A melatonin-based targetable fluorescent probe for screening of tumor cells and real-time imaging of glutathione fluctuations in tumor cells
Xiwei Li, Ciyun Liu,*, N Go, Wenlong Sheng, Bocun Zhu,*
a School of Water Conservancy and Environment, University of Jinan, Ji’nan 250022, China
b Biology Institute, Qilu University of Technology (Shandong Academy of Sciences), Ji’nan 250103, China
Keywords:Glutathione Reversible detection Fluorescent probe Tumor cells screening Melatonin
ABSTRACT Glutathione (GSH) is a key maintainer of cellular redox balance and plays an important role in many physiological effects.For example, GSH has been widely implicated in cancer initiation, progression and metastasis.Moreover, the concentrations of GSH in tumor cells can influence drug resistance.Given the serious harmfulness of cancer and the important roles of GSH in cancer, it has great significance to development probes for screening of tumor cells and real-time monitoring of GSH fluctuations in tumor cells.However, no targetable probe for reversible imaging of GSH in tumor cells has been reported.Herein,we constructed a melatonin-based targetable and reversible fluorescent probe (GR-MT) for screening of tumor cells and real-time imaging of GSH fluctuations in tumor cells.The probe uses coumarin as the skeleton, Michael addition reaction as the reaction mechanism, and melatonin as the targeted groups of tumor cells.The experimental results demonstrate this probe has many advantages including high selectivity, satisfactory sensitivity, excellent reversible ability, rapid reaction speed, and outstanding targetability of tumor cells.Therefore, this study provides a promising tool for tumor cells screening and real-time detection of GSH fluctuations in specific tumor cells.
Glutathione (GSH), the highest concentrations non-protein thiol,is found in mammalian tissues at concentrations of 1–10 mmol/L[1].It plays important role in maintaining the redox balance of cells [2].In addition, as one of the key signaling molecules in redox signal transduction, GSH plays a crucial role in cell proliferation and differentiation, cell apoptosis, and the occurrence and development of various diseases [3].For example, GSH has been widely implicated in cancer initiation, progression and metastasis [4–6].Moreover, the concentrations of GSH in tumor cells can influence drug resistance and the effect of treatment [7,8].While more and more life activities in cancer cells are attributed to GSH, many specific roles of GSH have not been systematically and clearly studied.Obviously, real-time detection of GSH can not only help us understand the role of GSH in the cancer, but also provide an effective tool for the evaluation of cancer treatment efficacy and drug development.
The treatment of cancer is a wide research subject and a century challenging topic.Currently, many new treatments are being developed, such as photodynamic therapy, photothermal therapy, and immunotherapy, but traditional surgery and chemotherapy with many limitations are still the gold standard for cancer treatment [9].This also illustrates the complexity and difficulty of cancer treatment.But it is well known that early treatment can greatly improve treatment outcomes [10].The premise of early treatment is to be able to achieve early diagnosis.Therefore, it has great significance to develop methods for early diagnosis of cancer.
Current detection and imaging methods mainly include computed tomography (CT), high-performance liquid chromatography(HPLC), capillary electrophoresis (CE), mass spectrometry (MS) [11–13].Compared to these detection methods, fluorescent probes have been widely developed and used to detect various analytes because of their non-invasion, high sensitivity, and easy operation [14–20].Today, a large number of GSH probes have been reported [21–28].Unfortunately, the vast majority of GSH probes are qualitative and irreversible.These probes cannot monitor the changes of GSH concentrations in real pathological and physiological processes.This results in the inability of the probes to study the specific roles of GSH.Even if some of reversible GSH probes are developed, there are still some problems, such as short wavelength, slow reaction speed that is difficult to reflect real-time changes in GSH concentrations, and inappropriate dissociation equilibrium constant that is unsuitable for detection of GSH in actual intracellular environment [29–33].Wanget al.developed the first reversible fluorescent probe for GSH based on coumarin conjugate and Michael addition reaction mechanism [34].Subsequently, other probes based the same design strategy were reported for optimizing the ability of the probe to enter the cell, the quantum yield, and the reaction speed [1,35-40].Additionally, several of probes were developed for targeting specific organelles [41–43].Meanwhile, other types of probes were also reported based on the xanthene skeleton [44].But, only one probe among them can be used to detect GSH in the concentration range of 1–10 mmol/L.Given the serious harmfulness of cancer and the important roles of GSH in cancer, the development of reversible GSH fluorescent probes with tumor cells targeting capabilities to enable real-time detection of GSH fluctuations in specific tumor cells is of great significance and necessity.Interestingly, the development of GSH probes with tumor cells targeting ability is consistent with the screening of tumor cells by targetability.However, such probes have not been reported.
Herein, we designed and synthesized a reversible GSH fluorescent probe (GR-MT) with melatonin as the targeting group of tumor cells.The double bond site connected by coumarin derivative and cyanoacetic acid is used as the recognition site of GSH, and the reversibility of detection is realized based on Michael addition reaction.The introduction of substituent (CN) greatly increases the reaction rate, so as to realize the real-time detection of GSH.On this basis, the tumor-targeting effect was realized by modifying melatonin (Scheme 1).As expected, the probe not only has an ideal reversible recognition site for real-time detection of GSH, but also has a ratiometric emission characteristic for quantitative detection.Its large emission wavelength shift (170 nm) can effectively reduce spectral overlap and achieve more accuracy for the detection of GSH.After the imaging experiments of normal cells and tumor cells, the probe has a good ability to screen tumor cells.Moreover, in order to verify that the targeting ability of the probe is attributed to melatonin, we synthesized the control probe GR-1 without the targeting group, which had no ability to screen tumor cells.Finally, probe GR-MT was successfully applied to the reversible detection of GSH in tumor cells.These results indicate the probe for screening of tumor cells has the potential for early diagnosis of cancer.More importantly, this targetable probe GR-MT has the potential for real-time reversible monitoring of GSH in specific tumor regions, and evaluating the therapeutic effect of drugs and related drug development.
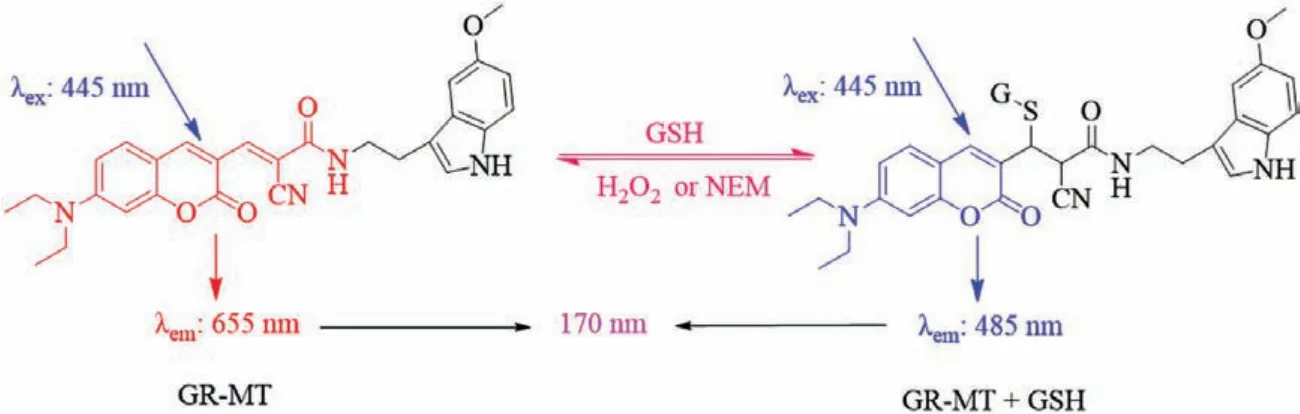
Scheme 1.Recognition mechanism of probe GR-MT for GSH.
The chemicals and instruments used in this work are detailed in Supporting information.In addition, the spectrometric measurements, preparation and imaging of cells are also displayed in Supporting information.
The synthesis of compound 1, control probe GR-1 and probe GR-MT is shown in Supporting information.
The importance of real-time reversible quantitative detection of GSH is self-evident.The key points of building such a probe are reversibility, the fast forward and reverse reaction rates, and the characteristics of ratiometric emission.To achieve these goals, we chose the Michael addition reaction as the recognition mechanism toward GSH.Meanwhile, the reactivity of double bond can be increased with the introduction of strong electron-withdrawing of substituent (CN).So that, the reaction rate between probe GR-MT with GSH can be improved, and the probe can reflect the changes of GSH concentrations in real time.Moreover, the strengthening conjugated structure results in the largely red-shifted emission spectra.After the GSH reacts with the double bond site, the double bond breaks, the conjugated structure decreases, and the probe molecule emits short-wavelength fluorescence.This mechanism achieves the target of quantitative detection of GSH by ratiometric fluorescence spectra (Scheme 1).Finally, 5-methoxytryptamine was modified on probe GR-MT.In this way, a targetable probe for reversible detection of GSH in tumor cells was constructed.
The ability to detect the actual concentration levels of GSH in the cells was the first prerequisite for the probe, so the ability of the probe GR-MT for detection of GSH at the millimolar level was tested.After the probe (5 μmol/L) was added to an aqueous solution (PBS 10 mmol/L, pH 7.4), GSH (0–15 mmol/L) was gradually added.It can be seen that the fluorescence spectra at 485 nm gradually increases, and then the fluorescence intensity at 655 nm synchronously decreases (Fig.1a).This is most likely due to the reaction of GSH with the double bond of probe GR-MT, resulting in the reduced conjugation structure and the release of short wavelength fluorescence of coumarin.Interestingly, unlike other GSH probes designed with coumarin and cyanide group as the skeleton whose long-wavelength emission peak at around 580 nm [1,35-43], the long-wavelength emission peak of probe GR-MT locates at 655 nm,such a large shift between the two peaks can reduce the spectral overlap and make the measurement with higher accuracy.In order to estimate the ability of the probe GR-MT to quantitatively detect GSH concentrations, the ratios (F485/F655) of the probe GR-MT was evaluated after adding GSH concentrations of 0–15 mmol/L, and the results showed that the ratios (F485/F655) of the probe GR-MT had a good linear relationship with GSH concentrations (linear equation:y=0.1553[GSH] (mmol/L)+0.1483,R²=0.9949) (Fig.1b).Moreover,when 3–10 mmol/L GSH was added, the fluorescence at 665 nm of probe solution was basically unchanged (Fig.S1 in Supporting information).This property may provide a highly accurate ratiometric quantification tool for GSH detectionin vivo.
Next, the reversibility and response speed of the probe GR-MT to GSH were studied to judge its ability for detection of GSH in real time.According to the intracellular actual environment, H2O2was used to decrease the concentrations of GSH in living cells.Therefore, 15 mmol/L of GSH was first added into the probe solution under the above test conditions, and then gradually added H2O2(0–20 mmol/L) after the probe fully reacted with GSH.It can be clearly seen that with the increase of H2O2concentrations, the fluorescence intensity at 485 nm gradually decreases, on the contrary,the fluorescence intensity at 655 nm gradually increases (Fig.1c).The results also showed that the ratios (F485/F655) of the probe GR-MT had a good linear relationship with H2O2concentrations(linear equation:y=-0.2809[H2O2] (mmol/L)+2.474,R²=0.9934)(Fig.1d).Consequently, these results demonstrated that the probe GR-MT possesses excellent reversibility.
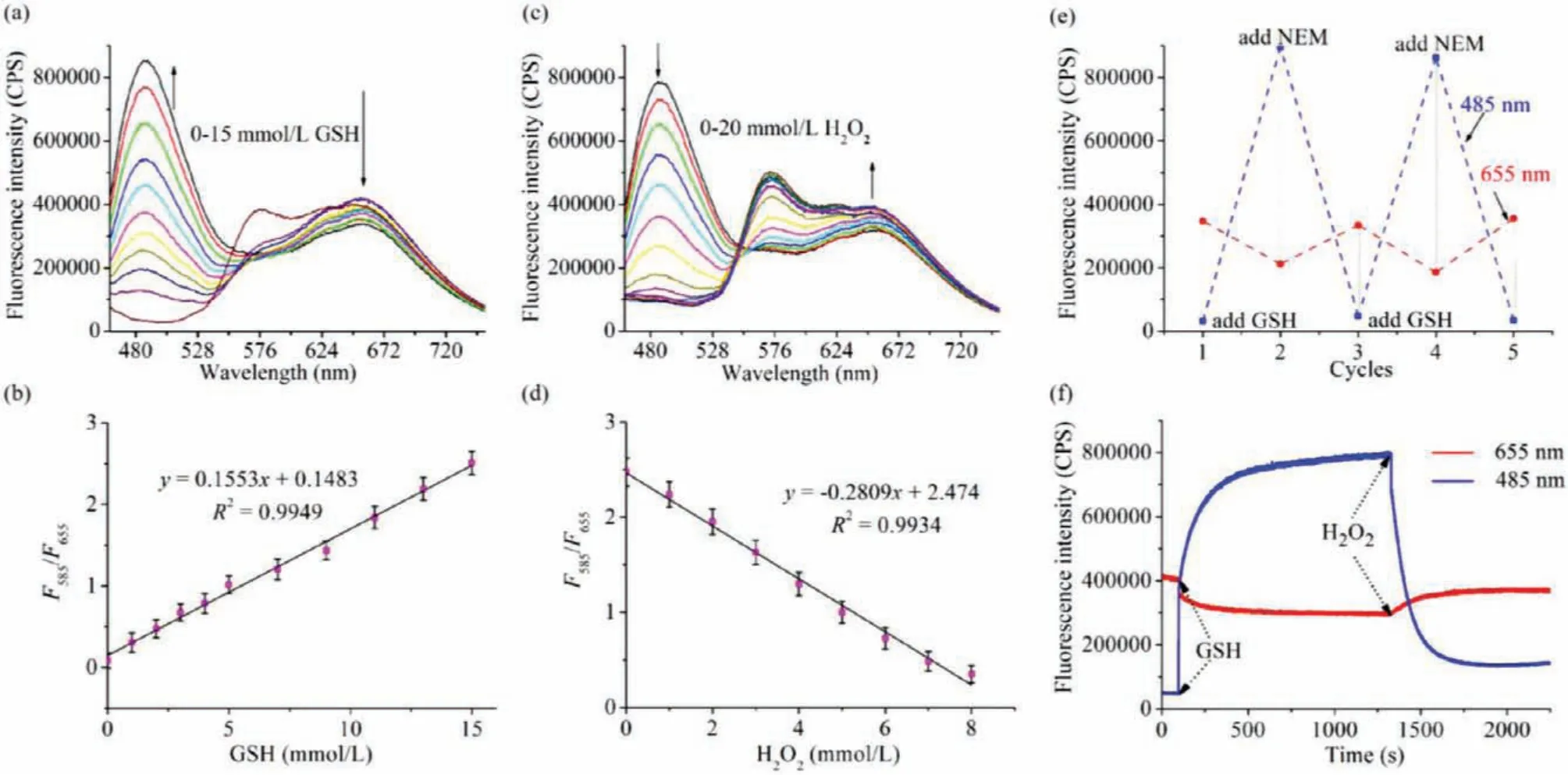
Fig.1.(a) Fluorescence responses of probe GR-MT (5 μmol/L) toward GSH.(b) Linear relationship between ratios (F485/F655) of the probe GR-MT and GSH concentrations.(c)Fluorescence responses of probe GR-MT (5 μmol/L) toward H2O2 after addition of 15 mmol/L GSH for 20 min.(d) Linear relationship between ratios (F485/F655) of the system of probe GR-MT containing GSH and H2O2 concentrations.(e) Reversibility cycles of probe GR-MT (5 μmol/L) with GSH and NEM.(f) The fluorescence intensity of the probe GR-MT changed with time after GSH (10 mmol/L) or H2O2 (20 mmol/L) was added. λex=445 nm.
In order to further evaluate the reversible ability of the probe GR-MT for GSH, GSH andN-ethylmaleimide (NEM) were alternately added to the probe solution, and it was found that the fluorescence of the probe could be well reversible (Fig.1e).
The reversible response rate of the probe to GSH is related to whether the probe can detect the changes of GSH concentrations in real time.Therefore, 10 mmol/L GSH was added into the probe solution at 100 s, and the fluorescence of the probe at 485 nm was rapidly enhanced (half time:<4 s).After the fluorescence intensity of the probe at 485 nm reached the platform, 20 mmol/L H2O2was added to the above solution, and the fluorescence signal can also change rapidly (Fig.1f).These results undoubtedly prove that the probe not only has good reversibility, but also has a very ideal reversible response rate.It laid a good foundation for real-time quantitative measurement of GSH concentrations in cells.
In view of the complexity of the actual biological environment,the following analytes (1 mmol/L) were added to the probe solution in order to evaluate the effects of other analytes on the probe GR-MT:H2O2, HOCl,1O2, O2-, histidine, L-asparagine, lysine,glycine, valine, DL-methionine, serine, aspartic acid, L-threonine,glutamine, arginine, proline.It can be clearly seen that, compared with the probe solution with 10 mmol/L GSH, the addition of other analytes (their concentrations are far higher than the actual concentrations in the organism) did not cause the significant changes of ratios (F485/F655) of the probe GR-MT (Fig.S2a in Supporting information).Subsequently, we also separately studied the effects of cysteine (Cys) and homocysteine (Hcy) on the probe for detecting GSH.As shown in the Fig.S2b (Supporting information), the effects of Cys (300 μmol/L) and Hcy (50 μmol/L) on the spectra are negligible compared with GSH (10 mmol/L).These results indicated that the response of the probe to GSH has good selectivity.
After determining its excellent spectral properties, the probe GR-MT was applied in the study of biological imaging.Before other research, the cytotoxicity of the probe was estimated by cell counting kit-8 (CCK-8).The obtained results showed that the cytotoxicity of probe GR-MT was negligible under the bioimaging conditions(Fig.S3a in Supporting information).Furthermore, an interesting phenomenon was found, when the probe concentrations increased to 30 μmol/L, the probe GR-MT showed significantly higher toxicity to MGC803 cells rather than other cells (Figs.S3b and c in Supporting information).This feature of selective toxicity can effectively kill MGC803 cells while maintaining low damage to normal cells.Therefore, probe GR-MT has the potential to be a specific anticancer drug.
Based on the fact that MT1and MT2are highly expressed in many tumor cells, attempts were made to use probe GR-MT modified with melatonin to achieve tumor cell screening [45–47].Then,two types of normal cells (RAW264.7 and HUVEC cells) and two types of tumor cells (A549 and MGC803) were selected.In order to remove the interference of the cell’s own background fluorescence,four types of cells incubated with the probe GR-MT (10 μmol/L)and those without the probe were compared.No detectable fluorescence was observed in cells without probe incubation, while strong fluorescence was observed in cells with probe incubation(Fig.S4 in Supporting information).Next, the fluorescence intensities of the blue and red channels in cells incubated with the probe were calculated.It can be seen that the fluorescence intensities of the two channels in two tumor cells were significantly higher than those in two normal cells (Figs.2a-e).Therefore, the probe GR-MT has an excellent capability of screening tumor cells.
Subsequently, the same experiment was conducted with control probe GR-1 without the targeting group of melatonin to verify that the excellent targeting performance of probe GR-MT was derived from the melatonin.As expected, there was no significant difference in the fluorescence intensities of the two channels in the four cells incubated with control probe GR-1 (10 μmol/L)(Fig.S5 in Supporting information).These experimental results not only demonstrated the excellent ability of probe GR-MT to target tumor cells, but also demonstrated the source of this targeting effect.
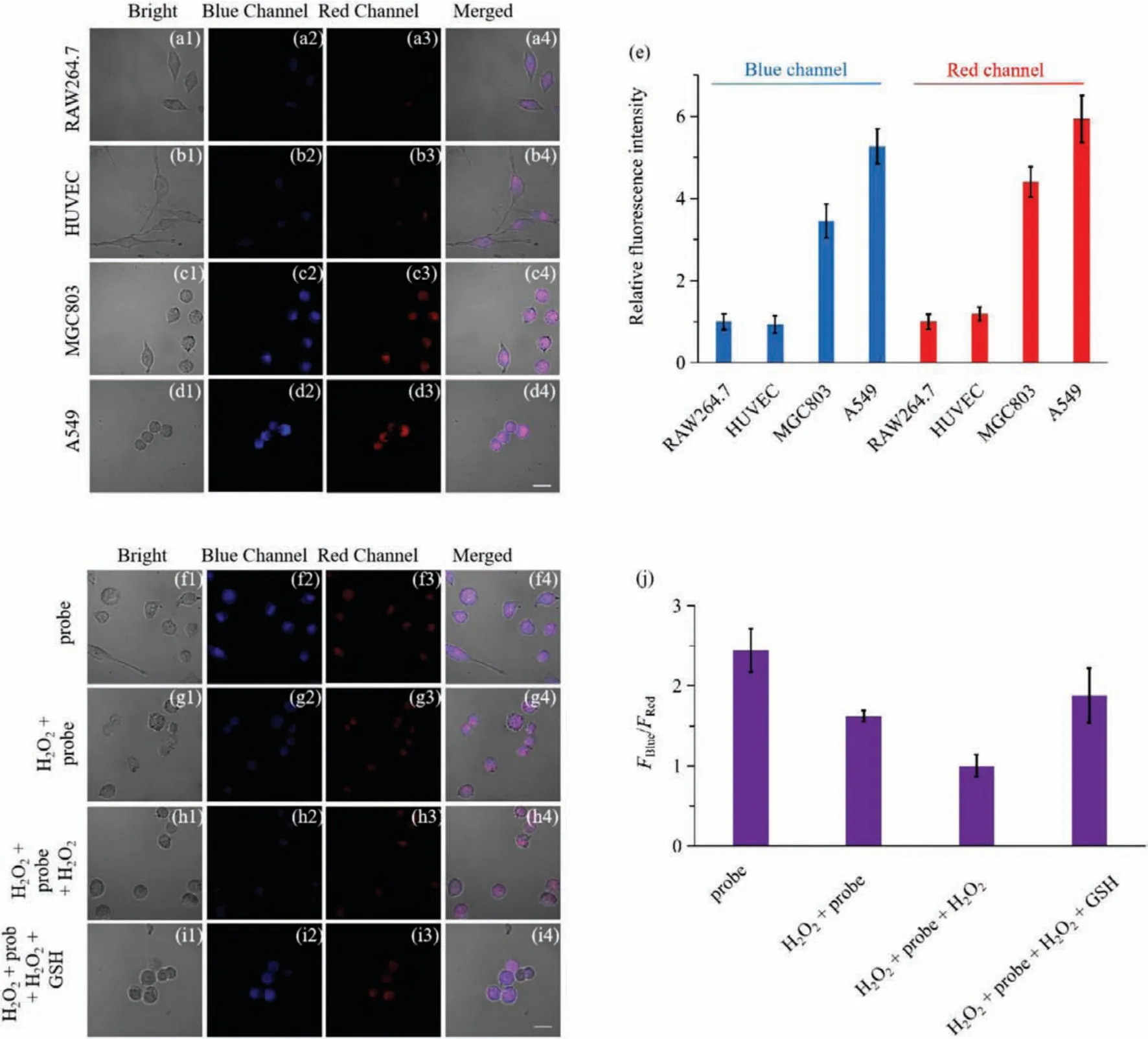
Fig.2.Fluorescence imaging of probe GR-MT (10 μmol/L) in RAW264.7 (a1-a4), HUVEC (b1-b4), MGC803 (c1-c4), and A549 cells (d1-d4).(e) Relative fluorescence intensities of blue (465–530 nm) and red (600–700 nm) channels of different groups.Fluorescence imaging of probe GR-MT (10 μmol/L) in MGC803 cells:(f1-f4) only probe; (g1-g4)incubation with 200 μmol/L H2O2 for 20 min after incubation with the probe for 20 min; (h1-h4) based on the second group, added 100 μmol/L H2O2 for 20 min; (i1-i4)based on the third group, added 10 mmol/L GSH for 20 min.(j) Ratios (FBlue/FRed) of the probe GR-MT in different groups.Scale bar=20 μm.
After the probe has been proven to possess the desirable targeting feature for tumor cells, the MGC803 cells were selected to study the ability of the probe GR-MT to detect GSH fluctuations in tumor cells.The MCG803 cells were first imaged after incubated with the probe for 20 min, and then imaged again after addition of 100 μmol/LN-ethylmaleimide (NEM, a known thiol-blocking agent)for 20 min.After NEM incubation, the ratio of fluorescence signal of the cells decreased significantly (Fig.S6 in Supporting information).Considering the actual situation of intracellular redox equilibrium, the following experiments were conducted.The first group cells were incubated with the probe (10 μmol/L) for 20 min (Figs.2f1-f4), and the second group cells were pre-incubated with 200 μmol/L H2O2and then incubated with the probe for 20 min for imaging (Figs.2g1-g4).On the basis of the second group of cells,100 μmol/L H2O2was added as the third group of cells (Figs.2h1-h4).Subsequently, the fourth group of cells was incubated with 10 mmol/L GSH for 20 min on the basis of the third group of cells for imaging (Figs.2i1-i4).Compared with the ratios (FBlue/FRed)of the probe GR-MT in the first group, it can be seen that the ratios (FBlue/FRed) of the second group decreased, and the ratios(FBlue/FRed) of the third group were further reduced.After GSH incubation, the ratios (FBlue/FRed) of the fourth group significantly increased (Figs.2f-j).These results fully indicated the probe GR-MT can accurately trace the GSH fluctuations in tumor cells.Therefore,the proposed probe provides a potential tool for monitoring GSH fluctuations in evaluating cancer treatment efficacy and drug development.
In summary, we designed and synthesized a melatonin-based targetable fluorescent probe for screening of tumor cells and realtime reversible imaging of GSH fluctuations in tumor cells.The probe not only has excellent spectral performances, but also has been proved to possess outstanding targetability to tumor cells by subsequent biological imaging experiments.The probe GR-MT was successfully applied to the screening of tumor cells and reversible imaging of GSH fluctuations in tumor cells.Importantly, we not only demonstrated the role of melatonin as a targeting group of tumor cells, but also opened the way for the development of targeted probes and drugs.This work also provides a potential tool for early screening of cancer and real-time detection of GSH concentrations in specific tumor regions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (Nos.21777053 and 22176070)and Shandong Province Higher Educational Youth Innovation Science and Technology Program (No.2019KJD005).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.080.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
