1,1′-Binaphthol annulated perylene diimides:Aggregation-induced emission enhancement and chirality inversion
Yng Zhng, Juejun Wng, Hongming Chen, Meijin Lin,,*
a Key Laboratory of Molecule Synthesis and Function Discovery, College of Chemistry, Fuzhou University, Fuzhou 350116, China
b College of Materials Science and Engineering, Fuzhou University, Fuzhou 350116, China
Keywords:Perylene diimides 1,1′-Binaphthol Aggregation-induced emission enhancement Chirality inversion
ABSTRACT Aggregation-induced emission enhancement and aggregation-induced chirality inversion are two individual phenomena for the enantiomerically pure organic dyes in the aggregates.Herein we reported for the first time that these two interesting phenomena could be observed simultaneously in the aggregated states of enantiomerically pure S/R-1,1′-binaphthol annulated perylene diimides, in which two perylene diimides moieties were bridged by S/R-1,1′-binaphthol (BINOL) at the bay positions.Owing to the rotatable C2 axes between two naphthol annulated perylene diimides moieties, both of them display intrinsic behaviors of aggregation-induced emission enhancements.At the same time, due to the steric hindrances in the imide and methoxy positions, the neighboring two π-systems of these two unique polycyclic aromatic imides in poor solvents are preferable to adopt a cross-stacking mode and thus form helical Xaggregates of opposite chirality (M/P) with chirality inversion characteristics in their circular dichroism and circularly polarized luminescence spectroscopic studies.
Organic conjugated luminescent materials have attracted great attention in the past years owing to their excellent opticalelectronic properties and wide applications [1,2].Nevertheless,these studies have always been plagued by the aggregation-caused quenching (ACQ) behaviors due to the formation of excimers or exciplexesvia π-πstacking interactions in the solid or aggregated states [3].To overcome these adverse behaviors, two close phenomena, aggregation-induced emission (AIE) [4] and aggregationinduced emission enhancement (AIEE) [5], have been reported by Tanget al.in 2001.Subsequently, tremendous AIE or AIEE molecules with rotatable C–C bonding structures have been synthesized [6–11].On the other hand, chirality inversion is an intriguing phenomenon for chiral materials [12,13].In general, it is rather difficult to achieve chirality inversion for optically pure organic compounds within the molecules [14,15] but possible in the aggregated states [16].Considering both emission enhancement and chirality inversion occur in the aggregates, the simultaneous observation of AIEE and aggregation-induced chirality inversion phenomena can be anticipated for chiralπ-conjugated systems with rotatable C–C bonding structures.Herein, we reported that this concept is indeed viable.
For the realization of this concept, we have synthesized a pair of enantiomerically pureR/S-1,1′-binaphthol annulated perylene diimides, R-BPDH and S-BPDH (Fig.1).Wherein, perylene diimides(PDIs) act as luminescent chromophore entities as their outstanding optical, electronic and light fastness properties [17–20], while 1,1′-binaphthol (BINOL) moieties play as the chiral units with the rotatable C–C bonding structures.Indeed, the BINOL derivatives have also been demonstrated to be an attractive class of organic dyes with remarkable AIEE and circularly polarized luminescence (CPL) properties [21–23].In addition, the incorporation of PDIs entities into BINOL units at the 5,6-positions to form helicalπ-clips with large dihedral angles, together with the introduction of steric substituents at the imide positions, which will help to adopt a cross-stacking mode [24] and thus form helical Xaggregates of opposite chirality (M/P).As expected, the AIEE phenomena are indeed observed in the fluorescent spectra of both two unique polycyclic aromatic imides in a mixture solvent of dichloromethane (DCM)/methylcyclohexane (MCH), while the circular dichroism (CD) and CPL spectroscopic studies show chirality inversion characteristics.
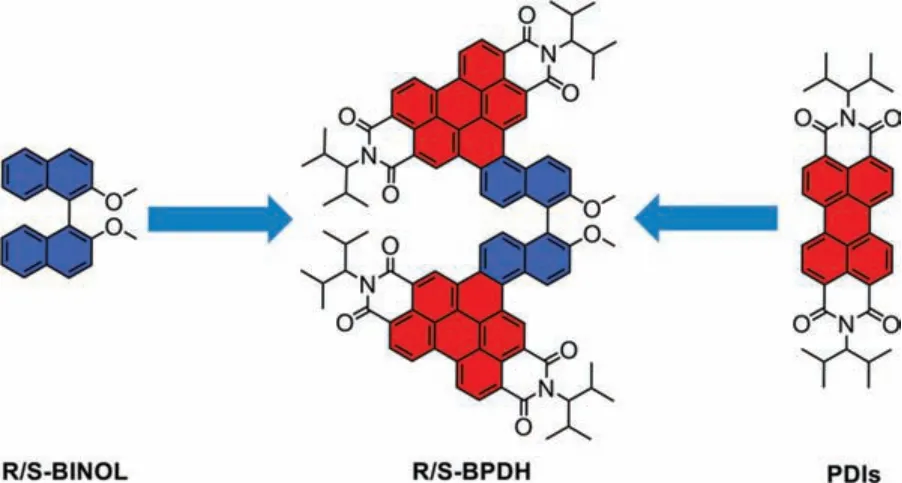
Fig.1.Chemical structures of R/S-BINOL, R/S-BPDH and PDIs.
As the known at bay area aryl-substituted PDIs that were usually prepared by transition metal catalysis [25], the present RBPDH and S-BPDH are synthesized in a yield of 79% and 82%, respectively (Scheme 1).They were obtained by the combination of mono-brominated PDIs [26] andR/S-1,1′-binaphthol [27] through a palladium-catalyzed Suzuki cross-coupling reaction followed by a photocyclization in the presence of catalytic amount I2in toluene solutions upon the irradiation of a mercury lamp for 45 min.The chemical structures of R/S-BPDH have been assigned by 1- and 2-D NMR spectroscopy (Figs.S1-S12 in Supporting information) as well as by high-resolution mass spectral analyses (Figs.S13-S17 in Supporting information), which unequivocally confirm their conjugated structures withR/S-1,1′-binaphthol annulated at the bay positions of perylene diimides.
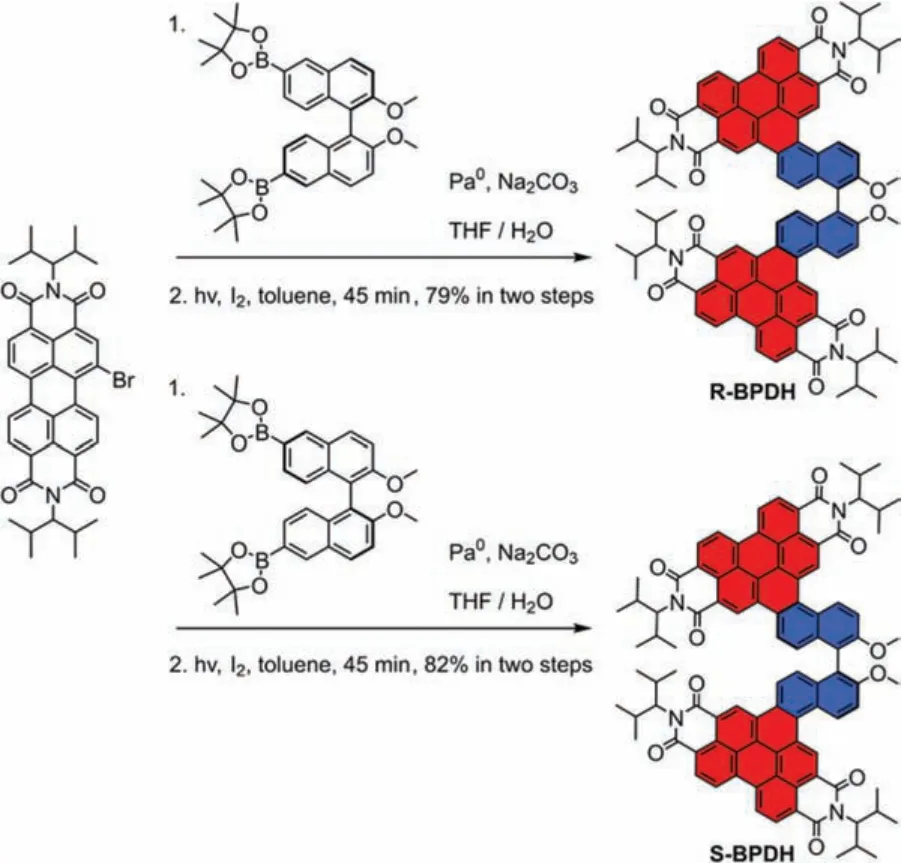
Scheme 1.Synthesis of enantiomerically pure R/S-BPDH.
The steady-state optical properties of R/S-BPDH in DCM have been explored by UV–vis absorption and fluorescence spectroscopy(Fig.2 and Fig.S21 in Supporting information).At room temperature, the UV–vis spectra of R/S-BPDH show very close absorption maxima at 505 nm, which are shifted hypsochromically byca.827 cm-1in comparison to that of the parent PDI without bay substituents (absorption maximum atca.527 nm [28]).And it is consistent with the spectral shift of bay-extended PDIs [29].Besides,due to theπ-extension at the bay position, the maximum molar extinction coefficients of 124,397 L mol-1cm-1in the R/S-BPDH are almost twice to PDIs for 69,298 L mol-1cm-1.The most remarkable feature of the absorption spectra is, however, the partial loss of vibronic fine structure for both R/S-BPDH.The UV–vis spectra in different polarity solvents show remarkable solvatochromism with different absorption maxima and spectral shapes (Figs.S19 and S20, Tables S1 and S2 in Supporting information).Especially in the polar solvent methanol, almost no vibronic fine structure could be observed.Accordingly, the absorption properties are mainly related to two factors:(1) a significant intramolecular charge transfer (ICT) from the electron-donating methoxyl substituents of BINOL units to the electron-deficient PDI core (the dominant factor);(2) the helical distortion of the aromatic system evoked by sterical congestion bridged with BINOL units in the bay area (the cooperative factor).
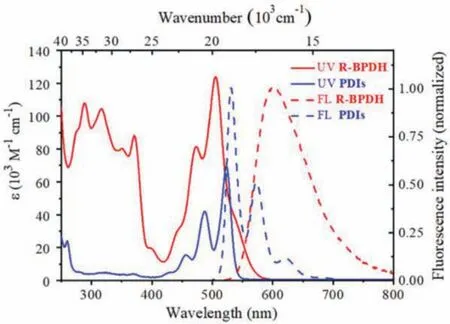
Fig.2.UV–vis absorption (solid line) and normalized emission fluorescence (FL)spectra (dotted line, λex=470 nm, 5×10-6 mol/L) of R-BPDH and referenced PDIs in DCM.
Similar conclusions can be drawn from the fluorescence spectra of R/S-BPDH that exhibit relatively broad emission bands with large Stokes shifts at 601 nm, and fluorescence quantum yields of 72% and 73%, respectively.The fluorescence quantum yields of R/SBPDH in solid state are recorded too (Tables S1 and S2).And it could be further corroborated by their electrochemical and theoretical studies.As shown in Fig.S18 (Supporting information), the cyclic voltammetry (CV) curves of R/S-BPDH in DCM exhibit close features with two reduction potentials at -1.07 and -1.27 Vvs.Fc/Fc+, which are slightly decreasing compared to those of bayunsubstituted PDIs (-0.95 and -1.17 Vvs.Fc/Fc+, respectively).Thus, the fusion of BINOL into PDIs at the bay positions results in decreasing reduction potentials and increasing LUMO energy levels, thus enlarging the HOMO and LUMO energy gaps.This result can be proved by the density functional theoretical calculations (DFT).Compared with the bay-unsubstituted PDIs [30],the R/S-BPDH presented a higher energy of HOMO orbits from-6.09 eV to -5.83 eV, while those of LUMO orbits from -3.60 eV to -3.19 eV.As a result, theEgap(Egap=ELUMO-EHOMO) of R/SBPDH are increasing to 2.64 eV in comparison to that of bayunsubstituted PDIs (2.49 eV).Furthermore, the electrons in LUMO orbits of R/S-BPDH are mainly distributed in electron-deficient PDI cores (Fig.3), while those in the HOMO orbits are delocalized over the electron-donating BINOL scaffolds and partly over perylene units.From these separated molecular orbits, a significant ICT is anticipated upon irradiations, which helps for the understanding of their solvatochromism.
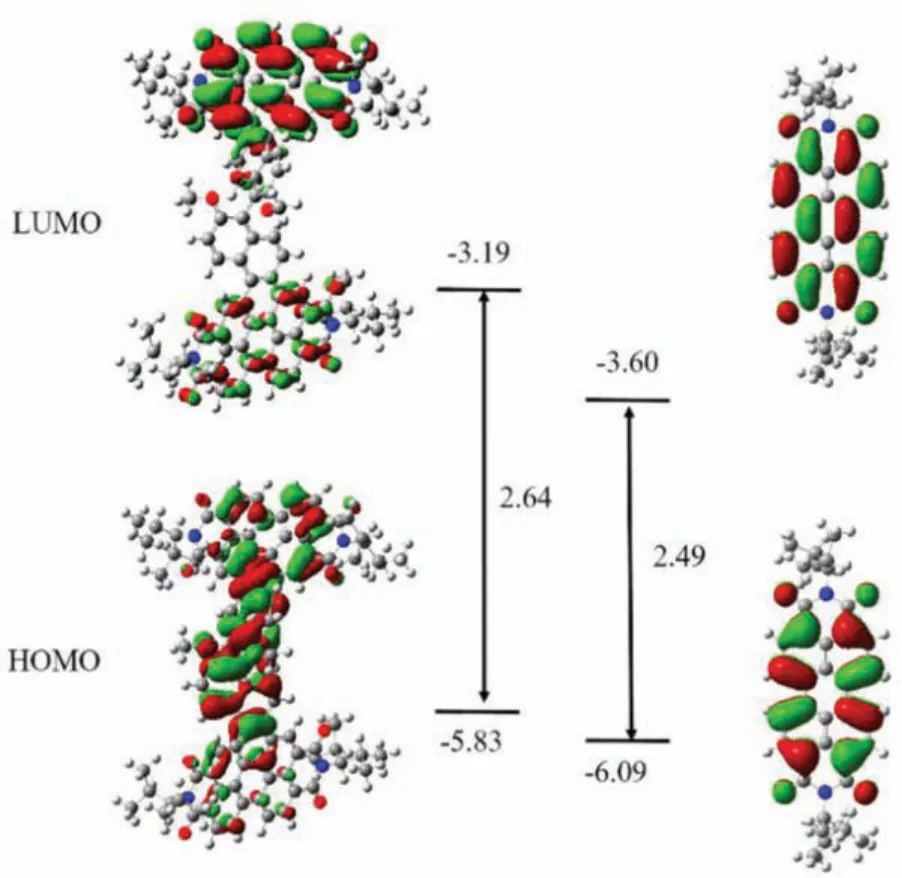
Fig.3.The frontier orbitals of R/S-BPDH and PDIs corresponding energy levels and energy gap calculated in Gaussian 09 at the B3LYP/6–31G(d, p) level by DFT.The structures of R/S-BPDH are optimized from conformational searching (unit:eV).
The enantiomerically pure structural characteristics of R/SBPDH have been investigated by the CD and CPL spectroscopy in DCM.As shown in Fig.4, a pair of clear mirror-image CD spectra could be observed for R/S-BPDH.In the region of 265–325 nm of the CD spectra, it belonged to the chiral BINOL unit exhibiting two characteristic CD signals at about 265 and 288 nm [31].However,in the longer wavelength region, one strong signal atca.341 nm with a crossover point at 386 nm, one weaker signal atca.400 nm as well as a moderate signal atca.550 nm, are mainly attributed to the helicalπ-system of naphthalene annulated PDIs.Accordingly, the incorporation of chiral BINOL units into the PDIs indeed leads to the chiral transfer from BINOL to BPDH molecules.Similar conclusions can also be drawn from the fluorescence spectra of R/S-BPDH that exhibit obvious mirror-image CPL signals with the emission maxima at around 600 nm (Fig.4).
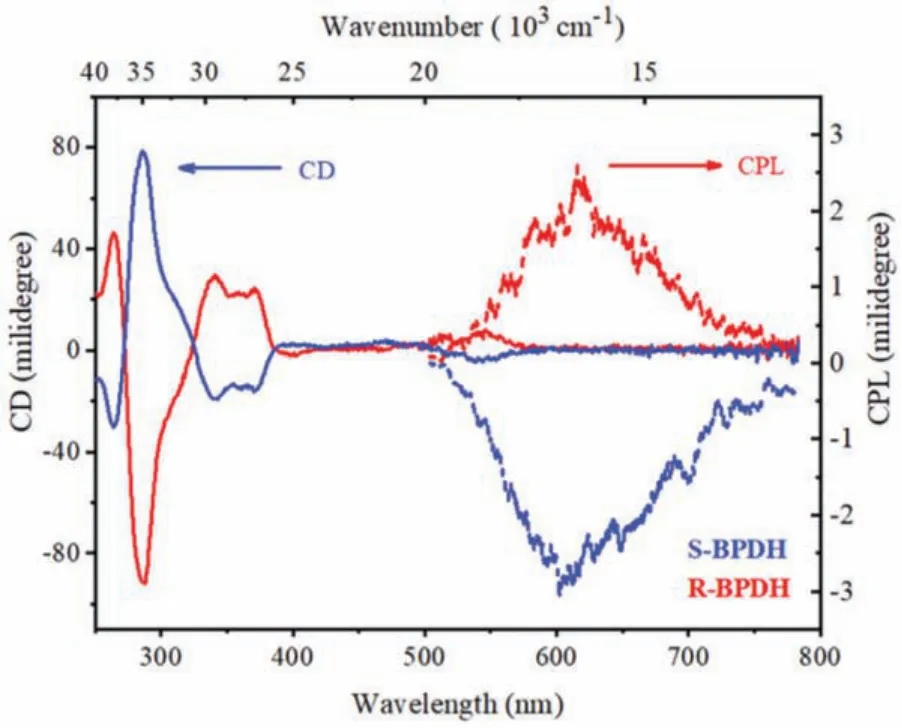
Fig.4.The CD and CPL spectra of R/S-BPDH in DCM (5×10-6 mol/L, λex=470 nm)at the room temperature.
As stated above, due to the rotatable C–C bonds in R/S-BPDH,the fluorescence intensities are quenching in the solvents, but possibly enhanced in the aggregates.To validate this speculation, their UV–vis absorption and fluorescence properties have been investigated in mixture solvents (5×10-6mol/L) of good solvent DCM and poor solvent MCH with different ratios.In the mixture solvents of DCM/MCH, with the increasing the MCH ratio from 0% to 90%, their absorption maxima are blue-shifted slightly.Meanwhile,their spectral shapes become sharper and sharper with the gradual appearance of their shoulder-bands at the longer wavelength and the intensity at the absorption maxima decrease gradually from 124,260 L mol-1cm-1to 102,988 L mol-1cm-1(Fig.S22 in Supporting information).These spectral changes might be attributed to two factors:one is the reduced ICT transitions in polar DCM solvent with the increasing ratio of nonpolar MCH, and another is the improved molecular rigidity upon the aggregations, which could be magnified in their fluorescence spectra.As shown in Fig.5 and Fig.S23 (Supporting information), with the increasing ratio of MCH from 0% to 90%, their emission maxima are blue-shifted remarkably from 601 nm to 562 nm, accompanied by the quantum yield increased gradually from 0.56 to 0.86.Accordingly, the blue-shifted emissions might be governed by the reduced ICT transitions, while the enhanced emissions are more likely by the improved rigidity,particularly the rotation restrictions of R/S-BPDH in the aggregated states.
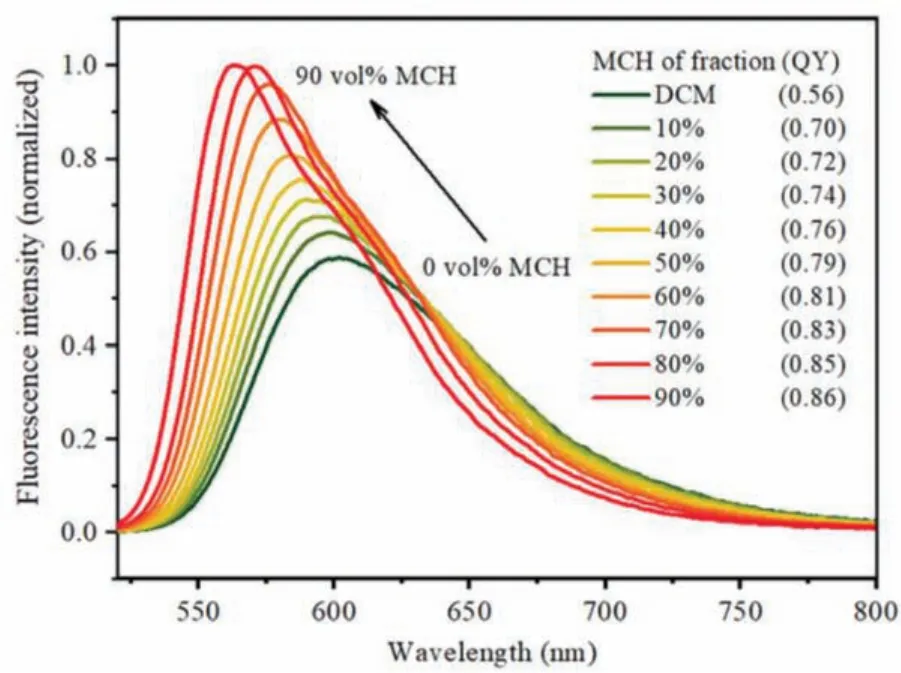
Fig.5.The fluorescence spectrum of R-BPDH in a mixture solvent of DCM and MCH with different ratios in a fixed concentration (5×10-6 mol/L).
Similarly, the CD and CPL properties of R/S-BPDH have also been studied in mixture solvents of DCM/MCH (5×10-6mol/L)with different ratio (Figs.S24 and S25 in Supporting information).As shown in Fig.6a, in a mixture solvent of DCM/MCH (1:9 in volume), the characteristic CD signals assigned to the BINOL units atca.288 nm became fewer for both R/S-BPDH in comparison to those in pure DCM.More interestingly, their CD signals are totally reversed in the region of 340–400 nm, accompanied by the significantly enhanced signals between 480 and 550 nm in the opposite direction.Obviously, the weakened signals atca.288 nm are attributed to the possibly smaller dihedral angles of R/S-BPDH in the aggregated states compared to those in the DCM [32], while the reversed signals, particularly those enhanced reversed signals are related to the generation of chiral aggregates with the opposite chirality to those of their monomers, respectively.Similar conclusions on the aggregation-induced chirality inversion can also be drawn from their CPL studies, which exhibit the reversed CPL signals upon addition 90% MCH into DCM (Fig.6b).Notably, the CPL intensities are much more enhanced, which is consistent with the enhanced reversed signal in their CD spectra.
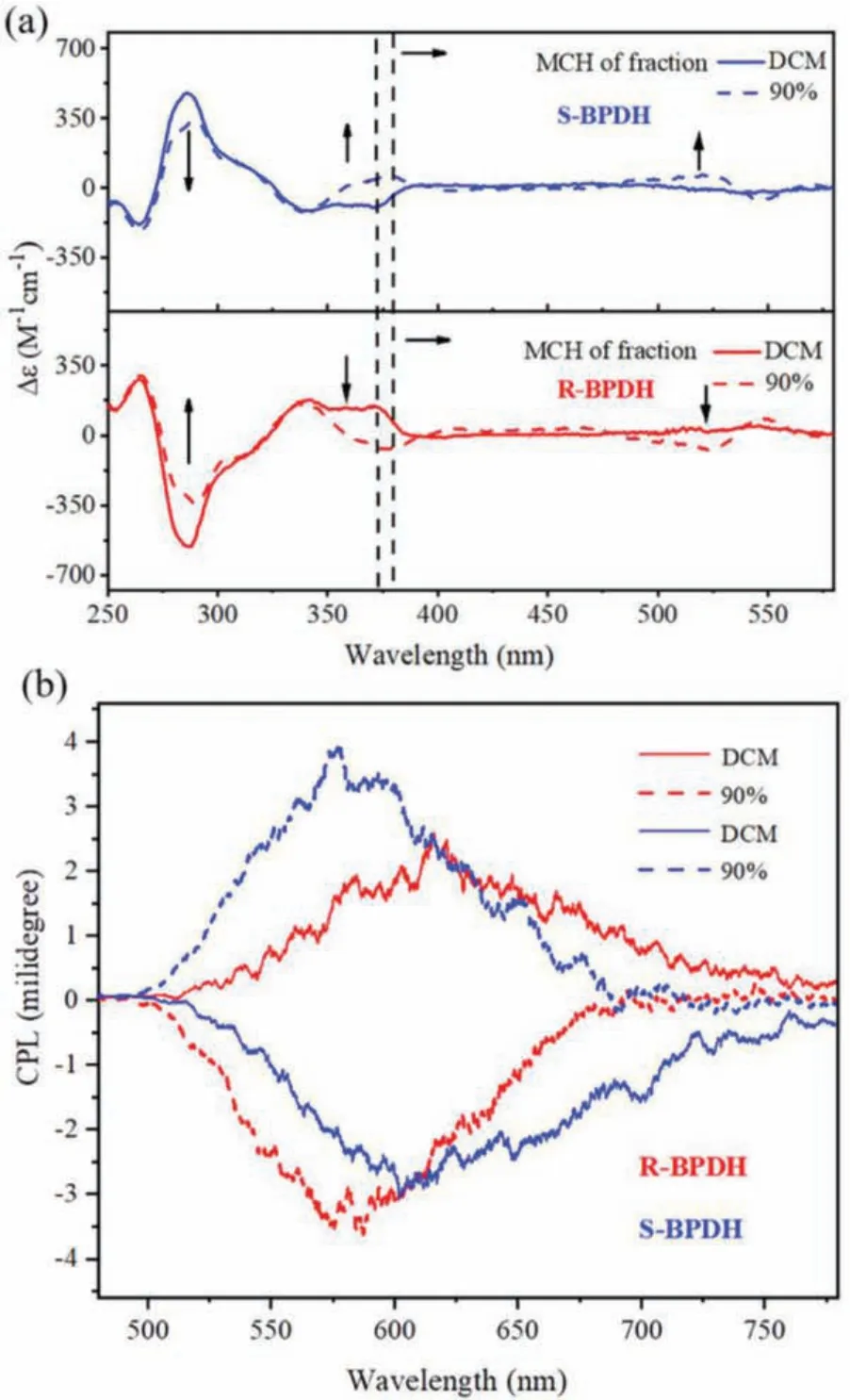
Fig.6.The CD (a) and CPL (b) spectra of R/S-BPDH in pure DCM and a mixture solvent of DCM and MCH with a volume ratio of 1:9 (5×10-6 mol/L).
To further explore the aggregated structures of R/S-BPDH, we have attempted to obtain their self-assembled samples from the DCM/MCH (1:9 in volume) solutions.Fortunately, a small amount of precipitation was collected for R-BPDH when the concentration increased to 5.6×10-5mol/L upon standing for several days.The transmission electron microscopic studies reveal that the precipitation is 1-D fibers (Fig.S26 in Supporting information).Due to the low resolutions, no chiral morphologies have been detected.In addition, the powder X-ray diffraction (PXRD) measurement of this precipitation indicate in the wide angle region, an obvious diffraction peak with ad-spacing of 3.16 Å was observed (Fig.S27 in Supporting information), demonstrating that there is strongππstacking in the aggregated R-BPDH [33].More importantly, the temperature-dependent UV–vis absorption spectral investigations of R/S-BPDH in pure MCH show that the spectral shapes are similar to those in pure DCM at high temperature (80°C).However, the remarkable feature is the gradual appearance of a new absorption band at the long wavelength side when cooling to low temperature(25°C).Together with the bulk substituent at the imide position,the new absorption band should be attributed to the cross stacking between two naphthalene annulated PDI unit from neighboring two R- or S-BPDH molecules (Fig.S28 in Supporting information).This deduction is indeed be substantiated by concentrationdependent1H NMR spectroscopy (Fig.S29 in Supporting information), which exhibits that two singlet peaks in the lower field(10.0–10.6 ppm) assigned to thepara-H close to imide positions are gradually shifted to the higher field due to the shielding effect.Therefore, a plausible molecular packing for R-BPDH in the aggregated state could be proposed.As shown in Fig.7, due to the bulk substituent at the imide position, the neighboring two PDI units from two R-BPDH molecules are inclined to adopt a cross-stacking mode.Besides, because of the steric hindrance of the imide substituent in the chiral BINOL corer (helicalπ-clips), two R-BPDH molecules are stacking against each other and thus forming a 1-D helical structure with opposite chirality (M).
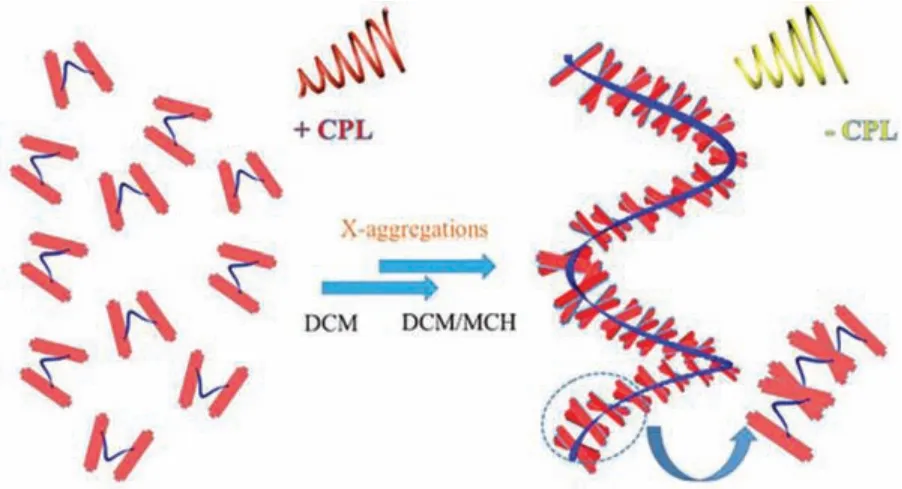
Fig.7.Schematic representation of the possible packing for R-BPDH in aggregated states.
In summary, we reported a pair of novel enantiomerically pure PDIs derivatives, R/S-BPDH by the annulation ofR/S-1,1′-binaphthol into perylene diimide dyes at the bay position.To the best of our knowledge, such axially chiral perylene dyes with red CPL emission is unprecedented.More importantly, the spectral studies reveal that two unusual phenomena, AIEE and aggregationinduced chirality inversion, were observed simultaneously for the first time.Thus, further studies on this unique class of chiral PDIs derivatives in various directions are well motivated.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (No.21971041) and Natural Science Foundation of Fujian Province (No.2020J01447).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.069.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
