Controllable fabrication of a supramolecular polymer incorporating twisted cucurbit[14]uril and cucurbit[8]uril via self-sorting
Wei Zhang, Yang Luo, Jie Zhao, Chao Zhang, Xin-Long Ni, Zhu Tao, Xin Xiao
Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China
Keywords:Cucurbit[n]urils Twisted cucurbit[14]uril Supramolecular polymer Self-sorting Host-guest interaction
ABSTRACT A linear supramolecular polymer with controllable features based on twisted cucurbit[14]uril (tQ[14]) and cucurbit[8]uril (Q[8]) was firstly fabricated via an effective self-sorting strategy.Herein we designed a monomer, 1–butyl–1′-(naphthalen- 2-ylmethyl)-4,4′-bipyridinium bromide (BNB), that contains bipyridyl,aliphatic butyl and aromatic naphthyl groups, simultaneously.Two host molecules, tQ[14] and Q[8] were employed to develop an effective strategy for constructing a linear supramolecular polymer with controllable features.The alkyl groups on both sides of BNB could insert into the two cavities of tQ[14], the naphthyl part of BNB via π-π stacking in Q[8] cavity, serving as the driving force for supramolecular polymerization.Through self-sorting of the monomer, tQ[14] and Q[8], led to the formation of the linear supramolecular polymer.Depolymerization could be achieved by addition of adamantane hydrochloride(AH) which driven two BNB guest molecules out of the Q[8] cavity.This self-sorting strategy has great potential, not only for designing supramolecular polymer materials with different controllable structures through introduction of multiple functional groups, but also for broadening the application of twisted cucurbit[14]uril in supramolecular chemistry.
Supramolecular polymer chemistry, an interdisciplinary research field, aims to construct highly complex polymer systems with desirable features by organizing arrays of components.The components are often connected by either covalent or noncovalent interactions such as hydrogen bonding [1–3],π-πstacking [4,5] and metal-ligand coordination [6,7].Because the intensity and orientation of interactions between components are adjustable [8–13], supramolecular polymers demonstrate attractive properties [14–18] regarding stimuli-responsivity, self-healing, and degradability.Over the last decade, supramolecular polymer materials have found potential applications in photovoltaic devices, drug delivery, porous membranes, catalysts,etc.[19–22].Several strategies have been employed recently to produce specific supramolecular polymers, including chain growth polymerization [23], seed polymerization [24] and self-sorting [25,26].Self-sorting is defined as the highly efficient recognition between molecules within complex mixtures, such that each type of molecule has its own corresponding unit [27].Since molecules have their own differing characteristics (structure, molecular size,etc.), the macrocyclic host can not only construct different intricate supramolecular polymers through host-guest interactions but can even precisely control them [28,29].
Cucurbit[n]urils (Q[n]s) [30–35], as important macrocyclic hosts with rigid cavity, distinct symmetry, and high stability, can bind a variety of guests, including neutral or positively charged molecules,with high binding constants in aqueous solution.Q[8] is unique because of its ability to form ternary complexes by encapsulating two hetero- or homo-guests in its large, rigid cavity.These complexes have been widely used as molecular handcuffs in the fabrication of various supramolecular polymers in the last ten years [36–39].Twisted cucurbit[14]uril (tQ[14], Scheme 1) [40], a new member of the cucurbituril family with one of the highest known degree of polymerization, has recently attracted much attentions.It contains 14 glycoluril units linked by 28 methylene bridges and is shaped like a distorted rubber band with two cavities.BecausetQ[14] almost always takes on a twisted shape similar to the Arabic numeral “8′′, it is capable of encapsulating two guest molecules to form ternary supramolecular compounds.Both Q[8] andtQ[14] are good candidates for macrocyclic supramolecular hosts.However,studies on the use oftQ[14] in the construction of supramolecular polymers are limited [41–42].
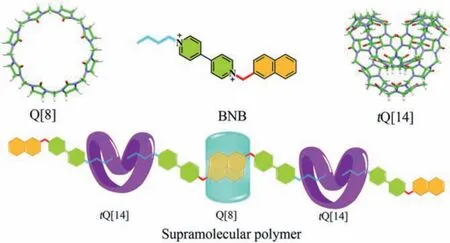
Scheme 1.Structures of tQ[14], BNB and Q[8] and illustration of supramolecular polymers.
The aim of this study is to construct a controllable supramolecular polymer system based ontQ[14] and Q[8] through self-sorting.A linear molecule, 1–butyl–1′-(1-naphthalenylmethyl)-4,4′-bipyridinium (BNB) composed of naphthyl, bipyridyl, and alkyl groups, is prepared (Figs.S1-S3 in Supporting information).By simply mixing BNB,tQ[14] and Q[8] in aqueous solution, Q[8] and the naphthyl part of BNB could interactvia π-πstacking, whiletQ[14] could bound to the alkyl chain parts of BNB, resulting in the formation of the linear supramolecular polymer.Interestingly,whether Q[8] ortQ[14] is added first or Q[8] andtQ[14] are added at the same time, BNB can form flexible chain-like supramolecular polymers based ontQ[14] and Q[8] through self-sorting.Therefore, the two key macrocyclic compounds cooperate to construct supramolecular polymers.
In order to verify that the supramolecular polymer is constructed by self-sorting, we first studies the method of constructing monomers by adding Q[8] on the basis of BNB.Q[8] naturally selects the BNB guest molecule containing conjugated naphthalene groups, and (Fig.S4 in Supporting information), forming aπ-πcomplex BNB@Q[8] of naphthalene moieties within Q[8]’s cavity, while the alkyl chain (butyl group) and bipyridine of the guest molecule remain exposed outside the cavity.As shown in Fig.1, whentQ[14] is subsequently added to BNB@Q[8] (2:1), the naphthyl group of BNB is not removed from the cavity of Q[8].On the contrary, the alkyl chain of BNB shifts to high field, which means thattQ[14] bind with BNB forming BNB@Q[8] (2:1), while the bipyridine part of BNB remains on the outside of Q[8] andtQ[14].Therefore, the strategy of adding Q[8] first and then addingtQ[14] is feasible to construct the flexible chain-like supramolecular polymers of BNB@tQ[14]@Q[8].

Fig.1.1H NMR spectra (400 MHz, D2O, 298 K, 5.0 mmol/L) of (I) free BNB, (II)BNB@Q[8] (1:1), (III) BNB@Q[8] (1:2) and with tQ[14] molar equivalents of (IV) 0.50 and (V) 1.00.
Subsequently, the method of initially addingtQ[14] and then adding Q[8] was also performed, and the same supramolecular polymer is also constructed.As shown in Fig.2 (I and II),tQ[14]binds tightly to the bipyridine and alkyl chain groups of BNB and constructs monomers of the type BNB@tQ[14], while the naphthyl groups with their larger molecular size are excluded (Fig.S6 in Supporting information).Upon adding Q[8] to the 1:1 mixture of BNB@tQ[14], Q[8] breaks the equilibrium state betweentQ[14]and BNB, producesπ-πstacking of BNB in its cavity, and forcestQ[14] to slide on the BNB molecule as well as changing the interaction mode of the BNB@tQ[14] from 1:1 to 1:2 to form the flexible chain-like supramolecular polymers BNB@tQ[14]@Q[8].It is worth noting that whether Q[8] ortQ[14] is added first, the interaction of the supramolecular polymer formed by BNB and Q[n]s is the same.
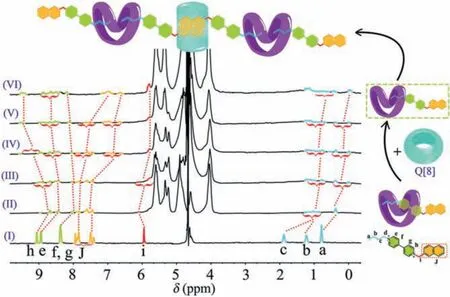
Fig.2.1H NMR spectra (400 MHz, D2O, 298 K, 5.0 mmol/L) of (I) free BNB, and BNB@tQ[14](NBNB:NtQ[14] = 1:1) with Q[8] molar equivalents of (II) 0.00, (III) 0.25,(IV) 0.50, (V) 0.75, and (VI) 1.00.
Finally, as expected, the same result is obtained by adding Q[8]andtQ[14] simultaneously on the basis of BNB (Fig.S8 in Supporting information).The 1:1 mixed Q[8] andtQ[14] solution is added to the BNB, and surprisingly it is found that Q[8] still specifically recognizes the naphthalene part of BNB;tQ[14] still binds with the alkyl chain part of BNB, while the bipyridine part of BNB always remains out of the cavities of both Q[n]s.The same supramolecular polymer can be prepared through the above three different addition orders, so it can be explained that the supramolecular polymer is constructed by self-sorting.
ITC is used to further elucidate the self-sorting process and the main driving force for the construction of the ternary supramolecular polymer (Fig.S9 in Supporting information).The binding constant of BNB@tQ[14] was (3.376 ± 0.121) × 105M-1, and that of the naphthyl part of guest BNB with Q[8] was as high as(5.963 ± 0.138) × 1011M-2[43,44] (Fig.S10 in Supporting information).Thus, the mechanism of this supramolecular polymerization can be described as Q[8] tending to bind with the naphthyl groups of BNB due to its higher binding affinity, leavingtQ[14] to complete the ternary structureviabinding to the alkyl chains of BNB.ITC data shows that interactions between the hosts and BNB appear to be driven by favorable enthalpy changes accompanied by small negative (unfavorable) entropy changes.
Since BNB is not fluorescent in aqueous solution, UV spectroscopy is an important means to examine host-guest interactions.The 1:2 stoichiometry of host Q[8] with guest BNB (Figs.S11 and S12 in Supporting information) and the 1:2 stoichiometry oftQ[14] with BNB (Figs.S13 and S14 in Supporting information) are determined by the mole ratio and Job’s methods.UV spectroscopy is also used to examine the supramolecular polymer.Fig.3 shows that although the absorbance of BNB@tQ[14] (2:1) at 262 nm changed sparingly, a red shift is evidenced with the continuous dropwise addition of Q[8].At the same time, absorbance at 224 nm initially drops then roses, with the inflection point occurring at a BNB@tQ[14]:Q[8] ratio of 1:1 (Fig.3, inset).This change is accompanied by a slight blue shift.
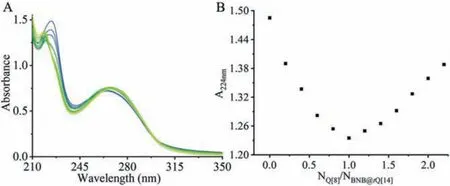
Fig.3.UV absorption spectra of (A) BNB@tQ[14] (NBNB:NtQ[14] = 2:1, 20.0 μmol/L) in the presence of increasing Q[8] concentrations.(B) The plot of vs. NBNB@tQ[14]/NQ[8]at λ = 224 nm.
The1H NMR, ITC, and UV spectroscopy data together demonstrate the process of this supramolecular polymer formation:the original host-guest structure of the BNB@tQ[14] complex is destroyed by the addition of Q[8], forcingtQ[14] to slide out of the bipyridine site of BNB.The system reaches a new equilibrium as a supramolecular polymer chain whose basic structure is two cavities oftQ[14] enclosing two alkyl chains and the cavity of Q[8] enclosing twoπ-πstacked naphthalene molecules.To further characterize this formation, large numbers of interlaced flexible lines are observed by SEM (Fig.4A), which is consistent with our inferred polymer structure.Meanwhile, particle size characterized by DLS (Fig.4B), increases from 54.79 nm (BNB) to 109.46 nm and 128.26 nm after formation of the BNB@tQ[14] complex and BNB@Q[8] complex, further to 348.32 nm on formation of the supramolecular polymer BNB@tQ[14]@Q[8].
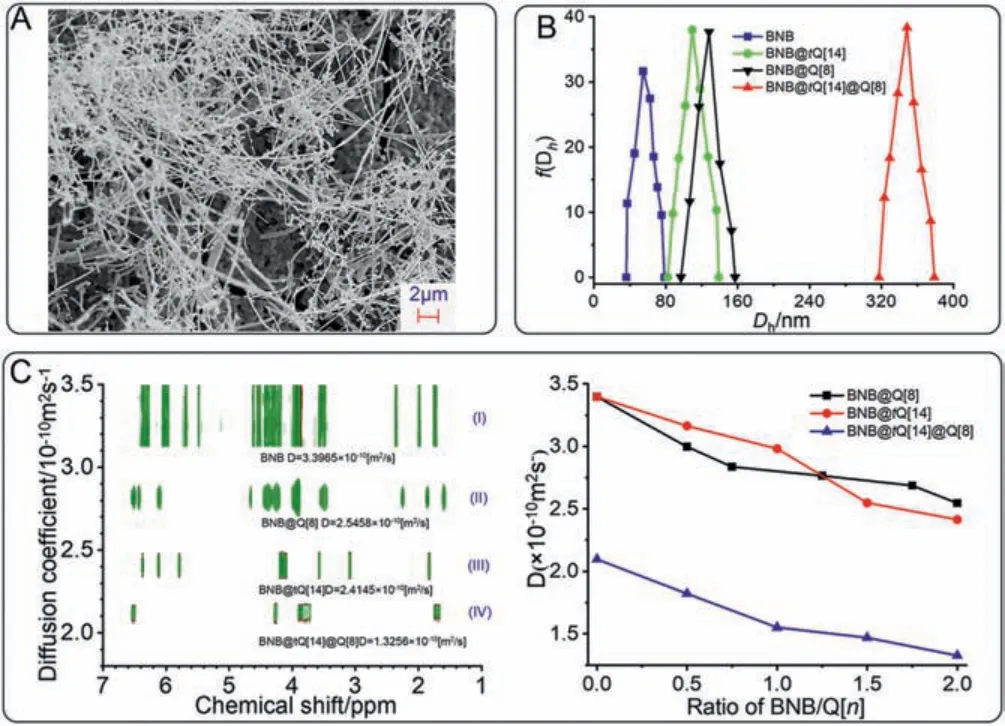
Fig.4.Scanning electron micrograph (A) of the BNB@tQ[14]@Q[8] (5.0 mmol/L)supramolecular polymer structure.DLS data (B) of BNB (blue), BNB@tQ[14](green), BNB@Q[8] (black) and supramolecular polymer BNB@tQ[14]@Q[8] (red),(C) DOSY spectra (400 MHz, D2O, 298 K, 5.0 mmol/L) of (I) BNB, (II) BNB@Q[8](NBNB:NQ[8] = 2:1), (III) BNB@tQ[14] (NBNB:NtQ[14] = 1:1), (IV) BNB@tQ[14]@Q[8](NBNB:NtQ[14]:NQ[8] = 2:1:1).
The existence of the supramolecular polymer structure is also confirmed by DOSY spectrum.As shown in Fig.4C, the diffusion coefficient of BNB in D2O) is 3.3965 × 10-10m2/s, while the coefficients of BNB@Q[8] and BNB@tQ[14] decreases significantly to 2.5458 × 10-10m2/s and 2.4145 × 10-10m2/s, respectively.The diffusion coefficient (1.3256 × 10-10m2/s) of BNB@tQ[14]@Q[8]significantly reduces when Q[8] is added to BNB@tQ[14].
Molecular modeling by quantum chemistry calculation is helpful to visualize the structure of supramolecular polymers.Density functional theory (DFT) calculations are used at the GFN2-xtb level to estimate the structures of BNB@Q[8] (Fig.S15 in Supporting information), BNB@tQ[14] (Fig.S16 in Supporting information), and the supramolecular polymer BNB@tQ[14]@Q[8] (Fig.5).Among them, the binding energy of BNB@tQ[14]@Q[8] is calculated as ΔE= -107.7495 kcal/mol, which indicates that the polymer can exist stably.
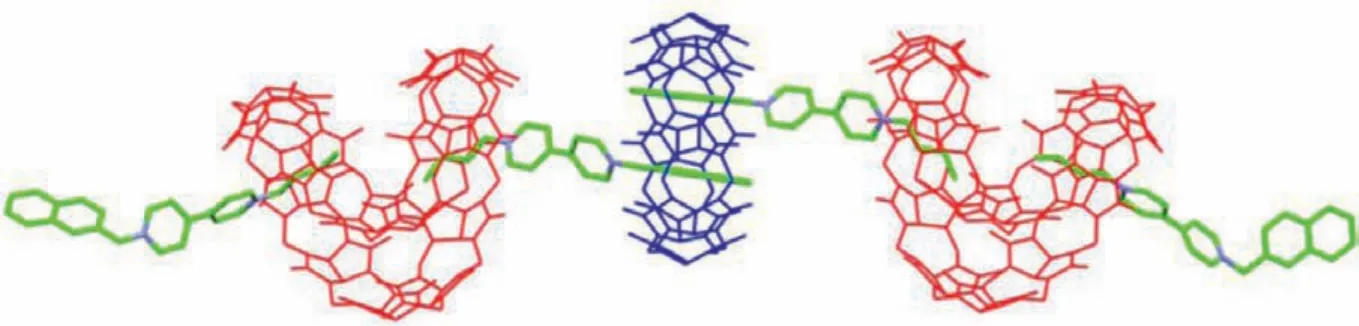
Fig.5.The molecular modeling of BNB@tQ[14]@Q[8].
This study demonstrates that supramolecular polymer formation can be easily controlled by the addition of Q[8] which disrupts the balance between BNB andtQ[14] and promotes the formation of BNB@tQ[14]@Q[8].Reversible transformation of the starting building blocks, especially for the guest species under certain conditions, is the primary feature of supramolecular polymers.This feature is evident in the present case.Due to the strong binding ability between Q[8] and adamantane hydrochloride (AH), the addition of AH as a competitive guest may dissociate the interaction of the BNB@tQ[14]@Q[8] polymer.As shown in1H NMR spectrum,AH forces naphthyl groups of BNB out of the Q[8] cavity and allow the bipyridyl groups to re-enter the cavity oftQ[14] (Fig.6), which indicates the successful dissociation of the BNB@tQ[14]@Q[8] polymer to the guest species and the concomitant formation of a new inclusion complex between Q[8] and AH.UV spectroscopy also confirmed this depolymerization.After addition of AH, absorbance and wavelengths reverts to approximately those of BNB@tQ[14](Fig.S18 in Supporting information).

Fig.6.1H NMR spectra (400 MHz, D2O, 298 K, 5.0 mmol/L) of (I) free BNB, (II)BNB@tQ[14] (NBNB:NtQ[14] = 1:1), (III) BNB@tQ[14]@Q[8] (NBNB:NtQ[14]:NQ[8] = 2:1:1)with the addition of AH, (IV) BNB@tQ[14]@Q[8] (NBNB:NtQ[14]:NQ[8] = 2:1:1), and (V)free AH.
In summary, a guest molecule (BNB) with three functional groups (naphthyl, bipyridyl and alkyl), and two hosts (tQ[14] and Q[8]) were employed to develop an effective strategy for preparing a linear supramolecular polymer with controllable polymerization.By simply mixing BNB,tQ[14] and Q[8] in aqueous solution,the host-guest interaction between the Q[8] and the naphthyl part of BNBvia π-πstacking are produced.Meanwhile, on combination with thetQ[14] that contains the alkyl chain parts of BNB,the linear supramolecular polymer was successfully constructed.Interestingly, whether Q[8] ortQ[14] is added first or Q[8] andtQ[14] are added at the same time, BNB can form flexible chainlike supramolecular polymers based ontQ[14] and Q[8] through self-sorting.Finally, depolymerization could be achieved by addition of adamantane hydrochloride which forces BNB out of the Q[8] cavity.This self-sorting strategy has great potential, not only for the preparation of supramolecular polymer materials with different controllable structures through the introduction of various functional groups, but also for broadening the study of twisted cucurbit[14]uril in supramolecular chemistry.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21861011, 21871064) and the Innovation Program for High-level Talents of Guizhou Province (No.2016–5657),the Graduate scientific research Fund of Guizhou Province (No.YJSCXJH-2019–011)
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.053.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
