Sn and Y co-doped BaCo0.6Fe0.4O3-δ cathodes with enhanced oxygen reduction activity and CO2 tolerance for solid oxide fuel cells
Nan Han, Rongzheng Ren, Minjian Ma, Chunming Xu, Jinshuo Qiao, Wang Sun,Kening Sun, Zhenhua Wang
Beijing Key Laboratory for Chemical Power Source and Green Catalysis, School of Chemistry and Chemical Engineering, Beijing Institute of Technology,Beijing 100081, China
Keywords:Solid oxide fuel cells Cathode Heterovalent co-doping Oxygen ion conduction Oxygen vacancy formation
ABSTRACT Applying mixed oxygen ionic and electronic conducting (MIEC) oxides as the cathode offers a promising solution to enhance the performance of solid oxide fuel cells (SOFCs).However, the phase instability in CO2-containing air and sluggish oxygen reduction activity of MIEC cathodes remain a long-term challenge for optimizing the electrochemical performance of SOFCs.Herein, a heterovalent co-doping strategy is proposed to enhance the oxygen reduction activity and CO2 tolerance of SOFCs cathodes, which can be demonstrated by developing a novel BaCo0.6Fe0.4O3-δ (BCF)-based MIEC oxide, BaCo0.6Fe0.2Sn0.1Y0.1O3-δ(BCFSY).In addition to improving the stability of BCF-based perovskites, this strategy achieves an optimized balance of ionic mobility and oxygen vacancies due to the synergies between the effects of the co-dopants.Compared with single-doped materials, BCFSY exhibits improved CO2 tolerance and considerably higher ORR activity, which is reflected in a significantly lower polarization resistance of 0.15 Ω cm2 at 600 °C.The results of this work provide an efficient tactic for designing electrode materials for SOFCs.
Solid oxide fuel cells (SOFCs), which can convert the chemical energy of fuels into electrical energy directly, have been deemed as promising power technology owing to their high chemical-toelectrical conversion efficiency, low environmental pollution, and the variety of potential fuels [1,2].However, the high operating temperature of SOFCs hinders their practical application which not only rapidly degrades the electrochemical performance but also challenges the compatibility between cell components.The key to reducing the operating temperature is to develop robust and stable cathodes because the sluggish oxygen reduction reaction (ORR)at low temperature (LT) (500–650 °C) and the poisoning by pollutants widely occurred in ambient air such as CO2are the major challenges for achieving high electrochemical performance of SOFCs [3,4].Therefore, there is a pressing need to develop suitable cathode materials with high ORR activity and extraordinary CO2tolerance.
The mixed ionic and electronic conductors (MIECs) that can broaden the fuel reactivity region from the three-phase boundary (TPB) to the entire cathode surface have gained extensive attention as cathode materials in which BaCo1–xFexO3-δ(BCF) perovskite oxides are the most popular materials.These MIECs have attracted extensive research attention as cathode materials and are often used as attractive parent constituents for numerous cobaltcontaining perovskite oxides [5,6].Because of the large size and low divalent state of Ba2+, these materials can offer adequate oxygen vacancies and lattice free volume to promote the migration of oxygen ions in the lattices of the perovskites [7].Moreover, because of the inclusion of the transition metals cobalt and iron,which possess alterable valence states, BCFs demonstrate excellent catalytic activity.However, the phase structure of BCF is unstable.Owing to the large mismatch in ionic size between Ba and Co/Fe,these BCF-based perovskites change from a cubic phase to a hexagonal phase at low temperature.The resulting poor stability hinders the development of BCF-based cathode materials [8].Consequently,stabilizing the phase structure of BCF-based materials is essential to fulfill the requirements of efficient cathodes for LT-SOFCs.
A mono-doping approach is often adopted to stabilize the cubic phase structure of BCFs.Cations with high or low valence have been doped into the partial B site to maintain the cubic phase structure of BCFs, such as Ta5+, Y3+, Sn4+, Nb5+[9–12].Doping with cations at the B site increases the electrostatic repulsion between BO6octahedrons, and thereby promoting the formation of the cubic structure [13].Unfortunately, although this mono-doping strategy demonstrates an effective approach to stabilize the structure of BCFs, it also leads to a high formation energy of oxygen vacancies and sluggish oxygen ion migration.The stable valence ions occupying the Co position will destroy the continuity of the Co–O–Co (Fe) bond, and inevitably reduce its oxygen electron–ion conductivity, which decrease the catalytic activity of the cathodes to ORR [9].The increase in the number of single dopants can improve the CO2tolerance at the expense of losing ORR activity.
Herein, we propose a new doping philosophy to enhance the ORR catalytic activity and the phase stability of BCF-based perovskite in CO2-containing air, which is demonstrated by doping heterovalent metal ions (Sn4+and Y3+) into the B site of BaCoFeO3materials [BaCo0.6Fe0.2Sn0.1Y0.1O3-δ(BCFSY)].The doping of Y can change the electronic affinity and promote the generation of intrinsic oxygen vacancies.Moreover, doping Sn at the B sites of BCFs effectively activates the M–O bonds and improves the migration of oxygen ions.The synergetic effect of heterovalent co-doping can generate an optimized equilibrium of ion mobility and the generation of oxygen vacancies and thus yield excellent ORR catalytic activity.Compared to doping with a single metal element, the strategy of heterovalent co-doping can not only achieve excellent durability in air containing CO2, but also provide superior oxygen ion conductivity, and thus embodies an advanced concept in the development of highly efficient cathodes for SOFCs.
To prove the successful incorporation of Sn and Y, X-ray diffraction (XRD) in air was first used to analyze the phase structure of the powders.All materials possess the perovskite crystal structure without any obvious impurity phases (Fig.1a).The (110) diffraction peak in BCFY, BCFSY, and BCFS is found to gradually shift toward higher angles, indicating a decrease in the lattice volume,which can be ascribed to the slight difference in ionic radii of Sn and Y (Sn4+= 0.069 nm, Y3+= 0.090 nm).To obtain more information on the crystal structures of these materials, the acquired XRD information of BCFY, BCFSY, and BCFS in air were analysed via the Rietveld method (Fig.1b, Figs.S1 and S2 in Supporting information).The low converged reliability factors (e.g.,Rp= 4.07%,χ2= 0.4206) illustrate that BCFSY can be divided into cubic perovskite structures (Fig.1c) based on the space group Pm-3 m [14],and the lattice constanta=b=c= 4.097 Å (Table S1 in Supporting information).The lattice volume increases in the trend BCFS<BCFSY<BCFY.Moreover, High-resolution transmission electron microscope (HRTEM) and their corresponding SAED patterns were used to further observe structural features (Figs.1d and e).The crystalline fringes possess a lattice spacing of 2.89 Å, corresponding to the (110) and (101) crystal plane of the cubic phase structure.As seen in Fig.1e, the SAED patterns near the center spot can be indexed to the (101), (211) and (110) planes in the view of the cubic unit cell, which further offer the evidence of the cubic structure of BCFSY.The results of the TEM and SAED show that the co-doping of Sn and Y can indeed stabilize the cubic phase structure of BCF.
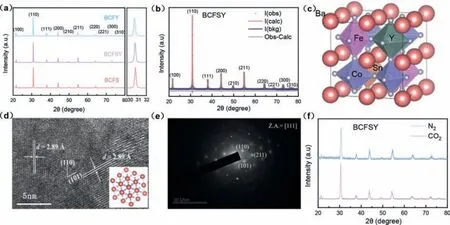
Fig.1.Powder X-ray diffraction patterns and crystal structure.(a) XRD patterns of BCFS, BCFY and BCFSY samples.(b) Rietveld refinement of BCFSY XRD data.(c) Crystal structure of optimized BCFSY.(d) HRTEM image of BCFSY.(e) Corresponding SAED pattern.(f) XRD patterns of BCFSY after sintering at 700 °C in 20% CO2 and N2 for 24 h.
The stability of the crystal structure, especially under low oxygen partial pressure and CO2atmosphere, is one of the important criteria for selecting suitable cathode materials [15,16].To check the stability of BCFSY, the XRD pattern of three samples obtained at 700 °C under N2/CO2for 24 h was compared.BCFSY still maintains its cubic perovskite structure when exposing in N2/CO2atmosphere, without other obvious impure phases (Fig.1f, Figs.S6 and S7 in Supporting information), confirming the phase stability of the BCFSY perovskite cathode.
The thermal expansion behavior is a crucial requirement for a stable performance of SOFCs.There should be a good match of the electrodes and the electrolyte in thermal expansion coefficient(TEC), otherwise the large difference in TECs between the SOFCs components may lead to internal stresses, which cause in performance degradation of the cathode.The TECs of BCFS, BCFY, BCFSY and SDC were measured (Fig.S9 in Supporting information).It can be seen that the TEC of BCFSY is 14.78 × 10-6K-1in the range of temperatures 150–1000 °C, which is lower than BCFS and BCFY.Besides, TEC of BCFSY is close to that of SDC (~12.6 × 10-6K-1)electrolyte, which is beneficial to the thermal stability of cathodes.
To investigate the electrochemical performance of BCFSY as an oxygen reduction electrode, symmetric cell configurations,i.e.,BCFSY|SDC|BCFSY, were constructed and tested in the temperature range of 500–650 °C (Fig.2a and Table S2 in Supporting information).It displays the typical electrochemical impedance spectroscopy (EIS) plots of BCFS, BCFY and BCFSY electrodes.The polarization resistances of BCFS, BCFY, and BCFSY at 600 °C is 0.21,0.29 and 0.15Ωcm2, respectively.It is clear that the polarization impedance (Rp) value of BCFSY is drastically decreased compared with those of BCFS and BCFY, revealing the outstanding ORR activity at low temperature.Moreover, the Arrhenius diagrams of the total polarization resistances of diverse samples (Fig.2b) shows that the activation energies (Ea) of BCFY, BCFS, and BCFSY are 1.17,1.15 and 1.10 eV, respectively.This strongly suggests that heterovalent co-doping can indeed significantly reduce the cathode polarization resistance, which is beneficial to the cathode ORR activity.
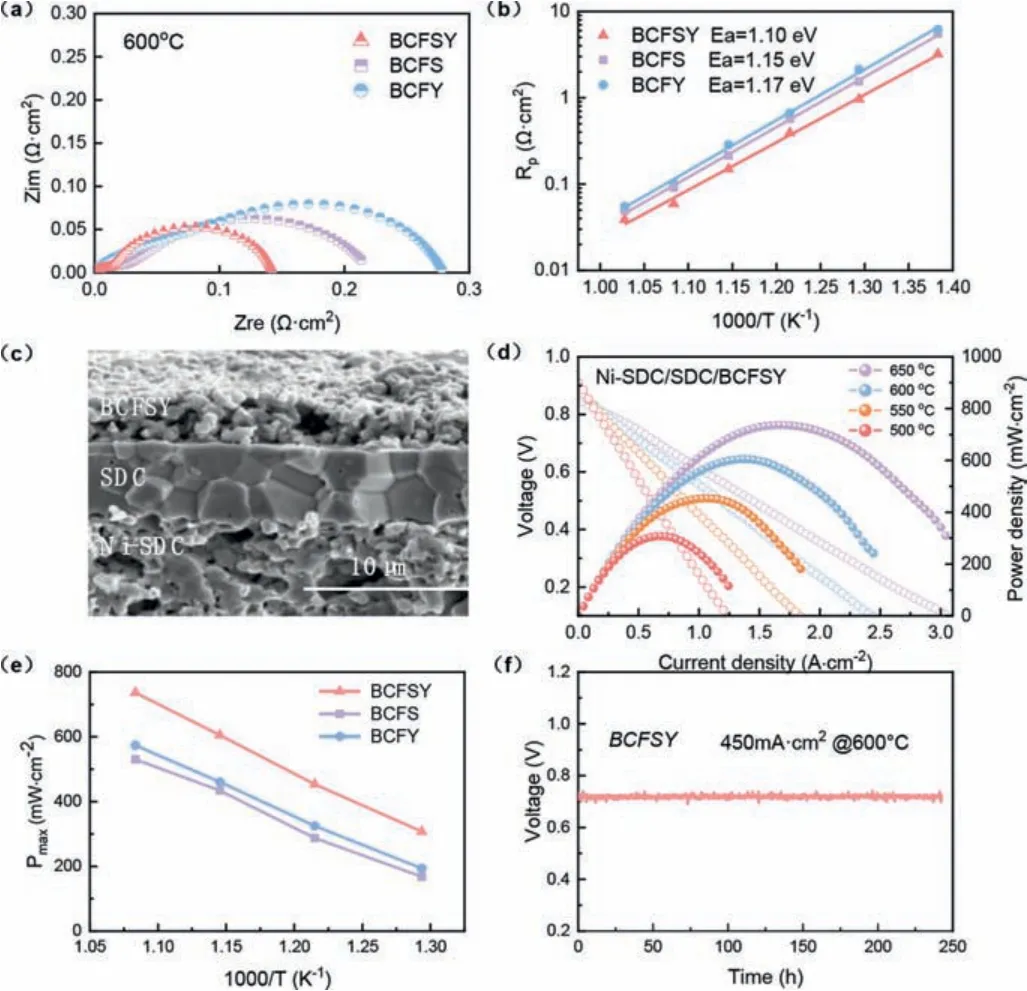
Fig.2.(a) Impedance spectra of three samples at 600 °C.(b) The Arrhenius plot of the total polarization resistance of different cathode materials.(c) A typical SEM image of an anode-supported single cell with a Ni-SDC anode, SDC electrolyte and BCFSY cathode.(d) Typical I-V-P curves of BCFSY-based single cells.(e) Comparison of the electrochemical performance of single cells based on BCFS, BCFY and BCFSY at different temperatures.(f) Full cell durability test for the BCFSY-based single cell operated at 600 °C.
In addition, a Ni-SDC-based anode-supported single cell employing an SDC electrolyte has been fabricated to estimate the output performance of the BCFSY cathode [17].The SDC electrolyte with a thickness of ~5 μm has a compact structure without connecting holes, ensuring a sealed operating environment (Fig.2c).The cell with the BCFSY cathode attains a maximum power density of 736 mW/cm2at 650 °C (Fig.2d), which is significantly greater than the power density obtained using the mono-doped cathodes(Fig.2e).In addition, the power density is also significantly better than the BCFT (120 mW/cm2) and BCFN (350 mW/cm2) reported in the literature (Table S3 in Supporting information).The single cell also shows low EIS values (Fig.S10 in Supporting information),thus increasing the power density and resulting in the excellent performance of the BCFSY.Notably, the single cell retains an extremely durable voltage output for 240 h under a steady polarization current density of 450 mA/cm2at 600 °C (Fig.2f), confirming that the BCFSY cathode can maintain outstanding ORR stability.
The performance of SOFC cathode mainly depends on the migration activity of O2-[18].In order to obtain a deep understanding of the superior ORR activity of BCFSY, the formation of oxygen vacancy and the activity of oxygen ion migration in the lattice of the materials were further studied.To analyze the formation mechanism of oxygen vacancy in BCFSY at room temperature,X-ray photoelectron spectroscopy (XPS) was initially carried out to acquire the information of O 1s in the three samples [19].As shown in Fig.3a, the peak at 528.3 eV is associated with the lattice oxygen (Olat), while the peak at 530.7 eV can be assigned to the adsorbed oxygen species (Oads) (Table S4 in Supporting information).The peak area of Oadsin the BCFY sample increases relative to BCFS and BCFSY.The increased proportion of Oadsindicates that the concentration of the adsorbed oxygen in BCFY increases,implying more oxygen vacancies are formed in BCFY [20,21].The reduction of lattice oxygen in ABO3materials at high temperatures is another way for the formation of oxygen vacancies.The formation of oxygen vacancy can be represented according to Eq.1:
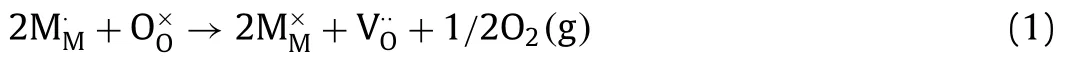
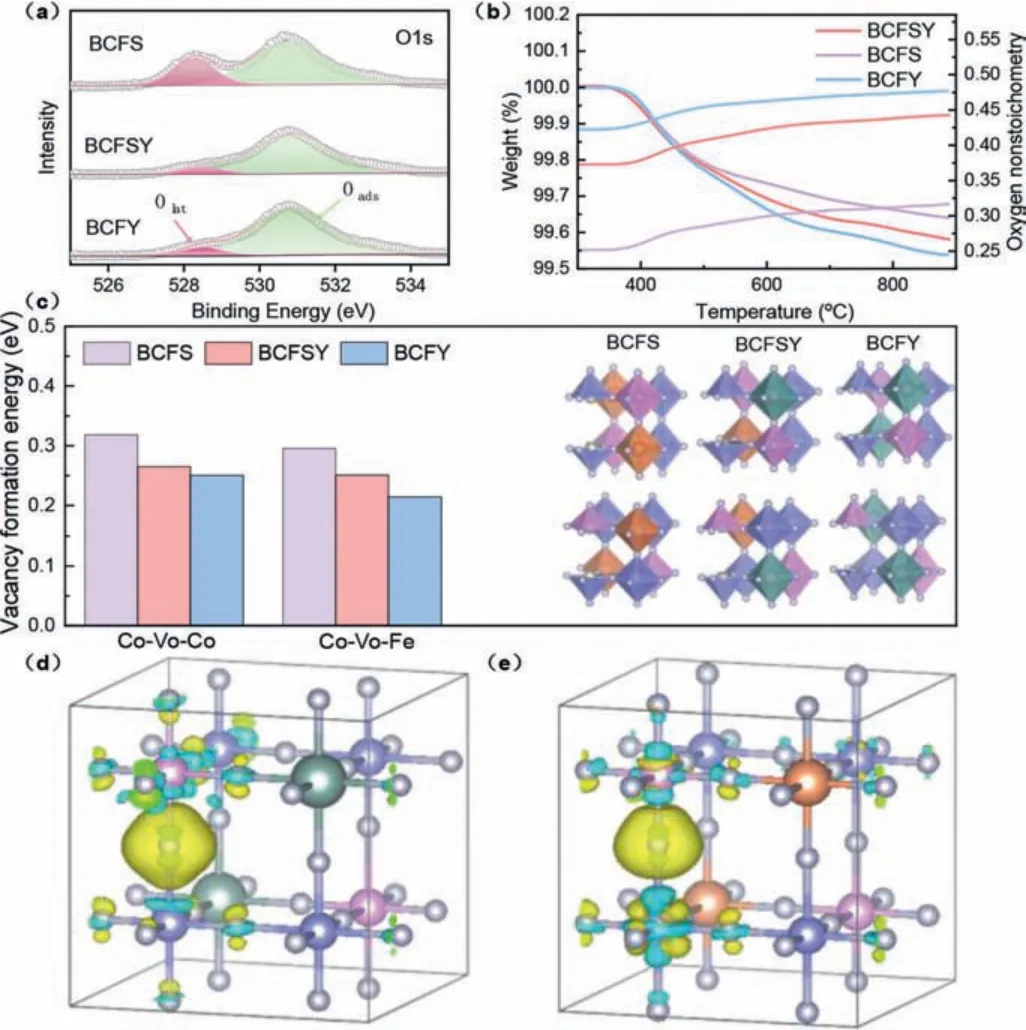
Fig.3.(a) O 1s XPS spectra of the samples.(b) TG patterns and oxygen nonstoichiometry in the temperature range of 300–900 °C in air.(c) Oxygen vacancy formation energy for different types by DFT computations.(d) The charge density distribution map in BCFY.(e) The charge density distribution map in BCFS.
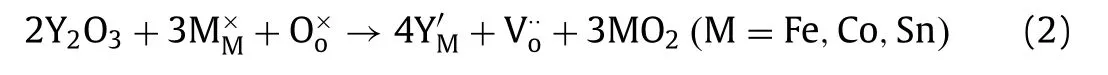
To understand the Y3+-doping on the formation of oxygen vacancies from an atomic point of view, first principles computations were implemented in BaCo0.6Fe0.2Sn0.1Y0.1O3-δ,BaCo0.6Fe0.2Sn0.2O3-δand BaCo0.6Fe0.2Y0.2O3-δ.The results ofEformare depicted in Fig.3c and Table S6 (Supporting information).It is obvious that the formation energy of oxygen vacancy is the lowest in BCFY regardless of at Co-O-Co or Co-O-Fe, implying that the formation ofis easiest in BCFY.We can explicate the cause of this trend by analyzing the changes in the electronic structure during the formation of[22].As one oxygen vacancy is formed, two electrons are left behind in the perovskite and the two electrons related to oxygen ions are rearranged.Accordingly, the formation of oxygen vacancies depends on the electron affinity of the metal ions involved.To visualize the electron rearrangement in BCFS and BCFY, the charge density difference based on the ground state of DFT was computed (Figs.3d and e).It is clear that the electronic density of the adjacent Co and Fe is changed by the leftover electrons from the oxygen vacancy.Hence, compared with BCFS, accepting electrons from the adjacent Co and Fe is easier owing to the presence of Y, which can explain why the oxygen vacancy formation energy of BCFY is the smallest.Combining the experimental results and theoretical calculations, we find that Y3+excites the formation of oxygen vacancies by adjusting valence and electron affinity.
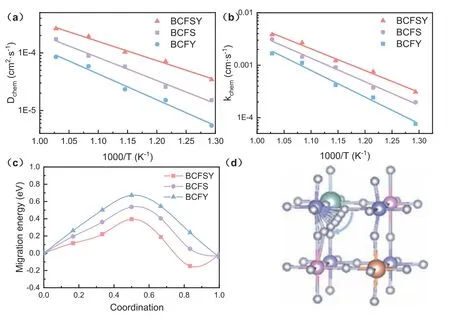
Fig.4.(a) Temperature dependence of fitted Dchem from 500 °C to 700 °C.(b) Temperature dependence of fitted kchem from 500 °C to 700 °C.(c) Calculated oxygen ion migration energy by DFT:migration barrier.(d) The migration path of the oxygen ion in BCFSY.
Identifying the oxygen-ion bulk diffusion coefficient (Dchem) and oxygen surface exchange coefficient (kchem), which are determined through the electrical conductivity relaxation (ECR) method, is essential for evaluating the MIEC behavior of layered perovskites and can be helpful in assessing both the migration rate of oxygen ions and the electrochemical activity of cathode materials[23].To illustrate the impact of Sn and Y heterovalent co-doping on MIEC performance,Dchemandkchemare assessed through the ECR method (Fig.S13 in Supporting information) and the data are shown in Figs.4a and b.In comparison, at 600 °C, theDchem(1.03 × 10-4cm2/s) andkchem(1.22 × 10-3cm/s) values of BCFSY are much higher than BCFS and BCFY, and are superior to the BCFS reported in the literature (Dchem:3.65 × 10-5cm2/s,kchem:2.23 × 10-4cm/s).Although the oxygen vacancy formation energy and oxygen vacancy concentration of the co-doped material BCFSY are not the lowest, we find that this sample shows the highestDchemandkchem, which indicates that the oxygen vacancy concentration is not the only factor that determines the mobility of oxygen ions.
Apart from electron affinity, M–O bond strength is another contributor to the migration energy of O2-.In order to explain how these factors synergistically effect the O2-migration in Sn and Y co-doped system, the oxygen ion migration barriers were calculated (Figs.4c and d, Table S7 in Supporting information) [24].It can be found that the energy barrier of BCFS is lower than that of BCFY, even though the formation energy of oxygen vacancy is higher than that of BCFY.These results suggest that the lower bond strength of Sn-O than that of Y-O bond is the main reason for the low energy barrier of BCFS.The lower bond strength of Sn-O can be explained by the higher d occupancy rate of Sn4+than Y3+, which is good to the formation of a weaker polar covalent,donor–acceptor Sn-O bond with lower bond strength.Due to the coexistence of Sn4+and Y3+in BCFSY sample, it can synergistically tune both the electron affinity and M–O bond strength, thus endowing BCFSY with the lowest oxygen ion migration barrier (Table S7) and most remarkable electrochemical performance (Fig.2b)among these three samples.
The high resistance to CO2poisoning of the SOFC cathodes matters a lot to the stable electrochemical output [25,26].To demonstrate the durability of the BCFSY cathode in the presence of CO2, the long-term stability of symmetrical cell configurations was tested in CO2-containing air at an operating temperature of 600 °C.As can be observed in Fig.5a, theRpvalues of BCFSY, BCFS, and BCFY electrodes remain steady at a CO2concentration of 1%–5%.Nonetheless, when the CO2concentration is increased to 10%, theRpof BCFS increases from 0.211Ωcm2to 0.253Ωcm2, and theRpof BCFY increases from 0.274Ωcm2to 0.329Ωcm2, whereas theRpof BCFSY only changes from 0.144Ωcm2to 0.147Ωcm2, which demonstrates the excellent stability of the BCSFY electrode.Moreover, when the CO2concentration is changed to 0, theRpof all the electrodes returns to their original values after a period of time,implying that CO2does not render an irreversible impact on the electrode structure under the working conditions of the fuel cell.Besides, the short-term stability test of the BCFSY electrode under CO2-containing air was further evaluated.As exhibited in Fig.5b, a symmetric cell with the configuration of BCFSY|SDC|BCFSY demonstrates a stableRpof around 0.147 after a 200 h operating period,clearly indicating that the BCFSY cathode has superior ORR activity and admirable CO2tolerance in 10% CO2-containing air at 600 °C.In conclusion, heterovalent co-doping perovskite BCFSY with stable phase structure was synthesized at low temperature.Comparing to BCFS and BCFY, BCFSY provides a favorable balance of oxygen vacancy content and ion mobility, which significantly improves the ORR activity.Accordingly, the highest power density, the distinguished durability in CO2-containing air, and the lowest Ea values were observed with the BCFSY cathode.Consequently, the cathode with high ORR activity and excellent durability in air with CO2of BCFSY holds great potential for application in SOFCs.This doping philosophy also provides new possibilities for the application of clean and efficient energy conversion technologies.
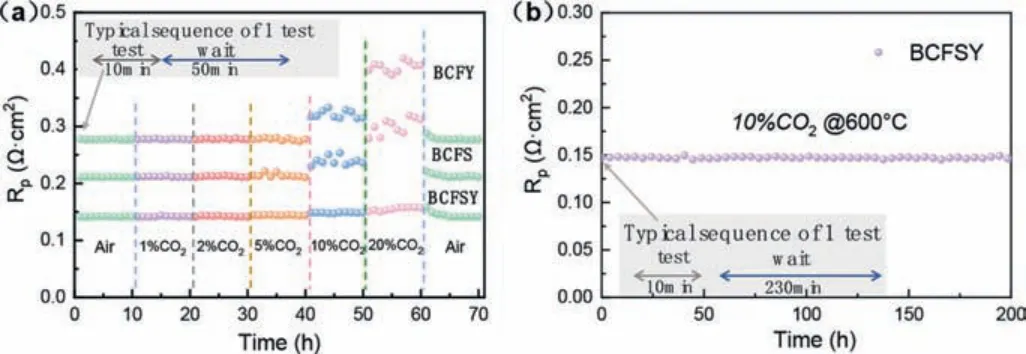
Fig.5.(a) Time-dependent Rp values of BCFSY, BCFY, and BCFS cathodes treated in different CO2 concentrations in air at 600 °C.(b) Durability test of a symmetric cell with the BCFSY cathode at CO2 concentrations in air at 600 °C for 200 h.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.22078022) and China Postdoctoral Science Foundation (No.2021M690379).We also thank Analysis & Testing Center, Beijing Institute of Technology for providing XRD equipment.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.100.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
