A copper-catalyzed three-component reaction of alkenes, cycloketone oximes and DABCO·(SO2)2:Direct C(sp2)-H cyanoalkylsulfonylation
Yting Liu, Luoyu Wng, Ling-Hui Zeng, Yun Zho, Tongho Zhu,*, Jie Wu,c,d,*
a School of Pharmaceutical and Chemical Engineering & Institute for Advanced Studies, Taizhou University, Taizhou 318000, China
b Department of Pharmacology, Zhejiang University City College, Hangzhou 310015, China
c State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
d School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
Keywords:Copper iodide Sulfur dioxide C-C bond cleavage C(sp2)-H Cyanoalkylsulfonylation Radical process
ABSTRACT A copper-catalyzed three-component reaction of alkenes, cycloketone oximes and DABCO·(SO2)2 is developed, which provides a convenient route for the synthesis of diverse (E)-cyanoalkylsulfonyl alkenes in moderate to good yields with excellent regio- and stereoselectivity.A broad substrate scope with excellent functional group tolerance is observed.A plausible radical pathway is proposed, which involves copper-catalyzed ring-opening C–C bond cleavage of O-acyl oxime and insertion of sulfur dioxide.During the reaction process, cyanoalkyl radical and cyanoalkylsulfonyl radical are the key intermediates.
Alkyl nitriles are particularly attractive and versatile building blocks in organic synthetic chemistry [1–4], due to the presence of useful cyano group, which can be readily transformed into other valuable functional groups.Additionally, aliphatic nitriles are important structural motifs of biologically active pharmaceuticals and natural products [5–10].Therefore, methods for the synthesis of alkyl nitriles are highly desired and remarkable progress has been made in recent years.Especially, radical-mediatedβ-C-C bond cleavage of cyclokteone oximes has been successfully applied to generate cyanoalkyl radicals, affording an attractive approach for the preparation of various aliphatic nitriles [11–30].
On the other hand, owing to the extensive application of sulfones in synthetic chemistry and medicinal chemistry [31–35], our group has continuously involved in the methods development for the synthesis of sulfonyl compoundsviathe fixation of sulfur dioxide by using DABCO·(SO2)2or potassium/sodium metabisulfite as the sulfur dioxide surrogates [36–60].Particularly, vinyl sulfones attracted our attention [61–69], since they were used as the neuroprotective agents for Parkinson’s disease therapy [70].In 2019, our group realized the cyanoalkylsulfonylationviathe cascade reaction ofβ-C-C bond cleavage of cyclokteone oximes with the insertion of sulfur dioxide, giving rise to a range of cyanocontaining sulfones [71].Prompted by this strategy and recent advance in the radical-based C–C bond cleavage of cycloketone oximes [72–82], we envisioned that vinyl sulfones could be generated through a direct C(sp2)-H cyanoalkylsulfonation of olefinsviaa radical-type insertion of sulfur dioxide.Herein, we report a copper-catalyzed three-component reaction of olefins, cycloketone oximes and DABCO·(SO2)2, providing a stereoselective pathway for the preparation of (E)-cyanoalkylsulfonated alkenes.
To verify the hypothesis above, initial studies were carried out for the direct C(sp2)-H cyanoalkylsulfonation of styrene 1a,cyclobutanoneO-benzoyl oxime 2a and DABCO·(SO2)2in 1,2-dichloroethane (DCE) at 80 °C.Fortunately, this reaction occurred in the presence of copper(I) iodide (10 mol%) as the catalyst and 1,10-phenanthroline (L1, 12 mol%) as the ligand, giving rise to the desired product 3aa in 63% yield (Table 1, entry 1).Further exploration showed that other copper catalysts were not as efficient as copper(I) iodide (Table 1, entry 2, for details see Supporting information).Subsequently, we shifted our focus to other ligands, and a range ofN,N-bidentate ligands were screened.Gratifyingly, the corresponding product 3aa was obtained in 72% yield by employing 4,7-dphenyl-1,10-phenanthroline (L2) as the ligand (Table 1,entry 3).However, the results were inferior when other ligands were used instead of L2 (Table 1, entry 4, for details see Supporting information).The solvent effect was then evaluated, and MeCN was demonstrated as the best choice (74% yield, Table 1, entries 5 and 6).Interestingly, comparable yields were afforded when theloading amount of copper(I) iodide and ligand L2 were reduced(Table 1, entries 7 and 8).The yield of product 3aa was decreased when the reaction temperature was reduced to 50 °C (Table 1, entry 9).A control experiment showed that no product 3aa was detected in the absence of copper catalyst and ligand (Table 1, entry 10).Another control experiment confirmed the necessity of ligand,giving rise to 3aa in 23% yield in the absence of ligand (Table 1,entry 11).
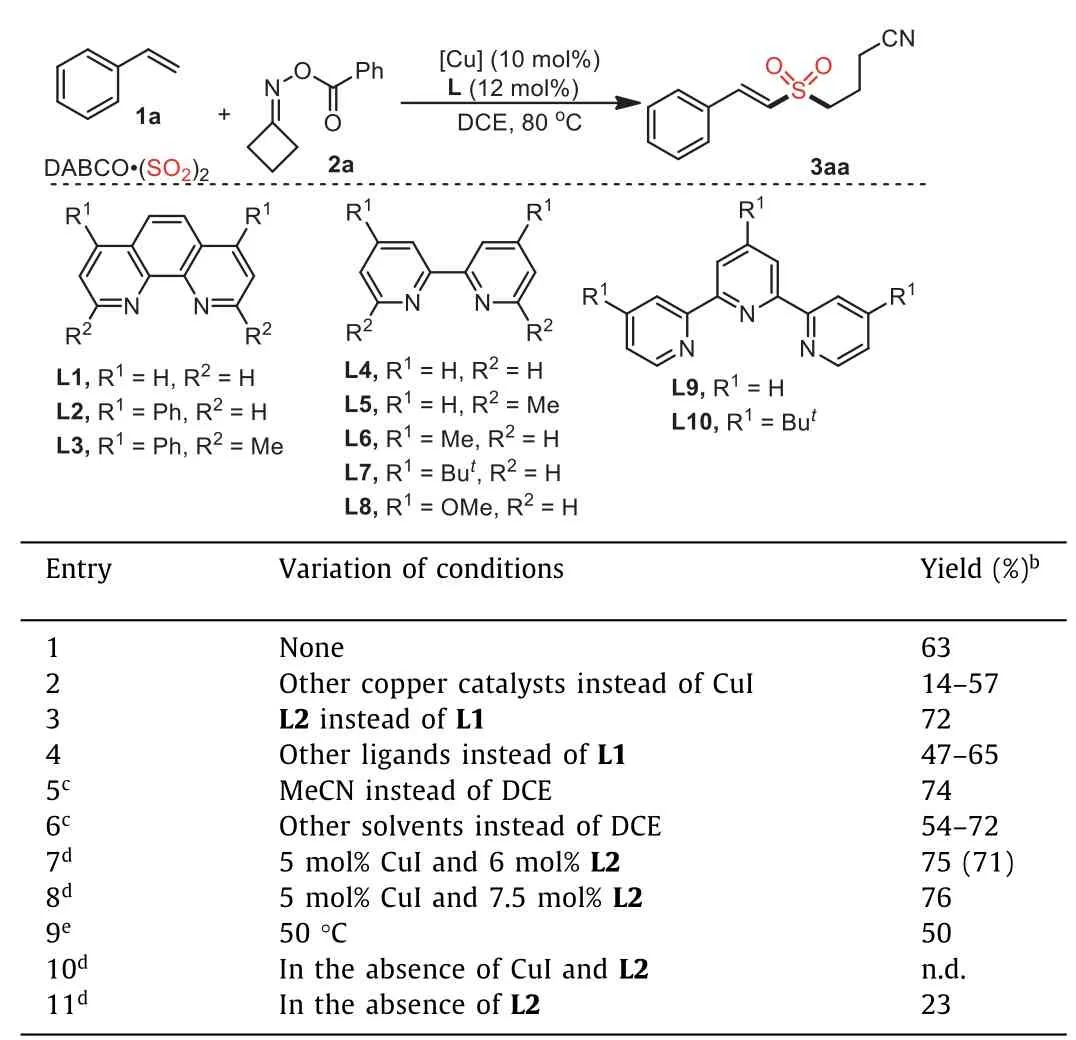
Table 1 Initial studies for the direct C(sp2)-H cyanoalkylsulfonation of styrene 1a, cyclobutanone O-benzoyl oxime 2a and DABCO·(SO2)2.a
Under the above optimized conditions, the substrate scope of alkenes was then explored.The results are summarized in Scheme 1.It was found thatpara-, meta-andortho-methyl phenylethylene derivatives could react with cyclobutanoneObenzoyl oxime 2a and DABCO·(SO2)2well, affording the corresponding products 3ba-3da in 60%-68% yields.Generally, alkenes bearing substituents of electron-donating (tBu, Ph, OMe, OAc,NMe2) and electron-withdrawing (CF3, COOMe, CHO, NO2, F, Cl,Br) groups on the aryl moieties were converted smoothly to the desired cyanoalkylsulfonated products 3fa-3ra in moderate to good yields.Notably, reaction of 1-methyl-2-vinylbenzene 1d or 1–bromo-2-vinylbenzene 1k could give rise to product 3da or 3ra in 68% and 45% yield, indicating the reaction was insensitive to the steric property of vinylbenzene.Additionally, reactions of alkenes incorporated withα- orβ-naphthyl group proceeded smoothly as well, leading to the desired products 3sa and 3ta in 66% and 60% yield.Alkenes bearing heterocyclic units were also compatible in this transformation, providing products 3ua,3va and 3wa in 70%, 35% and 36% yield, respectively.Furthermore, trisubstituted cyanoalkylsulfonated alkene 3xa could be afforded by using prop–1-en-2-ylbenzene 1x as the substrate.Unfortunately, alkyl substituted terminal olefins vinylcyclohexane 1y and 3,3-dimethylbut-1-ene 1z were not compatible for the standard conditions and the desired products were not detected.In addition,(Z)-1,2-di-substituted alkene 4a, (E)-1,2-di-substituted alkene 4b,trisubstituted alkene 4c and 1,3-dienes 4d were employed instead of styrenes, leading to the corresponding products in only trace yields.
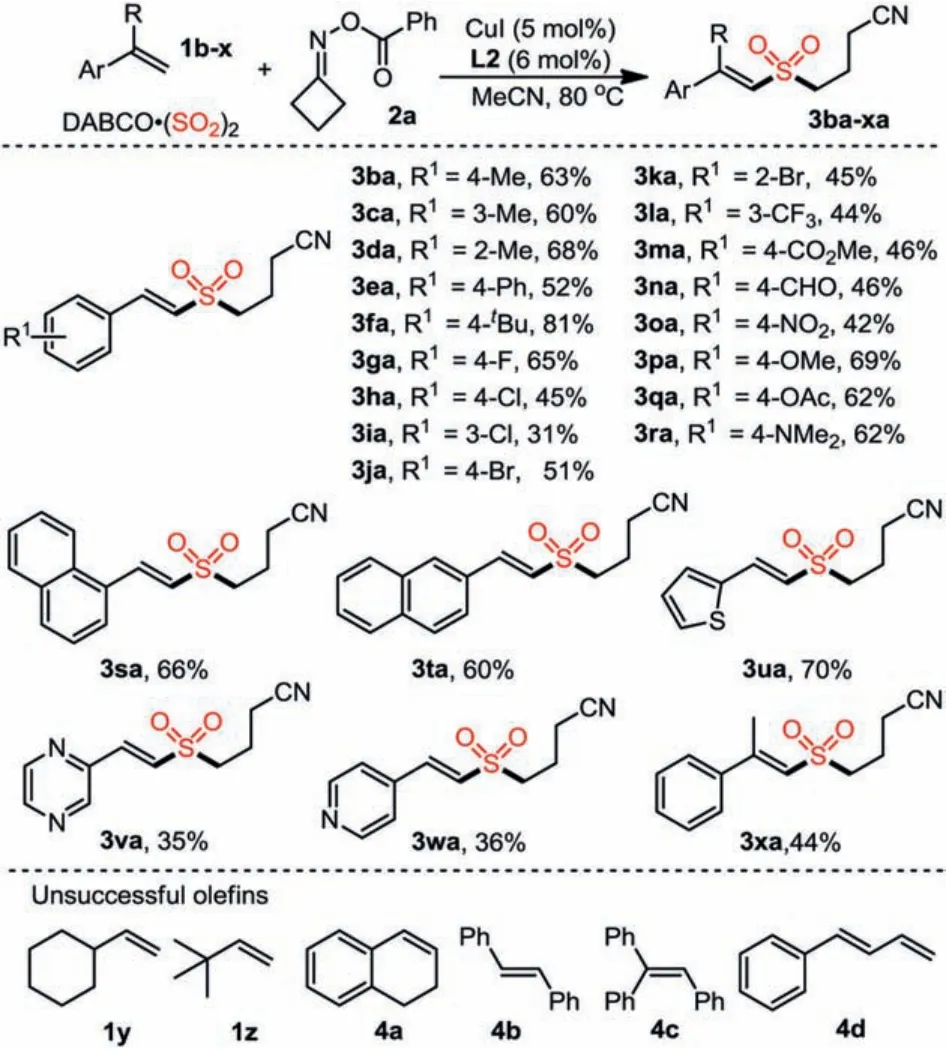
Scheme 1.Substrate scope of alkenes 1b-x.Isolated yield.
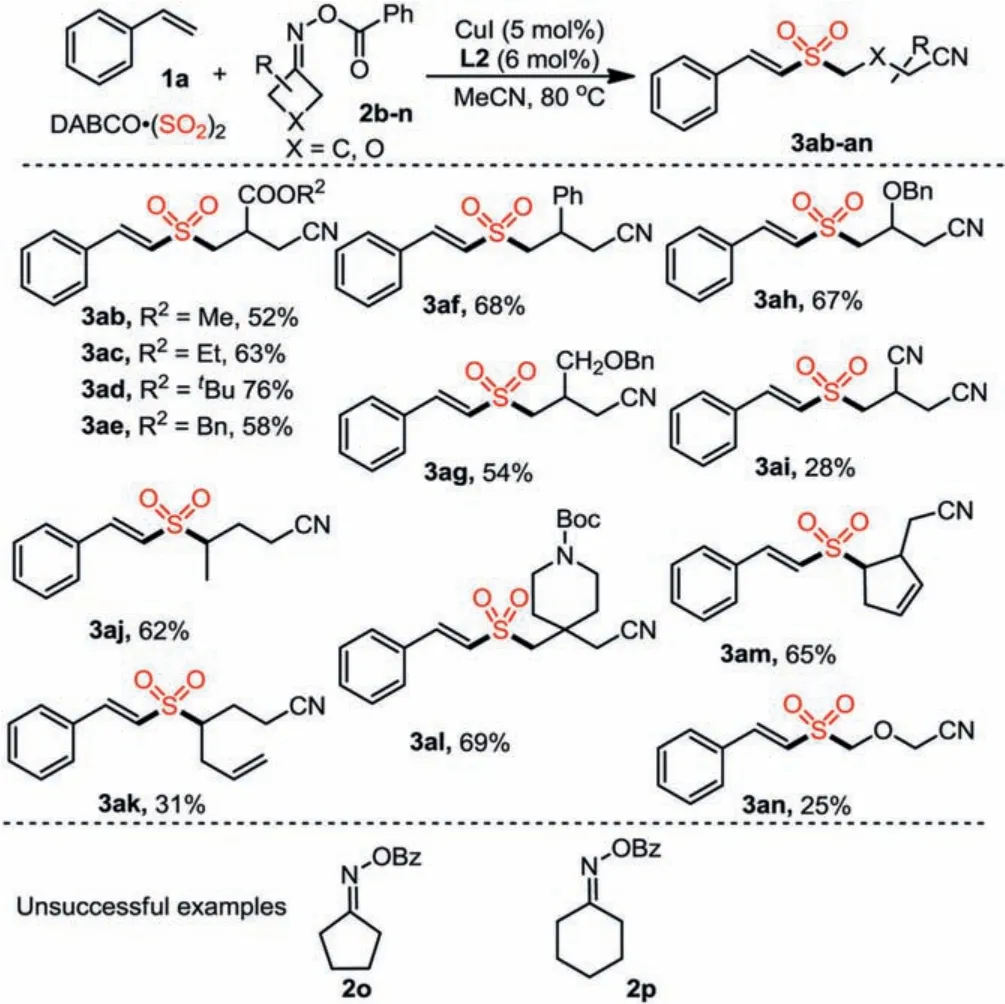
Scheme 2.Substrate scope of alkenes 1b-x.Isolated yield.
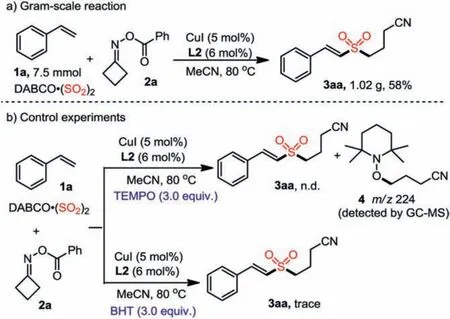
Scheme 3.Gram-scale reaction and control experiments.
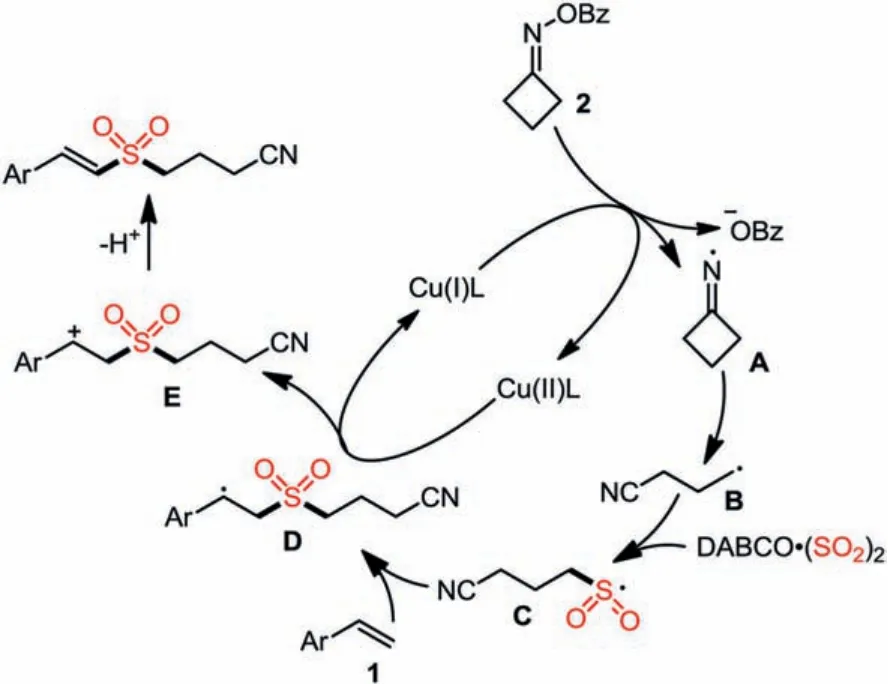
Scheme 4.Proposed mechanism.
We further evaluated the substrate scope by employing diverseO-benzoyl oximes with styrene 1a and DABCO·(SO2)2under the standard conditions.As shown in Scheme 2, a range ofO-benzoyl oximes 2b-2n with different substituents were well compatible,giving rise to the desired products in 25%-76% yields.It is noteworthy thatβ-substitutedO-benzoyl oximes could undergo selective C–C bond cleavage to form more stable secondary alkyl radical, thus leading to products 3ak and 3al.Interestingly, reaction of oxygen-containing substrate 2n was also workable, generating the target product 3an.Moreover, we also tested cyclopentanone oxime derivative 2o and cyclohexanone oxime derivative 2p under the standard conditions, but there were no desired products were detected.Besides, a gram-scale cyanoalkylsulfonylation of styrene was carried out, and product 3aa was obtained in 58% yield (1.02 g,Scheme 3a).
Since we initially hypothesized that this transformation might undergo a radical process, therefore, several control experiments were performed (Scheme 3b).The model reaction of styrene 1a,cyclobutanoneO-benzoyl oxime 2a and DABCO·(SO2)2was completely suppressed in the presence of 2,2,6,6-tetramethylpiperidin-1-oxy (TEMPO) under the standard conditions.The radical trapping product 4, which was formed by cyanoalkyl radical and TEMPO,was detected by GC–MS.Additionally, only a trace amount of product 3aa was detected when butylated hydroxytoluene (BHT) was added as the radical scavenger in the reaction system.
On the basis of the above observation and related reports [11-30,83], a plausible mechanism is proposed as shown in Scheme 4.We reasoned that initially, a single-electron transfer process betweenO-benzoyl cycloketone oxime 2 and Cu(I) species would lead to the formation of iminyl radical intermediate A and Cu(II) complex.Subsequently,β-C-C bond cleavage of iminyl radical intermediate A would occur, giving rise to cyanoalkyl radical B.This cyanoalkyl radical B would then react with sulfur dioxide, providing cyanoalkylsulfonyl radical C.Followed by the addition of cyanoalkylsulfonyl radical C to alkene 2, a more stable benzyl radical D would be formed.Then, an oxidative single electron transfer between benzyl radical D and Cu(II) complex would provide cation intermediate E with the release of Cu(I) species to complete the catalytic cycle.Further deprotonation of cation intermediate E would result in the formation of cyanoalkylsulfonyl alkene 3.
In summary, we have developed a copper-catalyzed threecomponent reaction of alkenes, cycloketone oximes and DABCO·(SO2)2, which provides a convenient route for the synthesis of diverse (E)-cyanoalkylsulfonyl alkenes in moderate to good yields with excellent regio- and stereoselectivity.A broad substrate scope with excellent functional group tolerance is observed.A plausible radical pathway is proposed, which involves copper-catalyzed ring-opening C–C bond cleavage ofO-acyl oxime and insertion of sulfur dioxide.During the reaction process,cyanoalkyl radical and cyanoalkylsulfonyl radical are the key intermediates.
Declaration of competing interest
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence the work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgments
Financial support from the National Natural Science Foundation of China (No.21871053), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No.2019R01005), and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (No.2020ZD04) is gratefully acknowledged.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.009.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
