A prototype of benzobis(imidazolium)-embedded conjugated polyelectrolyte:Synthesis by direct C–H arylation and fluorescent responses to anions
Chuangui Yu, Qinze Zheng, Linhua Wang, Tianbao Wang, Xuesong Zheng, Ge Gao
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China
Keywords:Conjugated polyelectrolyte (CPE)Benzobis(imidazolium)C–H arylation Fluorescent anion sensing Bisulfite
ABSTRACT We report the convenient synthesis of a benzobis(imidazolium)-embedded conjugated polyelectrolyte pBBI by a Cu-catalyzed direct C–H arylation of a cationic benzobis(imidazolium) monomer with a diiodide comonomer.pBBI shows weak fluorescence in solution due to rotation of the repeat units in the conjugated backbone, and enhanced fluorescence when electrostatically interacting with a variety of anions to form aggregates.Specially, pBBI responds to the bisulfite anion with intensified unique deep-blue fluorescence easily discriminated by naked eye.
Conjugated polyelectrolytes (CPEs) have received extensive attention due to their unique optical and electronic properties, which lead to various applications in optoelectronic devices, sensing and bioimaging [1–6].To date, most CPEs belong to the side-chain type,composed of a neutralπ-conjugated backbone, wherein excitons can move quickly along, and several ionic side chains to endow with solubility, processability as well as other functionalities.Their backbones are usually poly-para-phenylene [7–9], polystyrene [10–12], polyphenylene acetylene [13–16], polyfluorene [17–19], and polythiophene [20–25],etc., obtained by polymerization utilizing classical Suzuki, Heck, Sonogashira and Stille coupling reactions.
The other is namely the main-chain type, in which the ionic(usually cationic) segments are embedded in the conjugated backbones.Only limited main-chain CPEs have been reported due to synthetic challenges, although they possess different but appealing properties from the side-chain ones.The general strategy to construct main-chain CPEs is ionization after polymerization (Scheme 1a), but incomplete and irregular ionization are usually inevitable [26,27].Swageret al.subtly incorporated two pyridine rings and two quaternizing moieties into one monomer,and synthesized poly(pyridiniumphenylene) [28,29].Unlike sidechain CPEs, which generally respond to analytesviaa molecular wire effect, resulting in amplification of fluorescence quenching[30–32], Swager’s main-chain CPE exhibited fluorescence enhancement toward a range of electron-donating aromatics.It was assumedly caused by the analyte-induced change of the polymer structureviahydrophobic interactions [29].
A rare case reported by Tang and coworkers was the synthesis of a series of benzoquinolizinium-containing polyelectrolytes by a one-pot polymerizationvia in situgeneration of the benzoquinolizinium rings (Scheme 1b) [33].These main-chain CPEs possess color-tunable luminescence and aggregation-induced emission (AIE) properties.Direct polymerization of cationic monomers(Scheme 1c) is straightforward, but suitable cationic monomers and synthetic methodology are scanty.In 2019, Liu’s group utilized an ionic azaquinodimethane (iAQM) monomer to yield the CPEs with phosphonium groups directly bound to the main chains by the Stille polymerization [34].These CPEs showed broad NIR absorbance and capability as active agents in photothermal therapy.The fascinating properties found in the limited examples of mainchain CPEs indicate that there is a huge space waiting to be explored.Novel synthetic strategies to fabricate new main-chain CPEs are therefore in high demand.
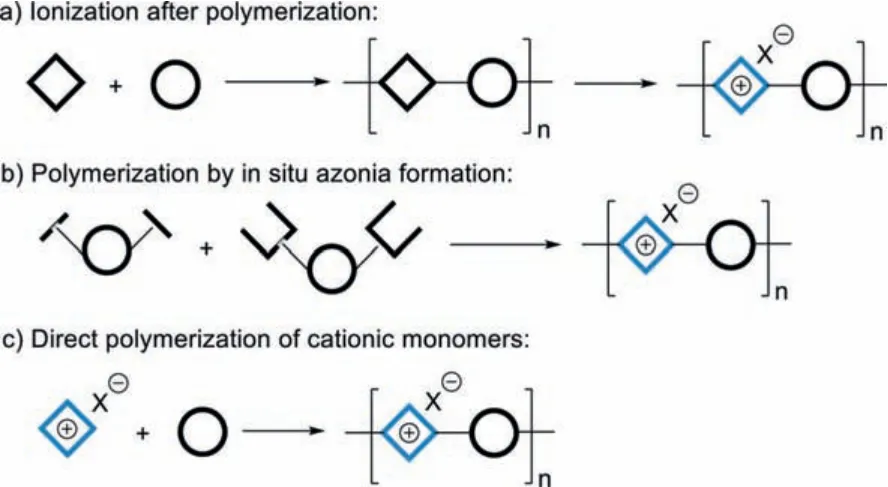
Scheme 1.Strategies to construct cationic main-chain CPEs.
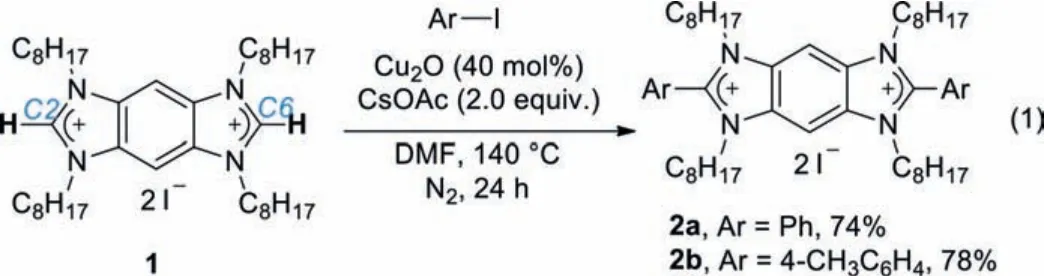
In 2008, Bielawskiet al.introduced benzobis(imidazolium) (BBI)salts as a new class of modular fluorescent organic salts [35,36],which feature an opposite bisimidazolium core with positive charges spreading within the rigid, planar andπ-conjugated skeleton.The fourN-substituents are tunable for solubility and functionality, and the two terminal C2 and C6 positions can be modified when suitable methodology is available.Therefore, BBI salts could be ideal ionic monomers to create new main-chain CPEs.Nevertheless, the known Pd-catalyzed C–H arylation of azoles[37,38] is generally not applicable because BBI salts are precursors of bis(N-heterocyclic carbene)s.In 2017, we developed a Cucatalyzed C–H arylation of imidazolium salts with aryl iodides [39],which provides a possibility to synthesize BBI-embedded CPEs by direct C–H polymerization of BBI salts with aryl diiodides.As part of our enduring efforts to develop novel ionic functional materials [40–45], we herein reported the proof-of-concept synthesis of this new type of cationic BBI-embedded CPE named pBBI.pBBI itself is weakly luminescent in solution, but responds to a variety of anions with enhanced fluorescence.Specially, intensified unique deep-blue fluorescence can be seen by naked-eye in the presence of the bisulfite anion.
We commenced our study with a model C–H diarylation reaction of a BBI salt 1 with iodobenzene.The diarylated product 2a was obtained in a low yield under our previous optimal conditions for the C–H arylation of imidazolium salts [39].The yield of 2a was finally optimized to 74% (2b was obtained in 78% yield when 4-iodotoluene was used) by using 40 mol% of Cu2O as the catalyst and 2.0 equiv.of CsOAc as the base to react at 140 °C in a nitrogen atmosphere for 24 h (Eq.1).
Under these conditions, however, C–H polymerization of 1 with diiodide compound 3 gave pBBI in 26% yield (Table 1, entry 1).Extension the reaction time could only increase the yield to 33%(Table 1, entry 2).The polymerization hardly occurred under the catalysis of other Cu(I) salts (Table 1, entries 3–5).Using KOAc as the base slightly lowered the yield, while using K2CO3retarded the polymerization (Table 1, entries 6 and 7).Varying other polar solvents such as DMAc and DMSO largely decreased the yield(Table 1, entries 8 and 9).In consideration of the rigid backbone and limited solubility of pBBI, 4 mL DMF was employed.To our delight, pBBI was obtained in 60% yield.Further increasing the volume to 6 mL diminished the yield (Table 1, entries 10 and 11),suggesting a crucial concentration effect.Moreover, elevating the reaction temperature to 150 °C had a deleterious effect on polymerization (Table 1, entry 12).Finally, the optimal conditions for the synthesis of pBBI were set to be 40 mol% of Cu2O and 2.0 equiv.of CsOAc in 4 mL DMF at 140 °C in a nitrogen atmosphere for 48 h (Table 1, entry 10).Excess 4-iodotoluene was employed to block the terminal carbene site on pBBI after polymerization (see Supporting information for details).
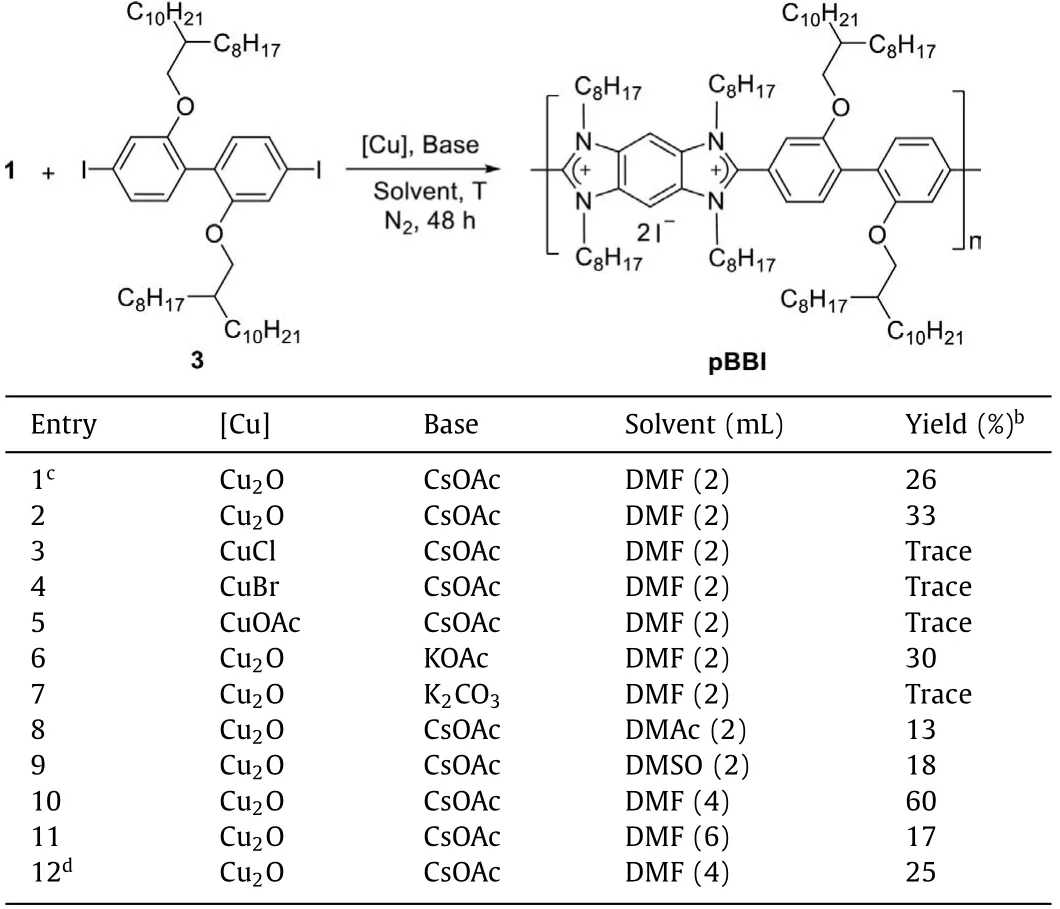
Table 1 Optimization for polymerization.a
The as-prepared pBBI is a mustard-colored solid with no luminescence, soluble in chloroalkanes, THF and DMF, but insoluble in water due to its rigid backbone with peripheral long aliphatic chains.The1H NMR spectrum showed disappearance of the C2 and C6 protons at 10.3 ppm and broadened peaks compared with the monomers (Fig.S1 in Supporting information), suggesting the success of polymerization by C–H arylation of the BBI monomer.Due to its polycationic nature and large polarity, the molecular weight of pBBI is not obtained by common GPC test.We therefore used1H NMR spectra in deuterated chloroform to conduct an end group analysis to approximate the number average chain lengths [7,14,46].In comparison with the chemical shift of the methyl group in 2b, the tiny peak at 2.55 ppm in the1H NMR spectrum of pBBI was ascribed to the methyl end group, which was integrated as 3.The methylene protons shown in the area of 3.5–5.0 ppm were integrated to be 222.According to the molecular structure, there are 12 methylene protons in each repeat unit.The ratio of the methylene protons in all and in each repeat unit was then calculated to be 18, suggesting that pBBI has 18 repeat units.Therefore, the molecular weight was estimated to be 28,944 (Figs.S1 and S2 in Supporting information).
The spectroscopic properties of pBBI were then studied in DMF(Fig.1).The DMF solution of pBBI (10 μmol/L) emitted weak blue-colored fluorescence.The spectra showed maximum absorption and emission at 325 and 460 nm, respectively, which shifted bathochromically relative to the model compound 2a, demonstrating extendedπ-conjugation along the main chain.The emission band was largely broadened and covered a long range of 250 nm from 370 nm to 620 nm.The Stokes shift was as large as 9030 cm-1, which was about double of that of 2a (5647 cm-1).These properties indicated multiple sub-energy levels in the excited state of pBBI, which was due to various twisted conformations of the backbone.The quantum yield of pBBI was measured to be 0.0078, significantly lower than that of 2a (Фf= 0.40).This could be attributed to nonradiative decays caused by rotations of the single bond-connected (hetero)aryl units.The weak fluorescence is believed to be a single chain behavior as the absorption and emission spectra showed gradual enhancements of the intensities with no change of the wavelength and shape in a concentration range of 1-30 μmol/L (Figs.S4 and S5 in Supporting information).Moreover, no particle formation was observed in the dynamic light scattering (DLS) measurement.However, by addition of a small amount of water (fw= 3 vol%) into the DMF solution, the emission band at 460 nm was largely suppressed and a new band at 520 nm emerged (Fig.S6 in Supporting information),corresponding to the change of fluorescence from blue to yellow color.Although the solution was still clear and transparent, the DLS measurement revealed the formation of aggregates in an average size of 18 nm (Fig.S8a in Supporting information).With increasing water fraction, the band at 520 nm first intensified and then attenuated.This indicated a gradual process from the formation of orderedπ-stacked aggregates enhancing the fluorescence to large compact aggregates quenching the fluorescence eventually.
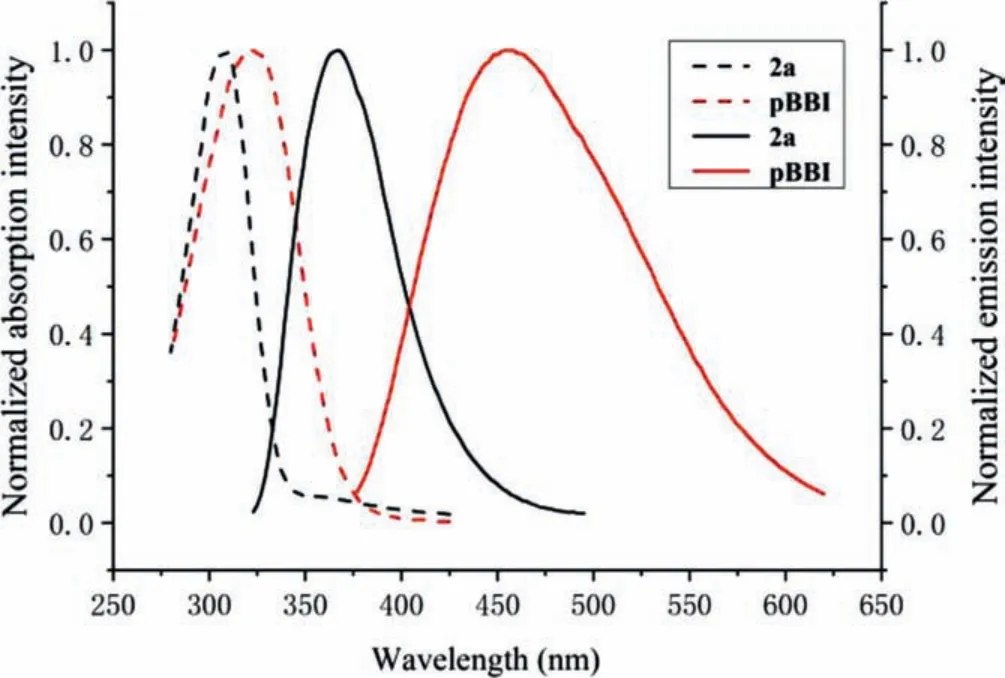
Fig.1.Normalized absorption (dashed line) and emission (solid line) of the model compound 2a and pBBI in DMF.Solution concentration:10 μmol/L.
Due to very limited examples, the sensing behaviors of mainchain CPEs are far less explored and understood.We then studied the fluorescence responses of pBBI toward various anions in DMF.A minimum amount of water (fw= 3 vol%) was used to ensure the solubilities of anion salts.The experiment was conducted by addition of 20 equiv.of different anion salts into pBBI solutions with a concentration of 10 μmol/L.It is clear to see in Fig.2a that the HSO3-anion led to a unique deep blue-colored fluorescence easily discriminated from the others by naked eye,indicating that pBBI can serve as a selective HSO3-anion sensor[47].It is worth noting that most of the tested anions led to enhancement of the fluorescence, except that the F-and HS-anions quenched the fluorescence completely and the PO43-and CO32-anions caused an ignorable variation (Fig.2b).This is distinctive from analyte-induced fluorescence quenching behaviors commonly observed for side-chain CPEs, suggesting a different sensing mechanism.Interestingly, a range of anions including SO42-, SO32-,HPO42-and HCO3-mainly caused fluorescence enhancement at 520 nm, among which HCO3-affected the most to induce a bright yellow-colored fluorescence.However, addition of the ClO4-, NO2-,NO3-, Cl-and Br-anions led to a slight increase of the band at 460 nm, resulting in a light blue-colored fluorescence.The spectrum in the presence of the H2PO4-anion showed an enhanced band centered at 430 nm with a shoulder at 520 nm.The broad emission still exhibited a light blue-colored fluorescence.In the presence of the HSO3-anion, the most intensified band centered at 430 nm was observed, displaying the unique deep blue-colored fluorescence.
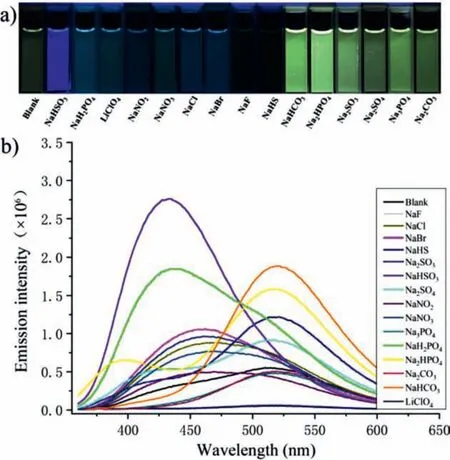
Fig.2.(a) Photograph of pBBI solutions (DMF with 3 vol% water) under irradiation of a UV lamp and (b) the corresponding fluorescence spectra in the absence and presence of 20 equiv.of different anions (excited at 340 nm).
The fluorescence titration of pBBI with the HSO3-anion was then performed (Fig.3).When 1 equiv.of the HSO3-anion was added, the emission around 430 nm arose immediately, suggesting the response toward the HSO3-anion is quick and sensitive.With a gradual increase of the HSO3-anion, this emission kept growing and the band at 520 nm related to the water-caused aggregates vanished.When 24 equiv.of the HSO3-anion was added, the fluorescence intensity reached the highest, corresponding to a 15-fold enhancement.The fluorescence response exhibited a rough linear relationship with the HSO3-anion concentration (Fig.3, inset), indicating that there is no amplification effect along the cationic conjugated backbone.
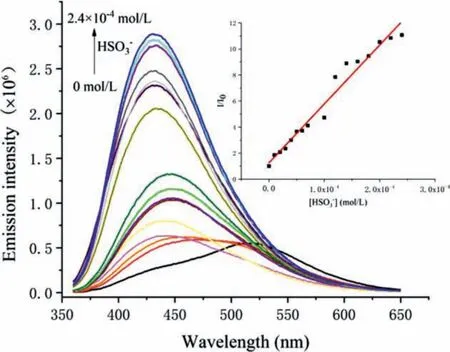
Fig.3.The fluorescence spectra of a solution of pBBI (10 μmol/L) in DMF with 3 vol% water titrated with NaHSO3 at room temperature.Inset:the relative fluorescence intensity (I/I0) of pBBI at 430 nm in the presence of NaHSO3 at different concentrations.
The dynamic light scattering (DLS) measurements were conducted for a few selected representative anion sensing experiments.It revealed the formation of various aggregates in sizes of 32, 24, 800, 13, 20, 110 and 18 nm in the presence of the HSO3-, H2PO4-, F-, Cl-, HCO3-, SO32-and CO32-anions, respectively (Fig.S8 in Supporting information).The fluorescence was obviously quenched when huge aggregates were formed with the F-anion.Different sizes of nanoaggregates were formed with the other anions, but the fluorescence responses were not sizedependent.In consideration of the complicacy of anion binding in water-containing environment [48,49], it is therefore reasonable to hypothesize that the electrostatic interaction between the cationic main chain of pBBI and anions with different ionic strengths,shapes and sizes induces ordered aggregates in various fixed conformations, resulting in different fluorescence responses.
In summary, we have developed a Cu-catalyzed C–H polymerization to synthesize the first prototype of BBI-embedded CPE named pBBI.The protocol would allow direct utilization of BBI salts to fabricate various main-chain CPEs by modification of diiodide comonomers.pBBI is weakly luminescent in solution, but responds to a range of anions with enhanced fluorescence, in contrast to the well-studied amplified fluorescence quenching behaviors of side-chain CPEs.Specially, the bisulfite anion causes a unique deep blue-colored fluorescence with a maximum 15-fold enhancement, which can be easily discriminated from the other anions.The fluorescence enhancement is believed to originate from ordered aggregates formed by electrostatic interactions between the cationic backbone and the anions.Future work should be directed to the precise design and synthesis of main-chain CPEs to control the ordered structures for specific functions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are thankful for the financial support from the National Natural Science Foundation of China (No.21772134) and the Fundamental Research Funds for Central Universities (No.20826041D4117).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.092.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
