Highly enantioselective construction of CF3-bearing all-carbon quaternary stereocenters:Chiral spiro-fused bisoxazoline ligands with 1,1′-binaphthyl sidearm for asymmetric Michael-type Friedel-Crafts reaction
Robert Li-Yun Bo, Lei Shi,b,c,d,*, Kng Fu
a School of Science, Harbin Institute of Technology, Shenzhen 518055, China
b State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China
c Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen 518055, China
d School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, China
Keywords:Chiral bisoxazoline ligand Chiral pocket Chiral spiro ligand Friedel-Crafts reaction Trifluoromethylated all-carbon quaternary stereocenter
ABSTRACT A novel class of chiral spiro-fused bisoxazoline ligands possessing a deep chiral pocket was prepared.The developed ligands have been employed in the nickel-catalyzed highly enantioselective Michael-type Friedel-Crafts reaction, affording the products bearing a trifluoromethylated all-carbon quaternary stereocenter with moderate to excellent yields (up to 99%) and good to excellent enantioselectivies (up to>99.9% ee).Moreover, a proposed model of chiral pocket revealed that the attack of indole from the Re-face of β-CF3-β-disubstituted nitroalkene was favorable.
Bisoxazoline ligands (box ligands), as one class of the privileged ligands [1,2], have made a great contribution to the progress of asymmetric catalysis over the past three decades [3–6].In the course of developing chiral box ligands, modifying the parent bisoxazoline ligands [7–9] has emerged as an efficient approach to seek novel chiral ligands.By using this strategy, a series of representative chiral bisoxazoline ligands were successfully designed and applied in the asymmetric radical addition reactions [10],Friedel-Crafts reactions [11], cyclopropanation reactions [12], aerobic oxidation reactions [13], and radical coupling reactions (Fig.1a)[14].The success of these representative chiral bisoxazoline ligands benefited from the C2symmetry of the ligands [15] and the good coordination ability of the bisoxazolines, providing valuable experience for the development of novel ligands.
Although plenty of representative chiral bisoxazoline ligands have been prepared to date, the development of novel chiral bisoxazoline ligands is still of central importance and highly desirable in the context of expanding the types of chiral ligands and solving the new synthetic challenges.Inspired by the structural features of the chiral spiro monooxazoline in the Spymox ligand (Fig.1b, left)[16–18] and the deep chiral pocket in the WingPhos ligand (Fig.1b,middle), [19] we designed a class of chiral spiro-fused bisoxazoline ligands based on the modification of the parent bisoxazoline ligands (Fig.1b, right).In our proposal, the chiral spiro-fused bisoxazoline ligands can be constructed by connecting the chiral binaphthyl moieties to the oxazoline rings.The developed chiral spirofused bisoxazoline ligands possess three characteristics:(1) The rigidity of the spirocyclic units can decrease the flexibility of these bisoxazoline ligands and their related complexes, which facilitates the control of enantioselectivity in asymmetric catalysis.(2) The C2symmetry of the chiral spiro-fused bisoxazoline ligands can reduce the number of possible transition states in asymmetric reactions[14].(3) Two chiral binaphthyl sidearms can protrude directly forward to the coordination site of substrate, generating a deep chiral pocket.In this communication, we describe the preparation and application of the chiral spiro-fused bisoxazoline ligands.
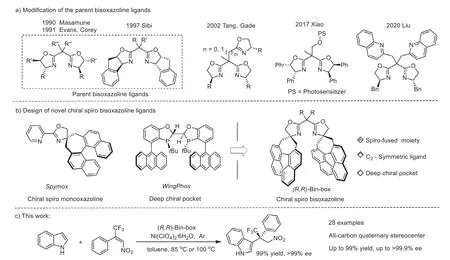
Fig.1.Design and application of the chiral spiro-fused bisoxazoline ligands.
All-carbon quaternary stereocenters exist in numerous pharmaceuticals and bioactive natural products [20–22].Although the chiral all-carbon quaternary stereocenters could be constructed effectively, the catalytically asymmetric synthesis of the trifluoromethylated all-carbon quaternary centers remains a synthetic challenge [23–27].The asymmetric Michael-type Friedel-Crafts reaction of indole with theβ-CF3-β-disubstituted nitroalkenes was a direct and efficient approach to prepare the active indole derivatives [28,29] bearing a trifluoromethylated all-carbon quaternary stereocenter.As a pioneering work, Gaoet al.utilized a Ni(ClO4)2-bisoxazoline complex as catalyst to prepare the active indole derivatives (up to 97%ee) [30,31].Similarly, Zhuet al.exploited a Ni(II)/(imidazoline-oxazoline) complex as catalyst to produce the trifluoromethylated bis(3-indolyl)methanes (up to 94%ee) [32].These two cases implied that the chiral catalysts based on the oxazoline ligands might have an advantage in this asymmetric Michael-type Friedel-Crafts reaction.Additionally, the studies from Akiyama group and Meggers group demonstrated that the chiral calcium BINOL bis(phosphate) complex [33] and the biscyclometalated iridium(III) complex [34] could be employed as catalysts to construct the trifluoromethylated all-carbon quaternary stereocenters as well (up to 98%ee).In order to assess the catalytic performances of the chiral spiro-fused bisoxazoline ligands and improve the enantioselectivity in the construction of the trifluoromethylated all-carbon quaternary stereocenters, the chiral spiro-fused bisoxazoline ligands were employed in the Michael-type Friedel-Crafts reaction of indole withβ-CF3-β-disubstituted nitroalkenes.
The chiral spiro-fused bisoxazoline ligands were synthesized according to the procedures modified from the route to prepare the Spymox ligands in Shibatomi’s reports.[16–18].The synthesis started from an amidation of the unnatural chiral 1, 2-aminol[16,35] with the disubstituted malonyl dichloride, which generated the desired bis-hydroxyamide compounds [9].Subsequently,the hydroxy groups of the bis-hydroxyamide compounds were converted to good leaving groupsviamesylation.Finally, the chiral spiro-fused bisoxazoline ligands (Fig.2, L1–L3), which were named as (R,R)-Bin-box ligands, were obtained under basic conditions (See the Section 2.1 in Supporting information for the synthetic details).
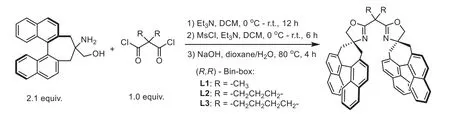
Fig.2.Preparation of the chiral spiro-fused bisoxazoline ligands.
With the chiral spiro-fused bisoxazoline ligands in hand,we studied their catalytic performances in the Michael-type Friedel-Crafts reaction using indole 1a and the (E)-1-phenyl-1-trifluoromethyl-2-nitroethene 2a as model substrates.Investigation of a series of parameters revealed that the Ni(ClO4)2.6H2O complex of the ligand L1 was the optimal catalyst.In presence of this catalyst, the reaction proceeded smoothly in toluene at 85 °C, delivering the product 3a with excellent yield (99%) and excellent enantioselectivity (>99%ee).The enantioselectivity was higher than that induced by the optimized ligand in Jia’s report [30], due to the unique effects of ligand structure and the noncovalent interactions in the ligand L1 (Table 1, entry 1).Although the ligands L2 and L3 were similar to the ligand L1, they were not suitable for this asymmetric reaction because the unique chelate bite angles and the sizes of the bridging substituents of L2 and L3 might decrease the reactivities and enantioselectivities (Table 1, entries 2 and 3)[36,37].Notably, only poor yield and poor enantioselectivity were achieved in the presence of L4, which implied that C2symmetric ligand L1 possessed great advantages over the chiral spiro monooxazoline ligand L4 in this asymmetric transformation (Table 1, entry 4).In addition, when Ni(OTf)2was utilized in this reaction,the yield would decrease obviously (Table 1, entry 5).Moreover,the enantioselectivity would decline slightly when the amount ofthe 1a was increased to 2 equiv.or the loading of ligand L1 was raised to 12 mol% (Table 1, entries 6 and 7).At last, if the reaction temperature was raised to 100 °C, the reaction time could be reduced to 12 h and the enantioselectivity only decreased slightly(98.6%ee), which indicated that higher temperature might be used as an alternative option in this asymmetric transformation.
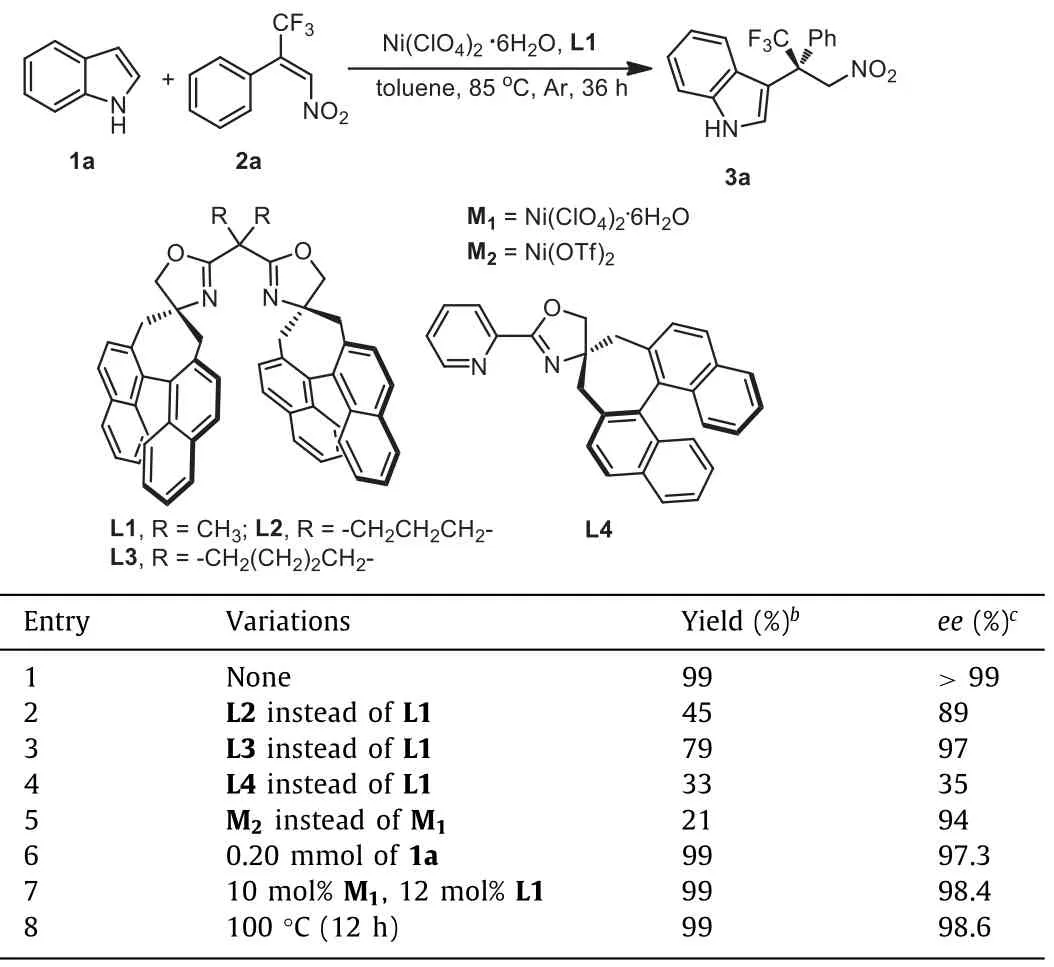
Table 1 Optimization of the reaction conditions.a
To evaluate the generality of this asymmetric Michael-type Friedel-Crafts reaction, we investigated the scope of the substituted indoles andβ-CF3-β-disubstituted nitroalkenes (Scheme 1).Initially, the indoles bearing a substituent at the C5 position were subjected to the reaction.The results demonstrated that indole with an electron-donating group (3b) possessed an advantage over that bearing an electron-withdrawing group (3c and 3d) both in the reactivity and enantioselectivity.Next, the effect of indoles bearing different functional groups at the C6 position was examined (3e–3k).To our delight, the catalytic system was highly compatible with diverse functional groups at the C6 position of indole ring and the corresponding products were obtained in good to excellent yields (87%-99%) with excellent enantioselectivities(>99%–>99.9%ee).In particular, the highly enantiopure products(3f and 3k) were observed (>99.9%ee) when 6-benzyloxyindole and methyl indole-6-carboxylate were subjected in this asymmetric reaction, respectively.Additionally, the 7-methoxy-1H-indole and 7-methylindole could deliver the products in excellent enantioselectivities as well, although the reactivities decreased because of the influence of steric hindrance (3i and 3m).
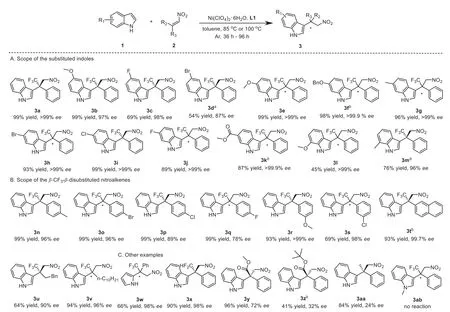
Scheme 1.Substrate scope of the asymmetric Michael-type Friedel-Crafts reaction.Standard reaction conditions:1a (0.15 mmol), 2a (0.1 mmol), Ni(ClO4)2.6H2O (11 mol%),L1 (10 mol%) in anhydrous toluene (1 mL) under Ar atmosphere at 85 °C for 36 h.The yields were the isolated yields.The ee values were determined by HPLC. a At 100 °C for 96 h. b At 100 °C for 36 h.
The scope ofβ-CF3-β-disubstituted nitroalkenes was also assessed under the optimal reaction conditions (3n–3v).When thepara-substituted (E)-1-phenyl-1-trifluoromethyl-2-nitroethenes were used in the reaction, different compatibilities were observed.The nitroalkene bearing an electron-donating group gave the product (3n) with excellent yield (99%) and excellent enantioselectivity (96%ee).On the other hand, the nitroalkenes bearing an electron-withdrawing group delivered the products (3o–3q) with good to excellent enantioselectivities (78%-96%ee), indicating that a stronger electron-withdrawing property would lead to a lower enantioselectivity.After that, the effect ofmeta-substituted(E)-1-phenyl-1-trifluoromethyl-2-nitroethenes were studied.The excellent enantioselectivities were observed whether the nitroalkene possessed an electron-donating or electron-withdrawing substituent (3r and 3s), while the reactivity would decrease obviously in the presence of the electron-withdrawing substituent(3s).Subsequently, it was found that theβ-CF3-β-naphthyl substituted nitroalkene could be converted to the desired product(3t) successfully at 100 °C in high yield (93%) with excellent enantioselectivity (99.7%ee).Furthermore, theβ-CF3-β-alkyldisubstituted nitroalkenes could also afford the corresponding products smoothly with moderate to excellent enantioselectivities(3u and 3v).
In order to expand the types of substrates, we also investigated the reactive properties of pyrrole,β-CF2H-β-disubstituted nitroalkene and alkyl substituted nitroalkenes, respectively.To our delight, pyrrole could deliver the product 3w in a moderate yield(66%) with an excellent enantioselectivity (98%ee), which suggested that the chiral Ni(II)-Bin-box complex has large potential in the enantioselective Friedel-Crafts alkylation of 4,7-dihydroindoles[38].Next, theβ-CF2H-substituted nitroalkene was investigated in the asymmetric reaction, affording the product 3x with 90% yield and 98%ee.The high yield and excellent enantioselectivity implied that the noncovalent interactions of the fluorinated groups were beneficial for the asymmetric induction [33].Additionally, other nitroacrylates and the methyl substituted nitroalkene could give the corresponding products (3y, 3z and 3aa) with obviously decreased enantioselectivities in the reaction, owing to the attenuation of noncovalent interactions between the substrates and chiral ligand.The outcomes of the 3y, 3z and 3aa suggested that the stereo control of the chiral spiro-fused bisoxazoline ligand was different with the chiral box ligands bearing two phenyl groups in the asymmetric alkylation ofα-substitutedβ-nitroacrylates [39] andβ-arylβ-alkyl nitroalkenes [40].In the end, 1-methylindole was subjected to the Michael-type Friedel-Crafts reaction, whereas no target product 3ab was obtained, indicating that N-H bond of indole was necessary in this nickel(II)-catalyzed asymmetric alkylation.
The absolute configurations of the known indole derivatives 3a–3d, 3i, 3m, 3n, 3p, 3r, 3t, 3u and 3x were assigned as (R) configuration and the known products 3y, 3z and 3aa exhibited (S) configuration, which were identified by comparing with the data of optical rotation from previous studies [30,33,34].
Apart from the nickel(II) catalyst based on the chiral ligand L1,four types of chiral catalysts have been utilized in the Enanti–Oselective synthesis of 3a [18–21].As shown in Fig.3, chiral catalyst Ni(II)/L1 had an advantage over other chiral catalysts in terms of the enantioselectivity (Fig.3, entry 8).By analyzing the results related to the chiral oxazoline ligands at 100 °C (Fig.3, entries 1–8), it was found that the ligand with a deeper chiral pocket could extremely enhance the confinement of substrate 2a, leading to a better enantioselectivity in this asymmetric reaction.
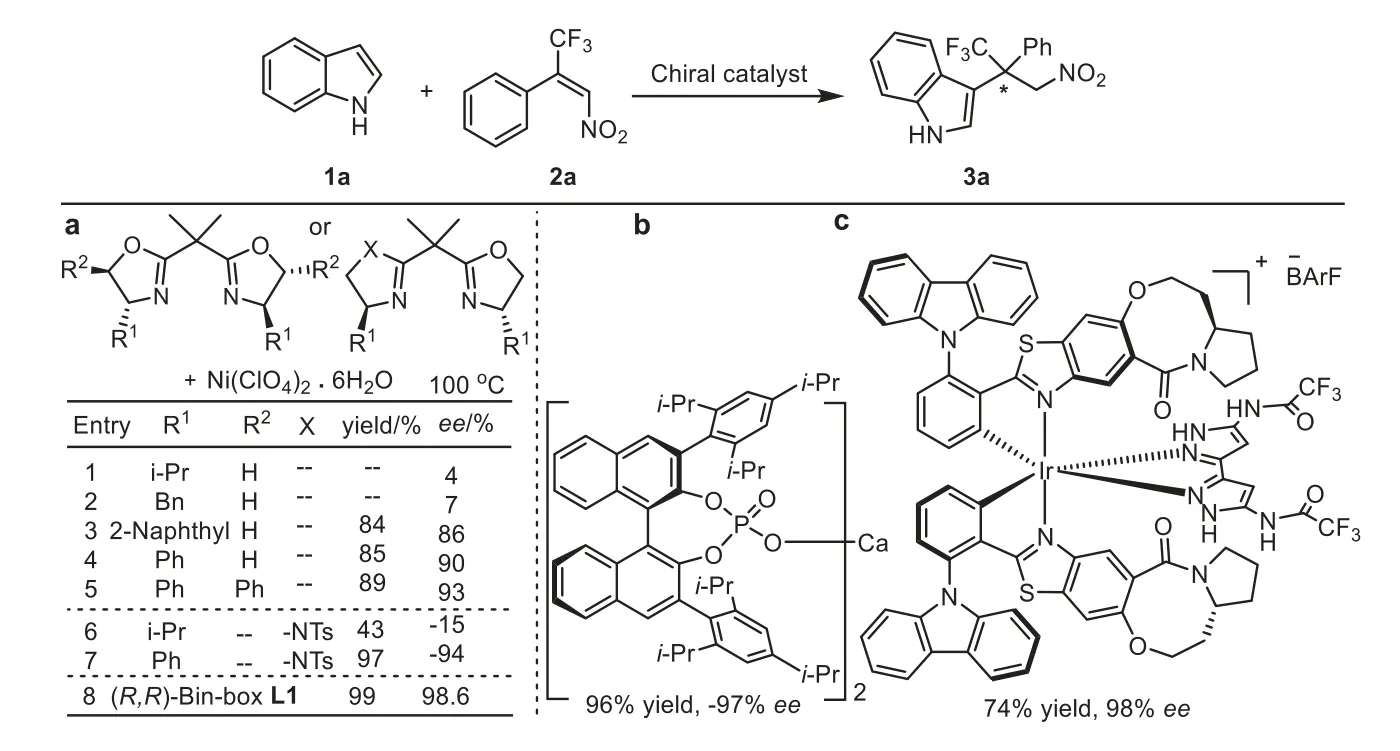
Fig.3.Chiral catalysts utilized in the enantioselective synthesis of 3a.
To understand the steric, electronic and stereochemical characteristics of the chiral spiro-fused bisoxazoline ligand L1, a model of chiral pocket was established according to the representation from Trost and Oslop [41–43], which might be effective in this asymmetric reaction.The naphthyl moieties with different stereochemical features played diverse roles, such as a flap or wall,forming a confined reaction space residing inside the deep chiral pocket (Figs.4a and b) [44].In the asymmetric addition of indole 1a withβ-CF3-substituted nitroalkene 2a, the conformation of the activated 2a was stabilized by the noncovalent interactions[45], which were possibly enhanced by the fluorine-πinteraction[46,47] between the naphthyl moiety D and substrate 2a (Fig.4c).Meanwhile, the deep chiral pocket reduced the number of possible trajectories of the incoming indole nucleophile, generating the desired product (R)-3a (Fig.4d).
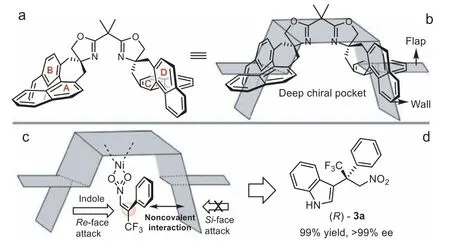
Fig.4.Proposed model of the deep chiral pocket for asymmetric induction.
In conclusion, we designed and synthesized the chiral spirofused bisoxazoline ligands, which have been successfully employed in the asymmetric Michael-type Friedel-Crafts reaction of substituted indole withβ-CF3-β-disubstituted nitroalkenes.A range of indole derivatives bearing a trifluoromethylated all-carbon quaternary stereocenter were constructed with moderate to excellent yields and good to excellent enantioselectivities.Moreover, a proposed model of stereoinduction suggested that the deep chiral pocket could shield the Si-face of theβ-CF3-β-disubstituted nitroalkenes effectively.The further applications of the chiral spirofused bisoxazoline ligands are ongoing in our lab.
Declaration of competing interest
The authors report no declarations of interest and the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (No.21871067), the Natural Science Foundation of Guangdong Province (Nos.2018A030313038 and 2021A1515010190), the Shenzhen Fundamental Research Projects (No.JCYJ20180306171838187), the Harbin Institute of Technology (Shenzhen) (Talent Development Starting Fund from Shenzhen Government), the Open Project Program of State Key Laboratory of Elemento-Organic Chemistry (No.202009)and the Guangdong Provincial Key Laboratory of Catalysis (No.2020B121201002).Robert Li-Yuan Bao also thanks Dr.Rong Zhao for the helpful discussion about the manuscript, and Prof.Min Wang (College of Materials, Chemistry and Chemical Engineering,Hangzhou Normal University) for the assistance on the measurement of the HRMS.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.062.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
