Seedless synthesis of gold nanorods with tunable plasmonic peaks beyond 1300 nm
Lingxi Zhu, Zhuoxun Lu, Liming Zhng, Nongyue He,c,*
a State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China
b Key Laboratory of Tropical Disease and Translational Medicine of the Ministry of Education & Hainan Provincial Key Laboratory of Tropical Medicine,Hainan Medical College, Haikou 571101, China
c Hunan Key Laboratory of Biomedical Nanomaterials and Devices, Hunan University of Technology, Zhuzhou 412007, China
Keywords:Gold nanorods Seedless method Hydroquinone Acid Aspect ratio
ABSTRACT Gold nanorods (AuNRs), as relatively common materials used in biomedical areas, have been synthesized by means of many methods.However, the conventional seed-mediated method is limited by complex operations and low yield.Besides, for further applications of AuNRs, well monodispersed AuNRs and tunable longitudinal surface plasmon resonance (LSPR) remain to be improved.Herein, we report a one-pot method for synthesizing AuNRs without seeding agents.In this method, we use phenols as reducing agents and hydrochloric acid and nitric acid are used to regulate the pH of the growth solution.AuNRs with the longest LSPR peak position reaching 1340 nm are prepared.Furthermore, by systematically optimizing concentrations of the reagents involved in the growth solution, different aspect ratios of AuNRs are synthesized.The facile synthesis, controllability aspect ratio, and long LSPR peak make our method promising for wider applications of AuNRs.
In the past few years, nanomaterials have found many applications in biomedical field [1–4].Among the widely researched biomedical nanomaterials, gold nanoparticles (AuNPs) have long been a topic of intense research, which were used for biosensing [5–7] and cancer treatment [8,9].Gold nanorods (AuNRs) are one of the most effective agents in all these AuNPs, have attracted much attention.Owing to their unique shape-dependent optical properties,i.e., longitudinal surface plasmon resonance (LSPR), they are widely applied in various areas [9–20].For example, when the aspect ratio of AuNRs changes, the color of the solution will change accordingly.Based on this, researchers use AuNRs to develop various biosensors to detect heavy metal pollution and pesticide pollution [21–23].Besides, AuNRs have high photothermal conversion efficiencies, making them good candidates for photothermal therapy and thermosensitive drug carriers [24–28].Due to their broad application prospects, many efforts have been devoted to the development of the synthesis techniques of AuNRs.
From past to now, the most conventional and wildly used method to synthesize AuNRs is the seed-mediated method, which is pioneered by Murphy and co-workers [29,30] and EI-Sayed and co-workers [31].In their method, ascorbic acid was severed as the reducing agent, and gold seeds were added into the growth solution to synthesize AuNRs.This method has several limitations:(1)Gold seeds need to be freshly prepared and cannot be preserved a long time; (2) the LSPR peak position of the prepared AuNRs is not longer than 850 nm.Considering many properties of AuNRs depending on their sizes [21,22,26], synthesizing AuNRs with tunable and long LSPR peaks is highly desirable.Researchers have proposed many strategies to prepare AuNRs with an LSPR peak at longer wavelengths.EI-Sayed and co-workers used a CTAB/BDAC surfactant to synthesize AuNRs with the LSPR peak position over 1000 nm [31,32].Murray and co-workers added aromatic additives into the growth solution to provide AuNRs with the LSPR peak position at about 1200 nm [33].To regulate the growth kinetics, Murphy and co-workers used HCl to decrease the pH of the growth solution and they prepared mini gold nanorods with the LSPR peak position>1000 nm [34].However, these methods are time-consuming and requirements on experimental parameters are strict, and the reproducibility and tunable LSPR peaks are not satisfactory.
Recently, another seedless method to synthesis AuNRs, which can also be called the one-pot synthesis method, has attracted much attention [35–38].Compared with seed-mediated method, it is more convenient.By adding CTAB, gold ion, silver ion, ascorbic acid, and NaBH4one by one into the reaction vessel, AuNRs are prepared.Although this method to synthesize AuNRs is simpler,the inherent limitations are relatively low monodispersity and untunable LSPR peaks [35,36].Jana and co-workers firstly used the seedless method to synthesize AuNRs [39], but there existed the problem of spherical impurities.El-Sayed and co-workers improved the monodispersity of AuNRs by adjusting the solution pH and the amount of NaBH4[37].Previously, we changed ascorbic acid with three different phenols (hydroquinone, 1,2,3-trihydroxybenzene,and 1,2,4-trihydroxybenzene) as reducing agents [40–43].Remarkable yields were obtained and LSPR peaks were adjustable across a broad range (550-1100 nm).The synthesis methods of gold nanorods were listed (Table 1).However, the wavelengths of the LSPR peaks of AuNRs previously synthesized from the seedless method were shorter than those of the AuNRs through the seedmediated method.Besides, LSPR peaks of AuNRs prepared by the seedless method were hardly tunable.Therefore, for further applications of AuNRs, it is necessary to develop some facile methods to synthesize high-quality AuNRs with LSPR peaks displaying longer and tunable wavelengths.
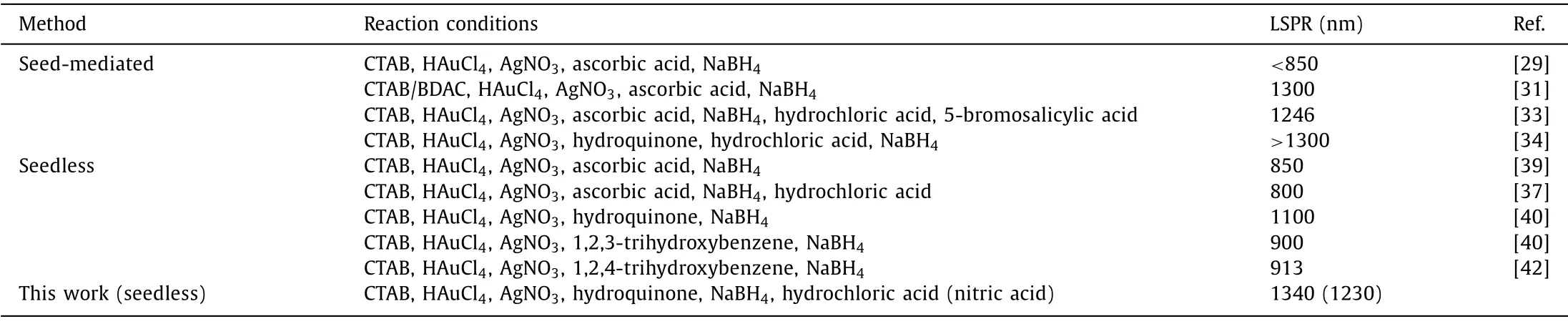
Table 1 Summaries of the synthesis methods of gold nanorods.
Herein, we report a facile and reproducible seedless synthesis method to prepare AuNRs.By using hydroquinone as a reducing agent, we apply hydrochloric acid and nitric acid to adjust the solution pH to synthesize AuNRs with the longest LSPR peak reaching 1340 nm.We also systematically study the effect of the concentrations of various reagents in the growth solution on the aspect ratios of the prepared AuNRs.
In this work, the growth solution was prepared by adding the surfactant CTAB, silver nitrate, and hydroquinone to the chloroauric acid solution, so that Au+or Au3+was reduced to gold atoms.Next, NaBH4was added to produce gold seeds.Under the induction of silver ions, the gold atoms were adsorbed on the gold seeds to obtain AuNRs.Hydrochloric acid (nitric acid) was used to adjust the pH of the solution to regulate the reducing ability of NaBH4and hydroquinone.Both the nucleation process and growth kinetics were influenced and the growth of AuNRs would be slower and thereby obtaining AuNRs with higher aspect ratios.
We first added nitric acid into the growth solution to synthesize AuNRs.In a water bath with a temperature of about 30 °C,chloroauric acid (0.01 mol/L), silver nitrate (0.02 mol/L), nitric acid(1 mol/L), hydroquinone (0.33 mol/L) were added into CTAB solution (0.11 mol/L) one by one, and then they were kept standing for 3-5 min.Then, NaBH4(0.498 mmol/L) solution was added into the growth solution and kept standing in a 30 °C incubator for over 12 h (final reactant concentrations are given in Fig.1).
Besides, we added hydrochloric acid into the growth solution to synthesize AuNRs.In a water bath with a temperature of about 30 °C, chloroauric acid (0.01 mol/L), silver nitrate (0.02 mol/L), hydrochloric acid (1 mol/L), hydroquinone (0.33 mol/L) were added into CTAB solution (0.11 mol/L) one by one, and then they were kept standing for 3-5 min.Then, NaBH4(0.498 mmol/L) solution was added into the growth solution and kept standing in a 30 °C incubator for over 12 h (final reactant concentrations are given in Figs.2-5).
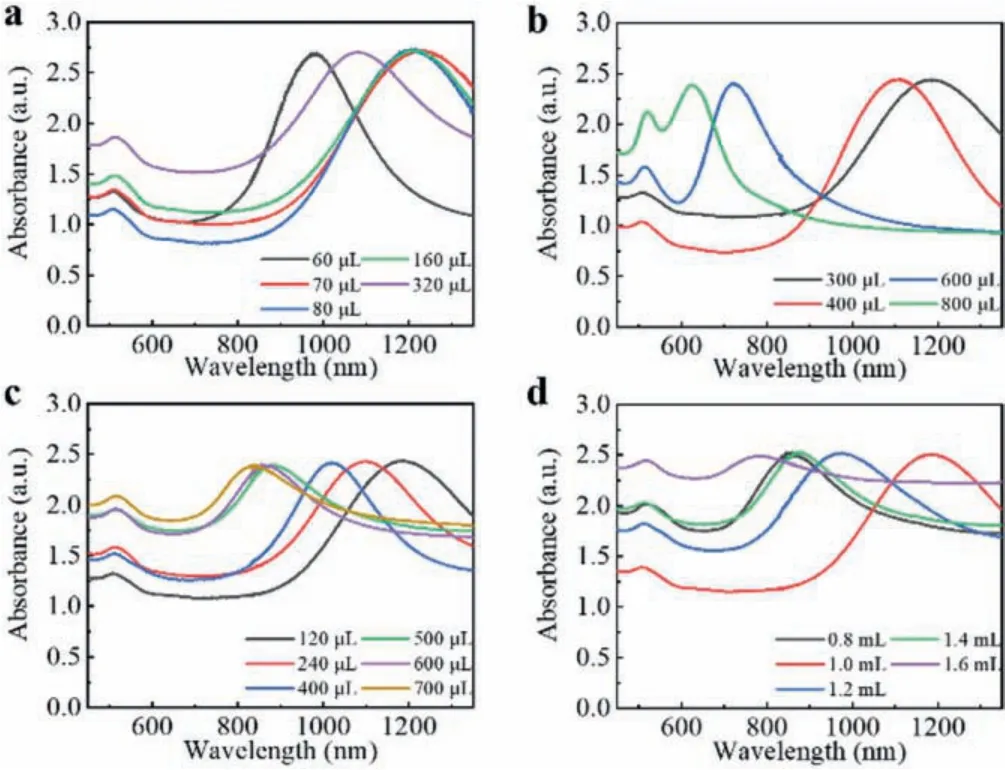
Fig 1.Vis-NIR spectra of AuNRs prepared by adding nitric acid into the growth solution with different amounts of (a) AgNO3, (b) HAuCl4, (c) hydroquinone, and (d)NaBH4.
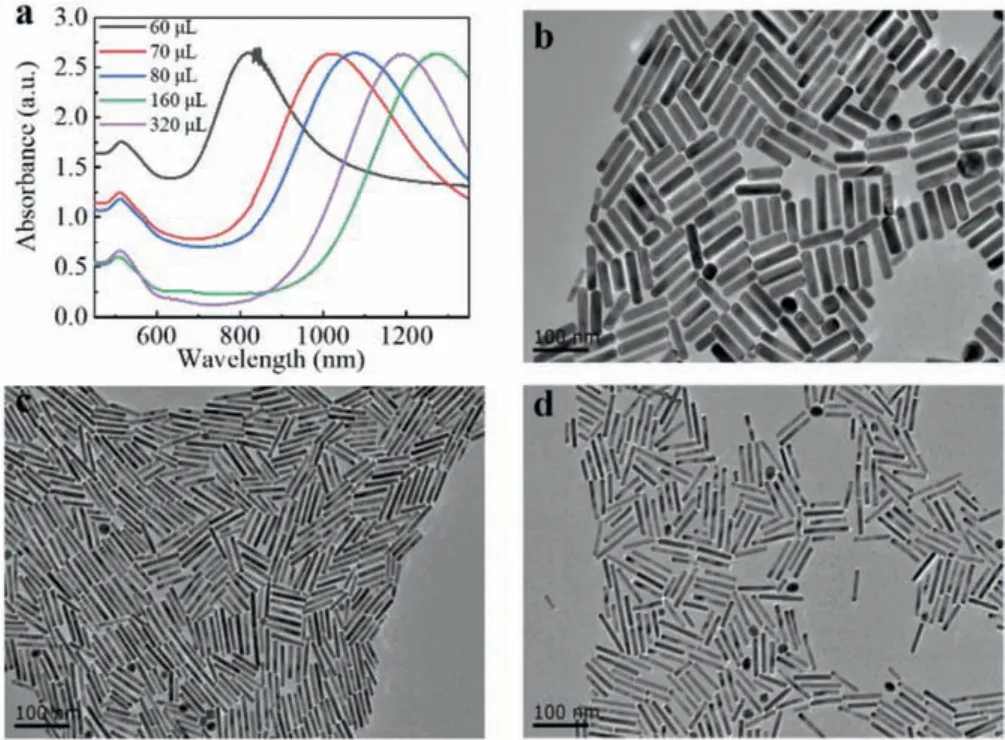
Fig 2.(a) Vis-NIR spectra of AuNRs prepared adding hydrochloric acid into the growth solution with different amounts of silver nitrate.(b-d) TEM images of AuNRs prepared adding silver nitrate (0.02 mol/L) with different volumes:(b) 60 μL, (c)320 μL, (d) 160 μL.Other parameters:CTAB (0.11 mol/L, 5 mL), HAuCl4 (0.01 mol/L,0.4 mL), HCL (1 mol/L, 45 μL), hydroquinone (0.33 mol/L, 120 μL), NaBH4 (0.498 mmol/L, 1 mL).Scale bar:100 nm.
As shown in Scheme 1, chloroauric acid, silver nitrate, acid, and hydroquinone were added into CTAB solution in a 30 °C incubator under slight shaking and then were kept standing for 3-5 min.Next, the freshly prepared was added into the growth solution and kept standing for over 12 h to synthesize AuNRs.We used hydrochloric acid and nitric acid to lower the reducibility of both hydroquinone and NaBH4, thus slowing down the growth of AuNRs,improving the monodispersity, and achieving higher aspect ratio AuNRs.
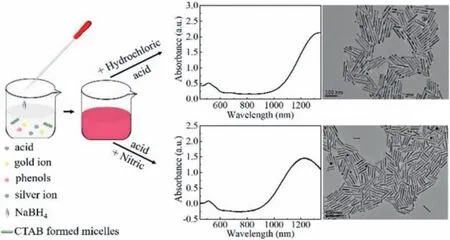
Scheme 1.Schematic of seedless synthesis of AuNRs.Scale bar:100 nm.
After centrifuging at 14000gfor 20 min and washing with distilled water three times, extra CTAB was removed and then purified AuNRs were collected.Then we used a spectrophotometer to measure the LSPR peak.It was found that in the case of adding nitric acid, the longest LSPR peak reached 1230 nm.By replacing nitric acid with hydrochloric acid, the LSPR peak suffered from a red shift and reached 1340 nm.TEM images of AuNRs were shown below and the aspect ratios of AuNRs were about 12 and 15.2, respectively.
As shown in Scheme 1, by adding nitric acid into the growth solution, the longest LSPR peak of synthesized AuNRs reached 1230 nm.As each parameter in the growth solution is vital to the preparation of AuNRs, we thus varied concentrations of different parameters and finely tuned LSPR peak positions of the prepared AuNRs.As shown in Fig.1a, when the concentrations of AgNO3increased from 0.18 mmol/L to 0.21 mmol/L, the LSPR peak redshifted from 980 nm to 1230 nm.However, when the concentrations of AgNO3increased to 0.24 mmol/L, 0.47 mmol/L, and 0.72 mmol/L, LSPR peaks were 1210 nm, 1207 nm, and 1080 nm, respectively.In Fig.1b, when the concentrations of HAuCl4were 0.45 mmol/L, 0.60 mmol/L, 0.87 mmol/L, and 1.13 mmol/L, the LSPR peaks were 1186 nm, 1108 nm, 719 nm, and 625 nm accordingly.In Fig.1c, as the concentrations of hydroquinone increased from 6.05 mmol/L to 32.46 mmol/L, the LSPR peak of synthesized AuNRs blue-shifted from 1185 nm to 839 nm.In Fig.1d, when the concentrations of NaBH4increased from 0.06 mmol/L to 0.11 mmol/L, the LSPR peaks red-shifted from 857 nm to 1185 nm, and then blueshifted to 780 nm, which had the same trend as shown in Fig.1a.
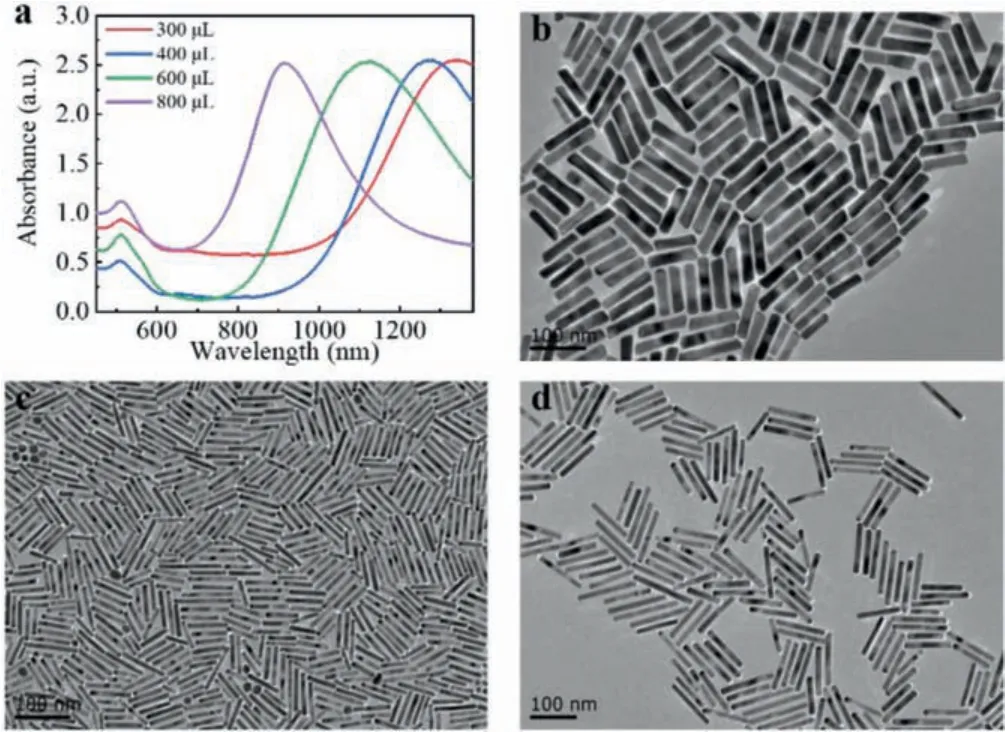
Fig 3.(a) Vis-NIR spectra of AuNRs prepared adding hydrochloric acid into the growth solution with different amounts of chloroauric acid.(b-d) TEM images of AuNRs prepared adding chloroauric acid (0.01 mol/L) with different volumes:(b)800 μL, (c) 600 μL, (d) 300 μL.Other parameters:CTAB (0.1 mol/L, 5 mL), AgNO3(0.02 mol/L, 160 μL), HCL (1 mol/L, 45 μL), hydroquinone (0.33 mol/L, 120 μL),NaBH4 (0.498 mmol/L, 1 mL).Scale bar:100 nm.
By adding nitric acid into the growth solution, we synthesized AuNRs with the longest LSPR peak wavelength reaching 1230 nm,which is longer than that of AuNRs prepared by our previous seedless method [40].In order to synthesize AuNRs with longer LSPR peak wavelengths, we tried to replace nitric acid with hydrochloric acid and finally, LSPR peak position of prepared AuNRs redshifted to 1340 nm.Then we tried to study the relationship between concentrations of different parameters and LSPR peak positions of AuNRs.
It is well known that the AgNO3concentration is closely related to the aspect ratios of AuNRs [29,33,44,45], so we attempted to synthesize AuNRs possessing higher aspect ratios and longer LSPR peaks by regulating the silver ion concentration.Fig.2 shows the vis-NIR spectra and TEM images of AuNRs in the presence of hydrochloric acid and various concentrations of silver ions.When adding 0.18 mmol/L AgNO3into the growth solution, the wavelength of the LSPR peak was the shortest (820 nm) and the aspect ratio was about 4.2, as shown in Fig.2b.After the concentrations of AgNO3increased to 0.21 mmol/L, 0.24 mmol/L, and 0.47mmol/L,the LSPR peak positions are 1020 nm, 1080 nm, and 1270 nm, respectively.The corresponding aspect ratios augmented to 8.5, 9,12.5.AuNRs with aspect ratios of 9 and 12.5 were shown in Figs.2c and d.However, when the volume of AgNO3was increased to 0.72 mmol/L, the LSPR peak suffered from a blue shift to 1190 nm.We guess that a high concentration of silver ions may inhibit the growth of GNRs in a longitudinal direction [40].Therefore, an appropriate silver nitrate concentration is of vital importance to the synthesis of well-shaped gold nanorods.
As an essential part of the growth solution, the concentration of chloroauric acid ions has a great effect on the growth of AuNRs.As shown in Fig.3, firstly we added 0.45 mmol/L chloroauric acid into the growth solution, LSPR peak reached 1340 nm and the aspect ratio was about 15.2, as shown in Fig.3d.When the concentrations of chloroauric acid were increased to 0.60 mmol/L, 0.87 mmol/L,and 1.13 mmol/L respectively, LSPR peaks blue-shifted to 1270 nm,1120 nm, and 915 nm, respectively, and the aspect ratios decreased to 12.5, 10.5, 5.5 approximately.AuNRs with aspect ratios of 12.5,10.5, 5.5 were shown in Figs.2d, 3c, and 3b.
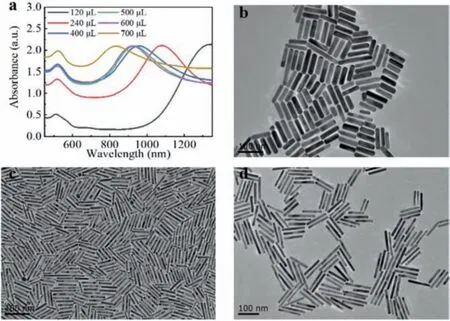
Fig 4.(a) Vis-NIR spectra of AuNRs prepared adding hydrochloric acid into the growth solution with different amounts of hydroquinone.(b-d) TEM images of AuNRs prepared adding hydroquinone (0.33 mol/L) with different volumes:(b) 700 μL, (c) 240 μL, (d) 120 μL.Other parameters:CTAB (0.1 mol/L, 5 mL), HAuCl4 (0.01 mol/L, 300 μL), AgNO3 (0.02 mol/L, 160 μL), HCL (1 mol/L, 45 μL), NaBH4 (0.498 mmol/L, 1 mL).Scale bar:100 nm.
Fig.4 shows the effect of hydroquinone concentration on AuNRs synthesis.As shown in Fig.4, we obtained a wide range of LSPR peaks from 830 nm to 1340 nm by varying the concentrations of hydroquinone.As shown in Fig.4a, when the concentration of hydroquinone was 6.05 mmol/L, the LSPR peak position of prepared AuNRs was the longest, 1340 nm and the corresponding aspect ratio was about 15.2.After we increased the concentration of hydroquinone to 32.46 mmol/L, the LSPR peak kept on decreasing till 830 nm and the aspect ratio was decreased to 4.5.Among them, when the concentrations of hydroquinone were increased to 11.74 mmol/L, 19.11 mmol/L, 23.55 mmol/L, and 27.86 mmol/L,LSPR peak positions were 1080 nm, 957 nm, 931 nm, 922 nm, and the aspect ratios were about 8.2, 6, 5.8, 5.6 accordingly.Figs.4bd showed the TEM images of AuNRs with aspect ratios of 4.5, 9,and 15.2.Compared with that AuNRs prepared by adding different amounts of ascorbic acid or other reducing agents have narrow LSPR peaks [46,47], hydroquinone is more appropriate for preparing AuNRs with tunable aspect-ratio-dependent LSPR peaks [40].
NaBH4solution is not stable in aqueous solution for an extended time and may lead to AuNRs with unsatisfactory morphology [40].To overcome this problem, we used freshly prepared NaBH4with ice water.In this seedless method, NaBH4is a strong reducing agent, which can govern the synthesis and monodispersity of final AuNRs [29].Therefore, we further studied the effect of NaBH4concentration on the synthesis of AuNRs.As shown in Fig.5a, when we added 0.06 mmol/L and 0.07 mmol/L NaBH4into the growth solution, the LSPR peak position wavelengths were as long as 1340 nm (Fig.5d).When the concentrations of NaBH4were increased to 0.08 mmol/L, 0.10 mmol/L, 0.11 mmol/L, and 0.12 mmol/L, the LSPR peak wavelengths were 1084 nm, 965 nm, 961 nm, and 890 nm.The corresponding aspect ratios were decreased to 8.3 (Fig.5c), 6.3, 6.1, and 5.2 (Fig.5b).
As we know, the color of the AuNRs solutions changes with the change of the LSPR peaks [21–23].Therefore, by observing the changes in the color of the AuNRs solutions, we can more intuitively recognize the changes in the position of the LSPR peaks.
Herein, we selected several different AuNRs solutions synthesized in this work, as shown in Fig.6.In Figs.6a-f, the positions of the LSPR peaks were 820 nm, 910 nm, 1080 nm, 1120 nm, 1230 nm, and 1340 nm respectively.
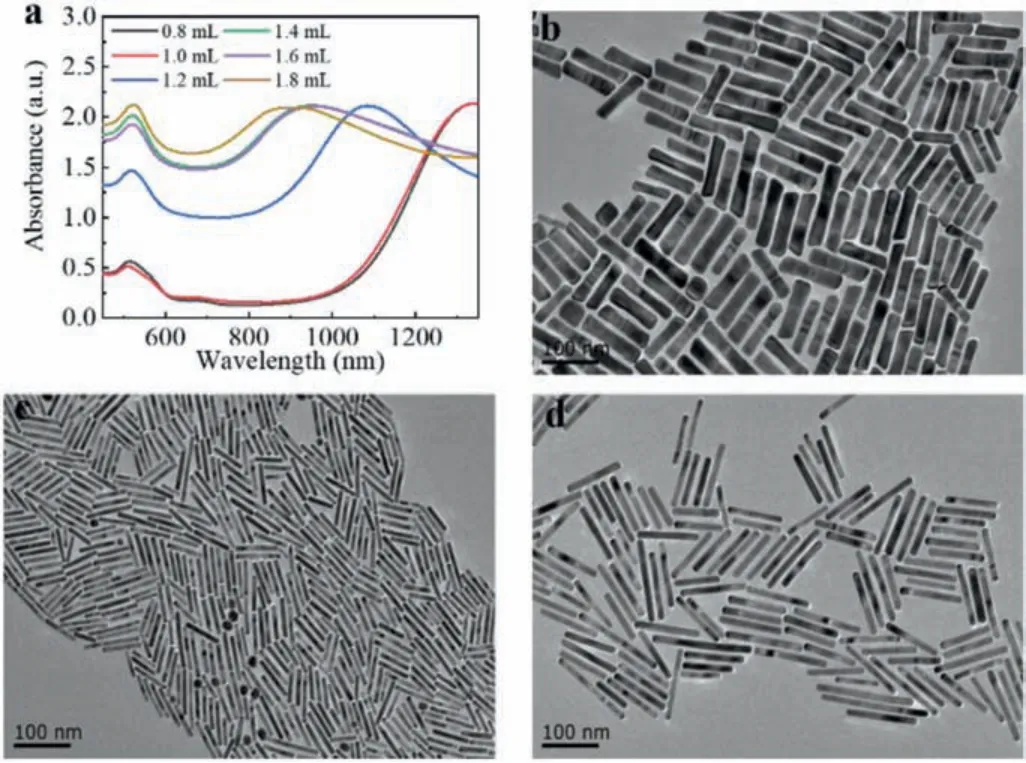
Fig 5.(a) Vis-NIR spectra of AuNRs prepared adding hydrochloric acid into the growth solution with different amount of NaBH4.(b-d) TEM images of AuNRs prepared adding NaBH4 (0.498 mmol/L) with different volumes:(b) 1.8 mL, (c) 1.2 mL,(d) 1.0 mL.Other parameters:CTAB (0.1 mol/L, 5 mL), HAuCl4 (0.01 mol/L, 300 mL),AgNO3 (0.02 mol/L, 160 mL), HCL (1 mol/L, 45 mL), hydroquinone (0.33 mol/L, 120 mL).Scale bar:100 nm.
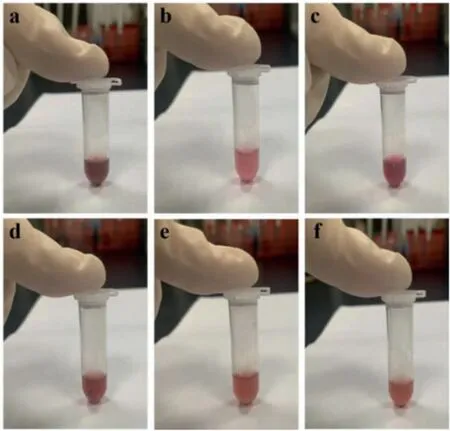
Fig 6.Pictures of AuNRs solutions with different LSPR peaks.
In summary, our proposed seedless method is efficient to synthesize monodispersed AuNRs.In our method, hydroquinone functions as the reducing agent, and hydrochloric acid and nitric acid are applied to regulate the pH of the growth solution.As a result, the longest LSPR peak wavelength of the prepared AuNRs is 1340 nm and the aspect ratio is about 14 accordingly.Besides, by optimizing the concentrations of different reagents in the growth solution, including AgNO3, HAuCl4, hydroquinone, and NaBH4, we finely succeed in tuning the size of the synthesized AuNRs and obtain a wide range of LSPR peak between 800 nm and 1340 nm.Considering the wide applications of AuNRs, we believe this seedless method has a great potential for mass production of highquality AuNRs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.62071119) and the Jiangsu Provincial Key Research and Development Program (No.BA2020016).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
