Total synthesis and biological evaluation of dracaenins A and B
Yaqiu Zhao, Shunli Xiao, Yun You, Zhi Zhang, Liansuo Zu, Luqi Huang,*
a College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing 210023, China
b School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China
c State Key Laboratory Breeding Base of Dao-di Herbs, National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences,Beijing 100700, China
Keywords:Total synthesis Natural product Dragon’s blood Dracaenin Hemostasis
ABSTRACT The first total synthesis of dracaenins A and B is achieved in four steps.The synthesis features the convergent coupling of three readily available fragments with minimized use of protecting groups.The chemical synthesis enables the discovery of their activity in stimulating platelet aggregation, and thus, sheds light on the possible origin of the hemostatic effect of dragon’s blood.
Dragon’s blood refers to the red resinous exudations from plant species and has been used as herbal medicine for more than 1500 years across many cultures.Modern pharmacological studies of dragon’s blood have revealed a wide array of bioactivities [1].Among them, it has been highlighted to have bidirectional regulatory effect of stimulating both blood circulation and coagulation.The clarification and identification of active components that account for these contrasting activities are crucial for the in-depth studies of this important herbal medicine.In this context, several phenolic compounds [2–4], such as loureirin B (5) and cochinchinenin A (6), isolated from dragon’s blood, have been shown to have the activity of promoting blood circulation (Fig.1).However, the active components that could stimulate blood coagulation (hemostatic effect) remain to be discovered.
Dracaenins A (1) and B (2) were isolated from the Chinese dragon’s blood,Dracaena cochinchinensis(Lour.) S.C.Chen, by Zhang and co-workers in 2012 (Fig.1) [5].Structurally, they feature the unusual chalcane-anthocyanidin dimers.Several anthocyanidins, such as dracorhodin (3) and nordracorhodin (4), have been isolated from dragon’s blood and are known to account for its intense red color (Fig.1).The related dihydrochalcones 5 and 6 have been reported to be able to inhibit platelet aggregation.Thus, the generation of such chalcane-anthocyanidin dimers by nature would be inspiring and useful as chemical probes to study the related biological activities.
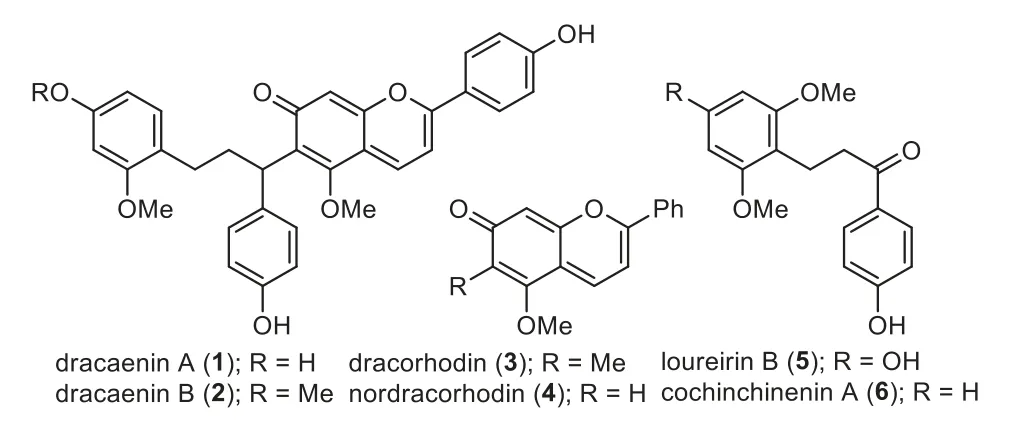
Fig.1.Dracaenins A and B and related natural products from dragon’s blood.
The isolation yield of dracaenin A from dragon’s blood is very low (23 mg dracaenin A was isolated from 3.0 kg of dragon’s blood).In addition, this herbal medicine is very expensive due to its beneficial values to human health, the wide usage and the shortage of natural sources.Total synthesis has long been a powerful tool in facilitating the biological studies of natural products by providing enough materials particularly when the nature supply is limited or not accessible [6].In addition, once the therapeutic potential of a natural product is identified, efficient chemical synthesis could offer a sustainable alternative for its production, thus alleviating the pressure on natural resource.While the structural complexity of dracaenins A and B is moderate, the concise assembly of these structures is not trivial.Attention should be paid to the electrophilicity of the anthocyanidin part in a synthetic design as demonstrated by the transformation of 3 to other more complex natural products by the group of Trauner [7].Moreover, there are 7 phenolic oxygens with varied substitution patterns and oxidation state.The successful introduction of them with minimal reliance on protecting groups is crucial for synthetic efficiency [8].Siteselectivity regarding the formation of the anthocyanidin part and chalcane-anthocyanidin connection are further concerns.Herein,we report our efforts to address these synthetic issues, which lead to the concise and convergent total synthesis of dracaenins A and B on only four steps.The chemical synthesis further enables the discovery of their activity in stimulating platelet aggregation, and thus, sheds light on the possible origin of the hemostatic effect of dragon’s blood.
It could be envisioned from the outset that a successful synthesis would afford dracaenins A and B in short synthetic sequence, in a convergent manner [9] and with the minimized use of protecting groups [8].To fulfill these standards, our synthetic plan was depicted in Scheme 1.We surmised that the chalcane-anthocyanidin connection of dracaenins A and B could be furnished by the direct Friedel-Crafts coupling of alcohol 7 and phloroglucinol derivative 8.It was hoped that the coupling could proceed without any protecting groups on the phenolic oxygens.Regarding the synthesis of the anthocyanidin part, several known methods have been reported [7,10,11].The most common approach requires the prefunctionalization of the precursor, for example the coupling products of 7 and 8, with an aldehyde group as the synthetic handle.We envisioned that such stepwise operations would pose significant challenges in controlling the chemoselectivity without using protecting groups.Thus, we turned to a direct annulation approach developed by the group of Chassaing using ethynyl ketone 9[12,13].In our synthetic plan, only ethynyl ketone 9 was protected as the TBS ether, which was introduced during its preparation and could be concomitantly cleaved under the acidic annulation conditions.In addition, our synthetic design features the convergent coupling of three fragments 7–9, which could allow for the synthesis of structural analogs, if necessary, by the facile variation of these fragments.
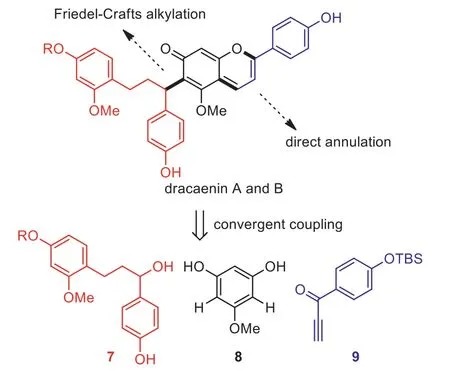
Scheme 1.Synthetic plan to dracaenins A and B.
We first selected dracaenin B as our synthetic target(Scheme 2).The coupling partner 7b was successfully synthesized in 2 steps by the Aldol type condensation of 10 and 11 followed by hydrogenation of the resulting chalcone.The coupling of 7b and phloroglucinol derivative 8 turned out to be challenging.Different acids that could potentially promote the Friedel-Crafts alkylation were screened (Table 1).In the presence of HCl orp-TsOH, the formation of the desired product was not observed.The switch to BF3.Et2O [14,15] at -10 °C led to the production of 12 in synthetically useful yield (48%; entry 4), albeit companied with its regio-isomer 12′(12:12′= 1.6:1).The use of diphenyl phosphate as the acidic promotor in acetonitrile gave similar yield of 12 with slightly lower site-selectivity (entry 6).In the currentinvestigation, we chose to use the BF3.Et2O condition for the synthesis of 12.
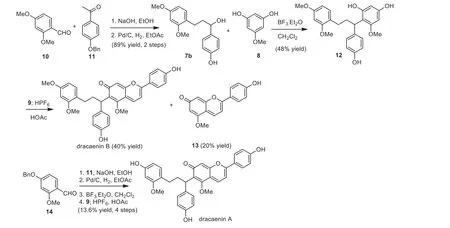
Scheme 2.Total synthesis of dracaenins A and B.
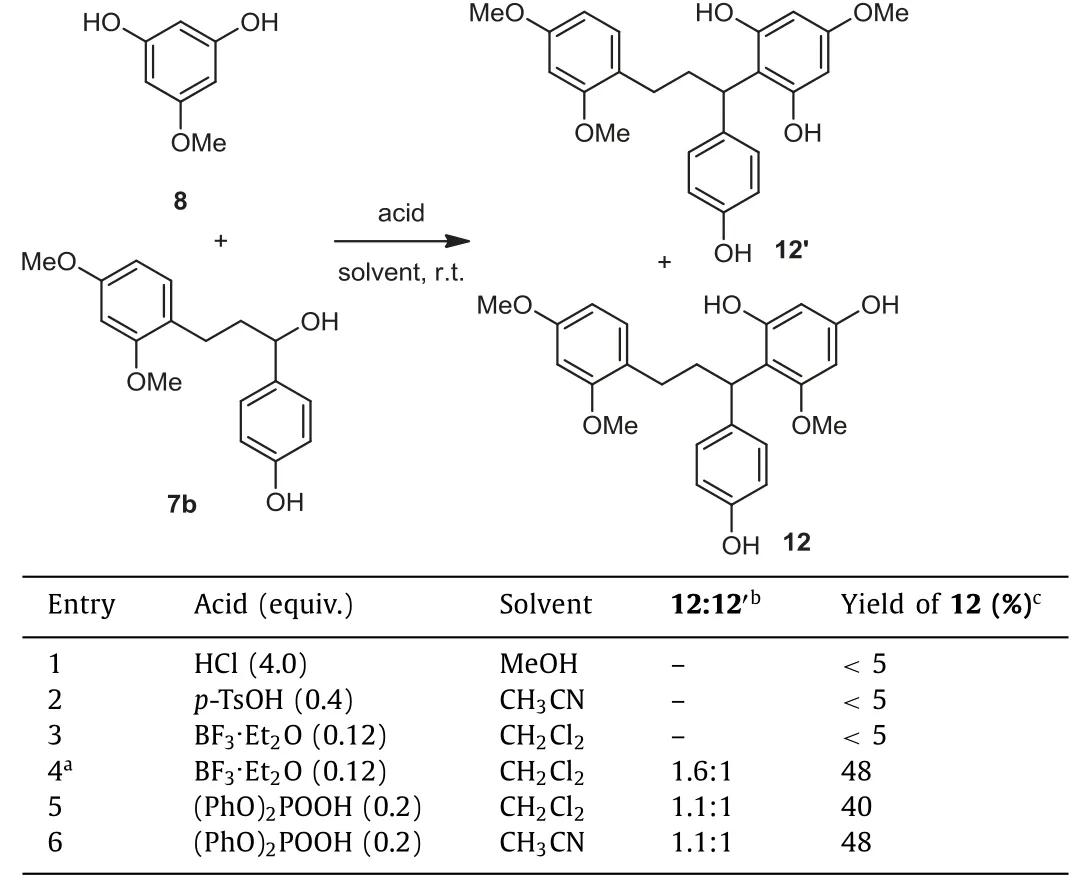
Table 1 Optimized conditions for the Friedel-Crafts coupling.
Finally, we turned to the direct annulation of 12 and 9 to afford the anthocyanidin moiety.The literature conditions using stoichiometric amount of HPF6gave less than 5% formation of the PF6-salt of dracaenin B [12,13].After further examination of the reaction mixture, the formation of 13 was observed as the major by product, indicating that theretro-Friedel-Crafts type cleavage occurred under the strong acidic conditions.While this side reaction pathway could be potentially inhibited by the appendant of suitable protecting groups, we did not prefer such a strategy as it would hurt the step economy of the synthesis [16].After systematic investigation of the amount of HPF6and the reaction concentration,we found dracaenin B could be formed in 40% yield in the presence of 12 mol% HPF6and at diluted concentration.Analogously,dracaenin A was produced from 14 in four steps in 13.6% overall yield.While the reaction yields of the coupling steps were not optimal at this moment, the rapid assembly of dracaenins A and B in only four steps, in a highly convergent manner and in good overall yields is noteworthy.
Dragon’s blood has been highlighted to have the bidirectional regulatory effect of stimulating both blood circulation and coagulation.The active components that could stimulate blood coagulation (hemostatic effect) remain to be discovered.With enough materials synthesized, the effects of dracaenins A and B on adenosine diphosphate (ADP), arachidonic acid (AA) or collagen induced platelet aggregation were evaluated by the light transmission method (Fig.2).As shown in 1b, ADP (2.5 μmol/L) mediated platelet aggregation was obviously stimulated by dracaenin B in a concentration-dependent manner, with average increasing rates of 8.02%, 9.15%, 13.87%, 19.76% and 114.18% at concentrations of 0.98, 2.9, 8.8, 26.3 and 79 μmol/L, respectively.Dracaenin B also showed marked stimulating effects in a concentration dependent manner with the increasing rates of 7.17%, 12.71%, 28.52%, 35.94%and 51.29% on collagen induced platelet aggregation, and 0.24%,1.10%, 2.22%, 43.95% and 71.04% increasing rates on AA induced platelet aggregation at concentration range from 0.98 μmol/L to 79 μmol/L (2b and 3b).The stimulating effect on platelet aggregation was also observed using dracaenin A (1a-3a), albeit less potent than that of dracaenin B.These results revealed the effects of dracaenins A and B in stimulating platelet aggregation, and shed light on the possible origin of the hemostatic effect of dragon’s blood.
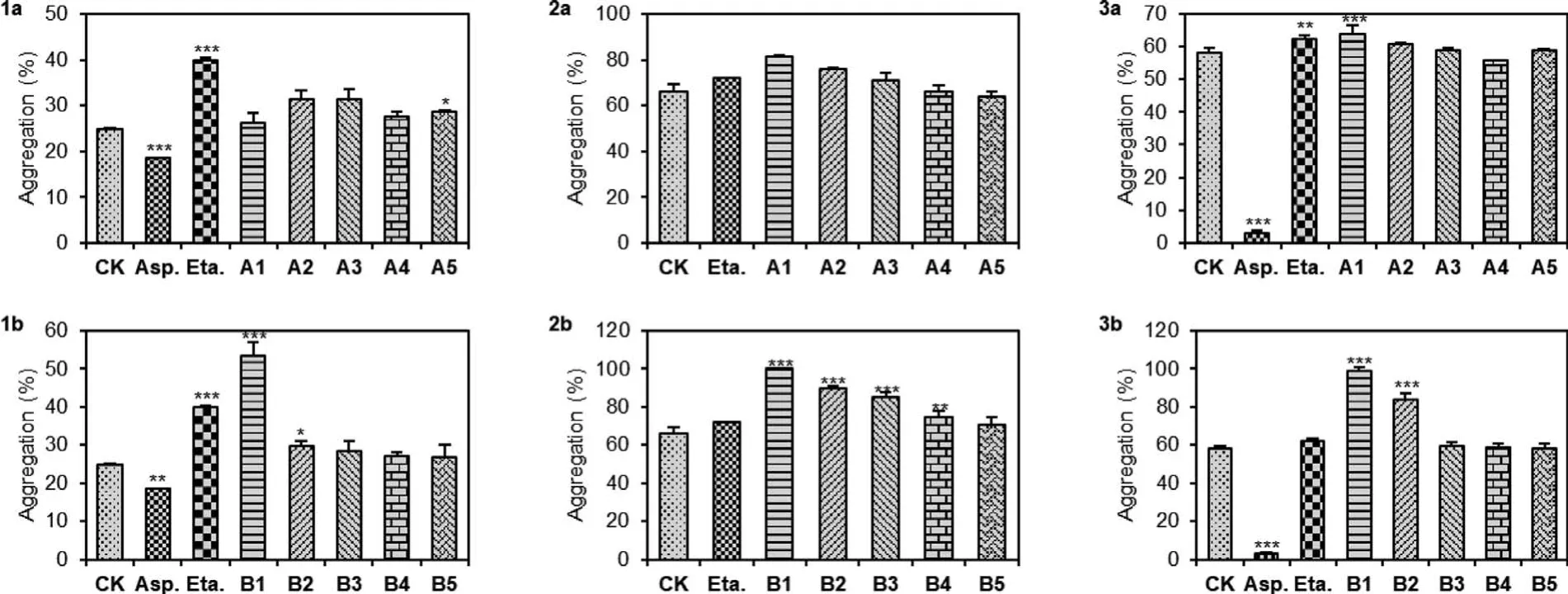
Fig.2.The effect of dracaenins A and B on ADP, collagen and AA-induced platelet aggregation; control group (CK) was treated with 0.1% DMSO; aspirin (Asp.17.90 μmol/L)and estamsylate (Eta.494.30 μmol/L) were used as negative and positive controls.The concentration of dracaenin A for group A1 to A5 is 55, 18.3, 6.1, 2 and 0.68 μmol/L respectively; the concentration of dracaenin B for group B1 to B5 is 79, 26.3, 8.8, 2.9 and 0.98 μmol/L respectively.The platelet aggregation was induced by 2.5 μmol/L ADP(1a & 1b), 3.0 μg/mL collagen (2a & 2b) and 150 μg/mL AA (3a & 3b), respectively.The bar graphs are a summary of each independent experiments; each column shows the mean ± standard deviation of at least three independent experiments.*P <0.05, **P <0.01, ***P <0.001 versus the control group.
In conclusion, dracaenins A and B represent the unusual chalcane-anthocyanidin dimers isolated from dragon’s blood.We have achieved the first total synthesis of these natural products in only 4 steps.Our synthesis features the convergent coupling of three readily available fragments with minimized reliance on protecting groups.The chemical synthesis enables the discovery of their activity in stimulating platelet aggregation, and thus, sheds light on the possible origin of the hemostatic effect of dragon’s blood.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.21871161), Key Project at Central Government Level for the Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (No.2060302) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (No.KYCX19_1256).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.067.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
