In-situ monitoring of cell-secreted lactate by electrochemiluminescence sensing under biomimetic microfluidic confinement
Yixin Liu, Yunyun Yo, Beibei Yng, Yn-Jun Liu, Bohong Liu,*
a Department of Chemistry, Shanghai Stomatological Hospital, and State Key Lab of Molecular Engineering of Polymers, Fudan University, Shanghai 200433,China
b Shanghai Key Laboratory of Medical Epigenetics, International Co-laboratory of Medical Epigenetics and Metabolism (Ministry of Science and Technology),Institutes of Biomedical Sciences, Fudan University, Shanghai 200032, China c College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China
d Department of Chemistry and Chemical Engineering, Nantong University, Nantong 226000, China
Keywords:Confinement ECL sensing platform In-situ monitoring
ABSTRACT Cell migration proceeds in 3D matrices in vivo, which can naturally switch to distinct phenotypes for better invasion in confined microenvironments.The studies of important metabolites under confinement are extremely meaningful for comprehensive insights into cancer metastasis.The integration of cell confinement device and analytical techniques is a key point for in-situ analysis of significant metabolites in vitro.Herein, an electrochemiluminescence (ECL) sensing platform was designed for in-situ monitoring of cellsecreted lactate in highly confined microenvironments.The 3-μm confiner was exactly fabricated via microfabrication and microfluidics technique, and cells in high confinement and low adhesion tended to be round with contractile blebs on cell margins.Significantly, in-situ monitoring of lactate was successfully achieved on the ECL platform with the catalysis of lactate oxidase, in which the levels in different time intervals were acquired in the luminol-hydrogen peroxide system.Furthermore, the results were verified by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology, which showed similar fluctuations with the ECL platform.This system offered an available avenue for metabolites analysis in highly confined microenvironments, which may advance deeper insights into metabolic mechanisms of cancer metastasis
Cell migration is a critical part in cancer metastasis, which plays an indispensable role in the process of detachment from primary lesions, circulation in microvessels, and further invasion into new sites for secondary tumor [1,2].The comprehensive insights into metabolic mechanisms in microenvironments were extremely significant for therapeutic strategies targeting cancer.Traditional studies towards rare cell analysis and cellular responses were generally based on two-dimensional (2D) modelsin vitro[3–11], which may offer some concepts on understanding of molecular mechanisms.However, the fact is that cancer cellsin vivoneed to cross through complex and variable microenvironments for efficient metastasis, especially narrow, confined three-dimensional(3D) regions in tissues.Significantly, researches have indicated that cancer cells can autonomously change their phenotypes in confinement for convenient migrationin vivo[12,13].Thereinto, recently,Piel’s group developed a confined microenvironmentin vitro[14],in which cancer cells could switch to fast amoeboid migration under strong confinement and low adhesion.However, the metabolic mechanisms in confined environments were rarely unexplored.
Metabolic disorders are obvious features in cancer microenvironments, and cancer metastasis can be effectively promoted through adjustable metabolites for suitable bioenergetics [15].Especially, the metabolism of migrated cells can be selectively regulated so as to form different metabolic traits for favorable migration in complex microenvironments [15].Among these metabolic pathways, lactate is an important metabolite generated from aerobic glycolysis, which is known as “Warburg effect”.It can be served as an important signaling molecule for effective protection against reactive oxygen species (ROS)in vivo, thus promoting cell migration in cancer metastasis.Additionally, the expression level of lactate is also a key indicator in clinic for health assessments.Thus,various strategies were widely developed for lactate detection in biological samples [16–18], however,in-situmonitoring of lactate is rarely studied in specific microenvironments.It is desirable to design a simple, sensitive and effective platform forin-situlactate analysis for deeper understanding of metabolic mechanisms under confinement.Electrochemiluminescence (ECL) has been applied as a hot technique for targets analysis due to its low background,high sensitivity, simplified equipment and flexible controllability[19–22], in which ECL signals are generated from electrogenerated species under certain voltages.The luminol-hydrogen peroxide system is a highly efficient ECL platform for biomolecules detection benefiting from its powerful luminescence efficiencies after electrochemical reactions, which offers great potential forin-situmonitoring of cell-secreted lactate in confined microenvironments.
Thus, in this work, an ECL sensing platform was designed forin-situmonitoring of cell-secreted lactate in confined microenvironments.As shown in Fig.1, cells were put into a confined microenvironment, in which exact polydimethylsiloxane (PDMS) microstructures were fabricated based on pre-designed templates.Remarkably, compared with non-confined cells, contractile blebs emerged on margins of the confined cells along with distinct deformations of the cell body and nucleus.Subsequently,in-situmonitoring of lactate in confined microenvironments was achieved by the ECL platform, of which the expression in different time intervals was measured based on luminol-hydrogen peroxide systems by means of lactate oxidase.Additionally, liquid chromatographytandem mass spectrometry (LC-MS/MS) technology was employed for further verification.The perfect combination of cell confinement device and ECL techniques offered a promising platform forin-situmonitoring of key metabolites in confined microenvironments, which may promote further studies on metabolic mechanisms of cancer in variable microenvironmentsin vitro.
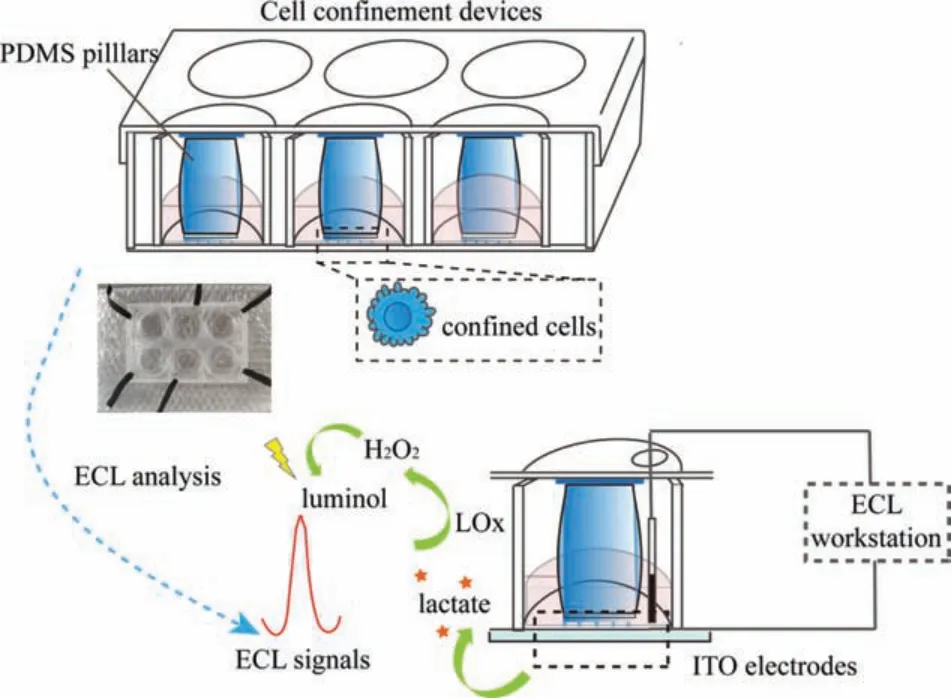
Fig.1.Schematic diagram of in-situ ECL monitoring of lactate in confined microenvironments.
As shown in Fig.1, confined microenvironments for HeLa cells were successfully engineered according to the previous report [23],and the detailed processes were provided in the supplemental files.Briefly, large PDMS pillars for main supports were acquiredviaa customized template.Similarly, the confinement slide was fabricated through a silicon mold, which was covered with regular holes with 440-mm diameters and 3-μm heights.Large PDMS pillars on the center of cover lids were applied to load with the confiner, and then covered on cells in a 6-well plate for constant force by use of adhesive tapes.The modification of pLL(20)-g[3.5]-PEG(2)(pLL-PEG) on coverslips was employed to form non-adhesive surfaces.The real-time monitoring of cell phenotypes in highly confined microenvironments was achieved in a microscope.As displayed in Fig.2B, cells in low adhesion and confined microenvironments exhibited distinct behavioral phenotypes.Compared to non-confined cells (Fig.2A), the cross-section of nucleus got bigger in effects of confined microenvironments (inset in Fig.2B), and the cell body showed rounded phenotypes with continuously contractive blebs around the cell edge.This was caused by increased cortex contractility in confined cells [24], which might further switch to amoeboid-like phenotypes in the complete medium under stable confinement.The distinct phenotypes of confined cells were directly relevant to the differential expression of metabolites.
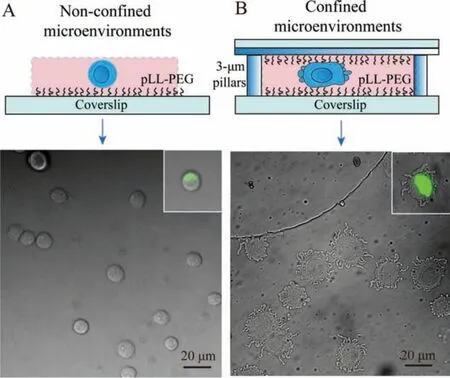
Fig.2.HeLa cells in non-confined (A) or confined (B) microenvironments on low adhesive substrates.Cells were cultured in PBS buffer solution (10 mmol/L, pH 7.4).The fluorescence imaging of nucleus in confined or non-confined cells was shown in the inset.
Considering application of the ECL platform in confined microenvironments, as exhibited in Fig.1, the glass for imaging in the 6-well-plate device was replaced by indium tin oxide (ITO)conductive substrates, and the cover lid of the 6-well-plate was perforated for introducing electrodes for next ECL analysis.In this work, lactate was investigated as a key metabolite in confined microenvironments.Thus, as shown in Fig.3A, the ECL platform showed well performance for lactate detection, of which ECL signals were increased with the concentrations of lactate with the catalysis of lactate oxidase, which showed a regression equation ofy= 11.22x- 16.66 (R2= 0.9952) in the range of 10-200 μmol/L with a detection limit of 5 μmol/L (Fig.3B).Therefore, the wellengineered ECL platform provided a possibility forin-situmonitoring of cell-secreted lactate in confined microenvironments.
For exploring metabolic differences in highly confined microenvironments, HeLa cells were cultured in phosphate buffered saline(PBS) solution forin-situmonitoring of secreted lactate on the ECL platform.The ECL responses were measured at different time intervals in the presence and absence of lactate oxidase.As exhibited in Fig.3C, confined cells showed contractive blebs around the cell edge along with time.The background in microenvironments(Fig.3D) may result from varied metabolism, while the signal differences in Fig.3E resulted from cell-secreted lactate gradually increased along with time, and the concentration reached ~5 μmol/L at 4 h.Simultaneously, signal differences of ECL responses in nonconfined microenvironment were hard to be monitored, which indicating stronger metabolisms of anaerobic respiration under confinement.
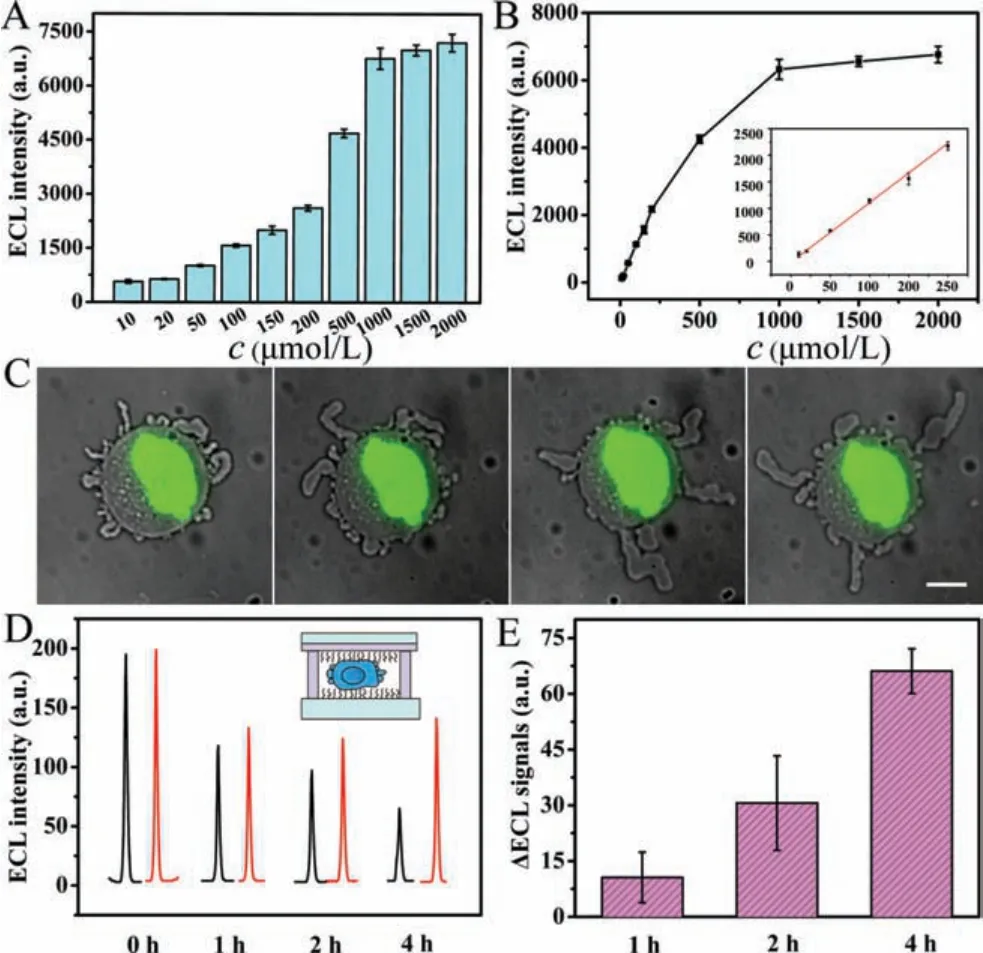
Fig.3.(A) ECL responses for the detection of varied concentrations (10, 20, 50, 100,150, 200, 500, 1000, 1500, 2000 μmol/L) of lactate in 10 mmol/L PBS (pH 7.4) with 200 μmol/L L012.(B) Intuitive exhibition for changed ECL signals, and the linear calibration curve for lactate detection was shown in inset.(C) Varied phenotypes in confined cells along with time.Scale bar is 10 μm.(D) ECL responses for cellsecreted lactate in confined microenvironments at different time intervals (0 h, 1 h,2 h, 4 h) in the presence (red lines) and absence (black line) of lactate oxidase.(E)The corresponding differences of ECL signals at different time intervals.
For verifying the fluctuation of lactate in confined microenvironments, as shown in Fig.4A, samples in different time intervals were collected through centrifugation for LC-MS/MS analysis.The lactate in samples was well detected in LC-MS/MS methods, and the heat map of samples in different time intervals (Fig.4B) was intuitively exhibited for the differential expression.The secreted lactate gradually increased along with confined time (Fig.4C),which was consistent within-situmonitored results on the ECL platform.The above results further demonstrated the accuracy of the ECL platform.
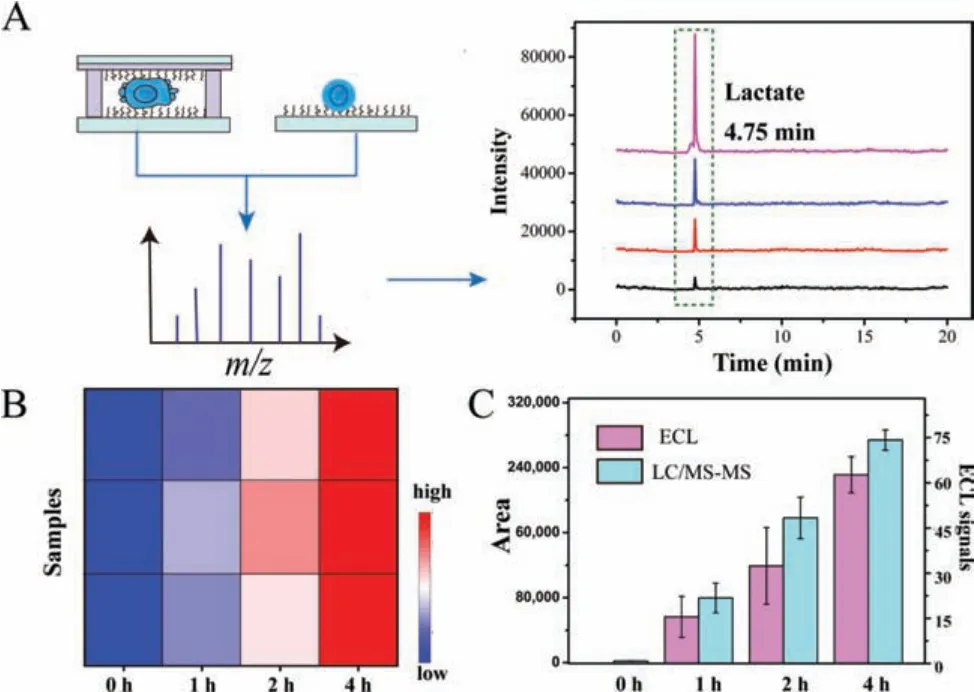
Fig.4.(A) The collected samples in different time intervals for LC-MS/MS analysis of lactate.(B) The heat map of samples for lactate detection.(C) The corresponding fluctuation of LC-MS/MS signals in different time intervals.
In summary, an ECL sensing platform was applied forin-situmonitoring of cell-secreted lactate under biomimetic microfluidic confinement.The highly confined microenvironments were well achieved by microfabrication and microfluidics technique.The cell body became round with retractile blebs on the cell margin, and the cell nucleus deformed under biomimetic confinement.Significantly, the lactate in confined microenvironments was successfully monitored in the ECL sensing platform by use of lactate oxidase, of which metabolic differences in different time intervals were obtained.The results indicated more active metabolisms in highly confined cells, which was further verified by the LC-MS/MS technology.This work offered a new perspective forin-situmonitoring of significant metabolites in biomimetic confined microenvironmentsin vitro, which may promote the development of molecular mechanisms of cancer metastasis.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21934001 and 31870978), the Natural Science Foundation of Zhejiang Province (No.LQ20B050002).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.074.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
