Nanobody-based polyvinyl alcohol beads as antifouling adsorbents for selective removal of tumor necrosis factor-α
Lichun Wng, Yu Ding, Nn Li, Ymin Chi, Qiyu Li, Yunzheng Du,Zhngyong Hong,*, Liling Ou,*
a The key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, Nankai University, Tianjin 300071, China
b State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Protein Sciences, College of Life Sciences, Nankai University, Tia njin 300071,China
Keywords:Nanobody Polyvinyl alcohol beads Immunosorbents Tumor necrosis factor-α Adsorption
ABSTRACT Highly efficient removal of tumor necrosis factor-α (TNF-α) from plasma by hemoperfusion for autoimmune disease therapy remains a challenge in the clinical field owing to the low adsorption capacity and poor blood compatibility of adsorbents.In this work, a new class of nanobody (Nb)-coupled antifouling polyvinyl alcohol (PVA) beads was constructed as an immunosorbent for the selective removal of TNF-α from plasma.Notably, our immunosorbent exhibited an exceptionally high specific TNF-α adsorption capacity of 416.9 ng/g in human plasma (at a plasma-to-adsorbent ratio of 300).More importantly, the obtained adsorbent beads showed outstanding blood compatibility.In addition, during in vivo experiments,the blood circulation device was constructed to remove TNF-α in rat models, proving that the beads had good removal performance (~85%/60 min).Furthermore, 95% of the original capacity was retained after 6-month storage, showed strong stability and prolonged storage of PVA-Nb.Above all, the results indicate that the novel PVA-Nb immunosorbent has possible clinical applications for treating autoimmune diseases in the clinic.
TNF-α, a crucial immunomodulatory cytokine produced by macrophages, plays vital roles in inflammation, antitumor responses, and infections [1].However, it is a double-edged sword[2].It can eliminate pathogens and promote tissue repair, but as the primary cytokine in inflammation, it accelerates the outburst of other cytokines and induces cytokine release syndrome (or a cytokine storm), which can exacerbate the inflammatory state and lead to immune dysfunction [3].Clinically, one fifth of corona virus disease 2019 (COVID-19) cases have been found to progress to severe illness, septic shock, and multiple organ failure owing to this cytokine storm [4].In addition, elevated levels of TNF-αin blood are associated with many human diseases, including sepsis, chronic hepatitis B, autoimmune diseases (such as inflammatory bowel disease and rheumatoid arthritis [RA]), insulin resistance and tumorigenesis [5].Thus, eliminating aberrant TNF-αfrom blood plasma has become a major focus in the treatment of the aforementioned ailments, including COVID-19 [6].Three FDA-approved therapeutic agents (adalimumab, infliximab, and etanercept) are effective for treating RA and other autoimmune diseases [7].However, all of them have obvious shortfalls:They are costly and associated with side effects, including neurological disorders and heart failure [8].Fortunately, hemoperfusion is an effective and economical technology that can inhibit TNF-α[9–11].One commercial extracorporeal hemoperfusion device, the CytoSorbTMadsorber (CytoSorbents Corporation, Monmouth Junction, NJ, USA) [12], has been successfully employed to rebalance the immune system and moderate cytokine storms as an adjunctive therapy for patients with COVID-19.CytoSorbTMcan remove a substantial proportion of most cytokines bothin vivoandin vitro.However, it can only remove 20%-30%of the trimeric form of TNF-α, which is the largest cytokine (at 51 kDa) [13,14].To address this, a wide variety of porous adsorbents have been crosslinked with anti-TNF-αantibodies and antibody fragments, and have shown exceedingly high selectivity and efficiency for the elimination of TNF-α[15,16].DiLeoet al.fabricated an anti-TNF-αhemoperfusion device combining CytoSorbTMwith TNF-αantibodies, which significantly enhanced the adsorption ability of TNF-α[17].Indeed, antibodies are employed as ligands with high specificity and strong binding affinity in hemoperfusion, however, poor stability and the high cost of antibodies are the major limitations to their further application in immunosorbent therapy, especially for hemoperfusion [18–21].Therefore, introducing more stable, cost-effective, and efficient ligands will undoubtedly lead to a more effective solution.
Scientists in Belgium discovered interesting heavy-chain-only antibodies (HCAbs) in camels; these contain a variable domain of heavy chain HCAbs (VHHs) and two conventional CH2and CH3regions [22].More importantly, as the smallest unit binding target antigen, VHH, known as the single domain antibody or nanobody(Nb), can be cloned independently and easily, and has shown exceptional structural stability and antigen-binding activity [23].Nb can be used in different formats such as monovalent, bivalent,and polyvalent.Among them, bivalent Nbs are coming to the forefront of therapeutic applications owing to their outstanding affinities and potencies [24].The unique biophysical and biochemical properties of bivalent Nbs make them ideally suited as ligands for hemoperfusion immunosorbents [25].
Polyvinyl alcohol (PVA) beads have been investigated as an ideal carrier owing to their outstanding mechanical properties, sufficient mesoporous structure, and fantastic biocompatibility, which are vital for hemoperfusion.Furthermore, a large number of hydroxyl groups exist in PVA, which can easily be functionalizedvianoncovalent and covalent chemical interactions.In addition, PVA beads are antifouling polymers with few adverse effects on blood components.
In this study, we developed Nb-coupled antifouling polyvinyl alcohol beads as immunosorbents.Ourin vitrostudy showed that the TNF-αlevel could be restored to a normal level within 10 min of the experiment.An extracorporeal blood circulation device was constructed to remove TNF-αin rat models, further proving that the beads had good removal performance (~85%/60 min).Furthermore, 95% of the original capacity was retained after 6-month storage, which showed outstanding stability of PVA-Nb.These results provide critical insight into how fabricate nanobody beads for hemoperfusion and their potential for the removal of TNF-αfrom plasma.
To construct the beads, bivalent anti-TNF-αNb was expressed overnight at 30 °C using the pPICZαA vector inPichia pastoriscells and purifiedvianickel-ion affinity chromatography.Amino acid sequence, production, and purification details are provided in the experimental section of Support information, while the characterization of Nb is shown in Fig.S1 (Supporting information).A yield of 100 mg Nb per liter culture was acquired and the bivalent Nb was analyzedviasodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig.S1a).The results of enzyme-linked immunosorbent assays (ELISA) indicate the excellent binding capacity of Nb to TNF-α, with aKDvalue of 4.36 nmol/L (Fig.S1b).According to matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) technology, the molecular weight of the Nb was 28,026.95 Da (Fig.S1c).
As shown in Fig.1a and Fig.S2 (Supporting information), covalent immobilization of Nb on the PVA beads depends on the reactive epoxy groups.The degree of epoxidation was detected by acidbase titration, and the epoxy value of PVA was 120–130 μmol/g wet matter, which fulfilled the immobilization requirements.
To investigate the morphologies of the constructed beads, a stereo microscope was used.Stereo microscope images of a few particles from both PVA and PVA-Nb revealed that the macroscopical morphology of beads was spherical with a diameter of around 75–120 μm (Fig.S3 in Supporting information).A closer examination of the scanning electron microscope (SEM, Figs.1b-e) showed spherical absorbents with a well-developed porous structure.This indicates that epoxidation and Nb immobilization did not affect the porous structure, as clear mesopores could still be seen on their rough external surfaces.
The chemical structure and elements of PVA and PVA-Nb were characterizedviaFourier transform infrared (FT-IR) spectroscopy(Fig.1f) and X-ray photoelectron spectroscopy (XPS) analyses(Figs.1g-i and Table S1 in Supporting information), respectively.After the functionalization with Nb, the PVA peak at 1086 cm-1had shifted to 1069 cm-1, probably due to the overlapping of the Nb peak at 1058 cm-1(the existence of methionine).This indicates that Nb was present in the PVA-Nb beads (more details are provided in the results and discussion section of Supporting information).Sulfur is a characteristic element of Nb in the PVA-Nb beads.XPS was conducted to investigate the chemical composition of PVA, in which the C, N and O elements are present.In the C 1s spectrum of PVA (Fig.1g), three deconvoluted peaks centered at 284.6, 285.9, and 289.2 eV could be assigned to C–C, C–N and C=O, respectively [26].The presence of C–N and C=O in PVA could be attributed to the functional groups in the crosslinker.It is also noteworthy that no sulfur was detected.Regarding PVA-Nb, C, N,O and S elements were observed.In the C 1s spectrum of PVANb (Fig.1h), three deconvoluted peaks located at 284.6, 286.1 and 289.1 eV could also be assigned to C-C, C-N and C=O bonds.However, one new peak was found at 287.7 eV, and this was assigned to the C-S bond [27].The presence of that bond confirms the existence of S as well as the successful conjugation of Nb.Fig.1i shows the S 2p spectrum of PVA-Nb.There were two deconvoluted peaks centered at 163.6 and 164.4 eV, which were assigned to the S-H and S-C bonds, respectively [28].The presence of these bonds is in good accordance with the functional groups in the Nb.These results again confirm that the Nb was successfully integrated into the sample.Moreover, the content of S determined by XPS was 0.08 at%.
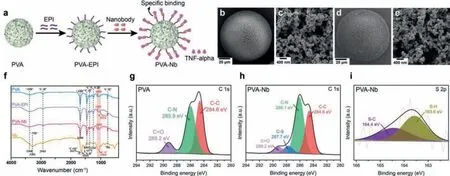
Fig.1.(a) Scheme of the PVA-Nb microspheres prepared by two reactions (substitute and opening-ring reaction) of epichlorohydrin (EPI).SEM micrographs of PVA (b, c)and PVA-Nb beads (d, e).(f) FT-IR spectra of PVA, PVA-EPI, PVA-Nb beads and Nb.(g) XPS C 1s high-resolution spectra of PVA.XPS C 1s (h) and S 2p (i) high-resolution spectra of PVA-Nb.
We also conducted fluorescence labeling to provide more direct evidence of the existence of Nb on PVA-Nb beads.Nb was labeled with fluorescein isothiocyanate (FITC).Fig.S4 (Supporting information) shows the images of FITC-Nb immobilized PVA compared with unmodified PVA beads.An obvious fluorescence signal is observed for the PVA-Nb but not the initial PVA beads.These data confirm the presence of Nb on the PVA-Nb beads.
In order to further understand the amount of Nb grafting on PVA, a BCA protein assay and a western blotting assay (Fig.S5 in Supporting information) were conducted.The amount of Nb immobilized on the carrier was determined by the difference between the amount of Nb in the solution before fixation and the amount of Nb in the eluent after fixation.From the BCA protein assay, the graft rate of Nb was as high as 63.59% (Fig.S5d).The result was also obtained in the western blotting assay (67.84% grafting rate)(Fig.S5c).
Next, we investigated their adsorption performance using human plasma with high TNF-αconcentrations (approximately 1000 pg/mL), in accordance with the levels in sepsis patients [29].To this end, we performed adsorption experiments, including assessments of time-dependent adsorption kinetics, plasma/adsorption ratios, adsorption isotherms, the effect of temperature, the preservation stability, and specific adsorption ability (Fig.2 and Figs.S7-S10 in Supporting information).PVA beads were used in control experiments.As depicted in Fig.2a and Fig.S7, the uptake of TNF-αby PVA-Nb is quite rapid and reaches equilibrium within 10 min, which can be explained by Nbs designed to remove TNFα.The final TNF-αadsorption rate of PVA-Nb reached 99.75%, with an adsorption capacity of 46.98 ng/g.By contrast, PVA alone had rates of only 13.93% and 6.20 ng/g, respectively.Next, we analyzed the adsorption kinetics of TNF-αat 37 °C onto three adsorbents (PVA, PVA-Nb, and CYT (a commercial adsorbent, USA))using two extensively used kinetic models (pseudo-first-order andpseudo-second-order models) [30].The equations used for nonlinear fitting, the relevant parameters (Qe,exp,Qe,calc,k), and the correlation coefficients (R2) are provided in the experimental section and Table S3 (Supporting information).Both models had highR2values (greater than 0.995).This suggests that whether hydrophobic and electrostatic action played an important role in the adsorption of TNF-αby PVA-Nb beads.However, theR2value ofpseudosecond-order model fit the experimental data better, with a much higherR2value.Interestingly, the equilibrium adsorption capacities of thepseudo-second-order model (Qe,calc) were much closer to the experimental values (Qe,exp), indicating that electrostatic action played the most vital role.To better mimic a feverin vivocondition, time-dependent adsorption kinetics at 42 °C were conducted (Fig.S8 and Table S4 in Supporting information).The uptake of TNF-αby PVA-Nb at 42 °C was also quite rapid and reached equilibrium within 10 min.The results are quite similar to those at 37 °C, which met the requirements of practical applications for hemoperfusion (the detailed discussion is provided in Supporting information).The plasma/adsorption ratios assay (Fig.S9a) indicated that the adsorption rate of TNF-αremained high (93.80%),with an adsorption capacity of 416.9 ng/g, at a volume ratio of plasma-to-resin of 300, owing to the high affinity of PVA-Nb for TNF-α.As shown in Fig.S9b, the adsorption isotherm for TNF-αof PVA-Nb is classified as the S-1 type, according to Giles’s method.The adsorption of TNF-αincreased slowly when the equilibrium TNF-αconcentration in plasma was lower owing to the competitive adsorption of other protein components in human plasma and increased quickly when a certain concentration range had been reached.This is because of the interaction between the free and adsorbed TNF-αfactors in plasma [2].With the rapid improvement of the adsorption rate and binding capacity, PVA-Nb became more efficient than the commercial CYT.Regarding temperature effects (Fig.2b), PVA-Nb exhibited remarkable TNF-αuptake values of 98.1% ± 2.02%, 98.07% ± 1.01%, 99.52% ± 0.36%, 98.47% ± 1.23%and 98.82% ± 0.24% at 8, 21, 37, 42, and 45 °C, respectively, while PVA had much lower rates of 0.42% ± 0.35%, 0.48% ± 0.22%, 12.94%± 1.00%, 3.12% ± 0.36% and 0.90% ± 0.41%, respectively.It is obvious that TNF-αuptake did not change much as the temperature increased, probably owing to the stability and specificity of the Nb-based immunosorbent.An ideal immunosorbent for clinical applications should be robust enough to allow prolonged storage in an economical and practical way.To test this, PVA-Nb beads were stored in normal saline for 6 months, and adsorption capacity was assessed each month.The beads maintained an extremely similar adsorption rate (greater than 95%) throughout the time period (Fig.2c), confirming its excellent preservation stability, and indicating that it is a more practical TNF-αadsorbent than other immunosorbents.To further examine the specificity of PVA-Nb, the beads were loaded with human plasma supplemented with several major plasma proteins, such as TNF-α, IL-1β, IL-2 and IL-6,whose concentrations before and after adsorption were measuredviaELISA.As shown in Fig.S10 (Supporting information), the material adsorbs TNF-αwith a high selectivity and without nonspecific adsorption to a variety of other blood components.It also showed excellent TNF-αselectivity in the hyperinflammatory plasma from a rat model of sepsis (Fig.2d).In addition, it had better adsorption than blank PVA beads and CYT beads.To further test the blood-cleaning capability of PVA-Nb beadsin vivo, we conducted dynamic hemoperfusion experiments in rats with sepsis (Fig.2e).The use of experimental animals was approved by the Animal Experiments Ethical Committee of Nankai University and the experiments were carried out in conformity with the Guide for Care and Use of Laboratory Animals.As we observed in ourin vitrostudies, the hemoperfusion device obtained significantly (P <0.0001)low levels of TNF-αfrom the septic rat’s blood within 1 h using PVA-Nb beads than those in infected animals treated with hemoperfusion either in the absence of added beads or using PVA beads(Fig.2f).Moreover, hemoperfusion using PVA-Nb beads did not affect the composition of blood, including white blood cells (WBC),red blood cells (RBC), hemoglobin (HGB), and platelet (PLT) (Fig.S11 in Supporting information).Finally, all of these results are consistent with thein vitroexperiments using human plasma.Taken together, these results imply that the PVA-Nb beads provide an excellent mesoporous structure with unexpectedly high stability and an enhanced adsorption rate and target–binding capacity, making them a more efficient TNF-αadsorbent than the CYT beads.
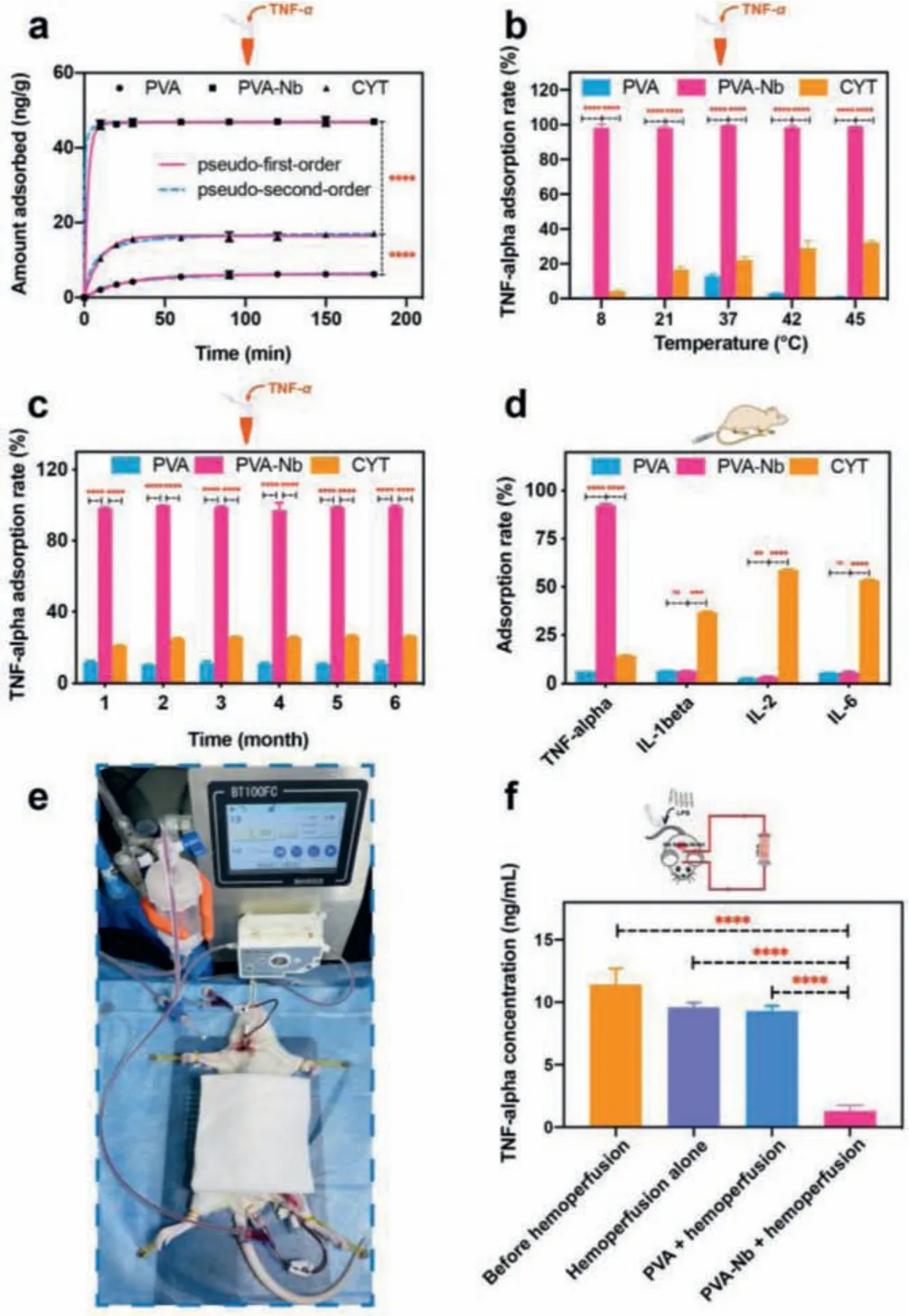
Fig.2.Adsorption characteristics of PVA-Nb in the plasma of human and rat models of sepsis.(a) Non-linear fitting of the adsorption kinetics of TNF-α onto the three adsorbents in human plasma (T = 37 °C, CTNF-α = 1417.19 ± 1.32 pg/mL).(b)Effects of temperature on the adsorption of TNF-α (t = 2 h, CTNF-α = 873.47 ± 1.18 pg/mL).(c) Stability of PVA-Nb (T = 37 °C).(d) In vitro study of the adsorption performance of PVA-Nb beads in plasma from a rat model of sepsis (t = 2 h, CTNF-α = 10.295 ± 0.28 ng/mL; CIL-1β = 1508.75 ± 0.38 pg/mL;CIL-2 = 1231.18 ± 0.14 pg/mL; CIL-6 = 143.429 ± 0.29 ng/mL).The plasma-toadsorbent ratio is 20 in all the above experiments, and all values are expressed as mean ± SD (n = 3).(e) Photograph of the experimental animal setup for extracorporeal hemoperfusion.(f) Depletion of TNF-α levels for the entire 1-h treatment period in a sepsis rat model (n = 5).ns:not significant, **P <0.01, ***P <0.001,****P <0.0001.
To assess the blood compatibility of the PVA-Nb beads and PVA beads, hemolysis experiments, routine blood tests, and coagulation assays were conducted [31].In the hemolysis assay, the hemolysis ratio for PVA-Nb beads was 0.26% ± 0.11% (<5%), indicating that the functionalization of Nb on the PVA beads exhibited little risk of facilitating hemolysis during application (Fig.S12 in Supporting information).The influence of PVA-Nb beads on blood components after 60 min of direct contact is depicted in Fig.3a.The beads had negligible negative effects on the majority of blood cells including WBC, RBC, HGB, and PLT compared to the normal group.The blood coagulation properties of PVA-Nb beads were further assessed using four clinical assays, including assessments of prothrombin time(PT), the activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (FIB) levels [32].Compared to the control,PVA-Nb beads did not show any significant changes in PT, APTT,TT, or FIB levels (Fig.3b), indicating their good anticoagulant properties.Altogether, these results demonstrate that the PVA-Nb has perfect blood compatibility.
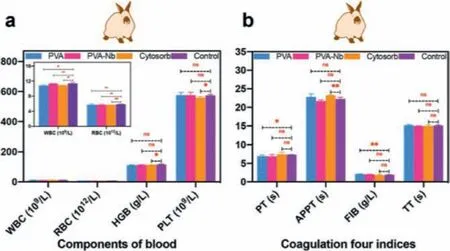
Fig.3.Blood compatibility of the PVA and PVA-Nb beads.(a) Routine blood assays after 60 min of contact with adsorbents.(b) Coagulation assays of adsorbents (mean± SD, n = 3).ns:not significant, *P <0.05, **P <0.01.
In conclusion, we prepared a TNF-αselective immunosorbent for hemoperfusion after covalent immobilization of anti-TNF-αNb.PVA-Nb beads exhibited biocompatible properties including low hemolysis rates (<1%), negligible changes in blood components,and reasonable effects on blood coagulation properties.In tests on human plasma, the beads achieved an ideal adsorption capacity of 416.9 ng/g (when the plasma-to-adsorbent ratio was 300).In addition, experiments using hyperinflammatory plasma from a rat model of sepsis confirmed the selective removal of TNF-αwithout a significant reduction in other blood proteins.Duringin vivostudies, the hemoperfusion device was constructed to remove TNF-αin rat models, further proving that the beads had good removal performance (~85%/60 min).Moreover, the adsorption performance did not change with an increase in temperature from 8 °C to 45 °C,indicating strong stability.In addition, the material was storable,as in tests, 95% of the original capacity was retained after 6-month storage.Therefore, PVA-Nb beads may have great potential for clinical application for efficient and safe TNF-αadsorption from blood.They may inhibit cytokine storms and thus improve the clinical outcomes of patients with sepsis and various inflammatory diseases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (No.2017YFC1104401), the National Natural Science Foundation of China (No.81771986), the Natural Science Foundation of Tianjin (No.18YFZCSY00860), and the Scientific Research Translational Foundation of Wenzhou Safety (Emergency)Institute of Tianjin University (No.TJUWYY2022004).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.087.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
