An injectable mPEG-PDLLA microsphere/PDLLA-PEG-PDLLA hydrogel composite for soft tissue augmentation
Yue Pan, Yao Xiao, Ying Hao, Kun Shi, Meng Pan, Zhiyong Qian
State Key Laboratory of Biotherapy/Collaborative Innovation Center for Biotherapy, West China Hospital, West China Medical School, Sichuan University,Chengdu 610041, China
Keywords:Soft tissue augmentation mPEG-PDLLA Microspheres Hydrogel Collagenogenesis
ABSTRACT Injectable filling material is a simple and efficient method for soft tissues reconstruction and is extremely popular in not only plastic surgery but also cosmetic industry.However, there is a lack of soft tissue fillers with perfect performance on the market currently.Here, we constructed a new microsphere/hydrogel composite and evaluated its potential as a candidate for soft tissue augmentation.mPEG-PDLLA microspheres were prepared by utilizing a SPG membrane emulsifier which endowed microspheres with good sphericity and particle size uniformity.PDLLA-PEG-PDLLA hydrogel which shared the same component with the mPEG-PDLLA copolymer acted as a carrier and fixed the microspheres at the injected sites.The mPEG-PDLLA microsphere/PDLLA-PEG-PDLLA hydrogel composite was flowable in room temperature and transformed into gel after being heated to body temperature.This feature is convenient for subcutaneous filling. In vivo assessment on mice showed good safety profile of the composite.Moreover, the density of collagen fibers increased over 13 weeks.Overall, this biocompatible microsphere/hydrogel composite involves simple component and no extra crosslinking agents, and has the ability to stimulate collagen production, thus, may be a candidate for soft tissue augmentation.
Soft tissue depressions or deformities may result from surgical resection, severe trauma, congenital disease, congenital human immunodeficiency virus infection or even the natural physiological aging process [1,2].Some of these soft tissue defects lead to malfunction of organs and create inconveniences to the patient’s normal daily activities such as snatching, chewing, speech, swallowing [3].While some, especially those involved faces, bring great negative impacts on the daily life, career and mental health of the patients [4].Therefore, the reconstruction and repair of soft tissue can not only help patients regain the physiological function of the original tissue, but also help to overcome the psychological problems caused by physical appearance and improve life quality,which is of great significance.
At present, the clinical treatments for the restoration of soft tissue depressions of different severity mainly involve surgeries.Allograft and autologous tissue transplantation are invasive surgeries which will inevitably leave scars.Besides, allograft requires one-to-one customization and processing of the implanted prosthesis according to the three-dimensional shape of the defective cave, and it has high risks of infection during and after the operation [5,6].While the operation and recovery time of autologous tissue transplantation are relatively long, and postoperative nursing needs to be very careful [7].Autologous fat transplantation is a non-invasive surgical method that has attracted much attention in recent years.However, this treatment is now facing problems of low survival rate of adipocytes and unpredictable filling effect [8–10], which restrict its development.Local injection of soft tissue fillers is another non-invasive procedure.It causes little damage to patients, so the postoperative recovery period is greatly shortened,and the risk of infection is also reduced [11–13].This method not only brings convenience to plastic surgeons and patients, but also is favored in the medical beauty industry because of its high costprice performance.
In-situforming hydrogel has played a very important role in tissue engineering, such as wound healing [14,15], hemostasis [16,17],bone repair [18,19], corneal repair [20], and dental cavity filling [21].Compared with the hydrogels formedin vitro, theinsituforming hydrogels can be implanted through injection, which cause less damage to patients, and thus improving the comfort and compliance of patients during the treatment [22].Besides, when thein-situforming hydrogel is used to fill the tissue cavities, it can automatically form a shape that fits the defective cavity, avoiding the complicated procedure of secondary processing due to the mismatch between the general mold and the filling void [14].All of these superiorities make it possible to employin-situforming hydrogel in soft tissue augmentation.
Soft tissue fillers like Sculptra®, Radiesse® and Artefill®, which consist of a carrier and particles, evolve from fillers represented by hyaluronic acids (HA) fillers.Instead of relying on entity volume alone, they work by the interactions between the body and the particles [23].In this process, an acute inflammatory response occurs first, which later transforms to a chronic inflammatory response.Monocytes, macrophages and fibroblasts will accumulate at the injection site, forming fibrous capsules surrounding the particles.As the particles degrade over time, these formed fibrous capsules are deposited, resulting in an increase in collagen to fill the space [24–27].This type of filler is more like a stimulator than a filler [28].It gets better outcomes, no matter from the perspective of longevity or aesthetics, than traditional bulking agents.
Based on these considerations, we developed an injectable composite consist of mPEG-PDLLA (PELA) microspheres and synthetic PDLLA-PEG-PDLLA (PLEL) hydrogel carrier (Scheme 1).PELA is a di-block copolymer polymerized by lactide and polyethylene glycol monomethyl ether.Due to the presence of hydrophilic PEG blocks,the polymer is less hydrophobic.We have learned from previous studies that the magnitude of the inflammatory response is related to the hydrophobicity of the foreign material [29,30].We expected that microspheres manufactured from this amphiphilic PELA can be used as a collagen stimulator with a lesser degree of inflammation initiation and good biocompatibility for soft tissue filling.PLEL is a copolymer composed of PEG and PDLLA segments.Both of the monomers are FDA approved.Appropriate concentration of PLEL aqueous solution can transform from sol to gel when the temperature increases from room temperature to body temperature.And it does not use cross-linking agents nor does it cause allergic reactions.So, shortcomings related to collagen and crosslinked HA fillers can be circumvented.In this study, PLEL copolymers were synthesized and characterized according to previous protocols in our groups and PELA microspheres were prepared by a SPG membrane emulsifier.After simply mixing these two components, we evaluated its thermo-sensitivity andin vitrocytotoxicity.Finally,animal experiments were carried out to explore its application for soft tissue filling in terms ofin vivosafety, inflammation, degradation and collagen generation.
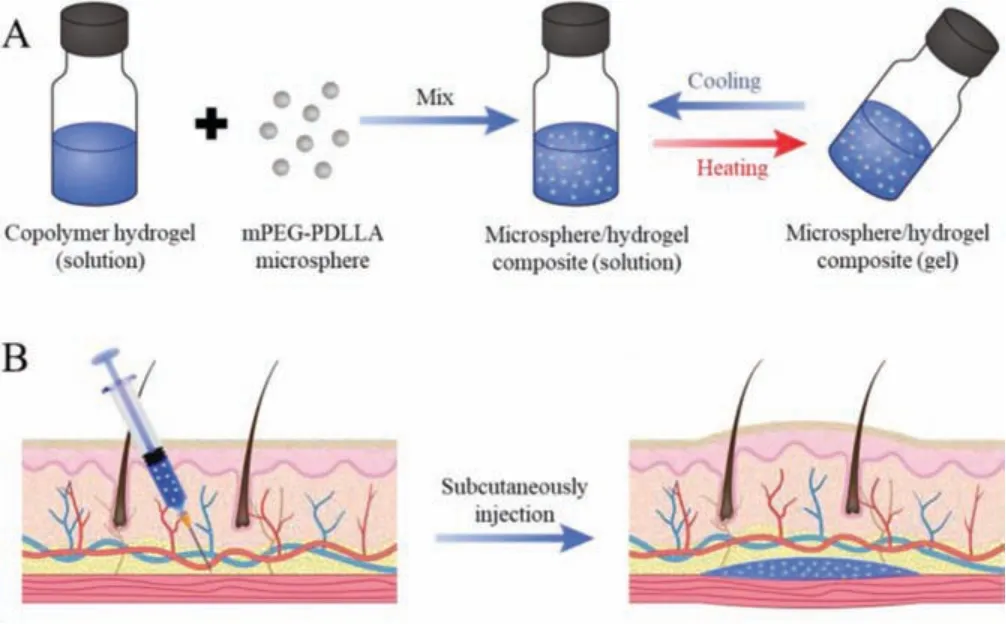
Scheme 1.Schematic illustration of this study.(A) Microsphere/hydrogel composite was obtained by mixing copolymer hydrogel and microspheres.(B) The thermosensitive composite was used for skin augmentation through subcutaneous injection.
Triblock PDLLA-PEG-PDLLA copolymer was synthesized through ring-opening polymerization between PEG and D,L-lactide in the presence of stannous octoate as catalyst (Fig.S1 in Supporting information).The absorption peak at 1747 cm-1(red line) in FT-IR spectra indicates the presence of the carbonyl group (C=O) which confirmed the reaction between PEG and lactide.1H NMR spectra of PLEL is shown in Fig.S1C (Supporting information).Signals at 5.10 ppm and 1.55 ppm were attributed to methyl group and methine in PDLLA segments respectively.Signals at 4.30 ppm and 3.70 ppm were resulted from methylene in PEG block.The1H NMR spectrum was similar to that of published work [31–33], which indicated the successful synthesis of PLEL copolymer.
Four kinds of PELA microspheres were prepared in assistance of a membrane emulsifier (Fig.1).As the SEM images depicted,all the PELA microspheres except those made from mPEG2000-PDLLA18kwere sphere.It was because the hydrophilic blocks in mPEG2000-PDLLA18kaccount for a large proportion in the material,leading to the low viscosity of the material and the inability to form stable microsphere structures.The surfaces of PELA microspheres exhibited porous morphology, which were similar to the findings in earlier reports [34].There were two possible explanations for this phenomenon [34,35].One was that the hydrophilic PEG blocks absorbed a large amount of water and extended on the surface of the microspheres.During the freeze-drying process,the water absorbed by the PEG blocks evaporated, thus resulting in large pores.The other reason was that the dichloromethane, which had a low boiling point, evaporated rapidly during the solidification stage, bringing in local "explosions" on the surface of the microspheres.This might also result in the holes.Apart from these, it could be observed that as the PEG block ratio decreases, the surface became less rough, and the pores became more compact.
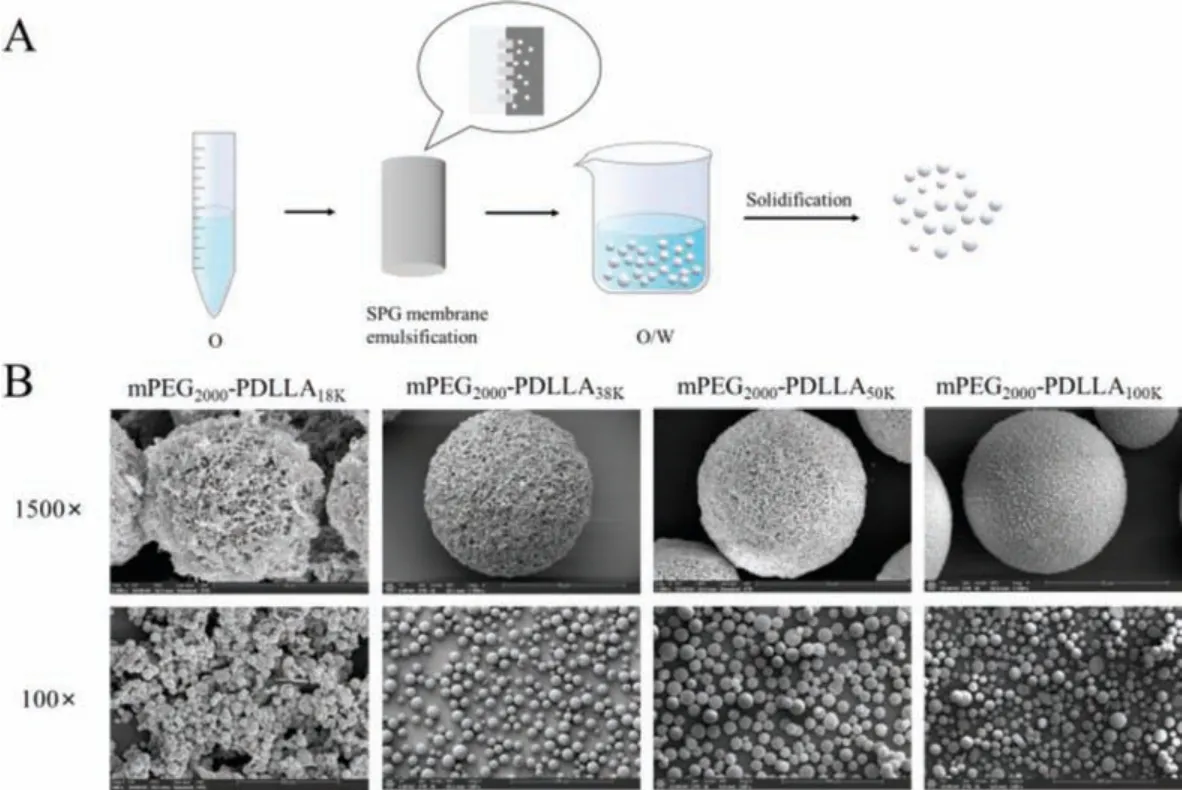
Fig.1.Preparation and characterization of PELA microspheres.(A) Schematic illustration of PELA microspheres preparation.(B) Representative SEM images of PELA microspheres showing their morphologies.
Since shaped microspheres could not be prepared from mPEG2000-PDLLA18K, PELA microspheres made from copolymers mPEG2000-PDLLA38k, mPEG2000-PDLLA50k, and mPEG2000-PDLLA100kwere employed for the following studies.They were named as 38 K PELA, 50 K PELA and 100 K PELA respectively for convenience.
PELA microspheres were incubated with HDF cells to investigate their cytocompatibility.As shown in Fig.S2A (Supporting information), after 24 h of direct contact with HDF cells, the cell viabilities were still above 90%, indicating that PELA microspheres had no obvious cytotoxicity to HDF cells.HDF cells were further treated with PELA microspheres for 48 h and 72 h to determine the influence on cell proliferation.As presented in Fig.S2B (Supporting information), after 48 h and 72 h, the cell viabilities of the 38 K PELA group dropped below 80% which might be attributed to a faster microspheres degradation rate resulting from a large proportion of PEG blocks and a smaller molecular weight.The acid produced by the rapid degradation of PELA affected the growth of cells.Compared with the 50 K PELA microsphere group, the viabilities of cells contacted with the 100 K PELA microsphere after 48 h culture decreased more obviously, which can be explained by the different degradation rate products of two type of PELA microspheres.Over the course of three days experiments, the cells in the 50 K PELA group exhibited good growth trend.
The potential to induce inflammatory cytokines on macrophages was studiedin vitro.Fig.S2C-(a) (Supporting information) showed the production of monocyte chemoattractant protein-1 (MCP-1).There was no significant difference between experimental group and control group after 24 h co-culture.While after 48 h, the MCP-1 secretion was much higher in the PELA microsphere groups than the blank control group.The produce of interleukin-6 (IL-6), tumor necrosis factorα(TNF-α) and other pro-inflammatory factors can reflect the potential of the biomaterials to induce acute inflammatory [29,36].As shown in Fig.S2C-(b) (Supporting information), after 24 h, TNF-αlevels in PELA microspheres groups increased more than those in the blank control group.This was because the macrophages were activated by the PELA microspheres, and secreted larger amounts of TNF-αsequentially.After 48 h treatment, the secretion of TNF-αin 100 K PELA microspheres group was twice as much as that in the other two microspheres groups, indicating that 100 K PELA microspheres could activate and induce inflammatory response on macrophage more than 38 K and 50 K PELA microspheres groups.High levels of IL-6 (Fig.S2C-(c) in Supporting information) and TNF-αproduced by macrophages incubated with 100 K PELA microspheres was most likely associated with its strong hydrophobicity.It had been reported previously that highly hydrophobic biomaterial surfaces were more likely to induce inflammatory responses [29,30].We can assume from these results that more hydrophobic PELA microspheres will induce stronger inflammatory responses.
Considering the surface morphology, influence on cell proliferation and potential to induce inflammatory cytokines on macrophages, 50 K PELA microspheres were selected to finish the following experiments.In subsequent experiments, PELA microspheres were defaulted as PELA microspheres prepared by mPEG2000-PDLLA50K, which was abbreviated to 50 K PELA, unless otherwise stated.
Fig.S3 (Supporting information) showed the thermosensitivity of microspheres/PLEL hydrogel composite.The microspheres/PLEL hydrogel composite was injectable at room temperature and transformed to gel at body temperature which laid a foundation for its application as injectable soft tissue filling material.
The cytotoxicity was evaluatedin vitro.The survival rates of all cells were all higher than 90% during 72 h incubation (Fig.S4A in Supporting information).While different leachates were studied,the cell viability decreased gradually with the increase of leachates concentration (Fig.S4B in Supporting information).But even at concentrations as high as 2 mg/mL, more than 80% cells survived.Besides, the fluorescent images (Fig.S4C in Supporting information) displayed no significance between the experimental group and the control group.To sum up, it can be concluded that the microsphere/PLEL composite showed no obvious cytotoxicity which was favorable for biomedical use.
The hemolysis rates (Fig.S5B in Supporting information) were below 5% for composites group of different concentration, which could be considered not to cause hemolysis [37].
All animal experiments were carried out complied with the NIH Guide Concerning the Care and Use of Laboratory Animals and were approved by the Animal Experimentation Ethics Committee of Sichuan University, Chengdu, China.The PLEL solution and microspheres/PLEL hydrogel composite were injected subcutaneously.Both of them formed hydrogelin situwithin 1 min.The oval protrusions could be clearly recognized (Fig.2A).The changes of implanted hydrogel were monitored over the experiments (Fig.2B).At week 5, the PLEL hydrogel were hard to be identified while the microspheres/PLEL hydrogel could be seen clearly.9 weeks after injection, PLEL hydrogel had been degraded completely whereas a few of the microspheres/PLEL hydrogel still remained until it disappeared at week 13.Thus, the PELA microspheres/PLEL hydrogel had a longer durationin vivo.
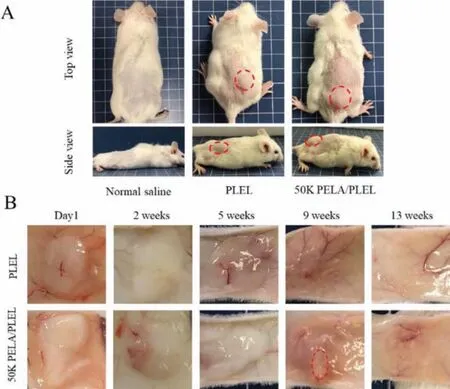
Fig.2.(A) Photographs of the mice before and after hydrogel injection.(B) Degradation of PLEL and PELA microspheres/PLEL hydrogel in vivo.
Several indicators were detected to evaluate thein vivosafety of microspheres/PLEL hydrogel composite (Fig.S6 in Supporting information).There was no significant difference in blood routine tests compared with normal saline group.Also, the indexes related to liver function and renal function was not found abnormal.Histological analysis of the distant organs showed no signs of migration of the implanted microspheres to the distant organs.Judging from these outcomes, we could come to the conclusion that the PELA microsphere/PLEL hydrogel composite and its degradation products injected did not cause harm to mice and had good safety.
Biomaterials usually trigger inflammatory responses in the host after implantation.As can be observed in Fig.3, there were a large number of inflammatory cells around and inside the filling material, the majority of which were neutrophils, indicating an acute inflammatory reaction occurred.This was due to the mechanical damage caused by syringes and the implantation of the foreign substance.At week 2 to 5, lymphocytes and neutrophils aggregation decreased rapidly and fibroblasts began to appear, suggesting a transition from acute to chronic inflammation.9 weeks after implantation, there were basically no inflammatory cells around the microspheres whereas the fibroblasts and collagen fibers grew in.No foreign body granulomatous reactions were observed around the PELA microspheres during the entire observation period.
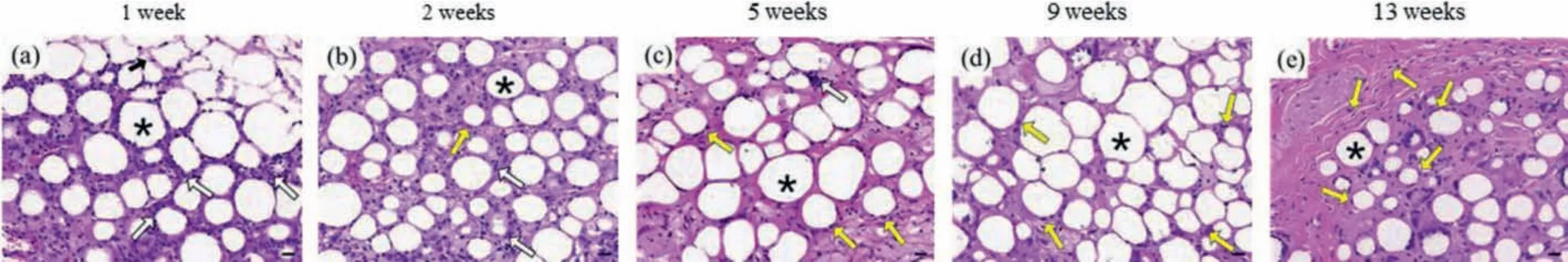
Fig.3.Histopathology images (× 40, H&E staining) of skin tissue obtained (a) 1 week, (b) 2 weeks, (c) 5 weeks, (d) 9 weeks and (e) 13 weeks after PELA microspheres/PLEL hydrogel was injected (*:PELA microspheres; black arrow:PLEL hydrogel; white arrow:inflammatory cells; yellow arrow:fibroblast).Scale bar:20 μm.
To observe the formation of collagen fiber after the implantation of PELA microsphere/PLEL hydrogel, Masson’s trichrome staining was performed.It could be seen from Fig.4A that 2 weeks after composite injection, fibers began to grow into the spaces between the microspheres.And the fibers become denser and gradually wrapped around the microspheres over time.By week 13,the collagen fiber occupied the space where the gel was filled.Quantitative statistics of collagen fiber density were exhibited in Fig.4B.Collagen fiber density was approximately six times that of one week after implantation at the end of animal experiment.
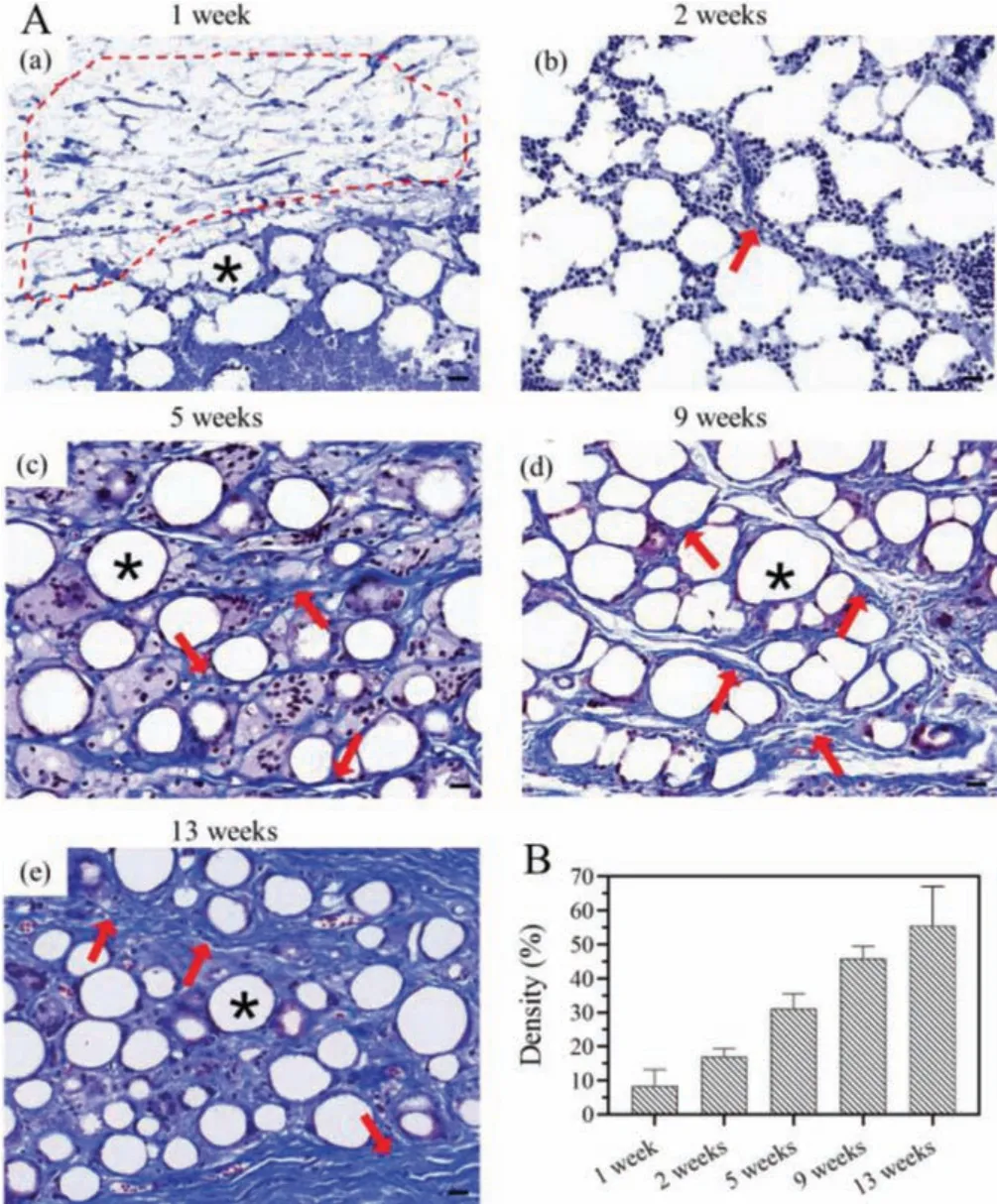
Fig.4.(A) Histopathology images (× 40, Masson’s trichome stain) of skin tissue obtained (a) 1 week, (b) 2 weeks, (c) 5 weeks, (d) 9 weeks and (e) 13 weeks after PELA microspheres/PLEL hydrogel was injected (*:PELA microspheres; red dashed line:PLEL hydrogel; red arrow:infiltration of collagen fiber).Scale bar:20 μm.(B) Changes of collagen fiber density over time.
Histological images demonstrated that the injection of PELA microsphere/PLEL hydrogel would not cause severe inflammation response and they could stimulate collagen produce which was the mechanism it functioned for soft tissue filling.
As there are no perfect soft tissue fillers that can meet all of the ideal characteristics, in this study, a composite consisting of PLEL hydrogel and PELA microspheres were constructed.The substances involved in this system were free from crosslinking agents which may carry potential risks and the biocompatibility of PELA microsphere/PLEL hydrogel composite had been confirmed bothin vitroandin vivo.The thermo-sensitivity of the composite enables easily injection for augmentation.In vivoresults showed nearly 3 months’longevity of the composite, during which no adverse action was detected, instead collagen fibers grew in and filled the space.Taken together, the PELA microsphere/PLEL hydrogel composite has the potential to serve as a soft tissue filler.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.NSFC31930067, NSFC31771096, and NSFC31525009), the National Key Research and Development Program of China (No.2017YFC1103502), the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No.ZYGD18002).And we thank Wang Hui (Analytical and Testing Center, Sichuan University) for her sincerely help in SEM tests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.093.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
