Keggin-type polycationic AlO4Al12(OH)24(H2O)127+ intercalated MoO3 composites for methyl orange adsorption
Qin Wng, Hongrui Tin, Zhong Zhng, Tinyi Dng, Wnyu Zhng, Jie Wng,Ying Lu, Shuxi Liu,*
a Key Laboratory of Polyoxometalate and Reticular Material Chemistry of the Ministry of Education, College of Chemistry, Northeast Normal University,Changchun 130024, China
b Department of Chemistry and Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen 518055, China
Keywords:MoO3 Keggin-type polycationic AlO4Al12(OH)24(H2O)127+Intercalation Adsorption Methyl orange dye
ABSTRACT Molybdenum trioxide (MoO3) can be employed as an excellent host for intercalation due to its 2D layered structure that connected by van der Waals interactions.Herein, a series of polyoxometalate-based MoO3 composites (Al13@MoO3) were successfully prepared by interpolating the Keggin-type polycationic AlO4Al12(OH)24(H2O)127+ (Al13) into MoO3 gallery.These composites can be applied to rapidly adsorb the anionic dye methyl orange (MO) through strong electrostatic interactions lead to compact and stable gathering in the surrounding of the numerous charged Al13.Adsorption behaviors of composites with the different amount of Al13 were determined, these results revealed that Al13-3.34%@MoO3 exhibited the most remarkable adsorption capacity.More importantly, the composite maintains superior adsorption capacity for five consecutive adsorption/desorption cycles, suggesting that Al13@MoO3 can be an efficient and durable adsorbent.
Methyl orange (MO) is a kind of azobenzene sulfonic acid dyes with complex aromatic ring, which can cause considerable damage to human health originated from carcinogenic and mutagenic effects.In recent years, a wide variety of methods have been employed to remove MO such as chemical oxidation [1], degradation[2,3], membrane separation [4], ion exchange [5] and adsorption[6,7].Among these methods, the adsorption-based process is one of the most favorable approach strategies in terms of versatility,simplicity, promptness, and efficiency.Traditional adsorbent like activated carbon faces poor efficiency and expensive price issues[8].Currently, novel generation materials are considered as excellent adsorbents, including zeolites [9], functionalized clays [10] and some composite materials [11].Within these substances, intercalation composites are emerging as promising adsorbent materials due to their potential advantages, including well-ordered layered structure, larger gallery space and controllability of interlayer species [12].
MoO3is a 2D layered structure with covalently bonded oxide layers separated by van der Waals interactions [13].In some cases,the 2D layered structure of the host lattice can slack as guest species are immobilized, forming intercalation materials with extension of the interlayer separation.In the previous works, small molecules such as acrylamide [14], nicotinamide [15] can be introduced into MoO3under room temperature stirring, while larger molecules cannot be inserted diametrically.A method is proposed that MoO3can be reduced to [Na(H2O)2]xMoO3using for ion exchange based on Na(H2O)2+is replaced by macromolecular to achieve intercalation [16–19].For example, Nazaret al.utilized Al13to exchange Na(H2O)2+further to obtain Al13pillared microporous MoO3hybrid materials, the gallery spacing enlarged to 17.35 Å[20].However, the combination process of [Na(H2O)2]xMoO3has been protected under N2and sample deposits in N2dry-box [21].Surprisingly, Pan took a simple and convenient strategy directly to interpolate different lengths alkylamine into MoO3layers [22].Among them, MoO3intercalation material (MoO3-DDA) through introducing dodecylamine (DDA) into MoO3improves the interval from 6.9 Å to 30.86 Å, and the larger space area is more conducive to import other guest molecules, which provides an exceptional technology for the preparation of intercalation composites.
Polyoxometalates (POMs), as one kind of significant metaloxide clusters with multiple charge have been employed in dyeadsorption [23,24].Regrettably, the high solubility in aqueous solution cannot realize selective separation and reuse.A series of outstanding works have been executed to incorporate POMs into layered compounds to prepare POMs-based intercalation composites [25,26].For example, Lesbaniet al.introduced[α-PW12O40]3–into Mg/Fe layered double hydroxides (LDH) host,extremely improved Cd2+adsorption [27].Similarly, Xuet al.reported [P2W17]10–and [CoW12]5–intercalated ZnAlFe LDH, which could enrich with the adsorption function for cationic dye methylene blue [28].Despite above progress achieved, cationic POMs like AlO4Al12(OH)24(H2O)127+(Al13) as guest to construct intercalation composites suffer setbacks, searching for an advisable host encapsulate cationic POMs as an imaginative point.
Herein, we successfully prepared a series of Al13@MoO3composites with different Al13loading by implanting Al13guests into extensive airspace of MoO3-DDA-5 which the molar ratio of DDA to MoO3is 5, as shown in Scheme 1.These composites demonstrate a certain adsorption capacity for MO due to the electrostatic interaction effects between cation Al13and anion MO.Among these,Al13-3.34%@MoO3exhibits superior performance with the rate up to 93% in 20 min as well as adsorption capacity to 357.2 mg/g,which is a useful adsorption material to eliminate MO.
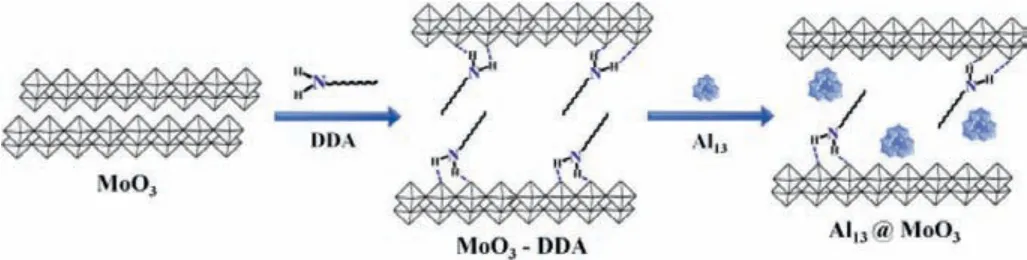
Scheme 1.Preparation methods of MoO3-DDA and Al13@MoO3.
MoO3-DDA-5 hybrid was synthesized by the literature method[22] and indicated 30.89 Å separation interstice (Fig.S1a in Supporting information).The van der Waals diameter of Al13cluster is 10.5 Å [20], therefore, it can migrate into the MoO3-DDA-5 passageway through the capillary phenomenon.Furthermore, replacement of Al13solution each 24 h results in Al13embedding easily in MoO3-DDA-5 thoroughfare derived from concentration disparity between inside and outside of the layer.Subsequently, Al13@MoO3composites with diverse Al13concentration are prepared, denoted as Al13-2.51%@MoO3, Al13-3.34%@MoO3, Al13-4.36%@MoO3, respectively.
The PXRD patterns indicated that Al13@MoO3appeared new characteristic diffraction peaks at 2θ= 3.30° and 2θ= 6.60° compared with MoO3-DDA-5 (Fig.1a).Layer spacing can be calculated by 2dsinθ=nλ, the consequent of 26.77 Å is narrower than 30.89 Å of MoO3-DDA-5.The variability of layer interspace could be associated with introduction of Al13due to the diameter of Al13is smaller than DDA molecular size.It is worth noting that Al13@MoO3exhibit two spacings of 30.89 Å and 26.77 Å, which corroborated incompletely deintercalated for DDA molecules.In addition, sharp peaks gradually enhance at 3.30° and 6.60° with increasing of immersion time, which can probably be attributed to the improved content of Al13.
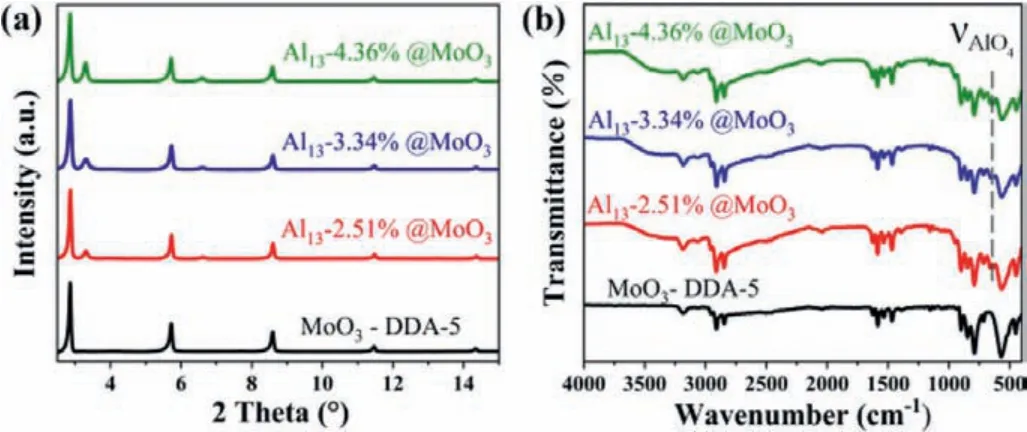
Fig.1.MoO3-DDA-5 and Al13@MoO3 with different Al13 con-centration:(a) PXRD patterns.(b) FT-IR spectra.
The FT-IR spectra of Al13@MoO3composites were obtained in the range of 4000-400 cm–1(Fig.1b).Al13@MoO3composites exhibit a new characteristic peak at 630 cm–1, which is attributed to the AlO4symmetric stretching vibration peak, and the characteristic peak at 3700–3400 cm–1correspond to the stretching vibration of the –OH on the Al13cluster surface [29], further verified that Al13is successfully encapsulated into MoO3-DDA.
To further explore the morphology of composites, scanning electron microscope (SEM) images were observed, as shown in Figs.S4a–e (Supporting information).MoO3-DDA-5 exhibits a special uniform flake morphology and smooth surface, but the size significantly shrinks than MoO3.The morphology of Al13@MoO3composites gradually changed.Due to immersing time influence of Al13, Al13@MoO3appear cracks and holes.To define the content of elements in the Al13@MoO3composites, energy dispersive spectroscopy (EDS) experiment was determined (Fig.S5 in Supporting information), suggesting that the weight percentages of Al in the three composites were 2.28 wt%, 2.93 wt% and 3.50 wt%, respectively.It is worth noting that the content of Al13is proportional with immersion time.The same conclusion can be further confirmed by elemental analyses (Table S1 in Supporting information).The elements of Mo, Al, C, N and O in Al13-3.34%@MoO3were corroborated by element X-ray mapping as shown in Fig.S4 (Supporting information), indicating Al and other elements are uniformly distributed on this composite.
We choose the typical anionic dye MO as the research substance to assess the capacity of these composites.Considering that MoO3and MoO3-DDA-5 also have ordered layer structures could accommodate pollutant, we investigated the adsorption performance for MO.The adsorption curves indicate that MoO3shows unobvious adsorption capacity (Fig.S7a in Supporting information),while MoO3- DDA-m (m represents the molar ratio of DDA to MoO3, m = 1, 2, 3, 4, 5) composites exhibit a little bit better adsorption behavior (Figs.S7b and f in Supporting information).We found that the amount of adsorbed MO increased as the ratios of DDA raised from 1 up to 5, reaching the adsorption rate value of 82% after 20 min by MoO3-DDA-5 (Fig.S7f).The possible reason for the different adsorption behaviors is that layer distance with accommodating MO has impact on capability.The above completion leads to MoO3-DDA-5 is selected as intercalation target for the immersing of Al13.
Owing to the electrostatic attraction exist between Al13and anionic MO.We further studied the adsorption properties of Al13@MoO3(Fig.2a).Expectantly, the results manifest that the adsorption ability enhanced with the amount of Al13less than 3.34%, the rate value of Al13-3.34%@MoO3reach 61% within 1 min, 87.3% after 5 min, attaining 93% after 20 min as adsorption equilibrium already appeared, exhibiting impressive ability toward quickly remove MO.However, with the amount of Al13above 3.34%, the adsorption rate and adsorption capacity of Al13@MoO3composites initiate to decrease unexpectedly (Fig.2b, Figs.S7c-e in Supporting information).The possible reason for the above phenomenon is believed to be that strong electrostatic interaction [30,31] between Al13with highly positive charges and anionic dye MO is propitious to absorb MO, which is incarnated on the raising adsorbed amount of MO as the content of Al13below 3.34%.With the amount of Al13further increased, excessive Al13will occupy the original internal area of Al13@MoO3for accommodating MO dye, and the remaining space is inadequate to effectively encase much number of MO molecules, so that the adsorption capacity declined.
In addition, the adsorption capacity of Al13@MoO3composites are investigated by the 100 mg/L concentration of MO dye (Fig.2c),suggesting that the adsorption capacities of composites are 320.6 mg/g, 357.2 mg/g and 295.1 mg/g after 2 h, respectively.We found that the amount of MO adsorption at the beginning get better with the increase of Al13loading, then decreases, the consequent is in fair agreement with the previous adsorption behavior.Compared with other adsorbents previously reported in the literature,Al13-3.34%@MoO3composites have higher MO adsorption capacity value (Table 1) [32–39].
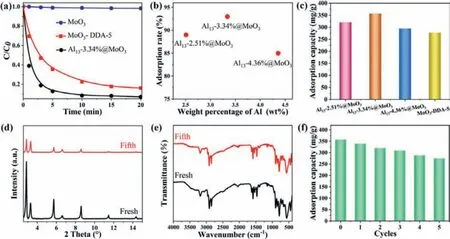
Fig.2.(a) The concentration changes of MO at different times (Adsorbent:40 mg, MO initial concentration:20 mg/L, volume:40 mL).(b) Adsorption rate of MO with Al13@MoO3 after 20 min (Al content measured by ICP).(c) Absorption capacity of Al13@MoO3 and MoO3-DDA-5 (Adsorbent:10 mg, MO concentration:100 mg/L, volume:100 mL).Al13-3.34%@MoO3 fresh and after five cycles:(d) PXRD patterns, (e) FT-IR spectra, (f) Absorption capacity.(Adsorbent:10 mg, MO concentration:100 mg/L, volume:100 mL).
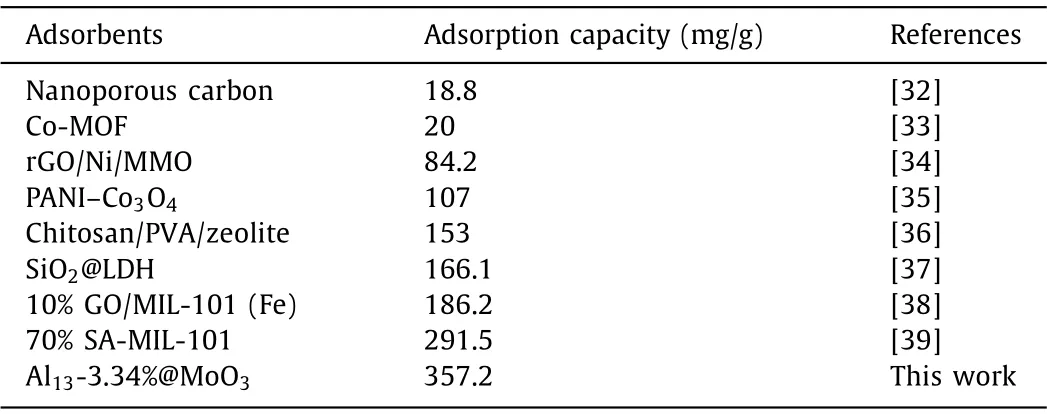
Table 1 The adsorption capacities of different adsorbents.
We have selected the material with the best adsorption effect to investigate its regeneration ability, PXRD patterns and FT-IR spectra indicate that the recycled adsorbent is consistent with fresh material (Figs.2d and e), the conclusion can be comprehended in terms of a recyclable material.The experiment result of the adsorption capacity for revived Al13-3.34%@MoO3indicates the amount of adsorbed MO decreased slightly (Fig.2f), which is attributed to the loss of Al13existing amid interlayers during multiple washing process.
In summary, we successfully prepared Al13@MoO3intercalation composites by encapsulating polycationic Al13clusters into MoO3layers area.These composites are utilized to evaluate the adsorption capacity of MO due to the electrostatic attraction interaction between cation Al13and anionic MO.The adsorption results show that Al13-3.34%@MoO3is provided with the noticeable amounts of MO adsorption capacity as 357.2 mg/g and the rapid rate reaches 93% after 20 min.Recycling experiments illustrate that the structure is pristine during the adsorption process, exhibiting favorable stability to MO removing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank the National Natural Science Foundation of China (Nos.21872021, 21671033, 22172022 and 22071019).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.079.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
