feature extraction from whole slide images.The appliance of machine vision in recognition of cells in biological samples becomes increasingly important.However, image segmentation in this technique suffers from the overlap between different cells.Lvet al.encapsulated single cells in emulsion droplets from a urine sample based on droplet microfluidics to settle this issue.They demonstrated that better image segmentation is achieved after cell encapsulation in micro-droplets, as the four parameters(Dice, Jaccard, precision, and recall) for evaluating image segmentation improved [78].
4.3.Cell-to-cell interaction
In nature, bacteria commonly live in synergistic communities with other species.The cell-to-cell interaction is crucial for maintaining the communities.Even though cell-to-cell communication can be investigated on culture plate or microfluidics with continuous fluids, studies at the single-cell level are still challenging.Droplet microfluidics provide a way to address the challenge.Typically, cell encapsulation in droplet microfluidics is a stochastic process obeyingPoissondistribution, in which the probability of a droplet containing only one cell and a cell pair of two distinct cell types is limited to 36.8% and 13.5%, respectively.Laguset al.achieved co-encapsulation of correct one-to-one pairing in droplets at rates on the order of 6 kHz, an accuracy of 64% (nearly fivefold improvement toPoissonco-encapsulation) [79].Parket al.encapsulated symbiotic pairs of bacterial strains that are incapable of growing alone due to both of them cannot synthesize one specific amino acid.By co-encapsulation based on a droplet microfluidic device, they displayed compensation for each other, live long and prosper, indicating the significance of cell-to-cell interaction on bacteria proliferation [44].
5.Application
5.1.Antibiotic susceptibility test
AST is an essential process in diagnosing and treating pathogenic bacteria, and has great significance for inhibiting the evolution and risk of antimicrobial resistance (AMR).Conventional AST, such as liquid media-based broth microdilution assay and solid media-based disk diffusion or Etest, demonstrate the overall result of a heterogeneous population.Besides, an isolation and a purification culture process are required before AST, and bacteria counting based on absorbance or visual determination of turbidity for AST needs multiple cycles of bacteria division, leading to a prolonged measurement time (about 1-5 days) and a delay in medication guidance.AST performed based on droplet microfluidics can efficiently shorten the measurement time to several hours ~several minutes, depending on the doubling time of the bacteria and the analytical assay.Both phenotypic and genotypic AST have been demonstrated to be viable for rapid AST and minimum inhibitory concentration (MIC) determination.
Phenotypic AST is a culture-based method.The presence of pathogens, morphological, biochemical, and molecular tests are being carried out to identify the bacteria.Kanget al.developed a rapid image-based AST (phenotypic AST) assay by a highly parallelized droplet microfluidic platform.This device can simultaneously screen four antibiotics/pathogens simultaneously and complete antibiotic sensitivity assessment in 15-30 min.They qualified doubling time and MIC of both Gram-positive and Gram-negative strains.The MIC determined in droplets was consistent with the MIC obtained from broth microdilution methods for all strains, indicating the reliability of this rapid and sensitive AST platform [80].
Genotypic AST is a nucleic acid amplification-based method.In genotypic AST, conventional PCR or isothermal amplification are commonly employed to amplify specific genes encoding antibioticresistant phenotypes due to their short assay time and high sensitivity.Genotypic AST only requires a short time for nucleic acid amplification.Therefore, compared with phenotypic AST, they have superiorities in shortening the assay time, and reducing the contamination risks and pathogens spread.Consequently, this approach is very suitable for infection treatment, especially for Grampositive bacteria [81].
5.2.Directed evolution
Directed evolution is a method used in protein engineering that guides proteins or nucleic acids toward a desiring function through mimicking the process of natural selection, which includes the creation of a library of variants, expression of the variants, isolation of members exhibiting the desired function, and amplification (generating a template for the following round selection).The advantage in high-throughput sorting of positive droplets makes droplet microfluidics an ideal platform for conducting directed evolution.Vallejoet al.established a fluorescence-activated droplet sorting (FADS) device to evolve enzymes for synthesizing and modifying artificial genetic polymers (XNAs).Using an enzymatic activity-responsive fluorescent sensor, the microfluidic system enabled droplet sorting at ~2-3 kHz [82].Zureket al.improved the enzyme activity assay sensitivity and DNA recovery by orders of magnitude by growth of initially singly compartmentalized cells in microdroplets, making the protein engineering easier for identification or evolution of new enzymes that can be applied in synthetic and chemical biology [65].
5.3.Environmental monitoring
Since bacteria are widespread in soil and water, droplet microfluidic-based bacteria analysis shows potentials in environmental monitoring [83-85].For example, Huet al.developed a high throughput MSP platform to expedite the isolation of microorganisms from deep-sea sediments.By picking individual droplets from MSP and scale-up cultivation, a total of 772 strains were successfully isolated from the deep-sea sediments, including 15 potential novel species.The MSP platform was validated to have superior ability in recovering microbial diversity than conventional agar plates.Therefore, it is a great potential for MSP to be applied in recovering rare microbial resources from various environments [83].
6.Summary and prospect
This article comprehensively and timely reviews droplet-based microfluidics for bacterial detection and analysis, which involves droplet generation, manipulation, bacterial detection and analysis,and their applications in bacteria-related fields that will be significantly promoted by droplet-based microfluidic techniques.
In droplet generation, methods of forming micro-droplets from bulk fluids based on microfluidic geometries (nozzle architectures,microfeatures), EWOD, surface wettability,etc.; methods of generating droplet arrays, such as SlipChip and MSP; droplets different interfaces and constitutions (W/O/W double-emulsion, lipid vesicles, hydrogel microparticles) are elaborated.Various methods and techniques have been developed to prepare droplets with different characters.Therefore, while developing a new platform for bacteria analysis using droplet-based microfluidics, the selection of a “suitable droplet” is the first but very crucial step, which includes decisions of using a continuous droplet fluid or an immobilized droplet array in either a close system or an open system.The decisions should be made by combining the condition, environment, process of assay with features of droplets.
In manipulation, both methods of droplet manipulation and indroplet particle manipulation, including fission, fusion, mixing and sorting, are introduced.A proper manipulation process can significantly improve the efficiency, accuracy, and sensitivity of the assay.Till now, plenty of advances have been achieved in this area.However, the integration level of many devices still needs to be improved, since interim intervenes are frequently involved in particular approaches.For instance, an off-chip cultivation process is commonly used in many FADS devices.More precise, compatible,simple methods and techniques are still in demand for completing complex processes on a compact platform.
In bacteria analysis, we briefly introduce what droplet-based microfluidics can do differently from the regular bacterial analysis,and how it is achieved by examples.Briefly summarising, dropletbased microfluidics possess superiorities of high throughput analysis, compatibility with rare and unculturable bacteria in conventional methods, rapid and sensitive detection due to the improved effective concentration in droplets.Besides, although it has not been widely exploited yet, applying the micro-droplets in machine vision for cell recognition has a great potential to be a hot topic in the future, because both the two techniques have properties of single-cell level detection, high throughput, and capable of digital operation.
Despite the widespread employment of current droplet-based microfluidic devices is limited due to their requirements of fluidic driving equipment and the lack of integration of droplet generation, bacteria culture and analysis on a single platform, they have tremendous potential of providing novel new solutions for many fields.In the final section, we list but are not limited to the application of droplet microfluidic-based cell analysis in AST, directed evolution, and environmental monitoring.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No.22104117), “the Fundamental Research Funds for the Central Universities” (No.JC2110), Wuhu and Xidian University special fund for industry-university-research cooperation (No.XWYCXY-012020012), and Open Fund of Zhijiang Lab(No.2021MC0AB02).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
- Encapsulation strategies on 2D materials for field effect transistors and photodetectors
