Prospering the biphen[n]arenes family by tailoring reaction modules
Kidi Xu, Zhi-Yun Zhng, Ziho Zhou, Chunju Li
a College of Science, Center for Supramolecular Chemistry and Catalysis, Shanghai University, Shanghai 200444, China
b Tianjin Key Laboratory of Structure and Performance for Functional Molecules, College of Chemistry, Tianjin Normal University, Tianjin 300387, China
Keywords:Biphen[n]arenes Macrocyclic arenes Synthetic receptors Supramolecular chemistry
ABSTRACT Reported here is the comprehensive investigation on the formation of biphen[n]arenes by tailoring reaction modules.Five new macrocyclic arenes and four oligomers were synthesized by the condensation of monomers possessing different multimethoxyphenyl reaction modules and paraformaldehyde.We proved that the number and sites of methoxy on reaction modules greatly affected the reaction activity, shape,and connection mode of macrocycles.Moreover, the triangular and saddle-shaped configuration of macrocycles were revealed by single crystal structures.The results provided a typical and fundamental guidance in designing new macrocyclic arenes.
The design and synthesis of new macrocycle with distinctive properties is one of the major driving force for the development of supramolecular chemistry.The serendipitous discovery and prosperous development of classic macrocycles are the best proofs [1–13].Therefore, much more efforts have been devoted to preparing new macrocycles and many excellent works have been reported over the past years [14–31].In the supramolecular toolbox, macrocyclic arenes which were readily prepared through the condensation of corresponding arene monomers and paraformaldehyde are important parts.More recently, a series of macrocyclic arenes with novel structures and peculiar properties were reported [32–40].
In 2015, our group reported a new macrocycle, biphen[n]arenesviaa one-pot reaction of alkoxy-substituted biphenyls monomer(M1) and paraformaldehyde in the presence of Lewis acid(Schemes 1 and 2) [41].Soon after, it was confirmed that the alteration of substituents sites from 4,4′ to 2,2′ (M3) resulted new macrocyclic compounds [42,43].More recently, tetramethoxy monomer was assessed (M4) and 2,2′,4,4′-biphen[n]arene was introduced into this family [44].Due to the high-efficiency and universality, 2,4-dimethoxyphenyl was proved to be a good reaction module for the synthesis of functional macrocycles (Schemes 1 and 2) [45].
Motivated by our ongoing interest in biphen[n]arenes, we proposed that it should be fundamental and significant to synthesize new macrocycles with dimethoxyphenyl and trimethoxyphenyl reaction modules and to evaluate the influence of number and sites of methoxys on the formation of macrocycle.
2,3-Dimethoxyphenyl module was first studied (Scheme 3a).The 2,2′,3,3′-tetramethoxybiphenyl monomer (M5) was synthesized by Suzuki-Miyaura reaction (Fig.S1 in Supporting information).As the most commonly used reaction condition for synthesizing biphen[n]arenes, the BF3·Et2O as a catalyst and the 1,2-dichloroethane (DCE) as a solvent were chosen to achieve the condensation between M5 and paraformaldehyde.However,it was nonreactive even after changing the solvents, such as dichloromethane (DCM), chloroform (CHCl3), 1,2-dichloroethane(DCE), and 1,1′,2,2′-tetrachloroethane (TCE).We further evaluated the catalysts of ferric chloride (FeCl3), aluminum chloride (AlCl3),andp-toluenesulfonic acid (TsOH) and solvents (DCM, CHCl3, DCE and TCE), it was still no reaction.Fortunately, a trace dimeric macrocycle (BP-1) was obtained under the combinations of trifluoromethanesulfonic acid (TfOH) and CHCl3which was proved by the high resolution mass spectra (HRMS) (Fig.S2 in Supporting information).Due to limited yield, further structural information was unavailable.
We speculated that inserting one phenyl unit into the skeleton of monomer would improve the flexibility and probably resulted higher yield.Indeed, the cyclization of M6 reached 38%yield under the same conditions, indicating a more favorable reaction (Scheme 3b).The1H NMR,13C NMR and HRMS verified the dimeric macrocycle (BP-2) (Figs.S5-S7 in Supporting information).The protons of 2,3-dimethoxyphenyl showed a large coupling constant (J= 8.5 Hz), revealing a 6,6′-connection.To further confirm the structure, HSQC and HMBC experiments were performed(Fig.1, Figs.S8 and S9 in Supporting information).The H and C were clearly assigned by the proton-carbon single bond correlation and multiple bond correlation.As the HMBC spectra mainly gave corrections between C and H that were separated by two and three bonds, the obvious C7-H9 correlation and absence of C6-H9 correlation indicated that the methylene group bridged on C8 but not C7.Moreover, the presence of C3-H9 three bonds correlation further proved such connection (Fig.1b).
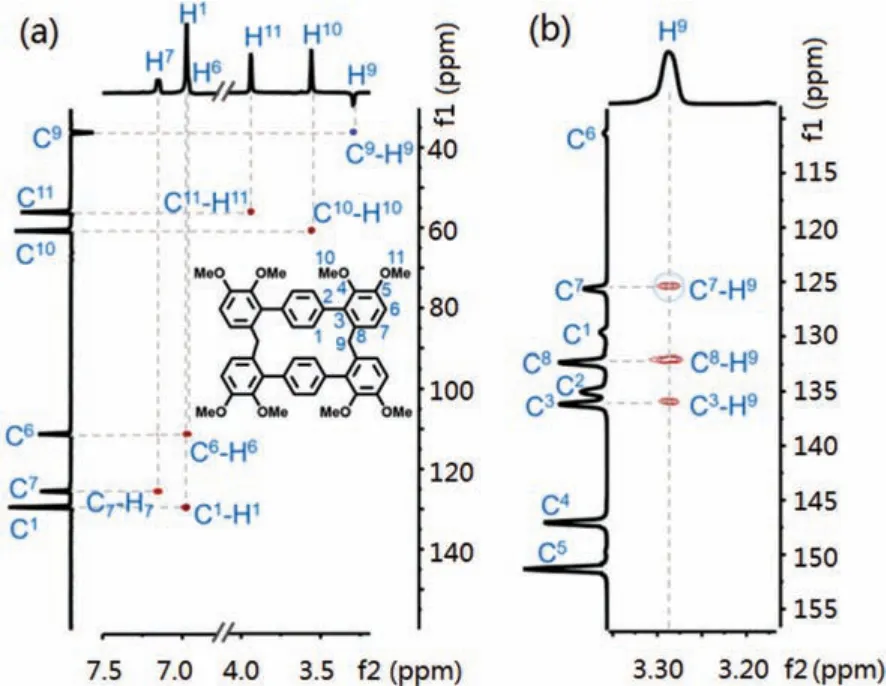
Fig.1.(a) HSQC and (b) partial HMBC spectra of BP-2.
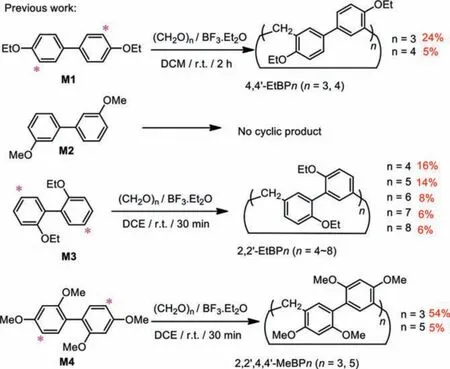
Scheme 2.The monomers of M1-M4 and corresponding products in previous work.
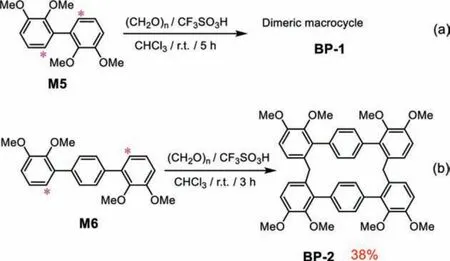
Scheme 3.The synthesis of cyclic products BP-1 and BP-2 from monomers (M5 and M6) with 2,3-dimethoxyphenyl reaction modules.
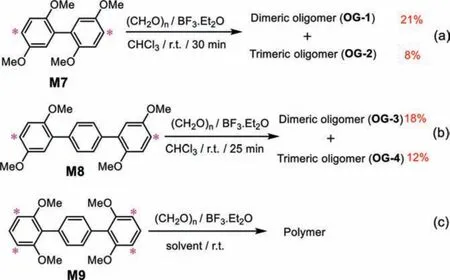
Scheme 4.The monomers (M7-M9) with 2,5-dimethoxyphenyl (a, b) and 2,6-dimethoxyphenyl (c) reaction modules and corresponding products (oligomer and polymer).
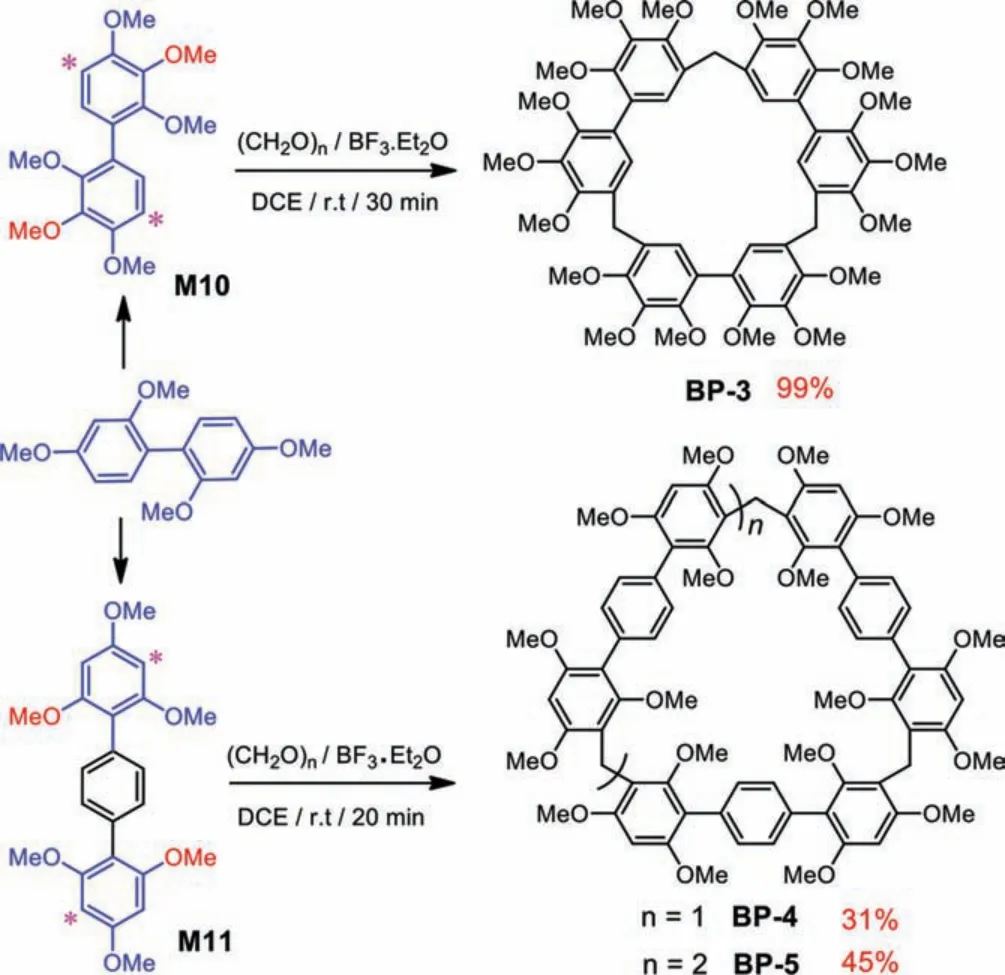
Scheme 5.The synthesis of cyclic products BP-3, BP-4 and BP-5 from monomers with 2,3,4-trimethoxyphenyl (M10) and 2,4,6-trimethoxyphenyl (M11) reaction modules.
As 2,4-dimethoxyphenyl (in M4) has been proved to be a good reaction module previously, we studied the remaining two modules:2,5-dimethoxyphenyl and 2,6-dimethoxyphenyl (Scheme 4).For 2,5-dimethoxyphenyl module, we evaluated the 2,2′,5,5′-tetramethoxybiphenyl (M7) and 2,2′′,5,5′′-tetramethoxyterphenyl(M8) monomer (Schemes 4a and b).The influence of catalysts(BF3·Et2O, FeCl3, AlCl3, TsOH, TfOH) and solvents (DCM, CHCl3,DCE, TCE, tetrahydrofuran (THF), and acetonitrile (CH3CN)) were investigated.Only the combination of BF3·Et2O and CHCl3could produce an oligomeric product without macrocycle and the rest of the conditions resulted polymerization.1H,13C NMR and HRMS proved that these oligomers were dimer and trimer (Figs.S10-S23 in Supporting information).We speculated that the cyclic products would form momentarily, but maybe the reversibility of Friedel-Crafts reaction made oligomers as dominant products which possessed lower ring tension.For 2,6-dimethoxyphenyl module(Scheme 4c and Fig.S24 in Supporting information), only polymer could be found after screening different catalysts (BF3·Et2O,AlCl3, FeCl3, TsOH, TfOH) and solvents (DCM, CHCl3, DCE, TCE, THF,and CH3CN), possibly because of the four equivalent reaction sites for one 2,2′′,6,6′′-tetramethoxyterphenyl monomer (M9).The additional reaction sites probably promoted the polymerization which was harmful for the cyclization.
Having above results in hand, it was anticipated that adding one more methoxy on the 2,4-dimethoxyphenyl reaction module would probably give higher yield and different macrocycles.Therefore, 2,3,4-trimethoxyphenyl module was introduced into the monomer (M10) (Scheme 5 and Figs.S25-S27 in Supporting information).Indeed, the cyclization yield reached 99% under the catalyzing of BF3·Et2O in DCE (Figs.S28-S30 in Supporting information).Comparing the 54% yield of M4 in the same conditions [44], the improved reaction yield for 2,2′,3,3′,4,4′-hexamethoxybiphenyl monomer probably arose from the additional electron-rich methoxy group.The additional methoxy was further moved from 3- to 6-position of the reaction module.The 2,2′,4,4′,6,6′-hexamethoxybiphenyl monomer was unavailable and the terphenyl monomer (M11) was synthesized as an alternative one (Figs.S31-S33 in Supporting information).Under the same catalyst (BF3·Et2O) and solvent (DCE), trimeric (BP-4) and tetrameric(BP-5) macrocycles were obtained in 31% and 45% yields, respectively (Figs.S34-S39 in Supporting information).Therefore, the results indicated that the addition of methoxy group could improve the reaction activity.Monomers with 2,4,5-trimethoxyphenyl(M12), 2,3,6-trimethoxyphenyl (M13), and 3,4,5-trimethoxyphenyl(M14) modules were also synthesized and evaluated (Figs.S40-S48 in Supporting information).But only polymers were obtained after screening catalysts and solvents.
Atomic-level structures were revealed by single-crystal X-ray diffraction analysis.Single crystals of BP-3 (CCDC:2061613) were obtained as colorless prism by slow vapor diffusion of isopropyl ether into a DCM solution of BP-3 during 7 days (Fig.2 and Table S1 in Supporting informaiton).BP-3 possessed a rigid triangular geometry with the size of 7.6 Å × 7.8 Å × 8.0 Å, incircle diameter of 4.6 Å and vertex angles of 114°, 115° and 116°, revealing its asymmetrical characteristic in crystal.The phenyl units in each monomer were mutually perpendicular and formed a bowlshaped structure with the depth of 4.9 Å.Moreover, the compound possessed a pair of enantiomers in solid-state (Fig.S49a in Supporting information).BP-3 aligned with each other with a portal-to-portal packing mode, resulting in the formation of one-dimensional channels by the hydrogen bonds and C–H···πinteractions (Figs.S49c and d in Supporting information).Surface electrostatic potential map showed that the cavity was electronegative and the terminal methoxys were electropositive (Figs.2b and d).
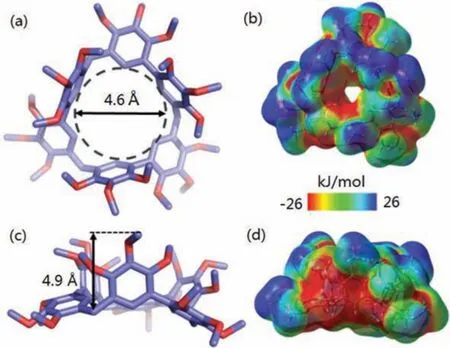
Fig.2.(a, c) Single-crystal structures and (b, d) electrostatic potential maps of BP-3(top view and side view).Note:red region represents low potential area with the characterization of an abundance of electrons.Blue region represents high potential area with the characterization of a relative absence of electrons.C, purple; O, red.Hydrogen atoms are omitted for clarity.
For BP-5, the single crystals (CCDC:2061614) were obtained as colorless block by slow evaporating a DCM solution of BP-5 during 4 days (Fig.3 and Table S2 in Supporting information).The macrocycle showed a saddle-shaped configuration with the external distance of 6.3 Å, middle distance of 9.1 Å and internal distance of 11.2 Å (Figs.3a and c).BP-5 assembled into a square-channel superstructure byπ…πinteractions and hydrogen bonds between 2,4,6-trimethoxyphenyl units of adjacent molecules(Fig.S50 in Supporting information).Surface electrostatic potential map showed that the cavity was electronegative and the terminal methoxys were electropositive (Figs.3b and d).
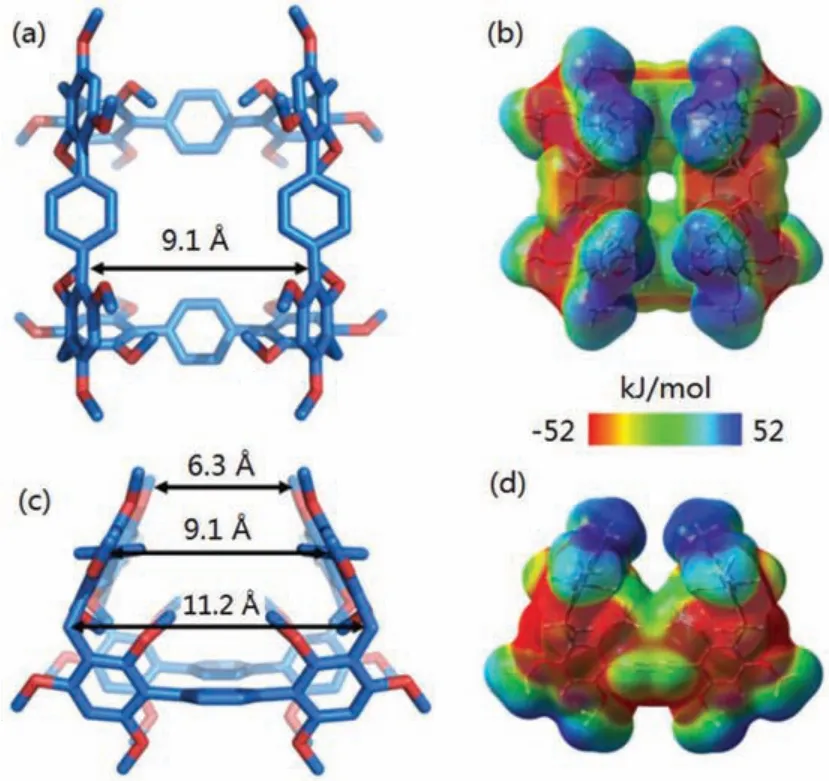
Fig.3.(a, c) Single-crystal structures, (b, d) electrostatic potential maps of BP-5(top view and side view).Note:red region represents low potential area with the characterization of an abundance of electrons.Blue region represents high potential area with the characterization of a relative absence of electrons.C, blue; O, red.Hydrogen atoms are omitted for clarity.
In conclusion, the influence of reaction modules on the formation of biphen[n]arenes was fully investigated.We found that the number and sites of substituents have great influence on the reaction activity, linker site, macrocyclic shapes, and yield.The addition of methoxys can improve the reaction activity and the cyclization yield.For dimethoxyphenyl reaction modules, the best one for cyclization is the 2,4-dimethoxyphenyl, followed by 2,3-dimethoxyphenyl.Monomer with 2,5-dimethoxyphenyl or 2,6-dimethoxyphenyl reaction modules was unable to form cyclic product.For termethoxyphenyl reaction modules, the 2,2′,3,3′,4,4′-hexamethoxybiphenyl monomer resulted trimeric macrocycle and the 2,2′′,4,4′′,6,6′′-hexamethoxyterphenyl monomer resulted trimeric and tetrameric macrocycles, and other monomers resulted polymers.The work on functionalization of these macrocycles are now in process.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (Nos.21971192 and 21772118) and the Natural Science Foundation of Tianjin City (No.20JCZDJC00200).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.096.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
