Synergistic activation of photoswitchable supramolecular assembly based on sulfonated crown ether and dithienylethene derivative
Conghui Wng, Ying-Ming Zhng, Horn Li, Jin Zhng, Yu Zhou, Guoxing Liu,Xiufng Xu, Yu Liu,*
a College of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China
b College of Computer Science, Nankai University, Tianjin 300350, China
c College of Sciences, Henan Agricultural University, Zhengzhou 450001, China
Keywords:Supramolecular assembly Crown ether Photochromism Energy transfer Conformational regulation
ABSTRACT Conformational regulation among two or more distant sites is not only one of the main pathways to accomplish multiple tasks in complex biological systems but also represents a powerful strategy to obtain stimuli-responsive supramolecular nanoconstructs with tailored physicochemical performance.We herein report the fabrication of a photochromic supramolecular assembly, which can be synergistically activated by the conformational regulation with bis(4,8-disulfonato-1,5-naphtho)-32-crown-8 and then reversibly switched by the through-space communication between restricted stilbazolium salt and photochromic dithienylethene.This work demonstrates that the synergistic conformational modulation via intra- and intermolecular interactions can be developed as a generalizable approach to construct more advanced biomimetic nanomaterials.
In natural ensembles, discrete biomacromolecules always adopt specific conformations in response to external stimuli, by which multiple tasks can be accomplished in a dynamic and reversible manner under physiological conditions, such as substrate binding,and cellular circuit, and information transmission [1,2].Inspired by the wisdom of nature, conformational regulation has also been employed by chemists to create artificially self-organized nanoarchitectures with tunable physicochemical properties [3,4].In this context, by precisely leveraging noncovalent interactions, supramolecular chemistry based on cavity-bearing macrocyclic hosts has been developed as a powerful method to drive conformational changes of encapsulated guests, thus enabling the transduction of environmental cues (including pH, light, ligand binding,etc.) into morphological conversion [5,6], signal processing [7,8], and task execution[9–11].As for the fabrication of these stimuli-responsive nanoconstructs, particularly, light input as energy supply is believed as an ideal candidate, due to its clean and renewable characteristics.Thus, the combination of light irradiation and macrocyclic complexation has been explored as a powerful strategy in regulating and improving photophysical performance of self-assembled superstructures, thus showed promising potentials in attaining intelligent luminescent materials [12,13].
Photochromism generally refers to the reversible change of color under light irradiation, usually accompanied by the structural changes of chromophores [14,15].There has been a profound evolution in this field during the past decades as a result of successful attempts to improve the photophysical properties of various photochromic molecules and to broaden the applicable horizon of photochromic materials in optical devices, molecular machines,and disease treatments [16–18].Among numerous photochromic substances, diarylethenes (DTEs) have been emerged into limelight, mainly due to their immense advantages of good photoresponsibility, excellent fatigue resistance, and high photoreaction quantum yield [19–21].Consequently, extensive endeavors have been devoted to exploring advanced diarylethene derivatives with tunable photophysical features, which have increasingly enriched the store of building blocks for development of photoresponsive materials and technologies [22,23].
In this work, intramolecular photochromism process and intermolecular inclusion complexation are married to induce the dramatic conformational changes of guest molecule and then maximize its spectral and energy-level matching between two distal functional sites in a photoswitchable supramolecular assembly.The energy transfer process is exclusively activated by the host–guest structural regulation, and the optically active and inactive states can be reversibly adjusted upon light irradiation at different wavelengths.Specifically, the 4-[4-(dimethylamino)styryl]-1-hexylpyridinium salt (DASP) could be tightly encapsulated in the ring of bis(4,8-disulfonato-1,5-naphtho)-32-crown-8 (DNC) as activator, accompanied by the more compact molecular conformation and dramatic fluorescence enhancement.Remarkably, the unique fluorescent emission arising from the DASP⊂DNC assembly could be reversibly and efficiently modulated by the photochromic conversion of perfluorocyclopentene-derived dithienylethene (DTEDASP).To integrate the tunable conformational and photochromic characteristics into a single supramolecular assembled entity, this obtained structurally-driven photoswitch may provide new insights in the design of bioinspired intelligent materials with tailored photophysical functionalities.
The photochromic properties of DTE-DASP were preliminarily explored by UV–vis spectroscopy.Compared to the free DASP as reference compound, the absorbance in the wavelength region from 400 nm to 550 nm was largely originated from the DASP moiety (Fig.S1 in Supporting informaiton).As discerned from Fig.2a, upon continuous UV light irradiation at 254 nm, DTE-DASP underwent photocyclization reaction, accompanied by the obvious color change from bright yellow to dark brown (Fig.2a, inset).Meanwhile, the UV–vis absorption spectra gave a new absorption maximum at 592 nm with a clear isosbestic point at 319 nm, corresponding to the photo-induced conversion of DTE core from the open-ring form (OF) to closed-ring form (CF).This photochromic switch showed satisfactory reversibility and no apparent deterioration could be observed during the repetitive switching cycles upon alternating UV (λ= 254 nm) and visible (λ >490 nm) light irradiation (Fig.2b).In addition, according to the chemical shift and integral ratio changes of aromatic proton (H3) upon 254 nm light irradiation, the conversion efficiency of DTE-DASP was determined to be 92% by1H NMR (i and ii in Fig.2c).In addition, the NMR spectra were completely restored after switching for several cycles,implying the using of light carried no deleterious effects (Fig.S2 in Supporting information).Meanwhile, no change was observed in the case of free DASP under the same light irradiation condition indicating the 254 nm light irradiation did not have a significant effect on DASP unit (Fig.S3 in Supporting informaiton).
As a classic type of photochromic compounds, DTEs possessedπ-conjugated thienyl group and electron-withdrawing perfluorocyclopentene counterpart, which renders them prime components for the fabrication of photo-controllable molecular assemblies[24,25].Moreover, in our case, the photochromic unit DTE was covalently bondedviaa hexyl linker to the push-pull-type DASP with an intramolecular charge transfer pathway from dimethylaniline donor to pyridinium acceptor upon excitation [26,27].The flexible alkyl chain with appropriate molecular length could ensure the complete complexation of stilbazolium salt with the crown ether’s ring and the subsequent energy transfer process.The aromatic protons of DNC showed obvious upfield shifts and became broadened upon complexation with DTEOF-DASP (iii in Fig.2c and Fig.S4 in Supporting information).Meanwhile, owing to the strong diamagnetic shielding effect of naphthyl rings, the resonance signals of central double bond and pyridinium ring in DASP exhibited upfield shifts, while the ones of dimethylaniline part gave downfield shifts, implying that the dimethylaniline part was located outside the crown ether’s ring.These chemical shift changes were in accordance with the host–guest complexation between DNC and free DASP (Fig.S5 in Supporting information).Moreover, the cyclization/cycloreversion reactions of DTE-DASP could efficiently and reversibly take place even in the presence of DNC (iv in Fig.2c and Fig.S6 in Supporting information) and the morphology of DTE-DASP⊂DNC complex was changed from regular nanospheres(around 200 nm) to irregular nanosheets after UV irradiation at 254 nm (Fig.S7 in Supporting information).In addition, crosspeaks were clearly assigned to the nuclear Overhauser enhancement (NOE) correlation between the aromatic protons of DNC and DASP in the two-dimensional1H NMR spectrum (Fig.S8 in Supporting information).Given that no chemical change or NOE correlation was observed for DTE pendant, the possible assembling mode involving the exclusive host–guest complexation of DNC with DASP moiety was presented in Fig.1.
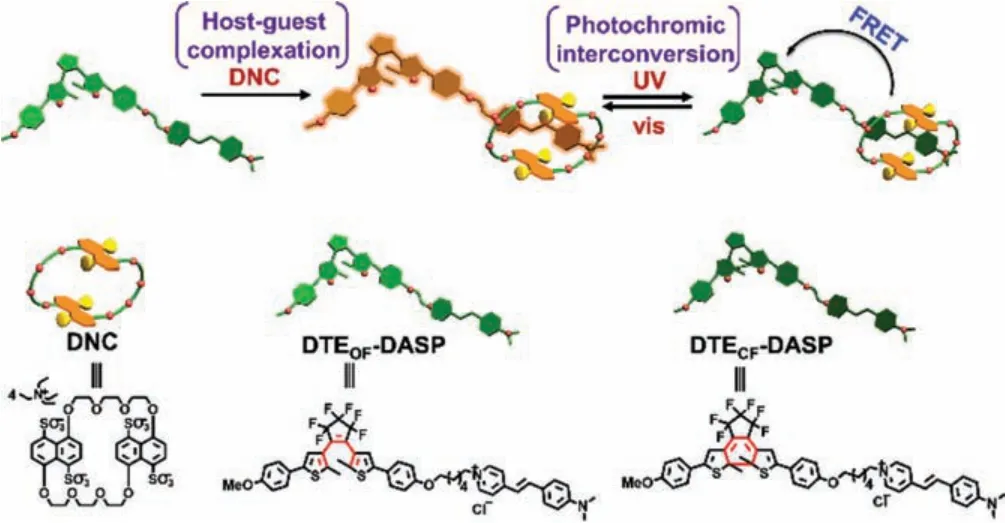
Fig.1.Schematic illustration and chemical structure of the supramolecular photoswitch.
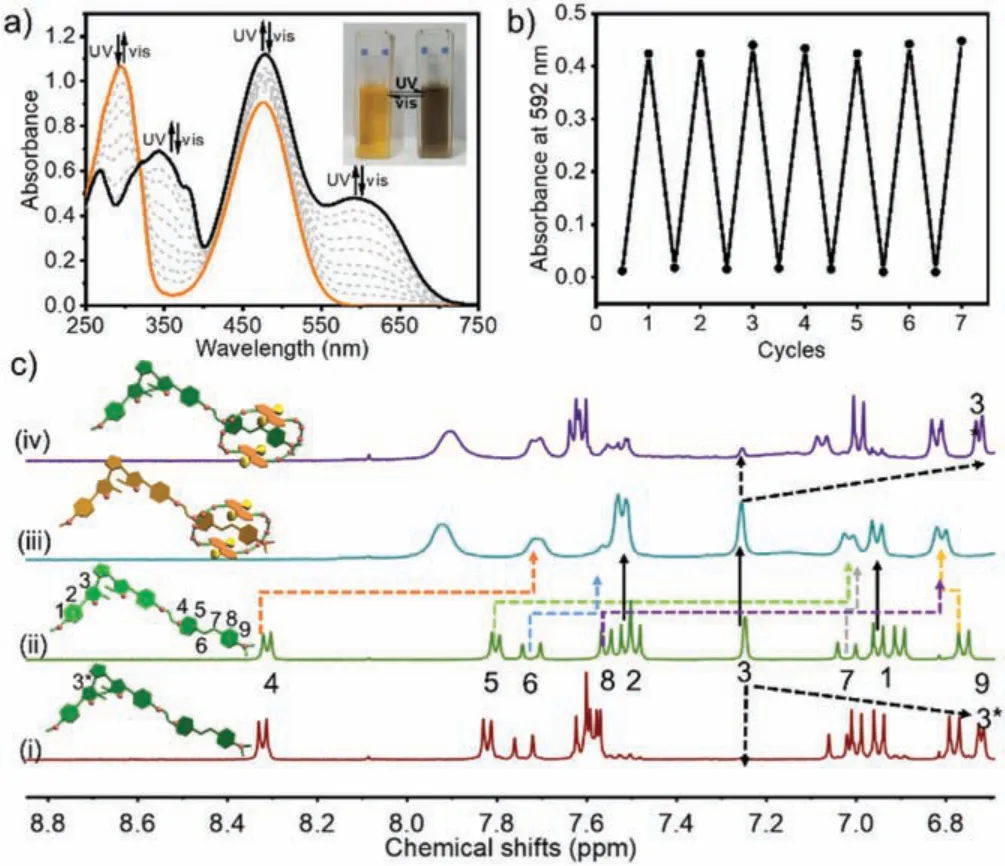
Fig.2.(a) UV–vis spectral changes of the photoconversion from OF to CF states upon light irradiation at 254 nm in CH3CN at 25 °C ([DTE-DASP] = 20 μmol/L).Inset:photographic images of DTEOF-DASP and DTECF-DASP.(b) Fatigue resistance of DTE-DASP upon alternating UV (λ = 254 nm) and visible (λ >490 nm) light irradiation.(c) Partial 1H NMR spectra (400 MHz) of (i) DTECF⊂DASP, (ii) DTEOFDASP, (iii) DTEOF-DASP⊂DNC, and (iv) DTECF-DASP⊂DNC assemblies, respectively, in CD3CN at 25 °C ([DTE-DASP] = [DNC] = 2.5 mmol/L).
More detailed investigations on the photophysical behaviors of DTE-DASP⊂DNC assembly came from the UV–vis and fluorescence spectroscopy.The absorption peak of DTEOF-DASP centered at 476 nm exhibited large hypochromatic shift to 465 nm upon addition of 1.0 equiv.of DNC, mainly due to the intermolecularπ-stacking interaction between naphthyl and pyridinium rings in the ground state (Fig.3a).The emission of DTEOF-DASP generally red-shifted as the solvent polarity increased resulting from the charge transfer characteristic (Fig.S9 in Supporting informaiton).More surprisingly, the fluorescence intensity of DTEOF-DASP pronouncedly increased in the presence of DNC, accompanied by a large hypochromatic shift from 619 nm to 577 nm (Fig.3b).This fluorescence enhancement and the changes of fluorescent emission intensity in the presence of competitive guest molecule convincingly demonstrated that the host-guest complexation with DNC was enormously beneficial to the visible emission of DASP from its locally excited (LE) singlet state with a more coplanar conformation, and extremely suppress the nonradiative decay from the charge transfer state with an intramolecular twisted conformation (Fig.S10 in Supporting informaiton) [28].This hypothesis was further confirmed by the following quantum chemical calculations.Moreover, after validating the 1:1 binding stoichiometry by Job plot, the binding constant (KS) was accordingly calculated as 2.6 × 105L/mol in the DTEOF-DASP⊂DNC inclusion complexation (Fig.S11 in Supporting information).Moreover, no obvious change inKSvalues was observed in the DTE-DASP⊂DNC complex before and after light irradiation, indicating that the photochromic process of DTE site could not make any negative impact on the DASP⊂DNC inclusion complexation (Fig.S12 in Supporting information).
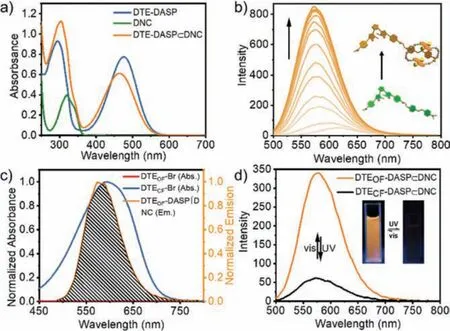
Fig.3.(a) UV–vis spectral changes of DTEOF-DASP with DNC in CH3CN at 25 °C([DTEOF-DASP] = [DNC] = 20 μmol/L).(b) Fluorescence titration spectra of DTEOFDASP (20 μmol/L) upon addition of DNC (0–180 μmol/L).(c) Spectral overlap between the absorbance of DTE-Br and the emission of DTEOF-DASP⊂DNC assembly.(d) Fluorescence spectral changes of DTEOF-DASP⊂DNC assembly upon UV light irradiation at 254 nm in CH3CN at 25 °C ([DTE-DASP] = [DNC] = 20 μmol/L).Inset:The photos of DTEOF-DASP⊂DNC and DTECF-DASP⊂DNC assembly.(λex = 450 nm).
After scrutinizing the spectroscopic data, it is noteworthy that the absorption spectrum of brominated intermediate DTE-Br in the CF state could perfectly fall into the fluorescence emission spectrum of DASP after the conformational fixation by DNC entrapment (Fig.3c and Scheme S1 in Supporting informaiton).Considering that the sufficient spectral overlap and the location of two chromophores in close proximity are indispensable design criteria required to achieve efficient energy transfer process, it is reasonable to anticipate that the through-space energy transfer behavior is more likely to occur in the DTECF-DASP⊂DNC assembly [29–31].As expected, a rapid decrease of fluorescent intensity was observed upon UV light irradiation at 254 nm, indicative of the formation of DTECF-DASP and the subsequent efficient energy transfer from DASP⊂DNC complex as donor to DTECFmoiety as acceptor(Fig.3d).Benefiting from the excellent photochromic property of DTE unit, the energy transfer process could be reversibly modulated by light irradiation.That is, when the solution was irradiated with UV light (λ= 254 nm), the bright orange fluorescence emission gradually disappeared, whereas the emission was recovered to initial level upon continuous irradiation by visible (λ >490 nm)light for 40 s.In addition, this process could be also readily distinguished by naked eyes (Fig.3d, inset), and the photoluminescent reversibility could be well reproduced for many cycles without color fading (Fig.S13 in Supporting information).By comparing the fluorescence intensity at both initial and photo-stationary states,the efficiency of energy transfer was determined as 82%.Comparatively, when simply mixing DTE-Br and DASP⊂DNC complex,the efficiency of energy transfer became rather low (Fig.S14 in Supporting information).Meanwhile, the DTE-induced fluorescence emission change of DASP was negligible without the DNC complexation in solution (Fig.S15 in Supporting information).These results jointly substantiate that the alkyl chain toggling between DTE and DASP units and the host–guest interaction with DNC play crucial roles in the conformational and photochromic control of energy transfer process.
To further excavate the structural origin and photoluminescence mechanism, quantum chemical calculations were performed on the free DASP, DTE-DASP, and their DNC-bound assemblies in the ground (S0) and first singlet excited (S1) states.It is known that the singlet excited state of push-pull-type molecule DASP spontaneously relaxes to the twisted intramolecular charge-transfer(TICT) state and hence keeps in fluorescent silence [32].Our calculations by the density functional theory (DFT) and time-dependent density functional theory (TDDFT) methods also revealed that the ground state of DASP moiety adopts a coplanar conformation,whereas the most stable singlet excited state of DASP moiety is twisted by the -92.2° rotation of dimethylaniline with respect to pyridinium unitviathe central chemical double bond, which is in consistent with the TICT relaxation mechanism (Figs.4a and b).Meanwhile, other possible conformations of DTE-DASP in the first singlet excited state were also considered but they were all higher in energy than the conformation in Fig.4b (Fig.S16 in Supporting information).The encapsulation in DNC ring could not change the coplanar conformation of DASP moiety in the ground state (the torsion angleφ= 179.3° in Fig.4c).In sharp contrast, the captured DASP completely retained the coplanar conformation (the torsion anglesφ= 177.2° in Fig.4d) in the ring of DNC even in the local excited (LE) state, which was co-stabilized by the multipleπstacking and hydrogen-bonding interactions.Consequently, through the cooperative host-guest stabilization in the S1state, the undesirable nonradiative quenching process from the susceptible TICT state is essentially blocked and the emissive channel from the S1state revives as the dominant pathway to dramatically augment the fluorescence efficiency.The transition energies of DASP and DTE from the S0to the S1state were also calculated to gain more insights into the energy transfer pathway.The energy difference between DTEOFand DTECFis fairly large at the S1state, which provides an opportunity to switch the energy transfer behaviors with a photosensitizer possessing suitable singlet energy to fill the gap[33].Indeed, the obtained transition energy of DASP (2.62 eV) is higher than that of DTECF(1.94 eV) but lower than that of the DTEOF(3.25 eV), suggesting that the energy transfer can only take place from DASP to DTECFrather than DTEOF.As a result, the fluorescence emission is accordingly switched off by virtue of the energy-level matching and the close range in the donor-to-acceptor contact between two chromophore centers (2.16 nm, Fig.4c).
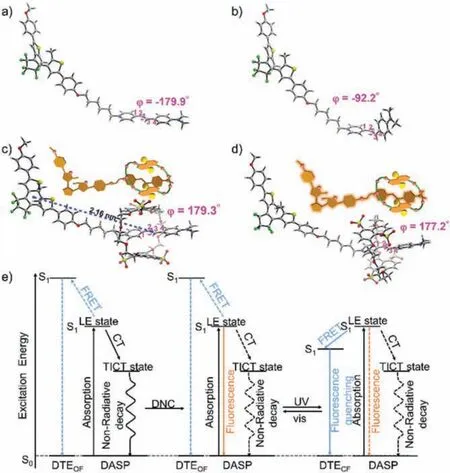
Fig.4.Optimized geometries for (a, b) DTE-DASP and (c, d) DTE-DASP⊂DNC assembly in the (a, c) ground states (S0) and (b, d) first singlet excited states (S1)in CH3CN calculated by the DFT and TDDFT methods, respectively, with the SMD solvation model.(e) Proposed fluorescence emission pathway and photoswitchable energy transfer mechanism in this system.The solid and dash lines represent the favorable primary and unfavorable secondary processes, respectively.
Therefore, combined the aforementioned experimental and computational results, a conformation–photochromism coupling mechanism is proposed in Fig.4e, which involves the supramolecular structural regulation and reversible photocyclization/reversion reaction.The DASP moiety consisting of dimethylaniline as donor and pyridinium ring as acceptor linkedviaa double bond undergoes transition from S0to LE states upon photoexcitation.However, the coplanar conformation in LE state is not stable enough in the polar solvents such as CH3CN.Under such circumstance,the excited molecule is preferentially located in the TICT state with a twisted conformation and the fluorescence emission is seriously weakenedvianonradiative decay processes.The host–guest complexation with DNC as activator can enhance the fluorescence emission intensity of DASP in its emissive LE state by the supramolecular structural regulation.Subsequently, the energy difference between OF and CF of DTE could be used to regulate the intramolecular energy transfer between DASP and the DTE,thus resulting in the photo-controlled FRET process in the DTEDASP⊂DNC assembly.
In conclusion, by combining the coupled conformational regulation with intermolecular inclusion stabilization and intramolecular photochromic switching, we have developed a facile strategy to fabricate a supramolecular photoswitch with tunable on-off photoluminescence behaviors.The crown ether DNC as modulator can greatly immobilize the molecular conformation of DASP and then trigger the extraordinary fluorescence enhancement at its LE state.Meanwhile, the fluorescence emission could be reversibly and efficiently modulated by the adjacent DTE moiety through the favorable photochromic interconversion.Thus, we can envision that the conformational co-regulatory effect on the structural fixation and energy matching in this study may provide more applicable nanoplatforms to continuously explore intelligent biomimetic systems for advanced catalysis, visual monitoring, optical data storage,and so on.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.21871154, 21772099,21861132001, and 21873051), and the Fundamental Research Funds for the Central Universities, Nankai University.We also thank Prof.Xiaoli Gong at College of Computer Science, Nankai University for his assistance in the preparation of this manuscript.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.106.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
