Discovery of the anti-influenza A virus activity of SB216763 and cyclosporine A by mining infected cells and compound cellular signatures
Ke Tang, You Wu, Shubing Chen, Yijing Xin, Ying Guo,*
a State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
b Department of Pharmacology, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
Keywords:Chemical perturbagen signature Host factor Transcriptome signature reversion Influenza A virus SB216763 Cyclosporine A Drug combination
ABSTRACT In this study, SB216763 and cyclosporine A were identified as anti-influenza A virus (IAV) agents by transcriptome signature reversion (TSR) analysis through deep mining of the cellular transcriptome of human airway and lung cell lines infected with 3 strains of IAV and the chemical perturbations library.A synergistic effect of SB216763 and cyclosporine A against influenza A was disclosed by quantification of the network-based relationship, which was validated in vitro.Along with burgeoning omics approaches,transcriptome-based drug development is flourishing, which provides a novel insight into antivirals discovery with comprehensive cellular transcriptional information of disease and chemical perturbations in multicomponent intervention.This strategy can be applied as a new approach in discovering multitarget antiviral agents from approved drugs, clinical compounds, natural products or other known bioactive compounds.
Omics allows comprehensive and dynamic investigation of the full physiological and pathophysiological landscapes, which is promoting a revolutionary transition in biomedical fields, including drug development [1-3].Cellular chemical perturbation signatures,representing whole-transcriptomic changes in the cellular state upon treatment with compounds, offer a more efficient and innovative perspective in drug discovery than the classical targetedbased approach [4,5].
Influenza A virus (IAV) is a highly contagious pathogen that poses a significant threat to public health due to annual epidemics and recurrent pandemics [6].All regimens used against influenza are directed against viral components, which pose a challenge of drug resistance due to high mutability [7-9].As an intracellular pathogen, IAV infection highly relies on host factors, which makes targeting cellular factors an attractive strategy for discovery of antivirals with less possibility of drug resistance emergence [10].Progress in omics offers various data-driven approaches for compound screening [11,12].Transcriptome signature reversion (TSR)is an emerging strategy characterized by mining transcriptomics to identify compounds with the capability of reversing the signature gene expression of the disease state, which has been performed for various diseases, such as neurological disease [13,14], inflammatory disease [15] and cancer [16,17].
TSR involves three steps:identification of the disease signatures, extraction of chemical perturbagen signatures and reversal signature matching between the disease and compound treatment.In this research, for extraction of the comprehensive characteristics of IAV infection, cellular transcriptome datasets (GSE106279 [18],GSE32139 [19] and GSE37571 [20], Table S1 in Supporting information) from three human airway and lung cell lines infected with 3 IAVs were collected from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) [21].Differentially expressed genes(DEGs) of each dataset were analyzed by the limma R package, and the threshold for upregulated DEGs was set asPvalue<0.001 and fold-change>2 [i.e., Log2FoldChange (Log2FC)>1]; for downregulated DEGs, the threshold wasPvalue<0.001 and fold-change<0.5 (i.e., Log2FC<-1); and three DEG lists were achieved (Tables S2–S4 in Supporting information).The final signature genes with IAV infection were obtained by acquiring genes appearing at least twice in the three DEG lists, which comprising 213 upregulated and 15 downregulated genes (Table S5 in Supporting information).Nevertheless, as the host response against IAV infectionneeds to be considered, especially the innate immune response,the host factors against IAV infection were investigated comprehensively from clustered regularly-interspaced short palindromic repeats (CRISPR) or RNA interference (RNAi) screening studies reported previously [22-30].Consequently, forty-six genes were identified, and the direction of three genes,IFITM3,HADHandRNF213,was adjusted (Table S5).
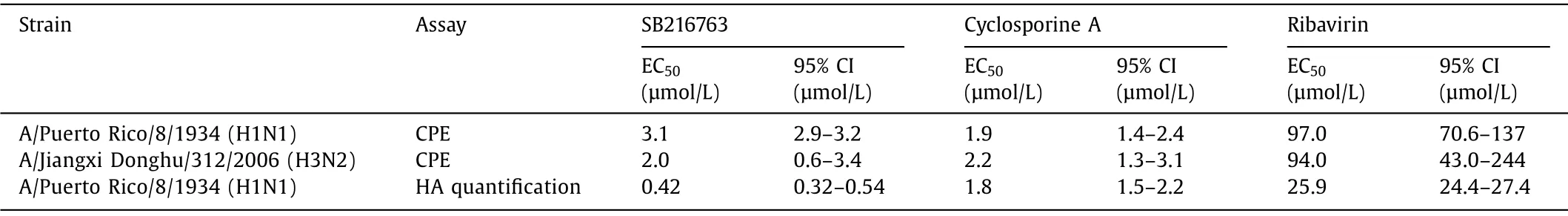
Table 1 Inhibitory effects of SB216763 and cyclosporine A on influenza virus infection (MOI = 0.02) in A549 cells.
To discover compounds with competence of the reversed regulation of DEGs during IAV infection, the characteristic direction (CD) signatures of 2044 instances from 1146 compounds on A549 cells were achieved from the L1000CDS2platform (https://maayanlab.cloud/public/L1000CDS_download/) [31].The genes in each instance were rank-ordered according to their CD values, and the enrichment scores (ESs) of each instance were calculated by the “fgsea” R package.All instances were ranked by ES in ascending order.The top 100 instances, including 91 compounds representing those most likely to reverse disease-associated gene expression, were deemed anti-IAV candidates (Table S6 in Supporting information).Thirty-one commercially available compounds in the list were used for further bioassays.
Initially, the cytotoxicity of the 31 compounds on A549 cells was determined by CellTiter-Glo assay [32] at final concentrations of both 10 and 30 μmol/L.The inhibitory activities of the 20 noncytotoxic compounds on the cytopathic effect (CPE) of A549 cells infected with A/Puerto Rico/8/1934 (H1N1) were evaluated, and two compounds, SB216763 and cyclosporine A, exhibited over 50% inhibitory activity (Table S7 in Supporting information),with half maximal effective concentrations (EC50s) of 3.1 and 1.9 μmol/L, respectively (Table 1 and Fig.S1 in Supporting information).Moreover, both SB216763 and cyclosporine A exhibited anti-H3N2 infection activities with EC50s of 2.0 and 2.2 μmol/L (Table 1 and Fig.S1).To quantitatively measure H1N1 in the supernatant,an enzyme-linked immunosorbent assay (ELISA) for the A/Puerto Rico/8/1934 hemagglutinin (HA) protein was performed (SinoBiological, KIT11684).Both SB216763 and cyclosporine A were able to reduce the virus generation with EC50s of 0.42 and 1.8 μmol/L, respectively, which implied that SB216763 and cyclosporine A may exert antiviral activity by inhibiting IAV replication (Table 1).In addition, text mining was performed by using the Request module (Python) for the 31 compounds in PubMed, and nine of them(29.03%) were reported with anti-influenza activities by others (Table S8 in Supporting information), which revealed the TSR used in this research much more powerful than that of the classical high-throughput screening (HTS), which usually one bioactive compound comes out from 1000 to 10,000 compounds [33].
To consider the experimental differences on cell lines, compound concentrations, and time points in this research and those performed by Library of Integrated Network-Based Cellular Signatures (LINCS), RNA sequencing of A549 cells treated with SB216763,cyclosporine A or the same amount of solvent was performed,and differential expression analysis was performed using DESeq2[34] and the DEGs were shown in Tables S9 and S10 (Supporting information).Subsequently, the Log2FC of signature genes of IAVinfected cells, along with the corresponding genes in SB216763-and cyclosporine A-treated cells, were extracted and are shown in a heatmap (Fig.1A), which shows that the signature genes of IAV infected cells are reversely regulated by the two compounds (Fig.1A).Thereafter, the DEGs due to SB216763 and cyclosporine A treatment were extracted (P <0.001 and Log2FC>1 or Log2FC<-1, Tables S9 and S10) for functional pathway analysis by the Metascape platform (https://metascape.org/) [35].The signature genes of cells with SB216763 treatment display significant enrichment (P <0.01) in cell morphogenesis involved in differentiation, blood vessel development, multicellular organismal homeostasis, circulatory system process and epithelial cell differentiation (Fig.S2A in Supporting information).Cyclosporine A produced significant enrichment in cell cycle-related pathways, including response to nuclear division, DNA replication, DNA conformation change, meiotic cell cycle process,etc.(Fig.S2B in Supporting information).
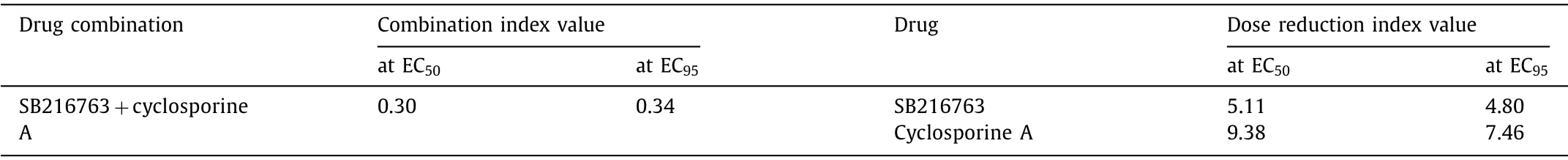
Table 2 The combination index values and dose reduction index values of the two-compound combination against A/Puerto Rico/8/1934 replication in A549 cells.
To further address the anti-IAV mechanisms of SB216763 and cyclosporine A, the signature gene list of cells infected with IAVs along with the signature gene list of cells treated with each compound were analyzed by Metascape for the common enriched pathways (Figs.1B and C).SB216763 is a potent glycogen synthase kinase-3 (GSK3) inhibitor with equivalent inhibitory activities against both GSK3αand GSK3β[36].As shown in Fig.1B,ten of the top 20 enriched GO clusters of cells infected with IAV or treated with SB216763 overlapped, including regulation of endopeptidase activity, apoptotic signaling, and regulation of cell adhesion (Fig.1B, a–j).According to previous studies, apoptosis and regulation of endopeptidase activity are critical cellular processes that affect influenza infection [37,38].This suggests that the anti-IAV activity of SB216763 occurs through the apoptosis pathway and the regulation of endopeptidase activity.In addition, the other overlapping pathways, including chemotaxis and multicellular organismal homeostasis, may also provide new perceptions for the elucidation of the anti-IAV mechanism of SB216763.Cyclosporine A, an immunosuppressant medication, has been reported to have anti-IAV activity by interfering with the late stage of the IAV life cycle through both cyclophilin A-dependent and cyclophilin Aindependent pathways [39,40].Here, the results of pathway analysis showed that four pathways were clustered in cyclosporine Atreated cells and overlapped with IAV-infected cells (Fig.1C, a–d), including the responses to bacterium pathway and endoplasmic reticulum stress pathway.It has been reported that the endoplasmic reticulum (ER) stress response pathway could affect IAV replication [41], which implies an additional anti-IAV mechanism of cyclosporine A.Taken together, our results suggest that SB216763 and cyclosporine A act through multiple pathways of the host response,indicating that the two compounds possess the capacity to reverse the gene expression of IAV-infected cells back to the normal state with multicomponent intervention.
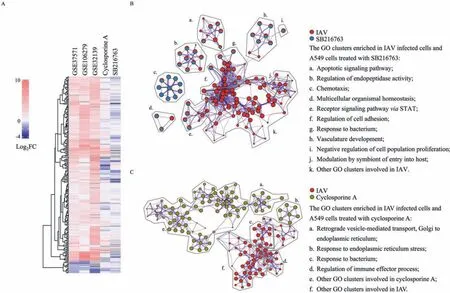
Fig 1.Differentially expressed genes analysis.(A) Heatmap of signature genes expression in IAV-infected cells, along with the corresponding genes in SB216763- or cyclosporine A-treated A549 cells.The color range represents the magnitude of the Log2FoldChange (Log2FC) of genes.(B, C) Functional pathway enrichment analysis of cells treated with SB216763 (B) or cyclosporine A (C) along with cell infected with IAV.The top 20 GO terms were selected as representative subsets, and their enrichment networks were visualized by colored nodes (IAV, red; SB216763, blue; cyclosporine A, olive green), where the size of the node and the sector of each pie were proportional to the number of genes enriched in that term and the counts of hit genes originating from the input signature gene list, respectively.Panels (B) and (C) were generated by using Metascape.The gene expression data of IAV infection were obtained from the three GEO datasets and analyzed by limma for signature genes of IAV-infected cells.A549 cells treated with SB216763 or cyclosporine A were collected for RNA sequencing, and signature genes of compound-treated cells were obtained by using the DESeq2 package.
Drug combination therapies are widely used for the treatment of infectious diseases due to the clinical outcome improvement from higher efficacy or lower individual dosages [42,43].In this study, a separation score was introduced to calculate the efficacy of the two compounds combination by network-based proximity measurement of compound-compound relationships [44,45].The separation score of two compounds represents the comparison of the mean shortest distance of the interactome between the signatures of each compound to the mean shortest distance between compound-compound signature pairs [44,45].As shown in Fig.2A, the IAV signature genes inversely regulated by SB216763 and cyclosporine A are listed as compound modules for separation score evaluation (the analysis details can be found in the Materials and methods section in Supporting information), which gives a score of 0.18 (Fig.2B), indicating a potential synergetic effect of the two compounds against IAV infection.To validate the prediction,an anti-IAV infection assay was performed for drug combination efficacy evaluation (Table S11 and Fig.S3 in Supporting information).The combination of SB216763 and cyclosporine A exhibited a strong synergistic effect with combination index (CI) values of 0.30(EC50) and 0.34 (EC95), respectively (Table 2).One of the greatest advantages of synergism is that it achieves a higher efficacy with low toxicity by dosage reduction.The dose reduction index (DRI)values, the dosage fold reduction for a single drug compared to that of the drug combination at a given activity, revealed that both compounds had a dosage reduction with a fold change ranging from 4.80 to 9.38 compared to that of each compound alone(Table 2).
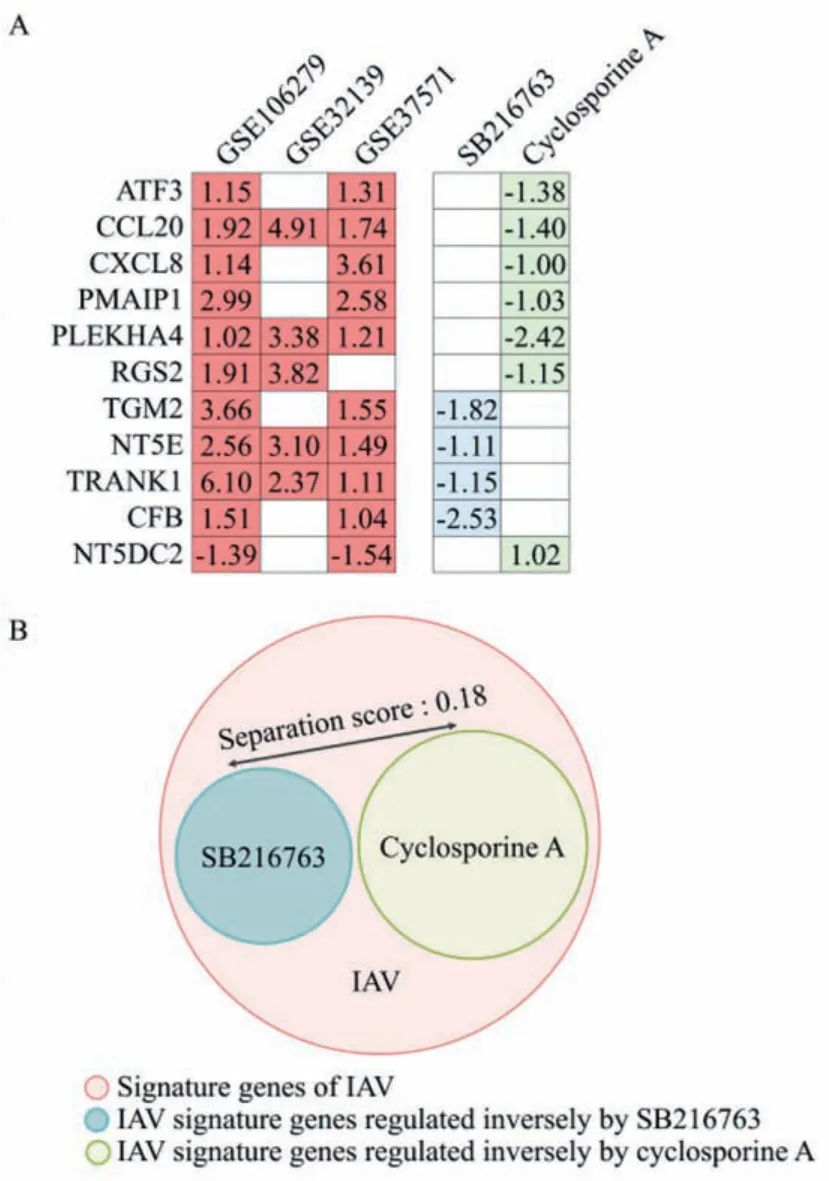
Fig 2.Network-based rational analysis of compounds combination.The IAV signature genes regulated inversely by SB216763 and cyclosporine A are listed (A) and were used as the compound modules for protein–protein interaction network topological relationship analysis by the separation score (B).Notes:The numbers in box(A) indicate the Log2FC of each gene; the separation score, which reflects their pharmacological relationships, was calculated as described in Materials and methods section in Supporting information.
In summary, the anti-IAV activity of SB216763 and cyclosporine A was disclosed by using TSR, and their synergistic activity was calculated by network-based proximity measurement and validatedin vitro.Compared to classical phenotype screening,the transcriptome-based strategy offers a novel, comprehensive and high-dimensional approach for bioactivity disclosure, synergetic analysis and target identification.With advances in disease databases and chemical perturbation libraries by data accumulation, the transcriptome reversal strategy will offer a more efficient approach in multitarget antivirals discovery from compounds with multicomponent intervention potential.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the CAMS Innovation Fund for Medical Sciences (Nos.2021-I2M-1-028 and 2020-I2M-2-010), the Opening Foundation of the State Key Laboratory of Bioactive Substance and Function of Natural Medicines (No.GTZK202109), the National Natural Science Foundation of China(No.81473256), the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (No.BZ0150), and the Disciplines Construction Project (No.201920200802).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.017.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
