NiCe bimetallic nanoparticles embedded in hexagonal mesoporous silica (HMS) for reverse water gas shift reaction
Hui Di, Siqi Xiong, Yongqing Zhu, Jin Zheng, Lihong Hung, Chngjin Zhou,Jie Deng, Xinfeng Zhng
a College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China
b Department of Chemical Engineering, Sichuan University, Chengdu 610065, China
c School of Chemistry and Chemical Engineering, Yancheng Institute of Technology, Yancheng 224051, China
d College of Pharmacy and Bioengineering, Chengdu University, Chengdu 610106, China
Keywords:Greenhouse gases Reverse water gas shift reaction CO selectivity CeO2 Hexagonal mesoporous silica
ABSTRACT Reverse water gas shift (RWGS) reaction is a crucial process in CO2 utilization.Herein, Ni- and NiCecontaining hexagonal mesoporous silica (Ni-HMS and NiCe-HMS) catalysts were synthesized using an insitu one-pot method and applied for RWGS reaction.At certain reaction temperatures 500-750 °C, Ni-HMS samples displayed a higher selectivity to the preferable CO than that of conventionally impregnated Ni/HMS catalyst.This could be originated from the smaller NiO nanoparticles over Ni-HMS catalyst.NiCe-HMS exhibited higher activity compared to Ni-HMS.The catalysts were characterized by means of TEM,XPS, XRD, H2-TPR, CO2-TPD, EPR and N2 adsorption-desortion technology.It was found that introduction of Ce created high concentration of oxygen vacancies, served as the active site for activating CO2.Also,this work analyzed the effect of the H2/CO2 molar ratio on the best NiCe-HMS.When reaction gas H2/CO2 molar ratio was 4 significantly decreased the selectivity to CO at low temperature, but triggered a higher CO2 conversion which is close to the equilibrium.
Since 1950, the concentration of CO2attributed to human activities has risen by 30% [1].CO2emissions directly cause global warming, projecting the global average temperatures to rise by about 1.5-4.5 °C by 2050 if left unchecked.Moreover, the burning of fossil fuels and excessive deforestation trigger global warming.The capture, utilization, and transformation of CO2into other energy sources is a potential approach to addressing the menace.The improvement of chemical processes aimed at reducing CO2emission is a highly effective strategy.In the past year, many countries have unanimously declared that their economies will be carbonneutral.The CO2transformation to other energy sources reduces CO2emissions and alleviates energy shortages [2].
CO2is a thermodynamically and kinetically stable molecule,making it harder to lose electrons and form CO2+, while easier to accept electrons and convert C=O bond into C–O bond.Therefore,addition reactions are more likely to occur [3].For instance, H2reacts with CO2to form hydrocarbons,i.e., CO2hydrogenation reduction.One useful reaction is reverse water gas shift (RWGS), which produces CO, a main C1 chemical raw material that can be converted into other high-value products [4].Meanwhile, this process is accompanied by other side reactions, including over hydrogenation to generate methane [5,6].RWGS is a major factor influencing CO selectivity in RWGS reaction, where the activation of CO2requires significant energy.RWGS reaction is typically processed at high temperature (e.g.,>500 °C), requiring high thermal stability of the used catalyst.For this reaction, noble metals including Pt [7], Pd [8], Rh [9] and Au [10] were applied as catalytic active sites and exhibited excellent performance.Nonetheless, these metals are found in relatively low quantities on earth and at relatively high prices, making them difficult for industrial use.Therefore, research and development of non-precious metals including Cu [11–13] and Ni [14–17] are essential.In recent years, RWGS reactions have widely used Cu-based catalysts, as a substitute for noble metal catalysts.Supported Cu catalysts suffered from low stability in this reaction.For example, the surface area of Cu/SiO2was significantly reduced after a long-time reaction due to the sintering of the activity components [18].In contrast with single metal Cu,Ni exhibits better thermal stability for long-term reaction [19–21].Nevertheless, Ni faces the drawbacks of coke deposition and deactivation.Luet al.[22] observed different forms of NiO particles for the NiO/SBA-15 catalysts, only separated single NiO particles improved CO selectivity.Methanation seemingly occurs in aggregated NiO species at lower reaction temperature.Therefore, highly dispersed active Ni particles promotes higher CO selectivity.
Rare earth metal oxide CeO2as a promoter regulates many reactions due to its redox properties.In the case of dry reforming of methane reaction, the metal support interaction between NiO and ZSM-5 is enhanced by adding Ce as a promoter, which has positive to the catalytic activity [23].Catalysts prepared with different morphologies of CeO2as support loaded with Cu have been applied for RWGS, where the amount of surface oxygen vacancies and Cu0after reduction by H2increased.The rate of catalytic reaction corresponded with the amount of surface oxygen vacancies for CeO2,participating in CO2adsorption and activation [24].The insertion of transition metal like Ni into CeO2lattice to form Ce–O–Ni solid solution prevents the production of byproduct CH4[25], however,bulk phase NiO existing in Ni/CeO2is the active center for synthesis of CH4[26].Therefore, the high dispersion of Ni nanoparticles causes higher selectivity of CO [27].
SiO2mesoporous materials like MCM-41, SBA-15 and hexagonal mesoporous silica (HMS) have drawn significant attention due to their merits including large specific surface area, uniform pore size, and excellent stability, and have been widely used as support in catalysts [28–30].Among them, HMS with wormhole framework structure incorporates metal into its thick framework wall through neutral amine surfactant and inorganic precursors [31], which is beneficial for the diffusivity of chemical species.Moreover, HMS demonstrated a better thermal stability compared to MCM-41 and SBA-15 [32], also, metals can be uniformly embedded in the HMS skeleton [33–36].Wanget al.synthesized Ni-HMS catalyst using anin-situone-step process, which could perform long-term test at 700 °C in dry reforming of methane for 100 h without significant reduction due to the high dispersion of Ni active sites on support [37].Sn-HMS catalyst was prepared by a similar method for propane dehydrogenation reaction and remained stable for 170 h after three times of regeneration [38].
This study introduced a timesaving, easy-handling, and energysaving method for the preparation and characterization of nickelbased catalysts and for the first time, described its application in RWGS reaction.The Ni-HMS and NiCe-HMS were prepared using HMS as support to investigate the influence of reaction temperature from 500 °C to 750 °C for their catalytic performance in RWGS reaction.Further, a series of catalyst characterizations were conducted to better understand the structure and composition of the catalysts as well as explain their catalytic activity.We purposed to prepare a type of RWGS catalyst with effective CO selectivity and better stability using a simple and easy operation method.
The pore structures of three catalysts were investigated by N2adsorption-desorption at 77 K (Fig.S1a in Supporting information).For Ni-HMS and Ni/HMS samples, a relative pressure range of 0.3–0.5 suggested a Type IV isotherm [39].For Ni-HMS, the hysteresis loops illustrated that it primarily has a 1D cylindrical channel of uniform mesoporous [37].Nevertheless, after adding Ce into the HMS structure, the hysteresis loop area was reduced and no clear vertical asymptote displayed a Type Ⅰisotherm, which could be attributed to the certain pores of the were loaded by Ce species [40].Combined with pore size distribution curves (Fig.S1b in Supporting information), the pore diameter was concentrated between 2–5 nm on mesoporous.Among the three catalysts, the Ni/HMS exhibited the largest surface area 782 m2/g, pore-volume 0.87 cm3/g,and diameter 3.5 nm.These physical data were reduced when Ni or Ce nanoparticles were embedded into the structure of HMS, due to the fact that the incorporation of Ni and Ce into the HMS framework occupied a part of the surface structure.
TEM characterization was conducted on the samples to analyze the nanoparticle distribution of catalysts from the microstructure.The results are shown in Fig.1.HMS supported presents the wormhole-like framework.Ni-HMS and NiCe-HMS catalysts show the metal oxide particles ordered insert into the hexagonal framework.For Ni/HMS catalyst, the NiO particles were distributed on the surface of HMS by counting the diameter of NiO particles ranging between 3 nm and 12 nm.Combined with the actual content of Ni tested by ICP-OES shown in Table 1, Ni/HMS the content was low while the grain was large.This accounts for the most of NiO particles distributed out of the pore.The average particle size of the three catalysts ranged from large to small,i.e., Ni/HMS (7.1 ±0.2 nm)>NiCe-HMS (6.7 ± 0.2 nm)>Ni-HMS (6.5 ± 0.2 nm).Ni/HMS unlike the other two had a weak metal-support interaction.As for NiCe-HMS, NiO and CeO2nanoparticles co-existed on support, while the average particle size was calculated by all the particles.EDX mapping analysis was used to confirm the successful addition of Ce into NiCe-HMS catalyst (Fig.S2 in Supporting information).In NiCe-HMS, Ni and Ce were highly dispersed on the carrier HMS, suggesting that the introduction of Ce enhanced the dispersion of Ni and thus showed a significant structural advantage.
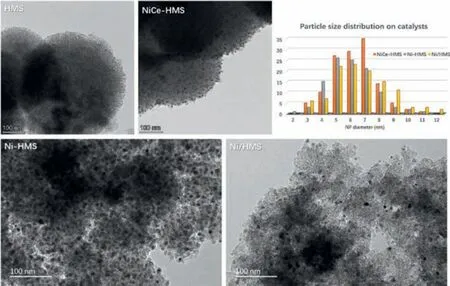
Fig.1.TEM images and nickel particle size distribution of the samples.
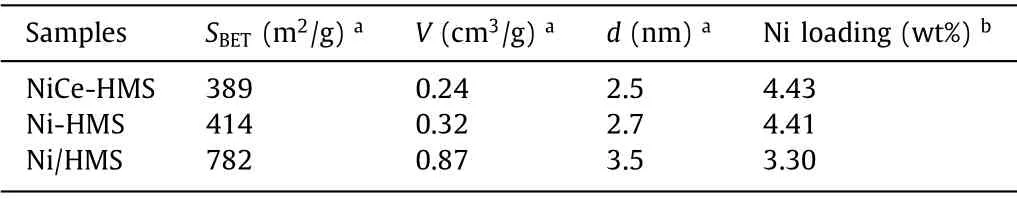
Table 1 Physicochemical properties of the catalysts
Characterization of the catalyst were performed by XRD(Fig.2a), and the typical peak at 22.9owas associated with supported HMS crystalline phase (JCPDS No.49-0623), which exists in three catalysts.In addition, others three peaks were belong to the(111), (200) and (220) crystal face of metal Ni, respectively (JCPDS No.04-0850) [41].For Ni-HMS and Ni/HMS, these peaks are relatively sharp, indicating that Ni species are relatively non-uniform and exhibit as larger particles.Combined with the actual content of Ni tested by ICP-OES showed in Table 1, the sharpest diffraction peak of Ni/HMS was found to have the smallest Ni loading of 3.3 wt%, illustrating that Ni nanoparticles are not well dispersed into the supported channel.The peak intensity of Ni-HMS with higher Ni content was obviously lower, indicating that the different preparation methods would directly influence dispersion of the active species.In contrast, NiCe-HMS had the highest loading of 4.43 wt%, while the diffraction peak was not obvious.Only Ni diffraction peak and no diffraction peak ascribed to the Ce metal was found,implying that Ce might increase the dispersion of Ni nanoparticles.All of the above results provide a good support for TEM observation.
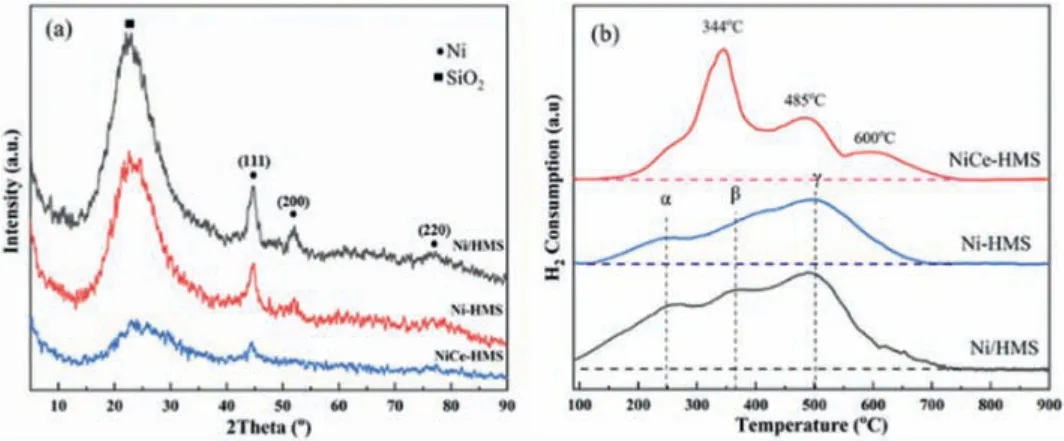
Fig.2.XRD partten (a) for the reduced catalysts and H2-TPR (b) profiles of the fresh catalysts.
H2-TPR test was used to explore the interaction between metal oxides and support (Fig.2b).By comparing all the three samples,the peak area of Ni/HMS was the highest.Combined with the previous analysis, the metal nanoparticles of the other two catalysts were partially embedded into the wall of support, causing a reduction of only a fraction of metal oxides.The reduction peaks of Ni-HMS and Ni/HMS exhibited similar temperature ranges, but different peak areas.The peak centered around 250 °C and 350 °C attributed the reduced surface of isolated bulk NiO particles and weak interacted with support, defined asα, β, respectively[41].The higher reduction peak range was represented asγ, ascribed to the reduction of NiO on the wall subsurface[37].In contrast with Ni-HMS and NiCe-HMS, the reduction temperature of Ni/HMS was relatively lower and intensity was relatively higher, deducing that the preparation method affects the interaction between the Ni and carrier [42].For NiCe-HMS catalyst, reduced peak at 344°C peak sharpness, attributed to NiO in close contact with CeO2and the chemisorbed oxygen in the oxygen vacancies of CeO2[43].500 °C-600 °C had the wide and lower intense reduction peaks,which belong to the simultaneous reduction of NiOxand surface CeO2[44].These two types of species were also the active sites of CO2.The high reduced temperature was ascribed to the reduction of metal particles embedded in mesoporous carriers [45].Therefore, thein-situpreparation method enhances the interaction between the active phase and support.The addition of Ce maintains the dispersibility of active metals, with lots of NiO distributed close to CeO2existing in the interface between Ni-Ce.
All the catalysts were applied in the RWGS reaction at the temperature range of between 500 °C and 750 °C.Fig.3 shows the conversion of CO2, selectivity of CO and CH4as a function of reaction temperature.Unlike NiCe-HMS, other catalysts revealed relatively poor catalytic performance in CO2conversion.Chenet al.[46], noted that the bare CeO2catalyst participates in CO2transformation in RWGS reaction, meanwhile they produced CO from the surface oxygen vacancies, causing a satisfactory catalytic performance of NiCe-HMS.Ni-HMS exhibits a CO2conversion of 3.7%in 500 °C, apparently higher than Ni/HMS.However, with increased temperature, CO2conversion of Ni/HMS was higher than Ni-HMS,suggesting that Ni/HMS exposed more Ni particles.For Ni-HMS, it had the highest CO selectivity of up to 99.9% among the three catalysts, benefiting from thein-situpreparation method that triggers uniform dispersion of Ni nanoparticles.The RWGS reaction was applied as an endothermic reaction, more favorable to enhance CO selectivity with the increase of reaction temperature.All the catalysts reached CO selectivity of 99.3% at approximately 750 °C.In contrast, the CO selectivity of Ni/HMS was low because the bulk Ni particles readily produced CH4[47].Conclusively, Ni-based catalyst prepared by thein-situmethod exhibited a higher CO selectivity than conventional prepared catalyst.Meanwhile, adding Ce increased CO2conversion.In combination with TPR, higher CO2conversion was ascribed to the neighboring oxygen vacancies forming the interface of Ni and Ce, active sites for CO2hydrogenation [48].A high dispersion of nanoparticles of NiCe-HMS increased the activity oxygen vacancies improving the efficiency in the RWGS reaction [48].
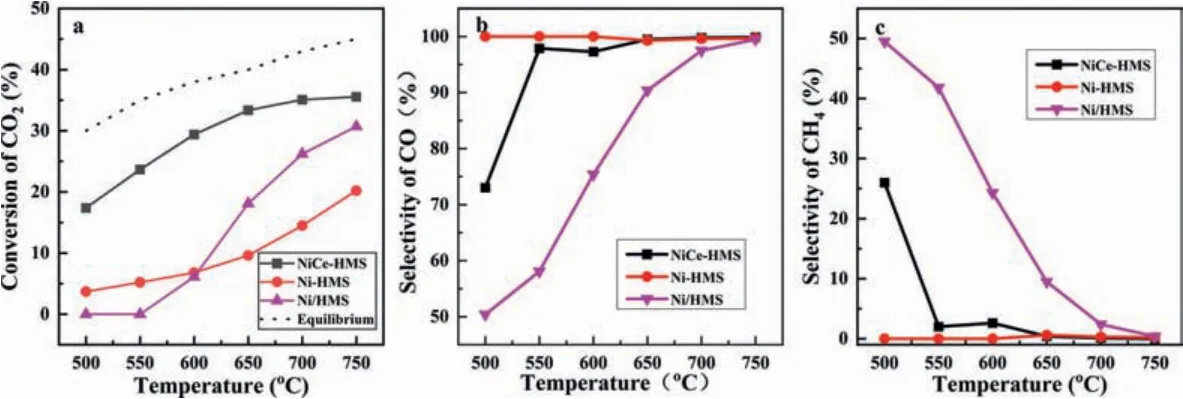
Fig.3.Catalytic activity of three catalysts applied to RWGS:(a) CO2 conversion; (b) CO selectivity; (c) CH4 selectivity.
The XPS in Fig.4a demonstrates the chemical states of Ni 2p for NiCe-HMS and Ni-HMS.Three peaks of Ni species were reported and the two lower binding energy represented different environments of surface Ni species.Binding energy around 854 eV consistent Ni(II)*species represented the bigger NiO particles.Binding energy at 856.9 eV defined as Ni(II), which was the presence of NiO highly dispersed on the support surface, created higher metalsupport interaction.The other broad peak was the shakeup satellite peak [49].Unlike the first two peaks intensity, Ni(II)*for Ni-HMS was significantly more than NiCe-HMS catalyst.Combined with the previous characterization analysis, this result once again confirmed that the doped Ce reduced the particle size of the active component.Meanwhile, it had better metal support interaction compared to the intensity of Ni(II).
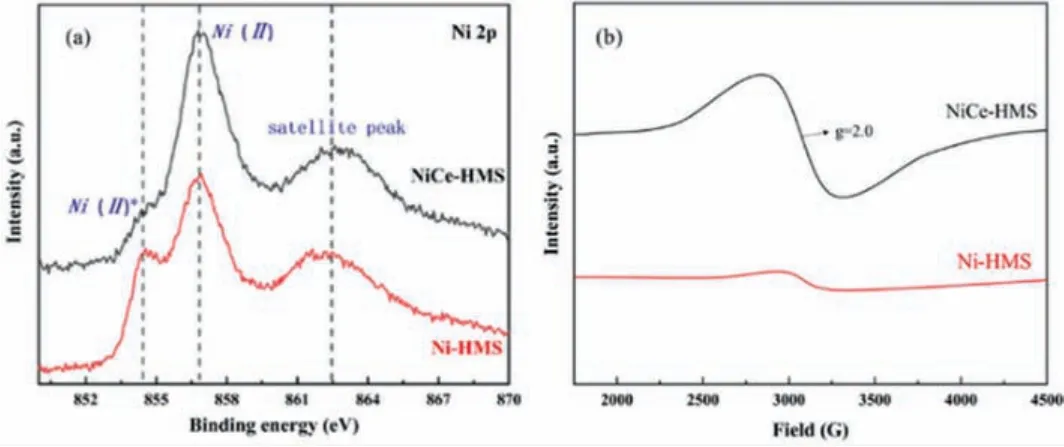
Fig.4.(a) Ni 2p XPS spectra of fresh catalysts NiCe-HMS and Ni-HMS.(b) EPR spectra of reduced catalysts Ni-HMS and NiCe-HMS.
EPR test was employed to further confirm the existence of oxygen vacancy.As shown in Fig.4b, at about g = 2.0, sample NiCe-HMS shows a higher EPR signal than sample Ni-HMS, which is a typical signal of oxygen vacancy (OVs) [50].The existence of oxygen vacancy prevents forming Lewis basic position, which may strongly adsorb and activate gaseous CO2and enhance the catalytic activity of RWGS.Which results also verified by CO2-TPD (Fig.S3 in Supporting information).
Effect of feed ratio on the catalytic activity, CO selectivity, and CH4selectivity were investigated on NiCe-HMS at 500–750 °C, and the results are shown in Figs.5a–c.As shown, with the increase of H2/CO2molar ratio from 1 to 4, CO2conversion increased, while CO selectivity decreased while CH4selectivity increased, specifically at lower reaction temperature [51], meanwhile, the CO2conversion was close to equilibrium.Ni demonstrated a better H2dissociation activity, when the H2feed ratio increased, a higher CH4formed specifically in 500 °C [52].The NiCe-HMS catalyst exhibited a high activity in RWGS with CO yield of up to 35.3% at a ratio of H2/CO2= 1, with a ratio of H2/CO2= 4 CO yield rising to 74.4% at 700 °C.Therefore, higher CO selectivity fed gas H2/CO2of 1, while, higher CO2conversion preferred the H2/CO2of 4.In order to demonstrate the stability of NiCe-HMS catalyst, it was used in reverse water gas shift reaction at 750 °C for 28 h.As shown in Fig.5d, the stability maintains well, albeit with high activity and stability for a long time.The conversion of CO2and selectivity of CO maintains stable, which can be attributed to the good thermal stability and resistance to carbon deposition of NiCe-HMS catalyst.Therefore, the NiCe-HMS catalyst may be a promising candidate for industrial application in the future.
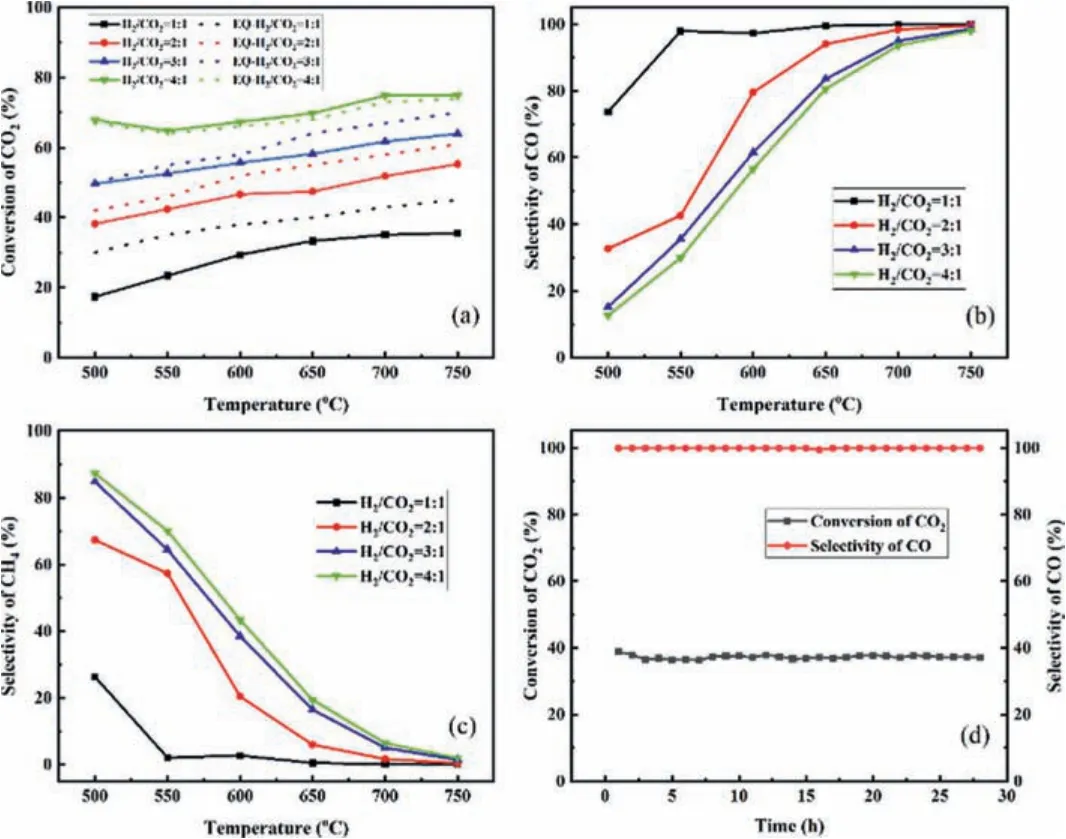
Fig.5.Catalytic activity of catalyst NiCe-HMS applied to RWGS at different inlet ratios:(a) CO2 conversion; (b) CO selectivity; (c) CH4 selectivity; (d) The catalytic activity of NiCe-HMS for 28 h at 750 °C.
In conclusion, NiCe-HMS and Ni-HMS were prepared by onepotin-situroute and applied in RWGS reaction at the temperature range of 500-750 °C.The selectivity to CO on these samples was significantly enhanced compared to conventionally impregnated Ni-HMS.This benefited from the highly dispersed Ni nanoparticles formed on the surface of the support, inhibiting the generation of CH4.In contrast with Ni-HMS, after adding Ce, the conversion of CO2increased due to the large amount of NiO nanoparticles existing close to CeO2.And a high dispersed Ni and Ce nanoparticles interface formed more active oxygen vacancies and efficient reaction sites for CO2hydrogenation.The catalysts prepared by the one-potin-situmethod outperformed those prepared by the traditional impregnation method.When the ratio of H2/CO2= 4, the conversion of CO2was close to the equilibrium.Our findings provide a promising strategy for the development of a bimetal catalyst system in RWGS.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
The authors thank to the Chengdu University of Technology Teachers Development Research Fund (No.10912-2019KYQD07266)and National Natural Science Foundation of China (No.21806015)for financial support.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.129.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
