Emerging 2D pnictogens for biomedical applications
Ruoyo Li, Zhengbo Zh, Zhohu Mio,*, Cheng-Yn Xu
a School of Food and Biological Engineering, Hefei University of Technology, Hefei 230009, China
b Sauvage Laboratory for Smart Materials, School of Materials Science and Engineering, Harbin Institute of Technology (Shenzhen), Shenzhen 518055, China
c Shenzhen Bay Laboratory, Shenzhen 518132, China
Keywords:2D materials Biomedical applications Anti-tumor Anti-inflammation Anti-bacterial
ABSTRACT Two-dimensional (2D) materials composed of single pnictogen element, namely, 2D pnictogens (e.g.,black phosphorus, arsenene, antimonene and bismuthine), have recently showed remarkable potential for biomedical applications, especially after the rapid development of black phosphorus.With unique optical and electronic properties, 2D pnictogens are considered as promising nanoagents for biosensors, diagnosis and therapy.In this review, after brief introduction of the structure, properties, synthesis strategies, and biocompatibility of 2D pnictogens, their biomedical applications including anti-tumor, anti-inflammation,anti-bacterial, neurodegenerative treatment and tissue repairing are reviewed.The major obstacles and opportunities of 2D pnictogens are also discussed.This review provides a short yet timely summary on the synthesis and biomedical applications of emerging 2D pnictogens.
1.Introduction
The successful isolation of graphene in 2004 has opened a brilliant world toward two-dimensional (2D) materials.Since then, a great deal of 2D materials beyond graphene have been successively proliferated [1], such as transition metal dichalcogenides(TMDs) [2,3], metal carbides and nitrides (MXenes) [4], and monoelemental 2D materials (Xenes) [5].Due to the high-percentage surface atom exposure and quantum confinement effects of atomically thin structure, 2D materials exhibit unique photonic, electronic, magnetic and catalytic properties, endowing impressive advantages for applications in the fields of optoelectronic devices,energy, and biomedicine.In particular, by virtue of excellent optical and electronic properties, 2D materials have been widely explored as promising nanoagents for biosensors, diagnosis (photoacoustic imaging (PAI), X-ray computed tomography (CT) imaging,etc.), photonic therapy (photothermal therapy (PTT) and photodynamic therapy (PDT)), depicting high prospect for biomedical applications [6,7].
Most recently, 2D materials composed of single pnictogen element, namely, 2D pnictogens, have captured broad attention, especially after the rapid development of black phosphorus (BP) in biomedicine.The pnictogen group shows a complete transition from non-metallic elements (N and P) to metallic elements (As,Sb, Bi and Mc) [8].Compared with other 2D materials, 2D pnictogens (e.g.,BP, arsenene, antimonene and bismuthine) have some intriguing inherent advantages.BP nanosheets (NSs) are degradablein vivoand P is one of essential elements for human body, endowing the biosafety of BP.Arsenene can be easily oxidized into arsenic oxide, which is an effective antitumor agent for acute promyelocytic leukemia in the clinic [9].In addition, both antimony and bismuth elements are the major components of a number of clinical drugs for more than two centuries [10].These revealed that pnictogen elements were closely associated with clinical drugs, indicating 2D pnictogens have high potential of clinical translation [11].
As a result, the biomedical applications of 2D pnictogens have been widely explored.Until now, several reviews have summarized the biomedical applications of one or two kinds of 2D pnictogens(BP NSs in most cases) [12,13].Thus, a short yet timely review on the biomedical applications of the family of 2D pnictogens is of urgence.Herein, after brief introduction of the structure, properties, synthesis strategies, and biocompatibility of 2D pnictogens, we presented a concise summary of their biomedical applications including anti-tumor, anti-inflammation, anti-bacterial, neurodegenerative diseases treatment and tissue repairing.At the end of this review, the major obstacles and opportunities of 2D pnictogens are discussed.
2.Structure and distinct properties of 2D pnictogens
Understanding the structure and properties of 2D pnictogens is beneficial for their potential biological applications.The most significant structural characteristic of BP NSs is the puckered structure(Fig.1) [14].Monolayer BP is arranged in two ways, including the double-layer structure in the zigzag direction and the folding structure in the direction of the armchair.For other 2D pnictogens, arsenene favors to form an orthorhombic (αphase) or rhombohedral(βphase) structure, while bismuthene and antimonene haveβphase structure [15].2D pnictogens have high specific surface area owing to buckled and wrinkled structure, providing a large number of surface-active sites for biomedical applications [16].Zhanget al.calculated and summarized the bandgap of 2D pnictogens [17].BP NSs are shown to have an adjustable direct bandgap, which depends on the number of layers of BP NSs.When the BP thickness decreased from bulk to monolayer, the tunable band gap of 0.3-2.0 eV could be adjusted.Arsenene and antimonene are cataloged as wide bandgap semiconductors, while bismuthene has a narrow bandgap.2D pnictogens have excellent optical properties and wide absorption in the visible and infrared regions, which is beneficial for their applications in the biomedical field.A catalogue summary of the structure and properties of 2D pnictogens are presented in Fig.1 [14–17].
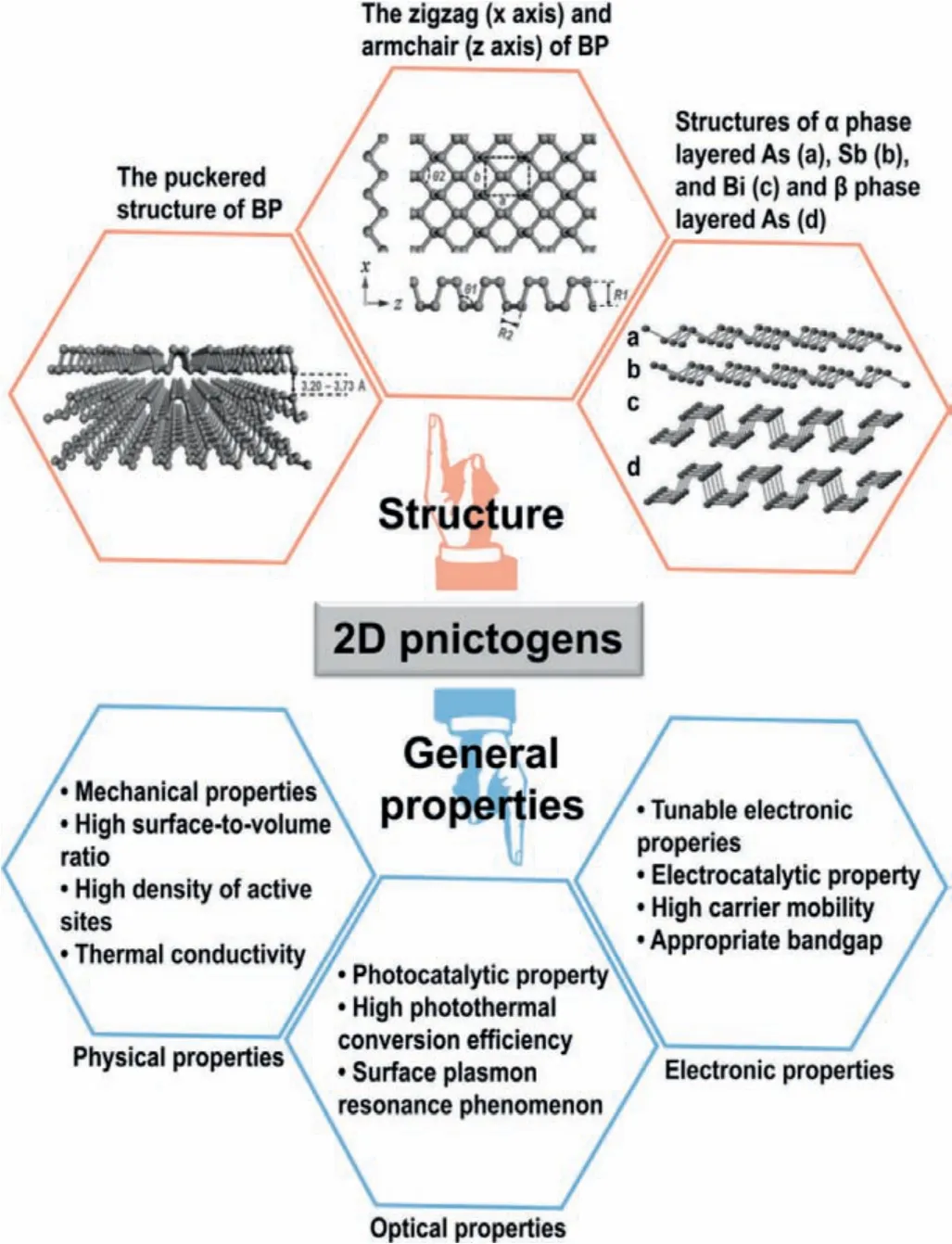
Fig.1.An overview of the structure and distinct properties of 2D pnictogens [14–17].Reproduced with permission [14].Copyright 2016, Wiley-VCH.Reproduced with permission [15].Copyright 2017, Wiley-VCH.
3.Synthesis strategies
The synthesis strategies of 2D pnictogens can be divided into two categories:bottom-up and top-down methods (Fig.2) [18].Bottom-up method can be further divided into molecular beam epitaxy (MBE), chemical vapor deposition (CVD), and solvothermal methods.The other approach, top-down approach, mainly includes mechanical, ultrasonic, electrochemical and ice-assisted exfoliation.
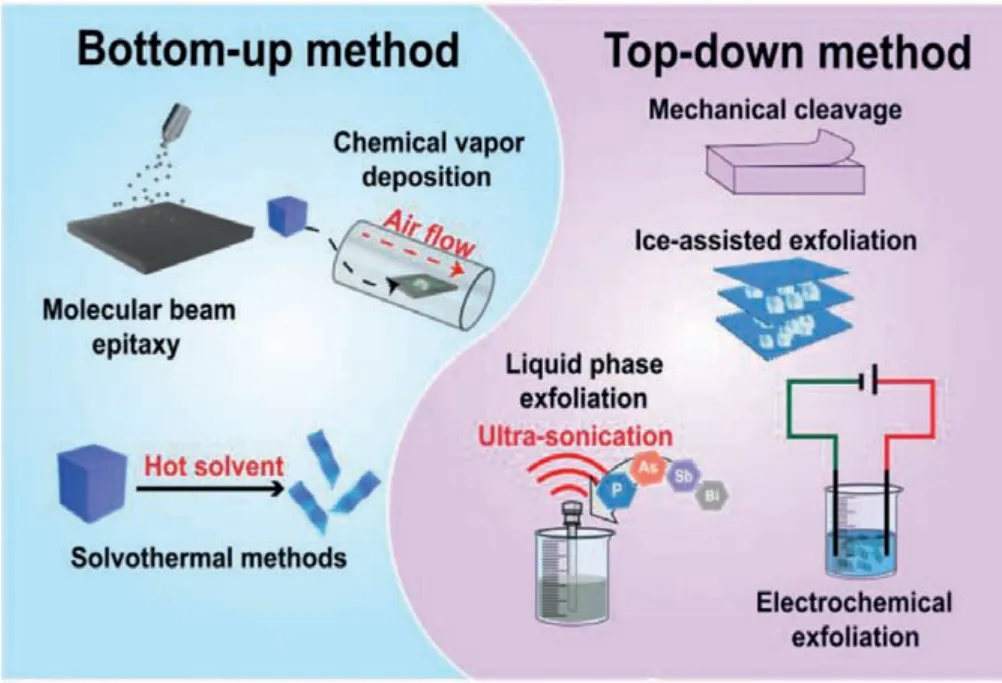
Fig.2.A scheme of various bottom-up and top-down approaches to the synthesis of 2D pnictogens.
The bottom-up method refers to directly synthesizing nanosheets by chemical reaction of small molecules [19,20].MBE is a slow growth process, and allows the precise control of major components, impurities and thickness [21].MBE has been proved to be effective for precise synthesis of phosphoene [22],antimonene [23,24] and bismuthene [25].CVD method is used to grow continuous, large and uniform 2D materials with few layers.BP NSs with thickness of approximately four layers (corresponding to thickness of 3.4 nm) were fabricatedviaCVD technology [26].Wuet al.reported the first successful CVD growth of single crystal antimony flakes on SiO2substrates [27].Solvothermal method is another effective method to synthesize 2D nanomaterials.The growth of 2D nanomaterials occurs in sealed vessel or autoclave.When the temperature rises beyond the boiling point of the precursor solvent, the precursor will be reduced to 2D nanosheets.Several articles show that solvothermal method is a reliable method to synthesize BP NSs [28,29].For example, Tianet al.synthesized few-layer BP NSs with typical 2D nanosheet-like structure and lateral size of 800–1000 nm, by using white phosphorus as raw material at low temperature (60–140 °C) for 12 h [30].
The top-down approach utilizes mechanical or other forces to break the bond between the material layers, resulting in the isolation of single or few-layer nanosheets.A typical example of mechanical exfoliation is the synthesis of antimonene.Areset al.successfully synthesized single/few-layer antimony flakesviaa modified mechanical cleavage stripping strategy [31].The film was obtained by repeated exfoliation with adhesive tape.Then, antimonene was transferred onto silicon substrate using viscoelastic stamp as the intermediate substrate for exfoliation, and the minimum antimonene layer thickness was only 1.8 nm.The strategy greatly improved the yield of conventional mechanical exfoliation.As one of the most common top-down approach, ultrasoundassisted liquid-phase exfoliation was proposed for the preparation of pnictogens nanosheets [32–35].Yasaeiet al.synthesized high crystallization BP nanoflakes by liquid-phase exfoliation [32].Aprotic and polar solvents, such as dimethylformamide (DMF) and dimethyl sulphoxide (DMSO), were explored as suitable solvents for the synthesis of atomically thin BP nanoflakes.Electrochemical method for the preparation of 2D materials involves the use of electrolytes and electric currents to facilitate the isolation of 2D materials by cathodic reduction or anodic oxidation of electrodes.In comparison with other synthetic methods, the advantage of electrochemical method is that the reaction conditions are mild,controllable, convenient and high yield [36].Kovalskaet al.demonstrated the electrochemical exfoliation of black arsenic for the preparation of arsenene with orthorhombic structure and thickness of 0.6 nm in anhydrous electrolyte media [37].Ice-assisted exfoliation is a newly-proposed exfoliation technique, and has attracted the attention because it can reduce the formation of structural defects in the exfoliation process.Both BP NSs and bismuthene have recently been synthesizedviathis method [38,39].
4.Stability and biocompatibility of 2D pnictogens
The stability and biocompatibility of 2D pnictogens are vital for biomedical applications.BP NSs were reported to present poor stability, severely limiting the biological applications [40].Liuet al.proposed a protective chemistry-based method based on protective chemistry to regulate the reaction stability of BP NSs.They used Al3+to reduce the electron density on BP NSs surface, and hydrophobic 1,2-catechol (BDT) was self-assembled on BP/Al3+to form an antioxidant/water-repellent layer [41].This protective step produced stable BP NSs with low degradation rate.In addition, the deprotection of BP/Al3+/BDT was also very simple.Upon chelating agent treatment, Al3+and BDT on BP NSs surface were removed to restore the electron density of BP NSs.
In addition, 2D pnictogens have unexceptionable biocompatibility by suitable surface functionalization.Guoet al.successfully synthesized BPQDs with high stability and low toxicity after polyethylene glycol (PEG) coupling [42].The obtained BPQDs could be effectively excreted through renal clearance due to small hydrodynamic diameter, avoiding the potential physiological toxicity of BPQDs.Moreover, the end product of BP NSs is biocompatible and non-biotoxic PxOyn-ions [43].As/AsxOyNSs-based nanoplatform was reported to be biocompatible based on both hematology assay and histology examination results [44].The nanoplatform had a strong ability to target cancer cells and could be degraded in the tumor microenvironment, which also greatly reduced the cytotoxicity to the host.Organobismuth compounds were used in the clinical treatment of various gastrointestinal diseases since the 18th century [45].Bi-based nanomaterials for cancer diagnosis and treatment, including Bi, Bi2S3and Bi2O3, were confirmed to be non-toxic to cancer cells and surrounding cells within safe dose ranges [46].Guoet al.coated bismuthene with polyethylene glycol imine (PEI), followed by the loading of siRNA (Bi@PP/siRNA) [47].The nanosystem reduced the risk of long-term toxicity through rapid clearance by the intestines and kidneys.Safe-by-design bismuthene had good biocompatibility and low toxicity.For antimonene, good biosafety and degradation of antimonenein vivohave been confirmed.As for antimonene, the biological distribution and biocompatibility of photosensitizer 5,10,15,20-tetrakis(4-hydroxy-phenyl)-21H,12H-porphine and PEG functionalized antimonene (Sb-THPP-PEG NSs) were evaluatedin vitroandin vivo[48].Sb-THPP-PEG NSs were mainly accumulated at the tumor site and excreted by the kidney.For major organs and tumor tissues,there was no obvious tissue damage or inflammatory reaction after Sb-THPP-PEG NSs treatment, demonstrating the good biocompatibility of Sb-THPP-PEG NSs.
5.Biomedical applications
2D pnictogens have witnessed the rapid growth for biomedical applications in recent years, especially the development of biocompatible BP materials.Due to the unique optical and electronic properties [49,50], 2D pnictogens have been widely used in biomedical fields, including anti-tumor, anti-inflammation, anti-bacterial, neurodegenerative diseases (ND) treatment, tissue repairing,etc.(see schematic category in Fig.3).
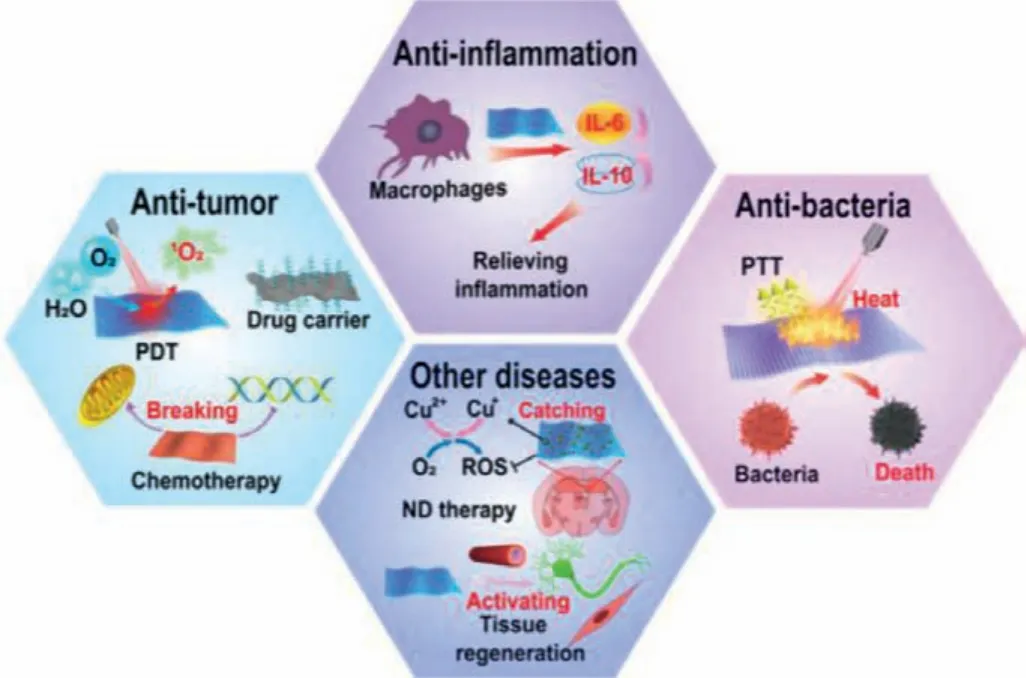
Fig.3.Typical biomedical applications of 2D pnictogens.
5.1.Anti-tumor applications
Cancer is one of the deadliest diseases in the world.The obstacles of cancer treatments mainly originated from the characteristic properties of tumor cells including unlimited replication, immune escape, and metastasis [51].
5.1.1.Chemotherapy
Chemotherapy is an effective method for cancer treatments by chemotherapeutic drugs, mainly through the influence of DNA replication, transcription, and tubulin synthesis.These biological events are critical to cell growth and proliferation.Both arsenene and BP NSs have been found to exhibit inherent chemotherapy function [11].BP NSs can be degraded into phosphate anions[52], and the concentration of phosphate ions can affect the hydrolysis of intracellular ATP (adenosine triphosphate), induce programmed cell death and strong inhibit the proliferation of cancer cells.Zhouet al.demonstrated that BP NSs were extremely selective and bioactive anticancer agents on account of their inherent chemotherapy effects [53].BP NSs depicted selective antiproliferation effect on Hela cells, while maintaining biocompatibility toward normal cells, and its selectivity was even better than that of doxorubicin (DOX).In cancer cell lines, the selective anti-proliferation effect of BP NSs was achieved by blocking the G2/M phase, contributing to apoptosis and autophagy-mediated cell death.
Arsenene, another 2D pnictogen material, also has inherent chemotherapy functionality.The most commonly used clinical arsenic compound is As2O3, which has achieved remarkable benefits in treating leukemia acute promyelocytic leukemia (APL) by promoting the degradation of the cancer-promoting promyelocytic leukemia protein PML-RARα[54,55].However, as a result of its low selectivity to normal cells and cancer cells [56], the toxicity of As2O3may not be completely avoidable, limiting its biomedical applications.Wanget al.synthesized selective arsenene nanosheets with chemotherapeutic function by liquid exfoliation(Fig.4a) [57].Arsenene nanosheets with planar structure were observed by transmission electron microscope (TEM) image (Fig.4b).In addition, arsenene had inhibitory effect on a variety of cancer cells.For example, arsenene was effective in inhibiting 82%of NB4 cells (Fig.4c).Proteomic analysis showed that arsenene down-regulates DNA polymerases including POLE, POLD1, POLD2 and POLD3 to impact the base removal repair, DNA replication, and pyrimidine metabolic pathways (Fig.4d).In addition, toxicity testin vivo viahaematoxylin and eosin (H&E) staining on the major organs of mice proved the biocompatibility of arsenene (Fig.4e).Compared with arsenic trioxide, arsenene had not exactly the same metabolic pathway for inducing apoptosis in NB4 cells, exhibiting significant cell inhibition.Arsenene is thus considered as a promising anti-cancer agent for biological applications.
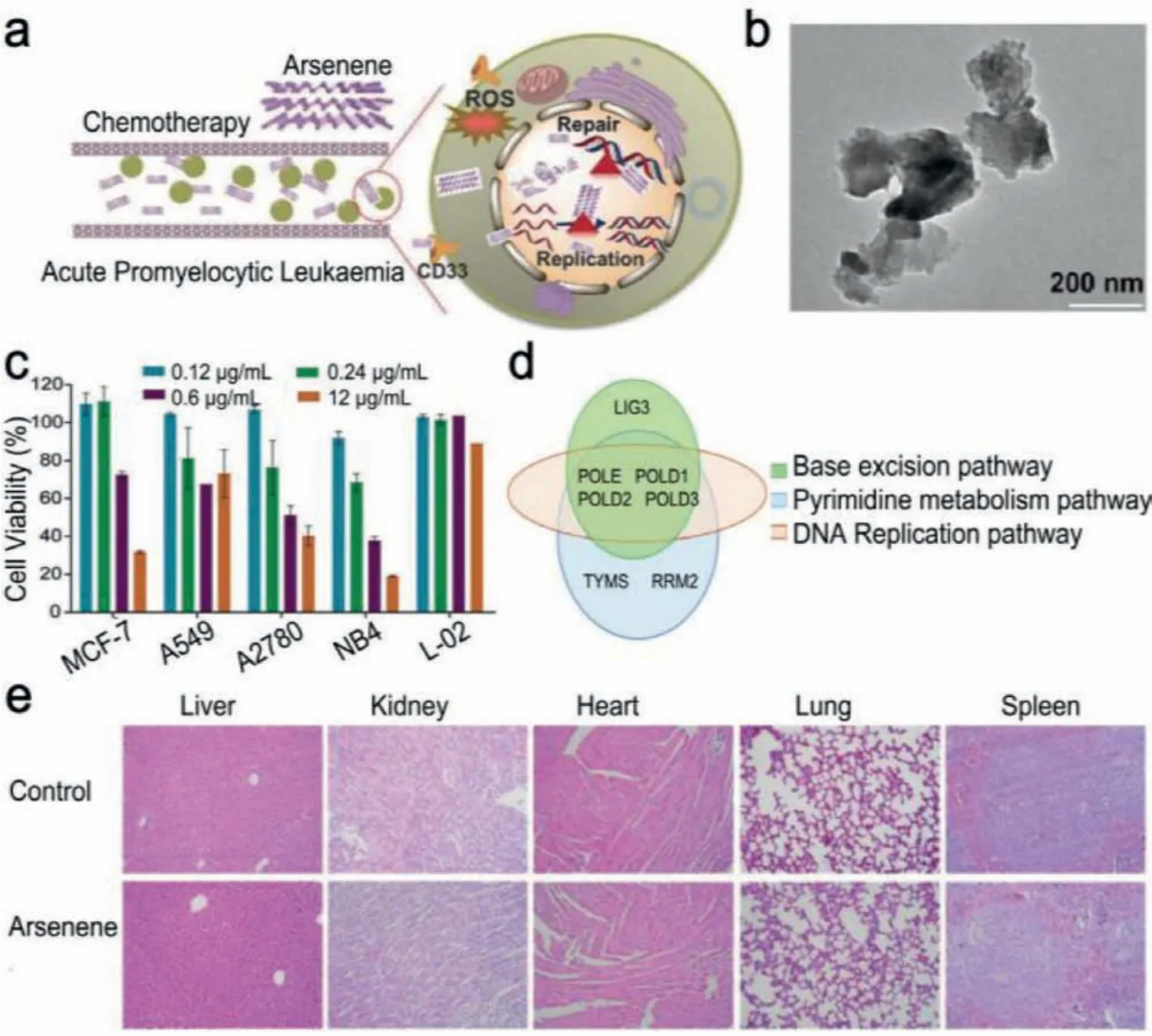
Fig.4.(a) Schematic representation of chemotherapy mechanism of arsenene.(b) TEM image of arsenene.(c) Cell viability after treating various cells with arsenene of different concentrations.(d) Common proteins in the three screening pathways.(e) H&E staining of the major organs of mice treated with arsenene after 14 days.Reproduced with permission [57].Copyright 2020, Wiley-VCH.
5.1.2.Photothermal therapy
Photothermal therapy (PTT), as an effective tumor treatment technology, has attracted extensive attentions due to its low invasiveness and laser target [58].However, the synthesis of ideal photothermal agents still remains challenges when considering the biodegradation and efficiency.The emergence of 2D pnictogens with exciting physical and chemical properties provides new opportunities.The reported 2D pnictogens have proven to exhibit excellent photothermal conversion capabilities [44,59].
To date, antimonene was considered as a new candidate for PTT of tumor on account of its strong light absorption capacity in the near infrared (NIR) region [59].Taoet al.successfully synthesized 2D antimonene quantum dots (AMQDs) using a controllable liquid exfoliation method [48].The physiological stability of AMQDs was improved by PEG coating.PEG-coated AMQDs not only had superior near-infrared photothermal property, but also showed rapid NIR-induced degradation.In addition, PEG-coated AMQDs demonstrated remarkable near-infrared light-induce MCF-7 tumor ablation and biocompatibilityin vivo.In addition to AMQDs, arsenene nanodots and bismuthene nanosheets have also been successfully demonstrated as efficient photothermal agents [44].
5.1.3.Photodynamic therapy
Photodynamic therapy (PDT) refers to the use of photosensitizers and laser irradiation to produce reactive oxygen species (ROS)inside cells for cancer therapy [60].In practice, most photodynamic agents at present are limited by the inherent oxygen starvation in tumor, and the insufficient oxygen supply in tumors leads to the incapacity of photosensitizers function.Fortunately, the reported 2D pnictogens NSs, such as BP, arsenene and bismuthine,have proven to exhibit excellent ROS production capacity under laser irradiation [61].Due to the abundant surface atoms as active sites and the low electron-hole recombination rate, ultrathin BP NSs exhibit a significant enhancement of singlet oxygen (1O2)generation [62].Liuet al.developed black phosphorus nanosheets(BPNs)-based photosensitizer [63].BPNs were modified with DNA double-stranded blockage using a blocker DNA of 5′-CY5-aptamerheme/3′-heme labeled oligonucleotidesand folic acid (Cy5-dHeme-BPNs-FA) (Figs.5a and b).Atomic force microscopy (AFM) image in Fig.5c showed the exfoliated few-layer BPNs had a thickness of approximately 2.5 nm.Cy5-dHeme-BPNs-FA inactivated peroxidase and activated the catalytic function by changing intracellular excess H2O2into O2, which is essential for maintaining BPNsmediated PDT (Fig.5d).Then, BPNs performed excellent PDT capability, producing sufficient1O2in anoxic tumor cells (Fig.5e).As a result, the efficacy of PDT was greatly improved in the treatment of hypoxic cells and tumors (8.7-fold and 7.5-fold, respectively) (Fig.5f).
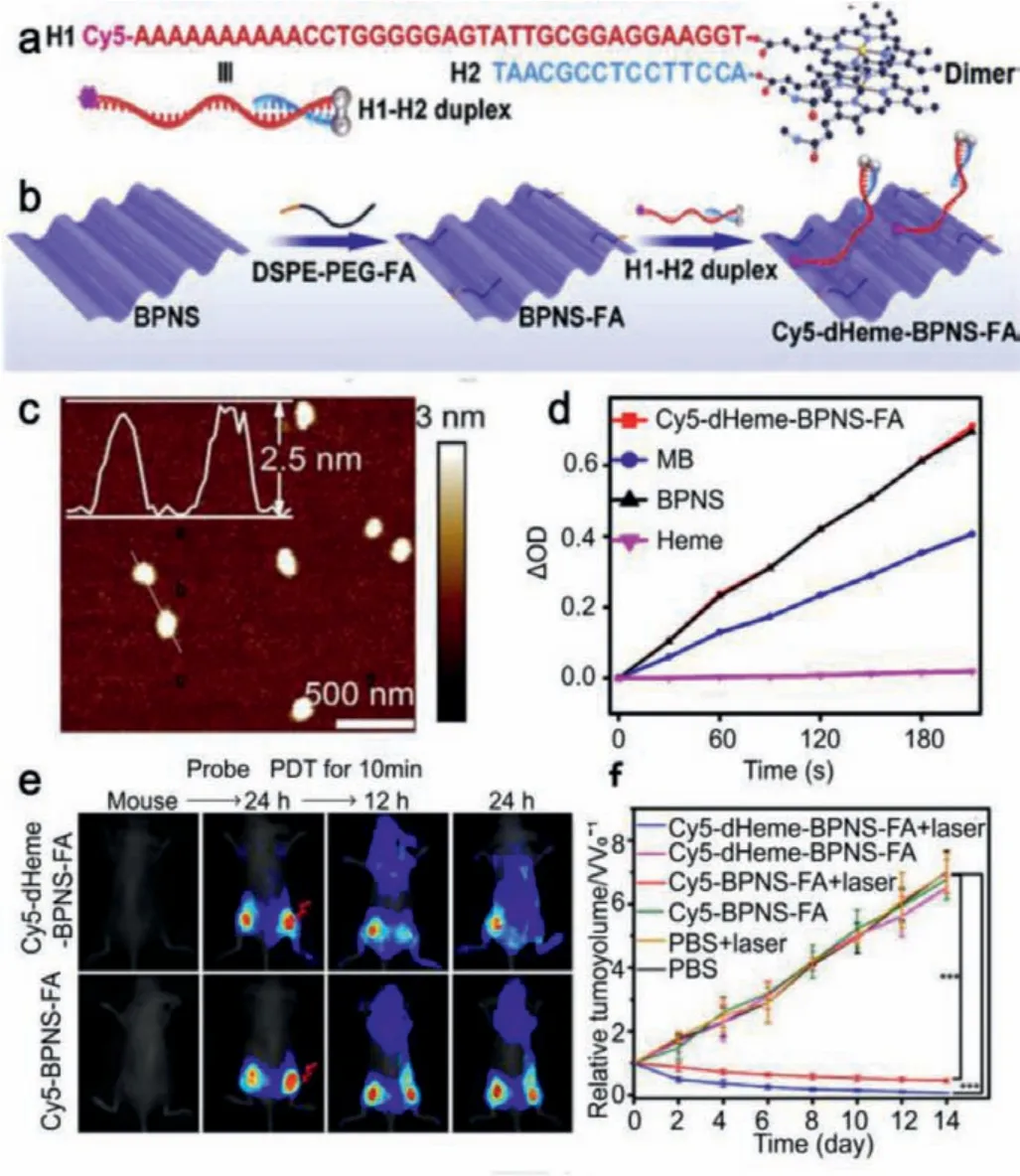
Fig.5.(a, b) Schematic illustration of the preparation and functionalization of BPNs.(c) AFM image and height profile of BPNs.(d) 1O2 generation monitoring by DPBF incubated with Cy5-dHeme-BPNS-FA, MB, BPNs and Heme.(e) In vivo imaging monitoring of PDT therapy on mice after treatment of Cy5-dHeme-BPNS-FA or Cy5-BPNS-FA.(f) Relative changes in mean tumor volume under different conditions.Reproduced with permission [63].Copyright 2018, Elsevier.
Bismuthene is another 2D pnictogen material that has also been proved to have photodynamic property [63].Wanget al.synthesized 2D ultrathin bismuthene nanosheets (BNs) through two crucial procedures:freezing thawing treatment by water-molecule and a NaBH4-triggered reduction process [39], followed by the modification of BSA (BNs-BSA) to ameliorate dispersion.BNs-BSA showed an excellent potent1O2production capacity as a result of photodynamic performance of ultrathin bismuthene.In addition, the photothermal conversion efficiency of BNs-BSA was up to 19.4%, which could effectively inhibit tumor in coordination with PDT, resulting in complete tumor eradication.Very recently, Konget al.reported the synthesis of surface oxidized arsenene nanosheets (As/AsxOyNSs)viaball-grinding and subsequent liquid exfoliation.Moreover,As in As/AsxOyNSs was proved to be an effective photosensitizer to produce1O2and superoxide (O2·-) bursts through energy conversion under 660 nm laser irradiation [44].
5.1.4.Immunotherapy
Tumor immunotherapy is an effective anti-cancer strategy that promotes the host’s immune system to fight against tumors [64].Consequently, the host acquires durable anti-tumor mechanisms.The checkpoint blockade antibody and chimeric antigen receptor T cell (CAR-T) have demonstrated excellent clinical efficacy in a variety of cancers [65].However, the high cost of neoantigens to identify individual mutations has hindered their clinical application,and the development of CAR-T-related vaccines had also stalled[66].2D pnictogens nanomaterials have provided alternative solution for cancer immunotherapy.Guoet al.designed a PD-Li siRNA release nanosystem based on bismuthene nanosheets as a unified strategy to achieve enhanced mild photothermal immunotherapy for tumors [47].Under artificially controlled NIR irradiation, the nanosystem not only induced the enhanced pathologic permeability and retention (EPPR) effect of tumor and increased the restrained tumor-infiltrating lymphocytes (TILs) recruitment, but also triggered procedural siRNA release to amplify immunotherapy.In addition, the nanosystem could be cleared quickly, reflecting superb safety of bismuthene.
In another work, Xieet al.developed a combination strategy of BP NSs-based photothermal therapy and anti-D47 antibody (aCD47)-based immunotherapy to synergically enhance tumor therapy [67].BP NSs combined with aCD47 could simultaneously activate innate and adaptive immunity to promote the occurrence of local and systemic anti-cancer immune responses.In addition, they found that BP-mediated tumor ablationviathe photothermal effect could improve the inherent immunogenicity of tumors, which greatly strengthened the effect of cancer immunotherapy.
5.1.5.Drug carriers
As far as drug carriers are concerned, 2D pnictogens nanomaterials with large surface area, unique surface chemistry and inherent optical properties, are regarded as one of the most promising and effective drug carriers for drug devlivery [68,69].Taoet al.reported that BP NSs could be used as drug carrier to realize effective loading of DOX [70].Chenet al.reported that the surface of BP NSs could be loaded with more DOX (950% in weight), higher than other 2D material system previously reported [71].Besides,BP NSs have a large number of periodic atomic slots on its surface,which are ideal binding sites for proteins and soft linear materials[72], suggesting their potential to load and deliver biomolecules.Chenet al.used small and thin BP NSs modified with PEG and polyethylene imine (PBP) as a new delivery system for human telomerase reverse transcriptase (hTERT) siRNA [73].The system had extremely high siRNA loading and cell uptake capacity, thus achieving targeted gene therapy of cancer.
Arsenene was considered as a potential drug carrier due to its layered structure, which is similar to the buckled honeycomb structure of BP NSs [74].PEG-PSMAA-modified arsenene (PMANs)was obtained by the modification of arsenene with polyethylene glycol (PEG) and prostate-specific membrane antigen (PSMA) antibody (PSMAA), and the obtained PMANs depicted good water dispersion, biocompatibility and tumor-targeting capacity [75].The tumor inhibitor DOX was loaded onto the surface of PMANs platform, which demonstrated excellent drug delivery and release capabilities.The maximum drug loading reached 140% (weight ratio),and DOX can be rapidly released at pH 5.0.These results suggested that arsenene can be used as a highly effective drug carrier.
Antimonene is also another drug carrier.PEG modified antimonene was explored to load DOX (AM-PEG/DOX NSs) [76].Due to its excellent photothermal conversion efficiency (41.8%), high loading rate (150.0%), and multi-responsive drug release capability including pH and near-infrared responses, AM-PEG/DOX NSs achieved significant antitumor efficacyin vitroandin vivounder the irradiation of 808 nm near-infrared laser through the combination of chemotherapy and photothermal therapy.
5.1.6.Biosensors
A wealth of literature indicates that 2D pnictogens materials have achieved great progresses in the field of biosensors [77–79].For instance, Xueet al.developed a ultrathin bismuthine-based sensing platform for specific detection of microRNAs (miRNAs)[77].Bismuthene was reported to be a highly efficient quenching agent due to the ground state weakly fluorescent charge transfer between bismuthene and the dye molecule, which could completely quench dye-labeled ssDNA fluorescence on bismuthine.When the target miRNA was present, the interaction between the dye-labeled ssDNA probe and the bismuthene surface was disrupted due to complementary binding between dye-labeled-ssDNA and the target miRNA, resulting in fluorescence recovery (Fig.6a).AFM image showed that the overall lateral dimensions of the bismuthine was more than 800 nm, and the thickness was about 1 nm, which corresponded to few-layer bismuthine (Fig.6b).Highresolution TEM (HRTEM) image demonstrated the crystalline structure of bismuthene and no obvious defects were observed (Fig.6c).As depicted in Fig.6d, a 2D diagram of transient absorption of FAM-SSDNA in the absence or presence of bismuthine showed the function of probe and wavelength delay up to 4 ns.It is worth noting that the detection limit of the platform was up to 60 pmol/L(Fig.6e).Thus, bismuthene could be used as a novel fluorescence quenchingagent for the detection of miRNA-21 biomarkers associated with lung cancer.
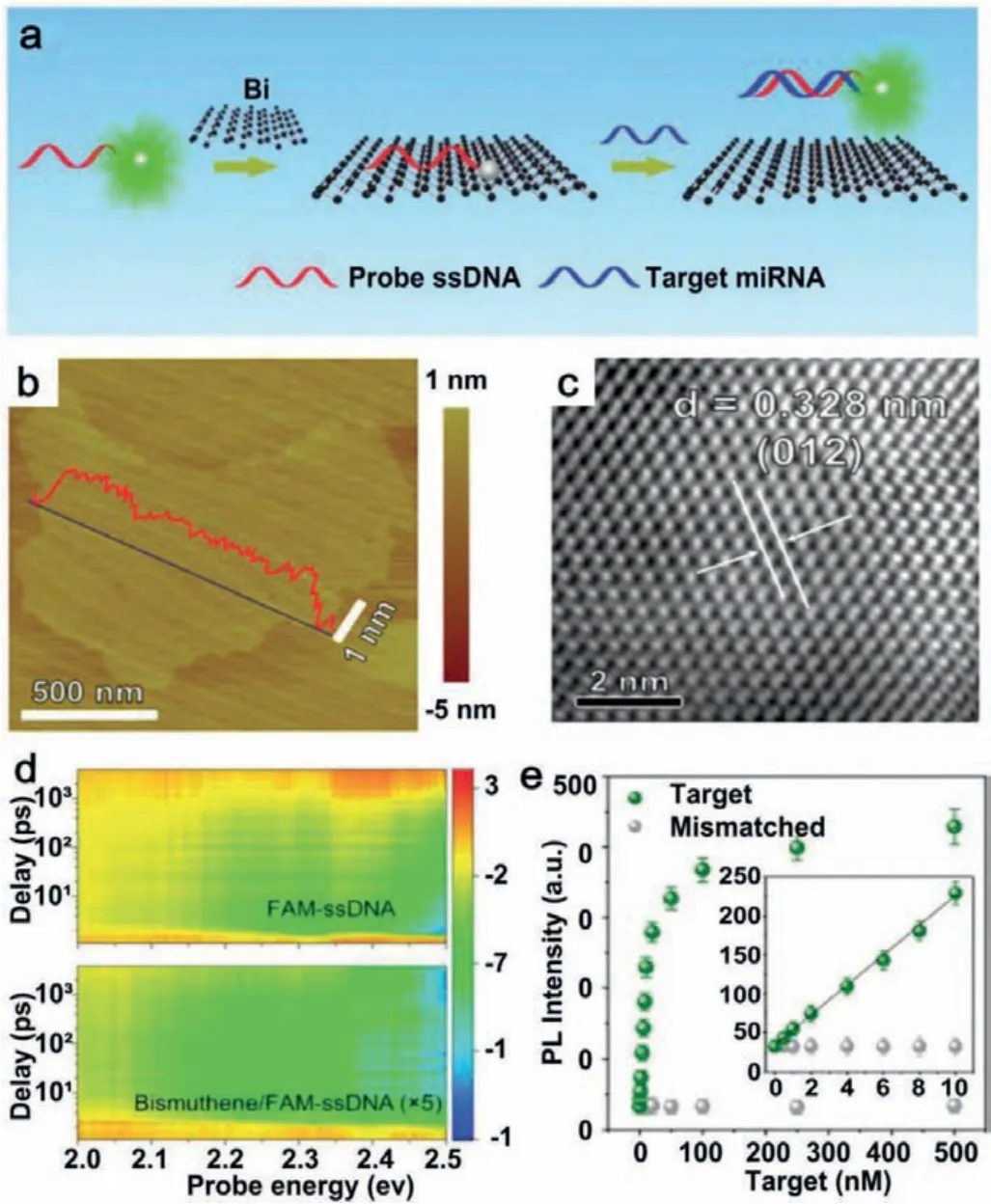
Fig.6.(a) Schematic representation of bismuthene-based miRNA detection.(b)AFM topography image of few-layered antimonene.(c) HRTEM image of few-layered bismuthene.(d) 2D images of transient absorption spectra of FAM-ssDNA and bismuthene/FAM-ssDNA.(e) Comparison of the weakly fluorescent charge transfer between dye molecules and mismatched.Reproduced with permission [77].Copyright 2020, Royal Society of Chemistry.
The large specific surface area of BP NSs facilitates the effi-cient adsorption of biomolecules due to its inclusion of armchair or zigzag structures [72], suggesting its applications in the field of biosensors.Huanget al.prepared nitrobenzene modified BP NSs-based biosensor (NP-BPs biosensor) for circulating tumor DNA(ctDNA) identification [80].Because of the nitrobenzene property,NP-BPs biosensor not only had a strong affinity for single stranded DNA (ssDNA), but also had a high quenching efficiency.Therefore,NP-BPs biosensor could be used to detect ctDNA sensitively and selectively.The detection limit of the biosensor was 50 fmol/L and the linear detection range was 50 fmol/L to 80 pmol/L.More importantly, NP-BP biosensor could accurately detect ctDNA in clinical serum samples.
5.2.Anti-inflammation
Inflammation is a physical disease caused by injury or infection in the body [81].Various nanomaterials have been regarded as foreign particles, which could often stimulate severe or sustained inflammatory effects and even damage normal organs or tissues.2D materials with outstanding scavenging capacity for ROS have been explored for anti-inflammation.Until now, BP NSs in the 2D pnictogens family have shown anti-inflammatory effect.Liet al.designed an intelligent drug delivery system based on black phosphorus quantum dots (BPQDS) [82].Both BPQDs and amikacin were encapsulated into PEGylated chitosan nanospheres (PEG@CS/BPQDS NPs), which exhibited excellent antimicrobial properties to improve the therapeutic effect of chronic obstructive pulmonary disease (COPD).Moreover, PEG@CS/BPQDS-NPs reduced the inflammatory symptoms of COPD.Cytokines associated with inflammation, including tumor necrosis factor-α(TNF-α), interleukin-1β(IL-1β), and interleukin-6 (IL-6), were significantly decreased after treatment with PEG@CS/BPQDS-NPs, which was an important aspect of COPD treatment (Figs.7a–d).In vivotherapeutic efficacy showed that inflammatory cell infiltration, bronchial mucus secretion, and other typical COPD symptoms were basically absent after the treatment of PEG@CS/ BPQDS-AM NPs (Fig.7e).
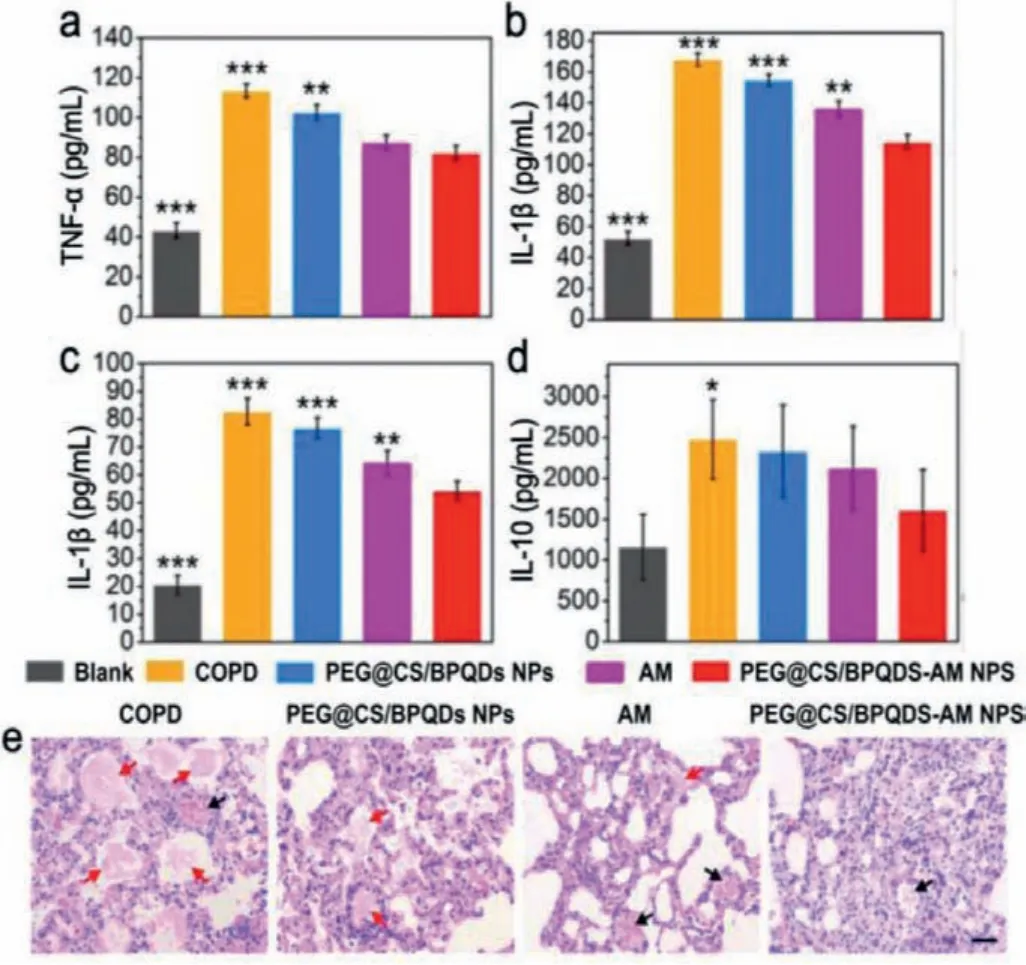
Fig.7.(a-d) Anti-inflammatory effect of black phosphorus nanosheets.Serological cytokine levels of (a) TNF-α, (b) IL-1B, (c) IL-6 and (d) IL-10 after the treatment.(e)H&E staining analysis of lung tissue (black arrows indicate inflammatory cells and red arrows indicate mucus secretion.Reproduced with permission [82].Copyright 2020, Wiley-VCH.
Zhaoet al.synthesized BP NSs usingN-methyl-2-pyrrolidone(NMP) as a solvent by liquid exfoliation and then mixed BP NSs with titanium sulfonate ligand (TIL4) for 15 h to produce TiL4@BPs[74].In vitroexperiments demonstrated that the exposed BP NSs induced significant inflammatory responses, while TiL4@BPs could evade cell uptake by macrophages, further reducing cytotoxicity and proinflammatory effects.This can be attributed that TiL4@BPs resulted in less cell uptake by macrophages.In addition, Rucciet al.studied the anti-inflammatory effect of BP NSs on cancerrelated inflammation [83].To investigate the role of the 2D BP NSs on inflammation and cancer, they proposed anin vitroco-culture model of osteosarcoma cells (SAOS-2) and healthy osteoblast cells(HOb).As a result, 2D BP NSs increased the production of antiinflammatory cytokines (i.e., IL 10) and inhibited the synthesis of pro-inflammatory mediators (e.g., IL-6) to prevent cancer-related inflammation.They not only demonstrated the inhibitory effect of BP NSs on pro-inflammatory mediators through the reduction of IL-6, but also suggested the anti-inflammatory potential of BP NSs,since BP NSs can increase IL-10 levels, thus reducing the inflammatory effects.
5.3.Anti-bacterial
Infections caused by pathogenic bacteria often lead to high morbidity and even mortality.More badly, due to the abuse of traditional antimicrobial drugs, a variety of drug-resistant bacteria, also known as superbugs, appeared in succession and spread rapidly [84].At present, nanomaterials, including 2D pnictogens materials, have attracted considerable attentions as potential candidates for antimicrobial agents [85].
Several reports have shown that BP NSs had excellent photodynamic effect [86,87], high surface areas [88], and outstanding biocompatibility [89], which undoubtedly provided a possibility for the anti-bacterial effect of BP NSs.Shawet al.revealed the broadspectrum antimicrobial activity of few-layer BP NSs, attributed to the production of ROS by layered BP NSs [90].BP NSs maintain highly antibacterial effect for a variety of bacteria and fungi, such as,Escherichia coli (E.coli),the fungal strainsCandida albicans (C.albicans), and Amphotericin B-resistantC.neoformans (C.neoformans (AR))cells.In another study, Lianget al.utilized a different strategy by anchoring silver nanoparticles (AgNPs) on the surface of BP NSs due to the puckered lattice configuration and the strong binding affinity between phosphorus and metal atoms [91].Although AgNPs showed a broad spectrum of antimicrobial activity, bare AgNPs tended to aggregate upon contact with bacteria,thereby reducing or even eliminating their antimicrobial activity.In contrast, the scaffold of BP NSs ensured the continuous and moderate release of AgNPs.On the other hand, AgNPs could be used as excellent electron receptors to improve the photodynamic effect of BP NSs.
Apart from BP NSs, the potential of antimonene for synergistic anti-bacterial applications is of interest to the biomaterial community.Liuet al.reported NIR responsive 2D antimonene nanosheet-based platform to achieve effective antimicrobial treatment [92].They constructed a composite hydrogel with excellent antibacterial properties by incorporating antimonene into a chitosan (CS) network structure (CS/AM NSs hydrogel) for the treatment of bacterial-infected wound.When co-cultured with bacteria,CS interacted with bacterial cell membrane and bonded to bacteria (Fig.8a).Under NIR irradiation, antimonene effectively converted NIR light energy into local heat and eliminated residual bacteria (Fig.8b).More importantly, CS/AM NSs hydrogels presented significant bacteriostatic effects against bothStaphylococcus aureusandE.coli.in infected wound mouse model, suggesting that antimonene had great potential for synergistic antimicrobial applications due to its excellent photothermal conversion efficiency.To sum up, 2D pnictogens NSs are beneficial in antibacterial field as a result of its high specific surface area and excellent photothermal effect.
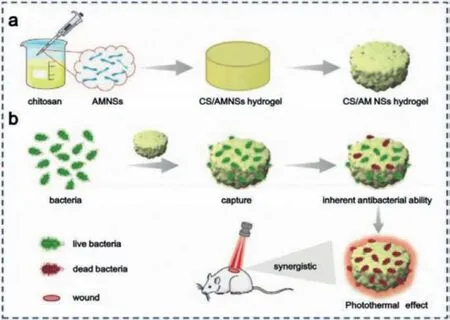
Fig.8.The anti-bacterial ability of antimonene.(a) The preparation process of CS/AM NSs hydrogel and (b) the application for synergistic PPT in treatment of bacterial infected-wounds.Reproduced with permission [92].Copyright 2020, Wiley-VCH.
5.4.Treatment of neurodegenerative diseases
Neurodegenerative diseases (ND), such as depression,Alzheimer’s disease (AD) and Parkinson’s disease (PD), usually occur with neurological dysfunction and progressive neuronal loss of the nervous system, which severely affects the patient’s memory function [93].ROS are a group of natural by-products produced by O2metabolism, including1O2, superoxide anion radical (O2·-), the highly reactive hydroxyl radical (·OH) and hydrogen peroxide (H2O2).ROS overdose has been reported as one of the main causes of ND induction [94].To date, BP NSs have been proven to have antioxidant properties [95,96].Transition metal homeostasis disorders have been shown to be a key factor in the development of ND.Chenet al.explored the application of BP NSs as neuroprotective nanomaterials in ND therapy [97].Resulting from the strong binding capacity between metal ions and phosphorus atoms, BP NSs could act as a nanocaptor to regulate copper ions concentration [98].BP NSs were directly obtainedviathe ultrasonication-assisted liquid exfoliation.TEM images in Figs.9a and b depicted that the BP NSs were well dispersed and about 200 nm in diameter.BP NSs acted as powerful antioxidant,effectively reducing the formation of cytotoxic ROS associated with copper ions homeostasis under physiological conditions (Figs.9c and d).Furthermore, BP NSs could reach the cerebrovascular endothelium and go across blood brain barrier (BBB) owing to excellent photothermal effect of BP NSs (Fig.9e).Therefore, BP NSs are a promising treatment option for ND.
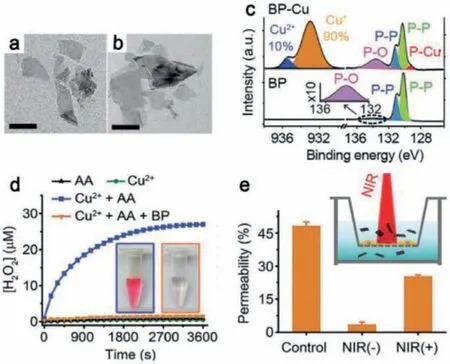
Fig.9.TEM images of BP NSs of (a) BP NSs and (b) BP nanosheets after copper ions capturing (scale bars are 200 nm).(c) XPS spectra of BP NSs before and after copper ions capturing.(d) Generation of H2O2 measured by Amplex red assay (Insert:the image on the left refers to Cu2+ + AA and the right refers to Cu2+ + AA + BP).(e)The blood brain barrier (BBB) crossing capacity of BP NSs promoted under 808 nm laser irradiation (Insert:Schematic diagram of BBB model in vitro).Reproduced with permission [97].Copyright 2020, Wiley-VCH.
Another interesting study showed that BP NSs were able to improve the treatment efficiency of depression [99].In this study, Jinet al.designed a BP NSs-based drug delivery system to implement the delivery of fluoxetine (Flu) and obtained BP-Flu.When exposed to NIR light (808 nm), BP-Flu significantly reduced the duration of treatment for depression, depicting good biocompatibility.
5.5.Tissue repairing
BP NSs can effectively repair damaged tissues on account of their excellent biocompatibility, biodegradability, and the essential element for human body.Maoet al.immersed CS hydrogel into BP NSs aqueous solutions to obtain CS-BP hydrogel.Due to excellent capacity to produce1O2, CS-BP hydrogel was proved to exhibit outstanding anti-bacterial efficacy [100].In addition, BP NSs promoted fibrinogen formation early in the process of tissue reconstruction, and simultaneously triggered the phosphoinositol 3-kinase (PI3K), protein kinase B phosphorylation (Akt), and extracellular signal-regulated kinase (ERK1/2) signaling pathways to enhance cell proliferation and differentiation.Qianet al.proposed a nanoscaffold composed of layer-by-layer of BP NSs [101].Their work demonstrated for the first time the capacity of the BP NSsbased nanoscaffold for promoting regeneration of severe nerve defects.The scaffold had excellent electrical conductivity, which enabled the stable release of BP into the surrounding microenvironment.In addition, they also found that the scaffold induced angiogenesis and neurogenesis under mild oxidative stress and stimulated calcium-dependent axonal and remyelinating regeneration.Xuet al.modified BP NSs with polydopamine (BP@PDA)to improve stcapacity.Then, BP@PDA was integrated into gelatin methacryloyl (GelMA) hydrogels matrix to produce biodegradable and conductive hydrogels (GelMA-BP@PDA biohybrid hydrogels)[102].BP@PDA improved electrical conductivity of hydrogels and promoted cell migration of mesenchymal stem cells within GelMABP@PDA biohybrid hydrogels.Most importantly, the scaffolds also significantly promoted the differentiation of mesenchymal stem cells (MSCs) into neuro-like cells under electrical stimulation.In conclusion, GelMA-BP@PDA biohybrid hydrogels could be used as degradable electroactive biomaterials for tissue engineering.
6.Summary and prospective
The recent advances of biomedical applications of 2D pnictogens are reviewed.Bottom-up and top-down methods are the two common strategies for the preparation of single or few-layer 2D pnictogens nanosheets.A large number of typical examples were listed to demonstrate the various biomedical applications of 2D pnictogens in detail.Benefiting from their intriguing optical and electronic properties, 2D pnictogens with large surface areas have been widely explored as promising nanoagents for photothermal therapy, photodynamic therapy, drug delivery,etc.Furthermore,considering antioxidation and physiological signal transduction, BP NSs have already produced a lot of biomedical research achievements in the anti-inflammation, neurodegenerative diseases treatment, and tissue repairing fields.
Although significant progresses have been made for the biomedical applications of 2D pnictogen materials in recent years,some obstacles and challenges remain.On one hand, there is still lack of standard methods for the mass production of high quality and reproduced 2D pnictogens nanoagents.Despite liquid exfoliation method was appropriate and beneficial for large-scale production, but the lateral size and thickness of obtained 2D pnictogens nanoagents are not uniform, which may not satisfy the clinical demands.On the other hand, the biodegradation mechanism of 2D pnictogens still remains unclear, along with their biocompatible activity.The biomedical applications of 2D pnictogens are still in their infancy, and their clinical translation requires more attentions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.31800834 and 52071120), the Open Foundation of Shenzhen Bay Laboratory(No.SZBL2019062801005), Fundamental Research Funds for the Central Universities (No.JZ2020HGTB0031), and the University Synergy Innovation Program of Anhui Province (Nos.GXXT-2019-045 and GXXT-2020-063).
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
