Synthesis and characterization of a sensitive and selective Fe3+fluorescent sensor based on novel sulfonated calix[4]arene-based host-guest complex
Ran Cen, Ming Liu, Jihong Lu, Weifang Zhang, Jingjing Dai, Xi Zeng, Zhu Tao, Xin Xiao
Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China
Keywords:Calixarenes Rhodamine B Metal ions Fluorescence probe Host-guest chemistry
ABSTRACT A novel fluorescent sensor was prepared from sulfonated calix[4]arene (SC4A) by the host-guest complexation method using the fluorescent dye rhodamine B (RB) as a structure-directing agent.The crystal structure of the host-guest complex (RB@(SC4A)3) was confirmed by X-ray diffraction studies while its performance and sensing mechanism for metal ion pollutants were characterized using fluorescence and nuclear magnetic resonance spectroscopies.The results showed that RB@(SC4A)3 had a triangular branch structure resulting from host-guest mediation of the interactions between the three SC4A host molecules and the three terminal groups of the guest molecule RB.The host-guest complex exhibited sensitive and selective sensing towards Fe3+ ions via a fluorescence quenching mechanism.The results indicated that RB@(SC4A)3 could be a promising sensitive and selective fluorescent sensor for metal ion pollutants monitoring.It also provided new insights into the synthesis of calixarene-based host-guest complex.
Trace metal ions such as Cu, Fe, and Zn are essential for the metabolism of plants and animals, while excess can cause harm to the human body and the environment.For instance, although Fe3+ions are active in cell metabolism (as absorbable Fe2+), oxygen transport, and electron transfer, an excess can damage nucleic acids and proteins [1–3].The detection and removal of heavy metal ion pollutants from water is a challenge for environmental control[4–9].Therefore, the development of rapid and effective technology for the detection of these hazardous ions in water has become a priority.In the past few decades, some of the conventional analytical methods developed for the detection of toxic ions were based on electrochemistry, ion chromatography, atomic absorption spectrometry, and fluorescence spectroscopy [10–13].Among the many detection methods, fluorescence has attracted considerable interest because of its short response time, high sensitivity, and simplicity.Recently, the use of photoluminescent materials as chemosensors for the detection of pollutants has been studied [14–17].Arunaet al.[18] synthesized bis(rhodamine) derivatives with pyridine and benzene bridging groups, and the materials were highly selective and sensitive turn-on fluorescent sensors for Fe3+.However,the relatively high cost associated with the production of these sensors has limited their application and the search for novel and cost effective materials remains a challenge.
Several researchers have reported the preparation of fluorescence sensors based on macrocycles such as cyclodextrins [19–20],calix[n]arenes [21–23], cucurbit[n]urils [24–28] and pillar[n]arenes[29–34].Calixarenes offer several advantages compared with other macromolecules, specifically:(i) a preorganized nonpolar cavity;(ii) a preorganized ion binding site; (iii) tunable functionalization of their lower and upper rims; and (iv) after functionalization,possess good water solubility or oil solubility, which expand the application area.The preorganization of the nonpolar cavities of calixarenes leads to the efficient size- and shape-specific binding of guests [35].Fluorescent calixarenes and their complexes have shown promising sensor performance in a wide range of applications.For example, Erdemir and Malkondu [36] synthesized a novel calix[4]arene derivative by covalent integration, and the sensor exhibited high fluorogenic selectivity towards Zn(II).Uttamet al.[37] preparedp–tert–butyl–calix[4]arene by covalent integration, and the material showed good F- sensing capability.However,many of the current approaches require covalent integration of either the donor or the acceptor, and sometimes both, limiting their synthetic accessibility.Recently, Fenget al.[38] successfully employed electrostatic complexation of tetraphenylethylene and calixarene to prepare a photosensitizer for tumor imaging and therapy (photodynamic therapy) applications.Zhenget al.[39] prepared a fluorescence displacement sensing array from calixarene derivatives (receptors) and eosin Y (reporter dye) for the chemometric discrimination of glycosaminoglycans.Yuet al.[40] established a facile low-cost method for the sensing and quantitative detection of trimethylamine species in the gut using intermolecular recognition of guanidinium-modified calixarene.Hence, the supramolecular strategy can avoid tedious synthesis and provides new opportunities to tune fluorescence sensing behaviors.
Here, we report the synthesis and characterization of a fluorescent sensor for Fe3+(aq.) from rhodamine B (RB) and sulfonated calix[4]arene (SC4A) using host-guest chemistry (Scheme 1).The main objective of this study was to assess the performance of the host-guest complex (RB@(SC4A)3) as a fluorescent sensor, and its application for the detection of various metal ions.
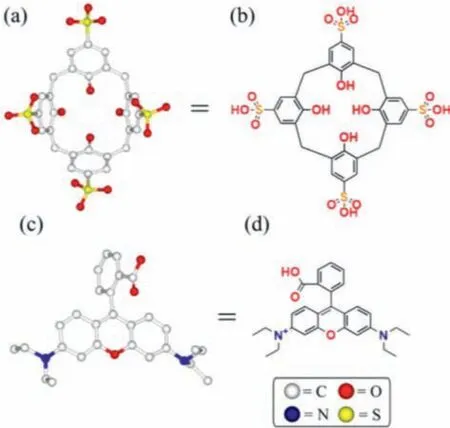
Scheme 1.Structures of SC4A (a, b) and RB (c, d).
The host-guest complex RB@(SC4A)3was prepared from SC4A and the aromatic dye RB using host-guest chemistry to direct the non-covalent interactions.Single-crystal X-ray diffraction (XRD) revealed that hydrogen bonding was the dominant interaction driving the formation of RB@(SC4A)3(Fig.1a).Table S1 (Supporting information) shows the crystal data and structure refinement for RB@(SC4A)3.The proposed initial interactions were as follows:C–H···πinteractions form between the ethyl group on RB molecule and the aromatic ring of the adjacent SC4A molecules (abbreviated as 1 in Fig 1b); the C–H···πand C–H···O interactions form between the RB molecule and a further SC4A molecules (abbreviated as 2 in Fig.1c) and here, the alkyl chain penetrates deeper into the chamber of SC4A and the intermolecular forces are stronger; C–H···πinteraction forms between the RB molecule and the remaining SC4A molecules (abbreviated as 3 in Fig 1d).Supported by these three intermolecular interactions, the RB molecule and SC4A molecules form a stable triangular structure (Fig.1a).Figs.1e and f show the relational interactions between RB@(SC4A)3and two additional macromolecular units resulting from dipole interactions between -SO3H and -OH of the adjacent SC4A molecules (1–3 in Fig.1a).In the initial interactions between the ethyl group of RB with the aromatic ring (1 in Fig.2b), the C–H···πdistances were 3.481 Å.Although the second type of intermolecular interaction was the same as the first type, its force was stronger and the corresponding C–H···πdistances were 2.685 Å.The third interaction between the phenyl group from RB molecules and aromatic ring of the three SC4CA molecules had a C–H···πdistance of 2.642 Å.The additional SO3H···OH interaction between the adjacent SC4A molecules (1–3 in Fig.1a) ranged from 2.401 Å to 2.689 Å in distance.Hence, a combination of these interactions resulted in the formation of the novel RB@(SC4A)3host-guest complex with different channels (Fig.S1 in Supporting information).
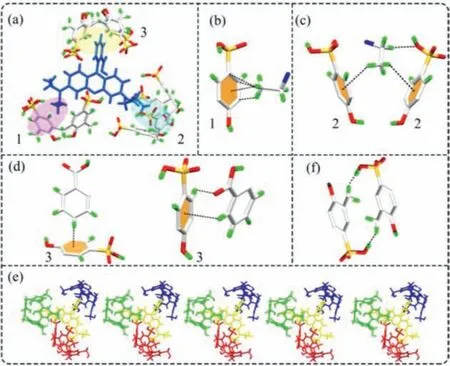
Fig.1.Crystal structures of RB@(SC4A)3.(a) Structure of the SC4A-based supramolecular unit prepared from SC4A and RB; (b-d) Intermolecular interactions between SC4A and RB of the supramolecular unit; (e) Interactions between RB@(SC4A)3 and two adjacent supramolecular units; (f) Intermolecular interaction between adjacent SC4A.
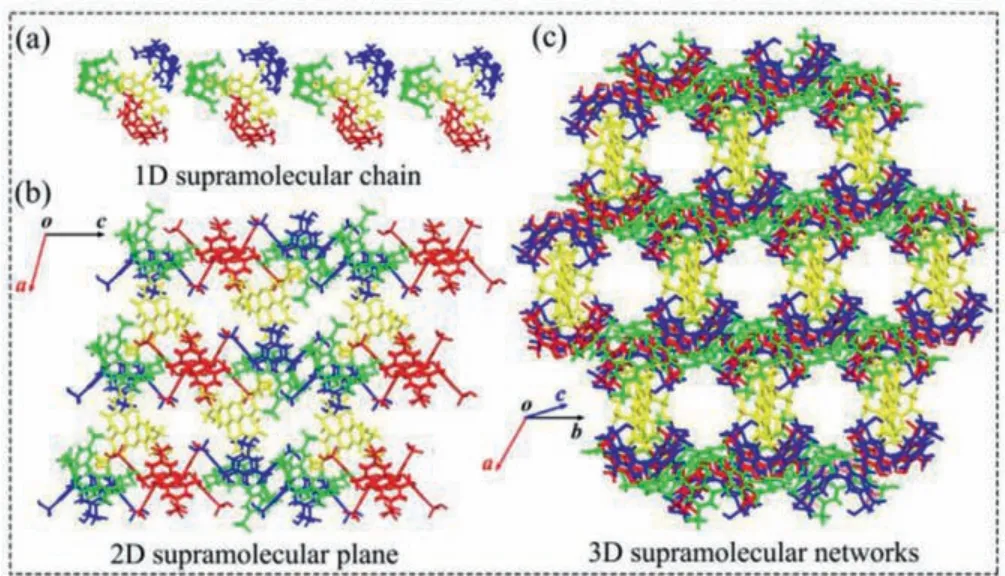
Fig.2.Crystal structures of RB@(SC4A)3:(a) 1D molecular chain; (b) 2D supramolecular plane; (c) 3D supramolecular networks.
The 1D, 2D and 3D structures of RB@(SC4A)3are shown in Fig.2.The triangular branches in the 1D supramolecular chain(Fig 2a) each comprised a RB unit and three SC4A units; the SC4A units formed a porous area with a high negative charge density resulting from the portal sulfonic acid groups.The pore formed by the adaptive cavity and portal size of SC4A, could accommodate the RB molecules.Driven by hydrogen bonding, the 1D supramolecular chains assemble into 2D planes and 3D networks(Figs.2b and c).Hence, these finding supported the successful synthesis of the 3D network of RB@(SC4A)3.
The interaction between RB and SC4A was determined by fluorescence (emission) spectroscopy.The emission spectrum of RB(Fig.S2 in Supporting information) gave a peak at 582 nm in aqueous solution at an excitation wavelength of 554 nm.Incremental addition of SC4A caused a decrease in the fluorescence intensity at 582 nm which reached a minimum at a molar ratio NSC4A/NRBof 3:1.Hence, RB@(SC4A)3was obtained by the simple mixing stoichiometric amounts (1:3) of RB and SC4A in aqueous solution.In aqueous solution, the intensity of the absorption (554 nm) and fluorescence emission (582 nm) peaks of RB@(SC4A)3changed over the pH range of 1–12.According to its pKa titration curve, the pKa value of RB@(SC4A)3was estimated to be 3.5 (Fig.S3 in Supporting information).In general, the RB derivative displays a red color and strong fluorescence in acidic solutions by activation of the carbonyl group in the spirolactone or spirolactam moieties.To study the binding interaction of RB with SC4A, subsequent investigations were carried out at a solution pH of 5.0.
The intramolecular binding behavior of RB@(SC4A)3was studied by1H NMR spectroscopy at pH 5.0.In D2O solution, the chemical shift (δ) assignments of each proton of RB were ascertained by1H NMR and 2D1H–1H correlation spectroscopy (Figs.S4 and S5 in Supporting information).The1H NMR spectra obtained from the titration of RB with SC4A are shown in Fig.3.When SC4A was added to the RB solution (RB:SC4A = 1:3), the1H resonance peaks corresponding to Ha, Hb, Hc, Hdon the benzoic acid group,together with Hh, Hion theN-ethyl group gradually shifted upfield (Δ:Ha, 0.09 ppm; Hband Hd, 0.02 ppm; Hc, 0.04 ppm; Hh,0.43 ppm; Hi, 0.64 ppm).Conversely, the resonances for He, Hf,and Hgprotons on the benzene ring of the aniline system gradually shifted downfield (Δ:He, 0.08 ppm; Hf, 0.24 ppm; and Hg,0.27 ppm).These results suggested that the benzoic acid group andN-ethyl group were situated within the SC4A cavity forming a triangular structure.
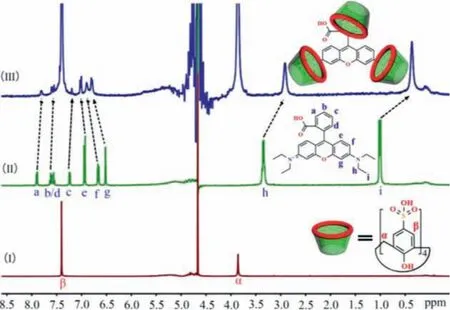
Fig.3.1H NMR spectra (400 MHz, D2O) obtained from the titration of RB with SC4A:(I) Pure SC4A; (II) Neat RB; (III) RB:SC4A = 1:3.
The fluorescence properties of RB@(SC4A)3indicated that it should be suitable for the rapid detection of metal ions.Hence,the fluorescence response of RB@(SC4A)3to a series of common metal ions including alkali (Li+, Na+, K+, Rb+, Cs+), alkaline earth(Mg2+, Ca2+, Sr2+, Ba2+), and various transition metal ions (Cr3+,Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg+, Fe2+) was determined (Fig.4).When Fe3+was added to an aqueous solution of RB@(SC4A)3, the fluorescence intensity at 582 nm was strongly reduced (Fig.4a).Except for Fe2+, which showed a slight reduction in intensity, the remaining 18 metal ions had no significant effect on the fluorescence intensity at 582 nm.These results are also illustrated in Fig.5 using the fluorescence images obtained from the corresponding solutions of RB@(SC4A)3containing various Mn+under UV light.Moreover, with the addition of Fe3+(aq.), the significant quenching in fluorescence intensity of the sensor could be observed visually (Fig.4b).These observations indicated that RB@(SC4A)3had high specificity towards Fe3+in aqueous solution.
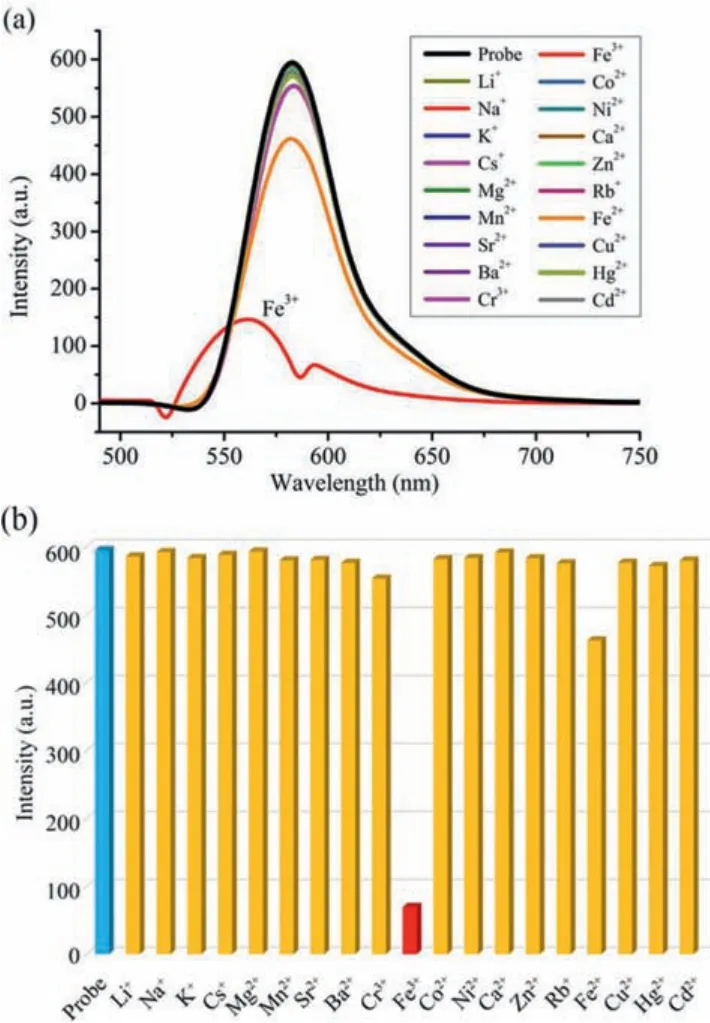
Fig.4.Fluorescence response of RB@(SC4A)3 (5 × 10-6 mol/L; λmax = 582 nm) to common metal ions (Mn+ (aq.), 50 equiv.):(a) Emission spectra of each Mn+ (aq.);(b) bar chart showing the intensities obtained from the addition of each Mn+ (aq.).
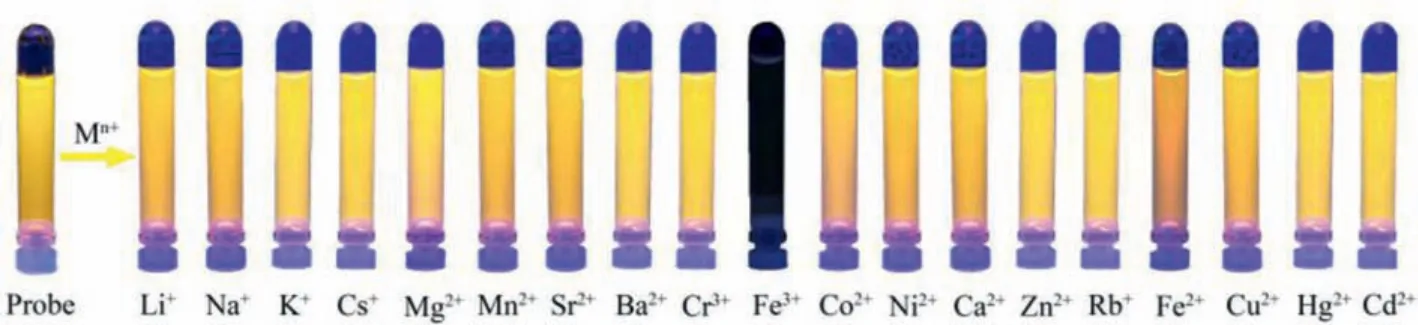
Fig.5.Photographic images of the RB@(SC4A)3 solutions containing various Mn+ under UV light (365 nm).
The effects of varying Fe3+concentrations on the fluorescence intensity of the complex were investigated.Fig.6a shows that the fluorescence maxima of RB@(SC4A)3at 582 nm decreased with increasing concentration additions of Fe3+(aq.) and was effectively quenched.Consistent with this, the maximum absorbance at 554 nm gradually decreased with increasing amounts of Fe3+(aq.);at NFe3+/NRB@(SC4A)3of ~20, further additions of Fe3+(aq.) did not produce any additional change in absorbance.The decay in fluorescence intensity as a function of the ratio of NFe3+/NRB@(SC4A)3is shown in Fig.6b.The change in fluorescence intensity exhibited a good linear relationship with Fe3+over the concentration range of 0–2.0 × 10-5mol/L (R= 0.993) (Fig.S6 in Supporting information) from which, a detection limit (DL) for Fe3+(aq.) could be calculated.The value obtained (3.08 × 10-6mol/L) was comparable to those reported elsewhere (Table S2 in Supporting information).These results demonstrated that the performance of RB@(SC4A)3was suitable for the detection and quantification of Fe3+.
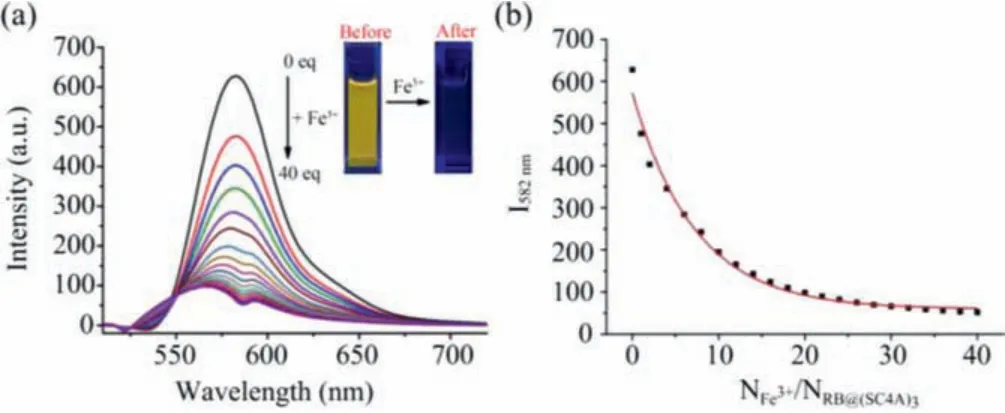
Fig.6.Titration fluorescence spectra of RB@(SC4A)3 (5 × 10-6 mol/L) with Fe3+(0, 2.0, 4.0,···40.0 equiv.).(a) Emission spectra; (b) change in fluorescence intensity with NFe3+/NRB@(SC4A)3.
Fig.S7 (Supporting information) shows that binary mixtures Fe3+and Mn+had no significant effect on the fluorescence intensity of RB@(SC4A)3.Furthermore, the Fe3+quenching influence of RB and SC4A was also determined.When sufficient Fe3+or SC4A was added to the free RB solution, the fluorescence intensity of RB slightly decreased (Figs.S2 and S8 in Supporting information)compared with RB@(SC4A)3, whilst the fluorescence intensity of RB and SC4A changed only very slightly after the addition of Fe3+under day light and UV light (Fig.S9 in Supporting information).
In aqueous solutions, RB@(SC4A)3demonstrated a unique affinity for Fe3+and high selectivity in the presence of other metal ions (Fig.7).To understand how the RB@(SC4A)3responded to Fe3+in solution, titration UV–vis spectrophotometry and1H NMR techniques were used to follow changes in the fluorescence and resonance properties of the RB@(SC4A)3inclusion complex respectively.Titration of RB@(SC4A)3(5 μmol/L) with Fe3+(aq.) resulted in the formation of isosbestic points confirming reaction between the two species (Fig.S10 in Supporting information).Fig.S11(in Supporting information) shows representative1H NMR spectra from the titration of RB@(SC4A)3with increasing the amounts of Fe3+(aq.).1H resonances due to bound RB and SC4A were observed throughout the titration process, confirming that the structure of RB@(SC4A)3remained intact and that it may form a cooperative interaction with Fe3+.On the one hand, the RB is the quinone form in acid conditions (pH<6) and it is in a state of protonation.On the other hand, the carboxyl group of the RB forms a hydrogen bond with the hydroxyl group on the sulfonate groups of SC4A.These make the host-guest complex of RB@(SC4A)3display strong yellow fluorescence, due to RB presenting a planar configuration in aqueous solution [41].Given the sulfonate groups on the upper edge of SC4A are negatively charged, they will bind with cationic metals (Fe3+) [41,42].With the addition of Fe3+, the hydrogen bonds between RB and SC4A have been broken, which changes the configuration from a planar quinone form to a vertical spiro ring structure, which leads to fluorescence quenching of host-guest complex.
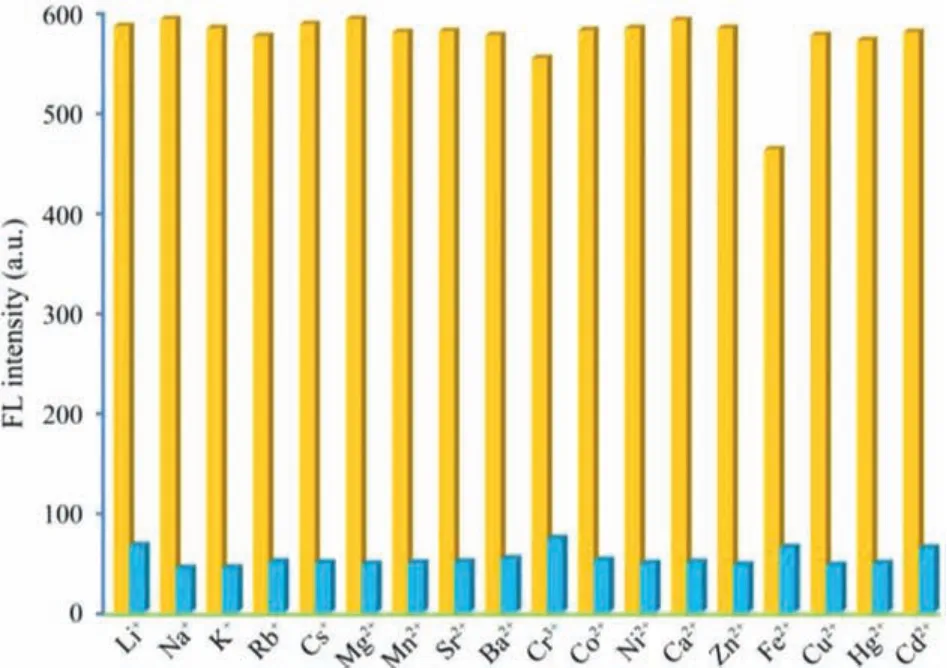
Fig.7.Effects of binary mixtures of Fe3+ and Mn+ on the fluorescence quenching of RB@(SC4A)3:yellow bars are RB@(SC4A)3 + Mn+ (Mn+ = metal ions other than Fe3+); blue bars are RB@(SC4A)3 + Fe3+ + Mn+.
A novel Fe3+(aq.) fluorescent sensor,i.e., RB@(SC4A)3, was successfully prepared from sulfonated calix[4]arene (SC4A) by the host-guest complexation method using the fluorescent dye rhodamine B (RB) as a structure-directing agent.Single-crystal XRD revealed a triangular branch structure mediated by host-guest interactions between the three SC4A host molecules and the three terminal groups of the guest molecule RB.The driving force responsible for the formation of RB@(SC4A)3was attributed to the intermolecular hydrogen bonding between SC4A and RB.In aqueous solution (pH 5.0), RB@(SC4A)3displayed an emission peak at 582 nm (excitation wavelength 554 nm).When RB@(SC4A)3was sequentially exposed to 19 different common metal ions, only Fe3+(aq.) produced a reduction in fluorescence emission, and the signal was rapidly quenched.In binary systems with 18 different metal ions, RB@(SC4A)3exhibited high selectivity and sensitivity towards Fe3+(DL = 3.08 × 10-6mol/L).The results from this study have given new insights into the design of metal ion sensors based on calixarenes.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
We thank the National Natural Science Foundation of China(NSFC, No.21861011), and the Innovation Program for High-level Talents of Guizhou Province (No.2016–5657) are gratefully acknowledged for financial support.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.005.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
