A two-step solvothermal procedure to improve crystallinity of covalent organic frameworks and achieve scale-up preparation
Xing-Ho Hn, Ji-Qi Chu,b, Wen-Zhung Wng, Qio-Yn Qi, Xin Zho,*
a Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China
b Shanghai Normal University, Shanghai 200234, China
Keywords:Covalent organic frameworks Synthesis Solvothermal condensation High crystallinity Scale-up preparation
ABSTRACT Covalent organic frameworks (COFs), as a novel class of functional polymers, exhibit versatile applications due to their crystalline porous structures and conjugated skeletons.However, synthesis of COFs with high crystallinity still faces great challenges, especially for scale-up preparation.Herein we report a two-step solvothermal process to improve crystallinity of COFs.The first step focuses on polycondensation of monomers with no need for optimizing crystallization conditions.In the second step, appropriate solvothermal conditions are used to facilitate crystallization of the COFs through defects correction and structural repairing.Furthermore, this strategy could also be applicable to scale-up synthesis of high quality COFs, which lays a foundation for their practical applications.
Covalent organic frameworks (COFs) are a class of crystalline porous polymers constructed by linking organic building unitsviacovalent bonds, presenting features of low density, permanent porosity, large surface area, and designable functionality [1–4].These characteristics endow COFs with superior application potentials in adsorption, separation, capture of harmful substances,energy storage, sensing, drug delivery, catalysis, and so on [5–10].During the past decade, various synthetic methods including solvothermal condensation [11,12], microwave synthesis [13],mechanochemical reaction [14], continuous flow approach [15] and even synthesis at ambient conditions [16], have been developed for COFs.Among them, one-step solvothermal synthesis is the most widely used one.To implement this method, usually mass screening of solvent systems is required to produce crystalline products.In the one-step process, polymerization of monomers and crystallization of frameworks occur simultaneously under the same condition, which thus is hard to balance.For this reason, the synthesis of COFs with high crystallinity is still challenging.Moreover, due to the crystallization problem, the reproductivity on crystallinity of a COF in different batches could not be always secured, even under an optimized condition.On the other hand, solvothermal synthesis of COFs is typically limited to small amounts (generally milligram scale) and scale-up synthesis usually results in a decrease or even loss of crystallinity, which becomes a bottleneck for COFs achieving practical applications.In this context, new strategies for scalable synthesis of high quality COFs are highly desired.
Among the COFs reported so far, imine-linked COFs are the largest category [17].The strong imine bond endows imine COFs with high thermal stability and moderate to high chemical stability, which makes them very promising for practical applications.On the other hand, the good reversibility of imine bond brings dynamic feature to COFs [18], providing the growing process of COFs with “self-healing and error correction” capabilities.Very recently such a dynamic feature has been exploited to facilitate the preparation of crystalline COFs in various respects [19–22].Moreover, recent studies have suggested that amorphous-to-crystalline transformation [23–25], or dissolution-recrystallization [26] occurred during the formation of crystalline frameworks, in which the dynamic feature of imine linkages played a crucial role.To further exploit this potential, in this contribution we have developed a two-step solvothermal procedure, which not only produces COFs with high crystallinity, but also is scalable.The design is based on a speculation that the one-step solvothermal condition might not be optimal for both bond formation and crystallization of COFs.Therefore, we divide the synthetic process into two steps, with the first step focusing on bond formation without optimizing crystallization conditions while the second step facilitating crystallization through solvent-assisted defects correction/self-repairing of networks.
To implement the above design, we chose two known iminelinked COFs, COF-ETTA-2,3-Dha and COF-DHTA [27,28], as representatives to explore the feasibility of the proposed two-step solvothermal synthesis (Scheme 1).To compare the crystallinity of the COFs obtained under different synthetic conditions, the intensities of (100) peak in their powder X-ray diffraction (PXRD) patterns recorded under the same data collection condition were used as a reference (BET surface areas were further used as another reference for reconfirmation, vide infra).COF-ETTA-2,3-Dha was selected as a representative to be studied in detail.For the first step,1,4-dioxane was used as the solvent to prepared COF-ETTA-2,3-Dha under solvothermal condition from the condensation of ETTA(500 mg, 1.3 mmol) and 2,3-Dha (423 mg, 2.6 mmol) in the presence of HOAc (aq., 9 mol/L) as a catalyst.The condensation reaction was conducted in a sealed tube at 120 °C for 3 days.After work-up,the as-obtained powder (named COF-ETTA-2,3-Dha-1st, 0.8 g) was characterized by powder X-ray diffraction (PXRD) (Fig.1g), which indicated formation of the predicted COF (vide infra).Compared to that of the sample of COF-ETTA-2,3-Dha previously prepared on a small scale (58 mg) [27], the intensity of the diffraction of its (100)peak decreases considerably, indicating that the scale-up synthesis gives the COF with lower crystallinity.In the second step, the powder was further treated in six different solvent systems, still under solvothermal conditions at 120 °C for 3 days.The PXRD patterns of the as-obtained products (named COF-ETTA-2,3-Dha-2nd)show that, after the second solvothermal treatment, the diffraction intensity of the (100) peak of the products varies depending on the solvents used (Fig.1 and Table S1 in Supporting information).The use of 1,4-dioxane again in the second step results in just a little increase in the intensity of the diffraction peak, suggesting that although 1,4-dioxane facilitates the Schiff base condensation,it is not optimal for self-repairing of the framework structure.In contrast, the uses of DMAC/o-DCB and DMAC/Mes result in much higher diffraction intensity than that of the pristine COF prepared in the first step, suggesting higher crystallinity of the products.
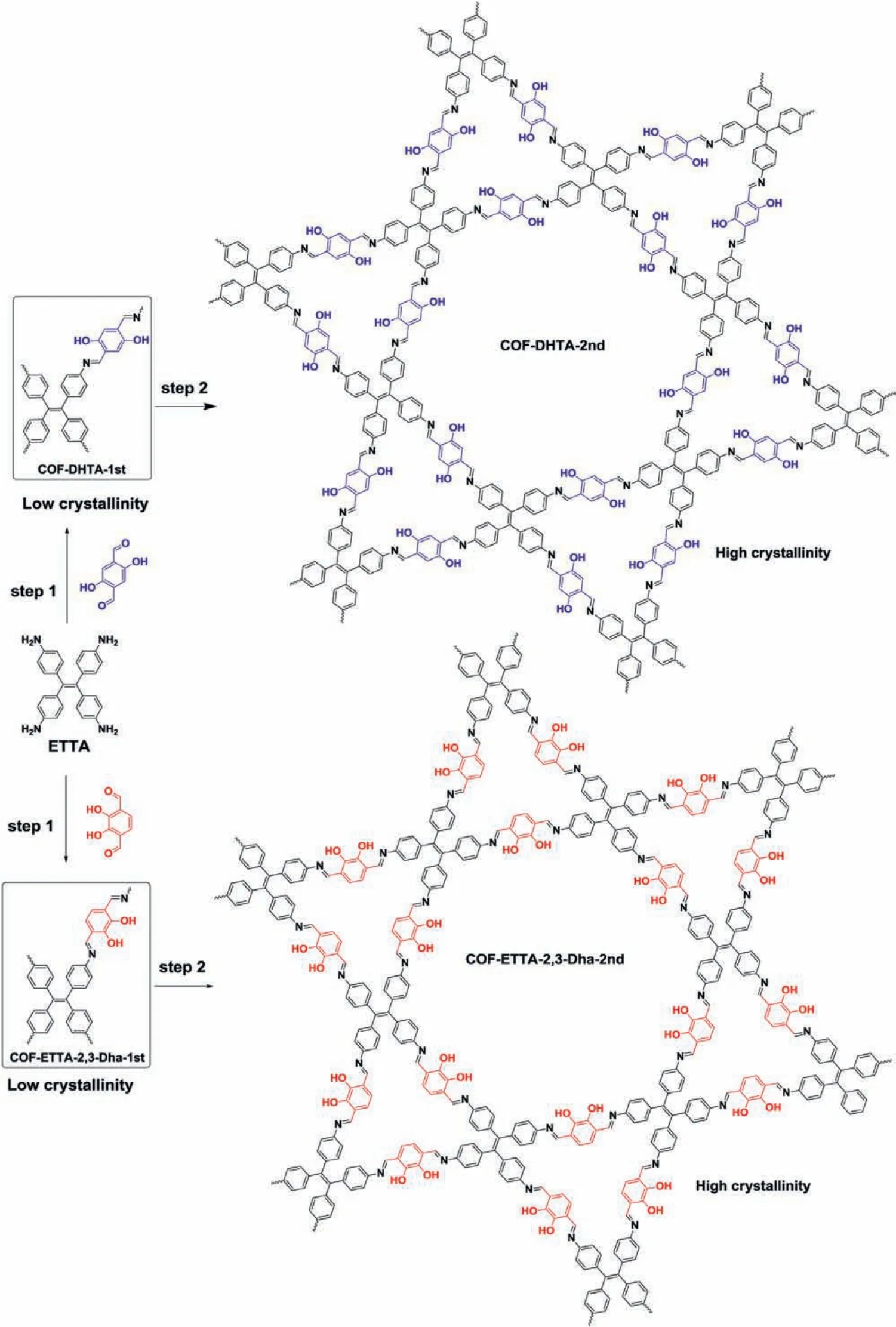
Scheme 1.Synthetic scheme for the preparation of COF-ETTA-2,3-Dha and COF-DHTA through the two-step solvothermal procedure.
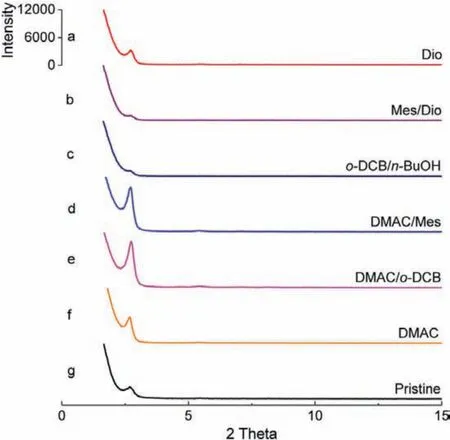
Fig.1.PXRD patterns of COF-ETTA-2,3-Dha-2nd obtained in different solvents.The scale of the ordinate of each diffraction pattern was kept the same.Abbreviations for the solvents:Dio = 1,4-dioxane, Mes = mesitylene, o-DCB = 1,2-dichlorobenzene, n-BuOH = butanol, and DMAC = dimethylacetamide.
The crystallinity of the COF could also be improved by the use of DMAC as a solvent for the second solvothermal treatment.The improvement of crystallinity of the products is attributed to favorable crystallization of the polymer through defects correction mediated by bond cleavage and re-formation in these solvents.Besides this, the PXRD result also indicates that the uses of Mes/Dio ando-DCB/n-BuOH cause decrease in crystallinity of COF-ETTA-2,3-Dha (Figs.1b and c), indicating that in these solvent systems the structural self-healing is not favored.The comparison between the FT-IR spectra of COF-ETTA-2,3-Dha-1st and COF-ETTA-2,3-Dha-2nd indicates that they display almost the same pattern, suggesting that bond formation has completed in the first step (Fig.S1 in Supporting information).The above results clearly indicate the two-step solvothermal synthesis, which separates structure repairing from polymerization, is effective to produce COFs with high crystallinity.The sample prepared from the second step solvothermal treatment was used as a representative to be characterized by infrared (IR) spectroscopy and PXRD, from which the predicted framework was confirmed (Figs.S1 and S3 in Supporting information).Its unit cell parameters were given by Pawley refinement to bea=b= 38.29 Å,c= 5.47 Å,α=β= 90°,γ= 120° (residualsRp= 2.23%,Rwp= 3.35%), which are consistent with the data previously reported [27].
We also investigated whether the solvents used in the first step made a significant influence on the crystallinity of the final products.For this purpose, COF-ETTA-2,3-Dha-1st was prepared in six different solvents, and the resulting powders were further heated in DMAC/o-DCB (1:1, v/v), in the presence of HOAc (aq., 6 mol/L)as a catalyst.The PXRD patterns of the as-obtained products (Fig.2 red and Table S3 in Supporting information) were recorded and compared with that of COF-ETTA-2,3-Dha-1st (Fig.2 black and Table S2 in Supporting information).It reveals that the intensity of the diffraction peaks of the products after the second solvothermal treatments (COF-ETTA-2,3-Dha-2nd) all increase dramatically,no matter how different crystallinity of the samples of COF-ETTA-2,3-Dha-1st is, indicating that the solvents of the first step have less effect on crystallinity of the product of second step.These results suggest that the monomers primarily polymerize in the first step while crystallization mainly occurs in the second step, again manifesting the feasibility of the two-step process in improving crystallinity of COFs.
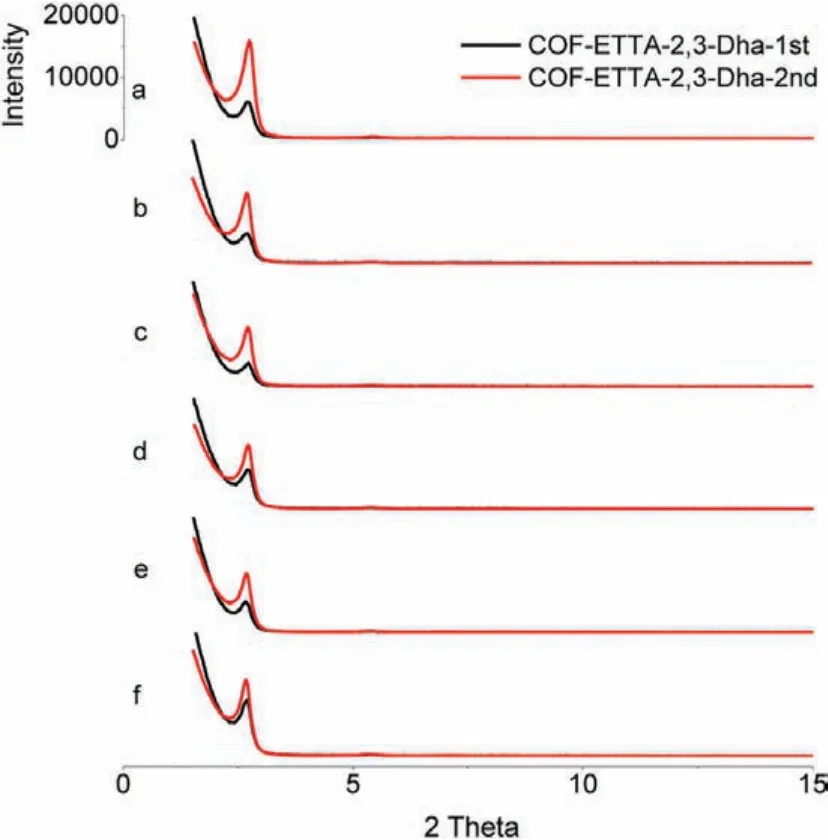
Fig.2.(Black) PXRD patterns of COF-ETTA-2,3-Dha-1st synthesized in different solvents:(a) Dio, (b) o-DCB, (c) o-DCB/n-BuOH (1/1, v/v), (d) Mes/n-BuOH (1/1, v/v),(e) Mes/Dio (1/1, v/v), and (f) DMAC/o-DCB (1/1, v/v).(Red) PXRD patterns of COFETTA-2,3-Dha-2nd obtained by using DMAC/o-DCB as the solvent for the second step solvothermal treatment.
The effect of condensation time on the quality of the COF products was also studied.It is found that a short reaction time leads to low crystalline products of COF-ETTA-2,3-Dha-1st and its crystallinity increases with the extension of the reaction time (Table S4 in Supporting information).However, it does not mean that a longer reaction time always leads to better crystallinity.The PXRD data of COF-ETTA-2,3-Dha-1st prepared in 1,4-dioxane at various condensation times indicate its crystallinity drops dramatically after 5 days.In spite of the fluctuation in the crystallinity of COFETTA-2,3-Dha-1st prepared from various reaction times, all these low crystalline products could be converted into high quality COFs after the second solvothermal treatment (Table S5 in Supporting information), suggesting that a long reaction time of the first step is not necessary.
The investigation on effect of acid concentration on the second solvothermal step indicated that acid concentration had little effect on the repairing process (Table S6 in Supporting information).Another experiment for COF-ETTA-2,3-Dha-2nd manifests that low temperature is not conducive to the self-repairing of the framework.In contrast, it even destroys the structure of the COF (Table S7 in Supporting information).To investigate the effect of reaction time of the second step on the crystallinity of COF-ETTA-2,3-Dha-2nd, the samples of COF-ETTA-2,3-Dha-1st were heated in DMAC/o-DCB at 120 °C for different time.The PXRD analysis revealed that the crystallinity of COF-ETTA-2,3-Dha-2nd gradually increased with the reaction time from 2 days to 5 days (Table S8 in Supporting information).However, since high quality COF products could be always obtained after the second step solvothermal treatments, a long time for structure repairing is not necessary.
The above results confirm our design that the two-step solvothermal process can efficiently convert a low crystalline COF into a COF with high crystallinity.To illustrate the generality of this method, preparation of another imine-linked COF, COF-DHTA, was carried out (Scheme 1).As shown in Fig.3 (Table S9 in Supporting information), in the first step which was conducted under three different solvent systems, COF-DHTA-1st was obtained as a product with extremely low crystallinity or even as amorphous products.Nevertheless, these materials still could be converted into the target COF with high crystallinity after the second solvothermal treatment (Fig.3 Red and Table S10 in Supporting information), again indicating the effectiveness of the two-step procedure.COF-DHTA-2nd was characterized with IR and PXRD (Figs.S2 and S4 in Supporting information), and the as-obtained data are consistent with the ones previously reported [28].
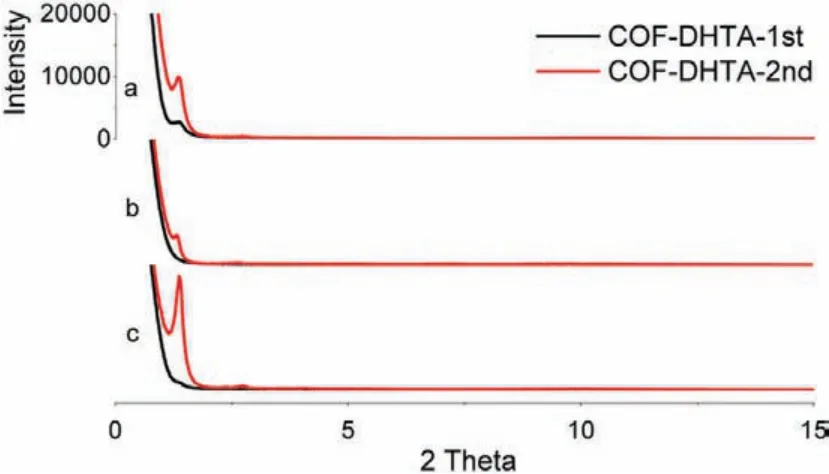
Fig.3.(Black) PXRD patterns of COF-DHTA-1st prepared in different solvents:(a)Dio, (b) Mes/n-BuOH (1/1, v/v), and (c) Mes/Dio (1/1, v/v).(Red) PXRD patterns of COF-DHTA-2nd obtained in the mixture of DMAC/o-DCB.The scale of the ordinate of each diffraction pattern was kept the same.
To further shed light on the advantage of this two-step process, scaled-up synthesis of the two COFs was carried out.For COF-ETTA-2,3-Dha, the first step process was performed in 1,4-dioxane, from which 1.65 g polymer was obtained.As revealed by Fig.4a, the as-prepared product in the first step exhibits very low crystallinity, with an intensity of (100) peak below 1500.In comparison with that of the sample prepared on small scale [27], the crystallinity of the 1.65 g scale product obtained from the one-step synthesis decreases significantly.However, after the second solvothermal process in DMAC/o-DCB/HOAc (aq.,6 mol/L) (1/1/0.1, v/v/v), the corresponding diffraction intensity of the resulting COF-ETTA-2,3-Dha product (98% yield) increases to 12,311, indicating a dramatic improvement of the crystallinity of the COF.The greatly increased quality of COF-ETTA-2,3-Dha through the second solvothermal process was also revealed by the N2adsorption-desorption experiment (Fig.4c).The Brunauer–Emmett–Teller (BET) surface areas were measured to be 405 and 1239 m2/g for COF-ETTA-2,3-Dha-1st and COF-ETTA-2,3-Dha-2nd(Fig.S5 in Supporting information), respectively, with total pore volumes (atP/P0= 0.99) being 0.30 cm3/g for the former and 0.81 cm3/g for the latter.The three-fold improvement of the BET surface area of COF-ETTA-2,3-Dha-2nd again confirms the effectiveness of the two-step solvothermal procedure on improving quality of the COF.For the gram-scale synthesis of COF-DHTA, similar results were obtained.After the second step solvothermal treatment,the intensity of the (100) peak increases from 8052 of COF-DHTA-1st to 21,216 of COF-DHTA-2nd, improving by around three times(Fig.4b).Its BET surface area increases more than two-fold (from 766 m2/g to 1775 m2/g), while the total pore volumes increase from 0.89 to 1.37 cm3/g (Fig.4d and Fig.S6 in Supporting information).These results fully demonstrate the effectiveness of the two-step process for scale-up synthesis of high quality COFs.
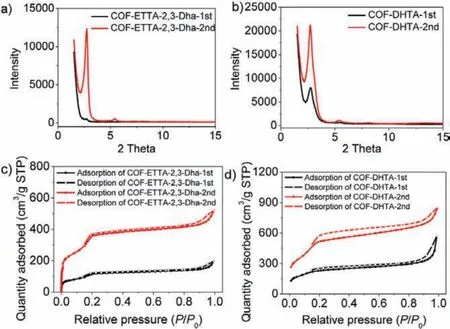
Fig.4.PXRD patterns of (a) COF-ETTA-2,3-Dha and (b) COF-DHTA synthesized in gram-scale.(c) N2 adsorption and desorption isotherm curves of COF-ETTA-2,3-Dha-1st and COF-ETTA-2,3-Dha-2nd.(d) N2 adsorption and desorption isotherm curves of COF-DHTA-1st and COF-DHTA-2nd.
In summary, a new method has been developed for the synthesis of COFs with high crystallinity and scale-up preparation.This two-step procedure operates polymerization and crystallization under different solvothermal conditions, with the second step mainly focusing on overcoming the crystallization problem through structure repairing and defects elimination of the crude products formed in the first step.As a result, scale-up preparation of COFs could also be achieved without a decrease in crystallinity.Thanks to its advantage, the second solvothermal treatment could be a remediation method to improve the quality of COFs prepared in low crystallinity.Since COFs are typically constructed on the basis of dynamic covalent bonds, this method should also be applicable to other types of COFs.Moreover, this work opens a new way to the scalable synthesis of high-quality COFs, which is crucial for implementing practical applications of this burgeoning class of crystalline porous organic polymers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China (No.21632004) and the Science and Technology Commission of Shanghai Municipality (No.19XD1404900) for financial support.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.066.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
