Facile fabrication of drug-loaded PEGDA microcapsules for drug evaluation using droplet-based microchip
Xindi Sun, Qirui Wu, Wei Li, Xiuqing Gong, Jun-Yi Ge, Jinbo Wu, Xinghua Gao
Materials Genome Institute, Shanghai University, Shanghai 200444, China
Keywords:PEGDA microcapsule Droplet-based microfluidic PC12 cells Drug evaluation
ABSTRACT Droplet-based microfluidic technology can be utilized as a microreactor to prepare novel functional monodisperse microcapsules.In this study, a droplet-based microfluidic chip with surface modification,which allowed the one-step preparation of double emulsion microcapsules.An O/W/O double emulsion using polyethylene (glycol) diacrylate (PEGDA) solution as the intermediate water phase was prepared by regulating the hydrophilicity and hydrophobicity of the chip surface, with PEGDA microcapsules prepared using UV polymerization.And then anti-tumor drug paclitaxel and neurotoxin 6-OHDA were encapsulated in microcapsules for drug and toxicology evaluation, respectively.Compared to controls, drug-loaded microcapsules caused a significant increase in the death rate of PC12 cells.This indicates that the obtained drug-loaded microcapsules could be used in drug evaluation and potentially in drug screening and delivery.
Microcapsules enclose or adsorb solid, liquid, or gaseous material in a microscopic hermetically-sealed capsule through the use of inert natural or synthetic polymers.With the rapid advance of biotechnology, microcapsule technology has been used widely within biomedicine, with applications including drug delivery, tissue engineering, and biosensor, amongst others [1-4].However,shortcomings such as inadequate mono-dispersity and function,limited repeatability, and poorly controllable morphology have limited the development and application of microcapsules in biomedical science.Droplet-based microfluidic technology provides precise control of fluid flow, and shows great potential in the controlled generation of monodisperse emulsion droplets [5,6].Such characteristics, combined with appropriate materials and manufacturing methods, allow us to create functional microcapsules with a variety of complex structures and morphologies; in turn, these enable active substances to be directly encapsulated in microcapsules with well-defined functions [7-9].Here, droplet-based microfluidic chip allowing the one-step preparation of double emulsion microcapsules was established.An O/W/O double emulsion using PEGDA solution as the intermediate water phase was prepared by regulating the hydrophilicity and hydrophobicity of the chip surface, with PEGDA microcapsules prepared using UV polymerization(Scheme 1).The microchip could be used repeatedly by hydrophilic and hydrophobic modification method.We have also probed the application of drug-loaded PEGDA microcapsules in drug and toxicological evaluation.
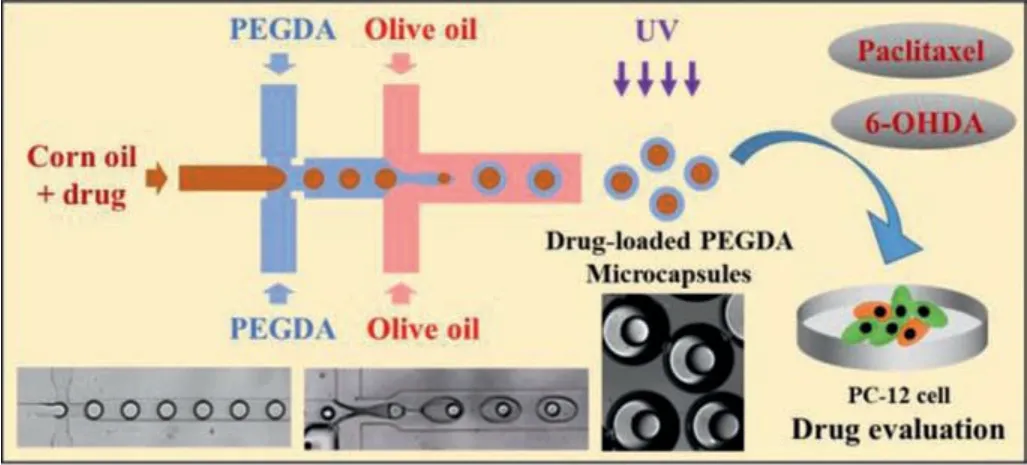
Scheme 1.Schematic diagram of preparation of drug-loaded PEGDA microcapsules using droplet-based microchip and its application in drug evaluation of PC12 cells.
In this study, droplet-based microfluidic chip was used to prepare O/W/O double emulsion in one step and then obtain microcapsules.A PDMS microchip was firstly prepared using soft lithography, as shown in Fig.1A.The microchip mainly comprises two convergence channels, with a height of 120 μm.Channel width on both sides of the first convergence decreases from 150 μm to 85 μm, while the channel width on both sides of the second convergence decreases from 250 μm to 150 μm.This design reduces the channel’s cross-sectional area at the point of convergence, speeding flow of liquid in the continuous phase on both sides, thus the continuous phases on both sides exert an enhanced pressure on the dispersed liquid in the middle channel, which stretches and fractures the dispersed phase to form droplets.We used 20% PEGDA as the water phase; this contains the photoinitiator 2-hydroxy-2-methylpropiophenone (2 wt%) and the surfactant Tween 20 (4 wt%).The corn oil and PEGDA/water phases together form O/W droplets at the first convergence and O/W/O droplets at the second convergence.After 405 nm ultraviolet light irradiation,the middle PEGDA/water phase of the O/W/O double emulsion solidified into a shell to form PEGDA microcapsules.
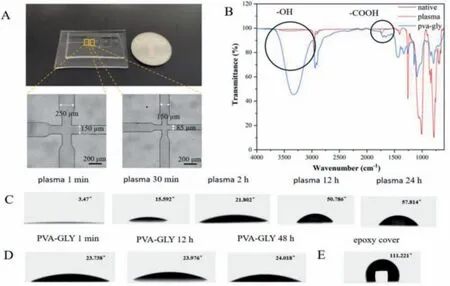
Fig.1.(A) Photograph image of the PDMS microchip and micrograph of two convergent channels; Scale bar:200 μm.(B) FTIR spectrum of PDMS after untreated, plasma treatment and PVA-GLY modified liquid treatment.(C) The change of contact angle between PDMS surface and water after plasma treatment with time.(D) The change of contact angle between PDMS surface and water after PVA-GLY modified liquid treatment with time.(E) The contact angle between the part covered by epoxy glue and water.
As far as droplet-based microfluidic technology is concerned,O/W droplets are easy to achieve, but O/W/O droplets have certain requirements for the hydrophilicity and hydrophobicity of the microchannel surface.We modified the microchannel surface to generate O/W/O droplets.Modification methods were evaluated,including plasma and chemical coating methods for hydrophilic modifications, and epoxy resin glue for hydrophobic modification.Generally, PDMS is hydrophobic under normal conditions, and the water contact angle is greater than 90° [10].First, plasma treatment was undertaken for 2 min on the PDMS surface, generating many surface active groups.Analysis using FTIR spectroscopy of the chip surface indicated the presence of hydroxyl and carboxyl functional groups (Fig.1B) [11], transforming the chip surface from hydrophobic to hydrophilic.The water contact angle simultaneously fell to almost zero.Fig.1C shows how the contact angle changed after different storage periods.We found that the PDMS surface could return quickly to its original hydrophobicity.This greatly limits the available time of the chip.To improve the effect of hydrophilic modification, we also modified the PDMS surface with chemical coating method:Polyvinyl alcohol (2 wt%) and glycerol (5 wt%) (PVA-GLY).Similarly, to plasma treatment, FTIR spectroscopy analysis indicated the presence of hydrophilic functional groups (Fig.1B).Fig.1D indicates that prolonged storage at room temperature maintains the water contact angle of the modified PDMS surface at approximately 24°, and that the PDMS surface was more hydrophilic.In addition, the hydrophilicity of the chip could be maintained for longer compared with plasma treatment.To modulate the relative hydrophobicity of the chip surface, epoxy glue was applied to the required parts of the chip and substrate before they were sealed.The contact angle between the part covered by epoxy resin and water was approximately 111° (Fig.1E).The pattern covering the epoxy resin on the chip can maintain the hydrophobic properties of the PDMS.Our experimental protocol can control the surface wettability of the PDMS chip, allowing different wettability on different areas of one chip.The microchip could be used repeatedly by hydrophilic and hydrophobic modification method.
After PDMS chip modification, fluorescence characterization of the hydrophilic and hydrophobic areas can be seen in Fig.2A.We injected a 0.026 mmol/L Rhodamin-123 (RH-123) solution into the microchannel and then remove it.No RH-123 solution remained on the hydrophobic regions (Fig.2B).However, RH-123 solution did remain on the hydrophilic areas (Fig.2C), which was indicated by the green fluorescence seen on the inner wall of the hydrophilic areas.After treating the chip surface, the innermost oil phases no longer adhered to the middle channel’s inner wall.Figs.2D and E show droplet formation before and after modifying the chip.No droplets can be produced on an unmodified chip.However, after modification, droplets can be produced smoothly and evenly.On this basis, we tried to generate one-core and two-core microcapsules, as shown in Figs.2F and G.
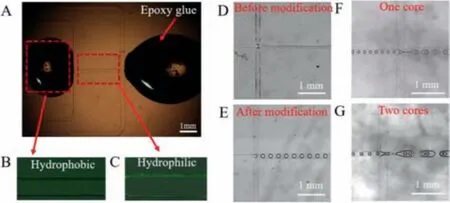
Fig.2.(A) Hydrophilic and hydrophobic characterization of modified chip.(B) Fluorescence characterization of hydrophobic regions.(C) Fluorescence characterization of hydrophilic regions.(D) Image without droplets generated on unmodified chip.(E) Image with droplets generated on modification chip.(F) Image of preparation of one core microcapsules.(G) Image of preparation of two cores microcapsules; Scale bar:1 mm.
In addition, during formation of double emulsion microcapsules, due to the interfacial tension between liquids, careful selection of a suitable outermost oil phase is needed to form a stable double emulsion.We experimented using mineral oil, FC40, and olive oil, finding that only olive oil generates stable double emulsions without adhesion (Fig.3A).Therefore, we used olive oil as the outermost oil phase.We also experimented with several inner oils and found that the type of inner oil had little effect on the system.Considering the biocompatibility, heat resistance and stability of corn oil, we finally used corn oil as the inner phase oil[12].An injection pump was used to adjust the flow rate of each phase, allowing us to adjust core and shell size and the number of cores.Results are shown in Figs.3B and C.For the first channel converges to form core droplets, while keeping the innermost phase flow rate (QI) constant, the core size decreases as the intermediate phase flow rate (QM) increases.For the second channel converges to form a double emulsion, while keeping the flow rate of the innermost phase (QI) and the middle phase (QM) constant,the shell size will increase as the flow rate of the outermost oil phase (QO) decreases.Moreover, an appropriate flow rate can be selected to generate the required core-shell size.By changing the flow rate, multi-core microcapsules can be prepared.If the outermost oil phase flow rate continues to decrease, multiple oil cores will accumulate before shearing, forming microcapsules with two cores (Fig.3D).PEGDA microcapsules were prepared using the double emulsion template method.Fig.3E shows dried PEGDA microcapsules observed using a scanning electron microscope.Fig.3F shows a microcapsule rupture image.Our results indicate we can prepare PEGDA microcapsules with a core-shell structure.We can successfully prepare O/W/O droplets after controllable modification of the chip surface.In addition, to characterize the release of smallmolecule drugs in microcapsules, we use coumarin 6, which has a molecular weight close to that of paclitaxel and 6-OHDA, as a dye for the drug to verify drug release.We encapsulated coumarin 6 in microcapsules, and the fluorescence intensity of the released coumarin 6 was measured every day, as shown in Fig.3G.The results showed that the normalized fluorescence intensity (Ii/Ib)increased gradually with time and reached balance in 7-10 days.In addition, for the stability of microcapsules, it is reported that PEGDA had good biocompatibility and stability [13].Our experimental results also showed that PEGDA microcapsules could stably release drugs.
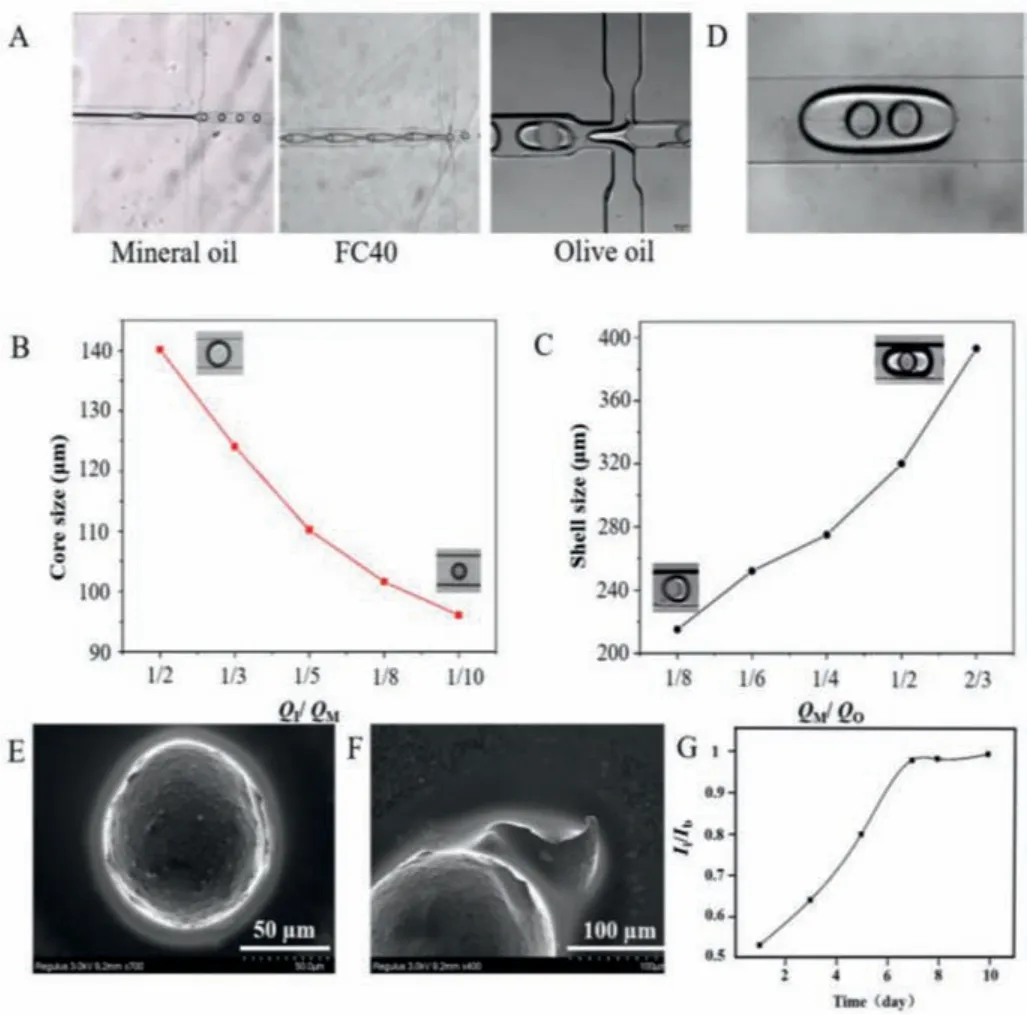
Fig.3.(A) Optical image of double emulsion formed by different oil phases as the outermost continuous phase.(B) Diagram of the relationship between core size and flow rate (QI/QM) for the first channel converges to form core droplet. QI was 1 μL/min.(C) Diagram of the relationship between core size and flow rate (QM/QO)for the second channel converges to form a double emulsion. QI was 1 μL/min, QM was 3 μL/min.(D) Image of two cores microcapsules.(E) SEM image of the complete shape of PEGDA microcapsules; Scale bar 50 μm.(F) SEM image of the microcapsule shell:Scale bar 100 μm.(G) The curve of drug release of coumarin 6. Ii was fluorescence intensity of coumarin 6 released from microcapsules under different measuring time. Ib was fluorescence intensity of coumarin 6 released from microcapsules at balance.
To verify the utility of preparing PEGDA microcapsules in drug evaluation and toxicology evaluation, we encapsulated the anticancer compound paclitaxel at a concentration of 16 μmol/L and the neurotoxin 6-OHDA at a concentration of 40 μmol/L in PEGDA microcapsules respectively.According to the size of the microcapsules, we estimated that the drug loadings of paclitaxel and 6-OHDA in the microcapsules were about 0.014 μg/mg and 0.013 μg/mg, respectively.PC12 cells are rat adrenal pheochromocytoma cells.Anti-tumor drugs are known to decrease the viability of PC12 cells.Neurotoxins can also damage PC12 cells [14,15].As shown in Fig.4A, PC12 cells were treated with pure PEGDA microcapsules,with approximately 1 × 103mL-1PEGDA microcapsules encapsulating paclitaxel, and with 2 × 103mL-1PEGDA microcapsules encapsulating paclitaxel.The rate of cell death after adding pure PEGDA microcapsules was below 5%, indicating that pure PEGDA has little effect on cells, and it can be used as a control group for cell experiments.Compared to control group, paclitaxel-loaded microcapsule gave rise to significant PC12 cell death (P <0.001).The 2 × 103mL-1paclitaxel-loaded microcapsule group had higher cell mortality (P <0.001).In addition, the effect of the drug is also related to time, and the death rate on the third day was significantly higher than that on the first day.The similar results also appeared in the 6-OHDA-loaded microcapsule experiment, the results were shown in Fig.4B.The cell death rate was proportional to the concentration and time of the neurotoxin 6-OHDA loaded PEGDA microcapsules.This indicates that PEGDA microcapsules can be successfully used as drug carriers.
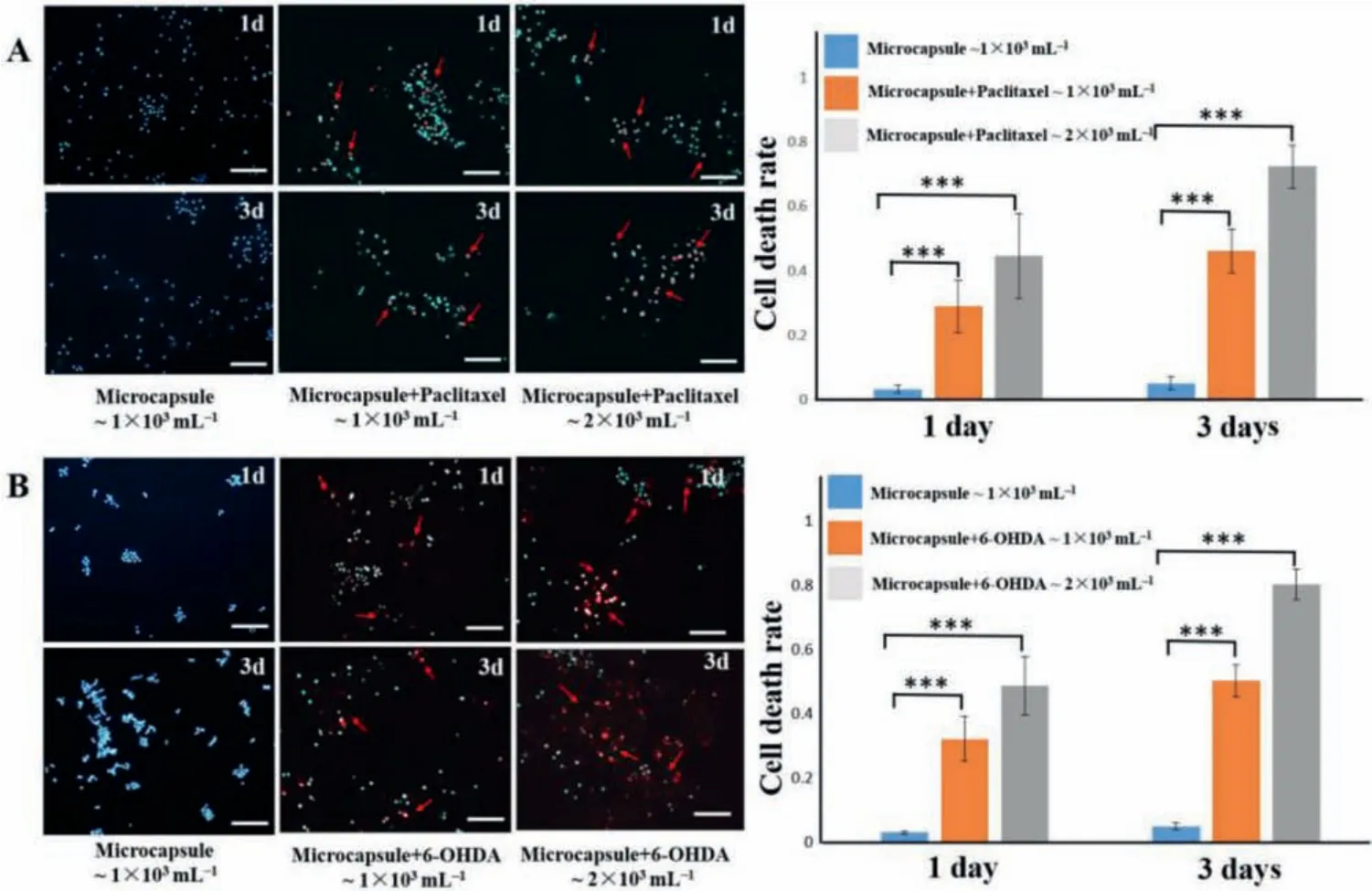
Fig.4.(A) Results of PC12 cells after paclitaxel-loaded PEGDA microcapsules treatment for 1 day (1d) and 3 days (3d).(B) Results of PC12 cells after 6-OHDA-loaded PEGDA microcapsules treatment for 1d and 3d.Blue:DAPI; Red:dead cells in PI staining; ***P <0.001; n = 9; Scale bar:100 μm.
In summary, a droplet-based microfluidic chip with a modified surface was established, which allowed the one-step preparation of double emulsion microcapsules.We used this approach to prepare O/W/O double emulsions, and then obtained PEGDA microcapsules with a core-shell structure by UV polymerization.Two kinds of drug-loaded microcapsules were prepared for drug and toxicology evaluation respectively, including anti-tumor drug paclitaxel microcapsules and neurotoxin 6-OHDA microcapsules.Compared to controls, drug-loaded microcapsules can cause a significant increase in the death rate of PC12 cells; resulting cell death was proportional to the concentration of the encapsulated drug, and at the same drug concentration, cell death was proportional to time.This indicated that the obtained drug-loaded microcapsules could be used in drug evaluation, and have potential to be used in biomedical related fields such as drug screening and delivery.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Nos.31800848 and 21775101).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
