Arsenic Accumulation in Rice:Sources, Human Health Impact and Probable Mitigation Approaches
Md Rokonuzzaman, Li Wai Chin, Man Yu Bon, Tsang Yiu Fai, Ye Zhihong
Review
Arsenic Accumulation in Rice:Sources, Human Health Impact and Probable Mitigation Approaches
Md Rokonuzzaman1, Li Wai Chin1, Man Yu Bon1, Tsang Yiu Fai1, Ye Zhihong2
()
The human body loading with arsenic (As) through rice consumption is a global health concern. There is a crucial need to limit As build-up in rice, either by remediating As accumulation in soils or reducing As levels in irrigation water. Several conventional approaches have been utilized to alleviate the As accumulation in rice. However, except for some irrigation practices, those approaches success and the adoption rate are not remarkable. This review presents human health risks posed due to consumption of As contaminated rice, evaluates different biomarkers for tracing As loading in the human body, and discusses the latest advancement in As reducing technologies emphasizing the application of seed priming, nanotechnology, and biochar application for limiting As loading in rice grains. We also evaluate different irrigation techniques to reduce As accumulation in rice. Altering water management regimes significantly reduces grain As accumulation. Bio- and nano-priming of rice seeds improve germination and minimize As translocation in rice tissues by protecting cell membrane, building pool around seed coat, methylation and volatilization, or quenching harmful effects of reactive oxygen species. Nanoparticle application in the form of nano-adsorbents or nano-fertilizers facilitates nano-remediation of As through the formation of Fe plaque or sorption or oxidation process. Incorporating biochar in the rice fields significantly reduces As through immobilization, physical adsorption, or surface complexation. In conclusion, As content in cooked rice depends on irrigation source and raw rice As level.
arsenic; rice; scalp hair; irrigation management; seed priming; nanotechnology; biochar; human health
Globally, 37% of terrestrial land is used for agriculture, and its major portion is used for rice production to feed more than half of the human population as a staple food (Smith et al, 2008; Islam et al, 2020a). Although more than a hundred countries currently produce rice worldwide, 14 Asian countries account for 90% of global rice production using groundwater as the significant irrigation source (Islam et al, 2016; Yu et al, 2020). Rice is a semiaquatic plant and requires a vast quantity of water starting from its establishment in the main fields (Mainuddin et al, 2020). Arsenic (As) is released into the groundwater both naturally and anthropogenically. The occurrence of As over its safe limit proposed by WHO (< 10 μg/L) and FAO (100 μg/L) for irrigation water is a significant concern worldwide (Chakraborti et al, 2018).
Of the total accumulated As in rice, inorganic As (iAs) accounts for up to 86%–99% (Rahman et al, 2014). Rice accumulates almost 10 times more As than other crops including barley, maize and wheat since reductive conditions prevail in rice fields (Williams et al, 2007), and naturally, this problem is even higher in Asian rice-producing countries such as Bangladesh, Indiaand China (Williams et al, 2005). Since approximately 54% of global rice production is used for local consumption and the rests are traded worldwide crossing the borders of the countries, which leads to place As enriched rice in the bowls of the daily meal of the people far from its country of origin (Meharg et al, 2009). Thus, rice consumption exemplifies a key route for As exposure in most nations, exclusively for populaces enjoying rice diets constituting up to 60% of their daily meal (Islam et al, 2016). Because of the daily consumption of rice, As accumulates and poses severe threats to the human body (Williams et al, 2005). Many researchers have revealed that chronic exposure to As may induce cancers and other diseases (Mazumder et al, 2000; Sobel et al, 2020).
To minimize the body loading of As through the consumption of contaminated rice, researchers suggested several mitigating strategies over the last few decades, focusing on their technical feasibility, practical applicability and efficiency (Kumarathilaka et al, 2020). Here,we evaluated human exposure with As through rice consumption and potential biomarkers used to trace the As, and also evaluated the impact of manipulating irrigation management, seed priming, some technical agronomic innovations such as nanotechnology and biochar application for limiting As loading in rice grains. Future research direction is also highlighted for producing safe rice.
As sources, and factors affecting As accumulation and speciation in rice
Although there are both natural and anthropogenic sources of As exposure of humans, drinking of contaminated groundwater, and consumption of fish and rice cultivated with contaminated groundwater are the major natural sources. Since As toxicity is associated with its solubility, which is actually influenced by pH differences and redox variations(Upadhyay et al, 2019). For example, the bioavailability of As increases with an increase in soil pH (Yao B M et al, 2021), and the inorganic form of As such as As(III) is the stable form under reducing conditions, i.e., anaerobic soil, while arsenate As(V) is the dominant form under aerobic conditions (Meharg and Zhao, 2012; Upadhyay et al, 2019). Among the organic forms, in addition to some other methylated arsenicals, monomethylarsonous acid [CH3As(OH)2, MMA(III)], monomethylarsonic acid [CH3AsO–OOH, MMA(V)], dimethylarsinous acid [(CH3)2AsOH, DMA(III)], and dimethylarsinic acid [(CH3)2AsOO–, DMA(V)] are regarded as the critical metabolites of As metabolism, where several thiolated arsenicals are also considered potential As species available in soils and crops (Upadhyay et al, 2019). However, inorganic forms of As are known to be more toxic than organic forms due to their bioavailability and toxicological and physiological effects, and the toxicity of As species occurs in the following order: DMA(V) < MMA(V) < As(V) < As(III) (Baig et al, 2010; Upadhyay et al, 2019), while Luvonga et al (2020) claimed the following order of toxicity: DMA(V) = MMA(V) < As(V) < As(III). On the other hand, Nookabkaew et al (2013) found that the major As species in rice followed the order: MMA(V) < As(V) < DMA(V) < As(III).
When considering the species of As based on the spatial differences, various As species prevail in rice at different origins. According to Batista et al (2011), As(III) (39.7%) prevails in Brazilian rice, followed by DMA(V) (38.7%), As(V) (17.8%) and MMA(V) (3.7%). Torres-Escribano et al (2008) claimed that Spanish rice contains an average of 62% iAs. As(III) (60%–80%) prevails in Switzerland rice, followed by DMA(V) (20%), As(V) (15%) and MMA(V) (< 3%) (Guillod-Magnin et al, 2018). The market basket study of Sarwar et al (2021), collecting rice from 14 cities of Pakistan (= 438), suggested that Pakistani rice contains more As(III) than As(V). Indian and Bangladeshi rice contains almost 80% iAs (Williams et al, 2005), similar to Chinese rice (78% iAs) (Zhu et al, 2008), while the European Union (EU) and USA (46% iAs) rice contains a higher percentage of DMA(V) (Zavala et al, 2008; Zhao et al, 2013). According to Nookabkaew et al (2013), brown rice in Vietnam contains the highest amount of As(III) (190.50 ± 1.00) μg/kg compared to brown rice (110.45 ± 8.01) μg/kg and red rice (105.47 ± 7.13) μg/kg in Thailand.
Correlation between As values in different parts of rice plants
As translocation from rice roots to grains involves several steps, which causes possible variations among genotypes, and higher accumulation in roots due to the transformation of As(V) to As(III) may minimize As translocation to aerial parts as well as grains (Islam et al, 2017; Suriyagoda et al, 2018). As accumulates in rice plants in the following manner: grain < husk < leaf < straw(stem) < root (Islam et al, 2016). However, Yao B M et al (2021) claimed that rice roots and leaves accumulate more As than stems and grains in the following order: grain < husk < stem < leaf < root. With rising soil As concentrations, plant As increases accordingly, and researchers have demonstrated overwhelming deviations in grain-As levels globally (Islam et al, 2016). Mukherjee et al (2017) reported that As in irrigation water is positively correlated with grain As. Soil-available As is also significantly correlated with As content in the roots (Bhattacharya et al, 2010a) and grains (Chohan et al, 2020), but no such relationship is observed between As in grain- and soil-total As (tAs) (Khan et al, 2010; Mukherjee et al, 2017), although soil-available As has a significant positive correlation with soil tAs (Kar et al, 2013). In contrast, Hossain et al (2008) explored a strong positive correlation between soil- and grain-tAs. According to Lu et al (2009), grain tAs levels usually increase with increasing soil tAs and reach a saturation plateau at > 10 mg/kg soil. However, Talukder et al (2012) claimed that straw As has a strong positive relationship with grain As for both boro (BRRI dhan29) and aman (BRRI dhan32) rice varieties. Similar findings were reported by Wu et al (2011).
Regression results on influence to grain As variances
Bogdan and Schenk (2009) revealed that soil tAs has a significant positive influence on the As content in rice straw and polished rice through linear multiple regression analysis. Considering grain As(V), DMA(V), As(III), arsenocholine (AsC) and MMA(V)as the dependent variables and soil tAs, root As species, straw As species and root tAs as the parameters for prediction, the stepwise multiple linear regression analysis of Ma et al (2017) showed that straw MMA(V) and straw As(V) explain 76% of the variance (2= 0.760) for grain As(III), where root arsenobetaine, straw MMA and straw As(III) explain 72% of the variance (2= 0.721) for grain As(V). The four predictors, root As(V), root tAs, straw MMA(V) and straw DMA(V) explain more than 85% of the variance (2= 0.854) for grain MMA(V); straw AsC, root As(III), straw DMA and soil tAs explain almost 90% of the variance (2= 0.907) for grain DMA, and 92% of grain AsC variance (2= 0.929) is explained by two predictors, root As(V) and straw AsC. According to Lin et al (2015), rice roots (2= 0.930) are a more significant determinant of grain As than rice straw (2= 0.560) and top soils (2= 0.000). However, Hossain et al (2008) and Lu et al (2009) in Bangladesh revealed a highly significant regression relationship between soil and shoot As, as well asbetween soil and grain As.
Interaction between As and other elements
The status of various elements is critical to modulate the biogeochemical cycle of As, and many biological functions that can affect As accumulation in rice grains. As(III) and silicic (Si) acid use the same transport routes to reach the rice root cells, therefore, more Si input poses a significant antagonistic impact on As(III) and limits its uptake and aboveground transportation in rice (Ma et al, 2008). Similarly, higher phosphate content is vital in limiting As(V) absorption and accumulation in rice since both elements are chemical analogues and use the same transporter, OsPT8, to enter into the rice roots from the soil solution (Wang et al, 2016). Research revealed the linkage of nitrate (NO3–) to oxidize As(III) to As(V) in the presence of denitrifying bacteria (Oremland and Stolz, 2003). Chen et al (2008) showed that KNO3incorporation to rice soils decreases tAs level in shoots and roots by up to 40%. The environmental coexistence of As and sulfur (S) facilitates sharing identical chemical as well as biological redox transformations and interlinked biogeochemical cycles (Hollibaugh et al, 2005), where S is reported to facilitate the whole redox cycle (Hollibaugh et al, 2006). Williams et al (2009) revealed a significant decrease in grain As content with the increase in grain selenium (Se). Norton et al (2010) reported that higher As-containing varieties accumulate lower Se, Cu and Ni contents in rice grains.
Analytical techniques for As determination
Hydride generation-atomic absorption spectroscopy (HG- AAS), HG-atomic fluorescence spectroscopy (HG-AFS),inductively coupled plasma mass spectrometry (ICP-MS),ICP-optical emission spectroscopy (ICP-OES), high- performance liquid chromatography coupled to ICP- MS (HPLC-ICP-MS), electrothermal-atomization atomicabsorption spectrometry (ET-AAS),graphite furnace- AAS (GF-AAS), neutron activation analysis (NAA) and test kit method (low cost field based method) all can be used to detect As content in rice. Given the limit of detection constraints, only HG-AFS and ICP-MS are extensively used for determining As content in rice grains, while HG-AAS and ICP-OES are appropriate for higher As content in grains (Meharg and Zhao, 2012). Though ICP-OES is equipped for chromatographic eluent acceptance, it is still lack of the capability as a detector functioning to determine species of grain As (Tyson, 2013). The detection efficiency of ETAAS for As is two magnitude inferior than that of ICP-MS, however, it is possible to measure tAs by ETAAS adequate precision but not the iAs since it cannot work to detect capillary electrophoresis or chromatography (Tyson, 2013). HPLC-ICP-MS is preferred for measuring As species with the benefits of detecting low limit (Rasmussen et al, 2013). According to de la Calle et al (2011), with a view to mediating the issue whether the determination of iAs and tAs depends on their analytical method or not, a proficiency test was organized by the European Union Reference Laboratory for Heavy Metals in Feed and Food entitled ‘Report of the seventh inter-laboratory comparison organized by the European Union reference laboratory for heavy metals in feed and food IMEP-107: Total and inorganic As in rice’. The IMEP-107 (International Measurement Evaluation Programme-107) took the endeavor with 103 laboratories registered from 35 countries where results for tAs and iAs came from 98 and 32 laboratories, respectively, using different instrumental techniques (NAA, ICP-MS and HG-AAS). Results showed the grain iAs concentration measured does not rely on the analytical method used to determine, while all the instrumental methods are come across to be dependable to characterize grain As concentration. However, this conclusive remark cannot be justified independently due to lack of available raw database.
Seasonal variation influencing rice As content
A significant variation in grain As content has been noticed with the variation in cultivation seasons, particularly, winter (boro) and monsoon (aman) seasons (Bhattacharya et al, 2010b). According to Imamul Huq et al (2011), the higher As loading in the rhizosphere and its rapid bioavailability during the winter season mainly due to the irrigation with As-contaminated groundwater in rice fields. Williams et al (2006) recorded the variability in rice growing seasons, boro and aman significantly determine the grain As content. Guo et al (2013) put forward an increased use of groundwater that might trigger dissociation of sediment-As into the groundwater facilitated by the iron oxyhydroxides dissolution or As(V)’s reductive desorption. In contrast, Chauhan et al (2009) argued no significant seasonal variance in groundwater As level in winter, monsoon and summer season. Cheng et al (2005) also claimed similar results. Roberts et al (2010) calculated a 13%–62% loss of soil As due to monsoon flooding accumulated during the winter crop cultivation. Such seasonal basis loss of soil As during rice cultivation has been reported in study of Lu et al (2009).
Human health risk due to inorganic As exposure
The quantity of daily As uptake via rice consumption is largely determined by the volume of rice in meals (Singh et al, 2015). According to the FAO (2004), the average consumption rate of rice for one person varies by up to 650 μg/din many Asian countries, where much lower consumption rates have been reported in some European and African nations. Out of the tAs content in rice, iAs accounts for approximately 96.8% (Roychowdhury, 2008), whereas rice from Asian countries contains up to 99% (Rahman et al, 2014). Exposure to iAs through rice consumption is responsible for internal and external cancers and many other diseases (Sobel et al, 2020). The daily intake of tAs through foods has been estimated in different countries. Mean daily tAs intake and its limit in rice and rice-based foods are presented in Table 1. The PTWI (Provisional Tolerable Weekly Intake) of As is 15 µg/kg body weight each week for everybody as established by FAO/WHO (Díaz et al, 2004). The NOAEL (No Adverse Effect Level) for chronic exposure through oral intake was established at 1µg/kg per day for everybody, where the LOAEL (Low Adverse Effect Level) for the same was established at 10–100 µg/kg body weight per day for everybody (Larsen, 1993;Díaz et al, 2004).
Presence of As in human biological samples
According to Mossop (1989), exposure to only 0.25 mg/kg iAs can generate poisoning symptoms in the human body. Just after ingestion, As is quickly metabolized and precipitously defecated in the urine, mostly following direct dietary exposure (Vahter, 2002). The rest of the As binds with the hemoglobin protein (Habib et al, 2002). Within 24 h, As present in the blood accumulates in various body organs, includingskin tissue, liver, spleen, bone, lung, kidney and muscle(Habib et al, 2002). Two to four weeks after absorption, the maximum As present in the body system is concentrated in the skin, nails and hairs, and gradually excreted (Habib et al, 2002).
Approaches to alleviate As accumulation in rice
Since As mobility in wetlands is controlled by the redox potential of soils, As mobility in rice fields and its uptake and transfer to rice grains can be minimized by altered water management (Zhao et al, 2010). Besides water management, seed priming (Moulick et al, 2016, 2017, 2018a, b), some technical agronomic interventions such as biochar (Wen et al, 2021) and application of nanotechnology (Maity et al, 2021) are some recently innovated techniques to limit As accumulation in rice.

Table 1. Mean daily total arsenic (As) intake and its limit in rice and rice-based foods.
WHO, World Health Organization; FAO, Food and Agriculture Organization of the United Nations; JFWCAC, Joint FAO-WHO Codex Alimentarius Commission; iAs, Inorganic As.
Arsenic alleviating altered irrigation management
Alternate wetting and drying (AWD) practice
Very few field trials have been conducted with AWD as an As mitigation approach. A study by Das et al (2016) with three treatments, AWD, non-flooded (NF), and conventionally flooded (CF) practices, explored insignificant differences in soil As levels and found a decrease in grain As in the order of NF < AWD < CF, while yield contribution was reported in the increasing order of AWD > NF > CF. Compared with CF, AWD and NF treatments reduces tAs concentration in rice grain by 49.7% and 53.0%, respectively. Further, significant (< 0.05) decreases in As(V) and As(III) levels in husks and grains are reported in NF and AWD than in CF. The enhanced phytoavailability of As in CF might be due to the enhanced reductive mobilization of As in flooded conditions (Roberts et al, 2010). Chou et al (2016) and Acharjee et al (2021) support this finding concerning As reduction in AWD compared with CF. Acharjee et al (2021) revealed significant As reduction in rice under AWD compared with CF. Chou et al (2016) investigated the effect of CF, aerobic (AR) and AWD irrigation practices on As loading status of two rice varieties Tainan 11 and Tainong 84, each in one season. The result reported that As(III) (AWD < AR < CF) prevails in brown rice in the both rice season, followed by As(V) and DMA(V). Grain tAs content reduces significantly in AR and AWD compared with CF because of the changes in oxidation and reduction process due to irrigation management.
While practicing single soil drying with ‘safe AWD’, Carrijo et al (2018) observed that grain As content does not decrease at ~0 cm soil water potential at 0–15 cm below the soil surface, but soil drying to -71 kPa or -154 kPa, marked as medium severity and high severity, respectively, reduces 41%–61% of grain As. They suggested that since the grain As level reductionlargely depends on soils reaching the unsaturated state, safe AWD allows continuous saturation state and therefore cannot perform well. This finding is comparable with the results of Islam et al (2019) and Yang et al (2019). The field trials of Rahman et al (2015) and Shah et al (2016) revealed AWD practice contributes significant grain As reduction with higher grain yield compared to continuous flooding.
The two-year field trial of Linquist et al (2015) in the USA with several irrigation treatments revealed that AWD accounts for a yield decline (< 1% to 13%), but the As content in rice grains under AWD decreases remarkably compared to that under CF. In contrast, when practicing AWD with fertilizer management, Islam et al (2020b) observed that early AWD practices reduce grain As by 66% without sacrificing grain yield. This sustained yield with AWD management agrees well with the previous studies (Qin et al, 2010; Islam et al, 2017). AWD’s success largely depends on the rice variety selected (Norton et al, 2019). Grain As levels are increased with the increasing number of identifying quantitative trait loci (QTLs) in selected rice varieties (Norton et al, 2019; Fernández-Baca et al, 2021). For example, Norton et al (2019) conducted a 2-year study with AWD irrigation management and some rice varieties to determine genotype by the interaction of water management with QTL for As accumulation. They observed a 15.7% grain As reduction in the first year when the declining percentage is 15.1% in the next year, and concurrently, 27% less As accumulation is recorded compared to continuous flooding practice. They reported that six QTLs exhibit stability in water treatments across years out of a large number (74) identified individual QTLs for As. On the other hand, Fernández-Baca et al (2021) identified seven QTLs influencing grain iAs accumulation such as C4_2481896, C4_27292997, C5_19872059, C8_5186967, C9_18034390, C11_2659978 and C12_824609.
Aerobic rice cultivation
Xu et al (2008) evaluated As accumulation in a greenhouse experiment in rice grains and shoots under aerobic and flooded conditions. Under CF condition, the As level in the soil solution was 7–16- and 4–13-fold higher than that under NF conditions, i.e., without As and adding As (added 10 mg/kg as arsenite or arsenate), respectively.CF enhances the reductive dissolution of iron oxyhydroxides, which facilitates the dissociation of the adsorbed As to the solution. However, the result represents 10–15-fold grain As and 10–15-fold grain yield reduction compared to CF. They also claimed that with increasing tAs level in rice grains, the proportion of DMA(V) increases while iAs (predominant species) decreases. Arao et al (2009) reported tAs, iAs and DMA reduction in rice grains (tAs from 950 to 100 μg/g; iAs, As(III) + As(V), from 450 to 120 μg/g; DMA from 480 to 10 μg/g) of Koshihikar rice variety in aerobic rice cultivation than those for flooded cultivation. The greenhouse experiment of Li et al (2009) and the pot experiment of Talukder et al (2012) support this finding. As a semiaquatic plant, rice cultivation typically needs a flooding state to ensure maximum yields, therefore, substantial grain yield losses have been recorded under aerobic irrigation practices (Sarkar et al, 2012). In contrast, Duxbury and Panaullah (2007) found a positive impact on rice yield while practicing aerobic irrigation regimens.
Intermittent irrigation practice
In a field experiment, Somenahally et al (2011) found grain As reduction upto 50% for As(V) and 5%–30% for As(III) inaddition to tAs in intermittent flooding (IF) than CF plots. According to Shrivastava et al (2020), IF gradually reduces grain As by 40%–63% in the consecutive years of 2013 to 2016. The possible explanation behind such grain As reduction in IF is the lower irrigation requirement and subsequent lower As bioavailability. Xu et al (2008), Sarkar et al (2012) and Spanu et al (2012) support the above findings. While comparing intermittent ponding with aerobic ponding, Sarkar et al (2012) argued a decrease in As content in roots, leaves and grains in the order of intermittent ponding > aerobic regimes. The only exception is Duxbury and Panaullah (2007), who could not generate a conclusive argument for the effect of deficit irrigation practice on reduced grain As content. The selection of suitable rice variety determines IF practice’s performance. For example, Hua et al (2011) revealed statistically similar (< 0.98) As accumulation on Cocodrie and Rondo rice varieties, while another variety, Zhe 733, accumulates much less As (< 0.07 or 0.12) in similar water management and soil As content.
Raised bed cultivation
Duxbury and Panaullah (2007) conducted a field trial with a boro rice variety BR29 with two irrigation regimens, conventional flooding and raised bed practice. The results indicated a reduced straw and grain As accumulation in aerobic practice than in conventionally flooded plots. This reduction is possibly due to the less-reducing state in the soil during the rice growth stage (Somenahally et al, 2011). Talukder et al (2011) observed that raised bed cultivation requires 30% less water use than conventional practices and simultaneously reduces straw and grain As by 86% and 62%, respectively, with an increase of 13% in grain yield. The possible reason might be that there remains a higher redox potential (Eh) value in raised beds than the conventional rice fields and thus contains low As content. Similar to permanent raised beds, furrow- irrigated periodically raised beds significantly decreasebioaccumulation of As compared with the conventionally flooded practice (Talukder et al, 2011). Iron- oxyhydroxides are pretty stable in the furrow-irrigated fields because of a comparatively oxidized state there, which ultimately keeps the soil As unavailable to the rice plants (Aide et al, 2016).
Sprinkler irrigation
Moreno-Jiménez et al (2014) compared flooded and sprinkler irrigation (SI) practices under the Mediterranean regime with rice variety Gladio. The results revealed that grain tAs decreases over seven years in successive SI to one-sixth (tAs decreases from 547 to 89 μg/g; iAs decreases from 138 to 25 μg/g) of it’s early concentration in the submerged system, while one-third (tAs decreases from 547 to 200 μg/g; iAs decreases from 138 to 70 μg/g) of grain As is reduced in only one cycle of SI, with no significant difference for yield between the two practices. On the contrary, iAs and DMA concentration in ricegrain are enhanced by two folds under CF compared with SI, which might be due to the methylation under CF condition. The field trial of Spanu et al (2012) with 37 genotypes revealed 50 times (tAs reduced from 95–235 μg/g to 1.3–5.1 μg/g) less grain As under SI than those under CF. However, for all genotypes, a total of 95.5% to 98.0% As is reduced. They added such extensive genotypic variability in As bioaccumulation suggests a ‘genetic variability effect’. Genetic effect on As bioaccumulationin rice is also evident (Norton et al, 2009; Ahmed et al, 2011). Since 9%–10% of the variability of grain As accumulation accounts for the genetic variation (Ahmed et al, 2011), the environment plays a crucial role by 69%–80% in the amount of As accumulation in rice grains (Norton et al, 2009).
Seed priming
There is a range of seed priming techniques, viz., osmopriming, hydropriming, nutripriming, hormonal priming, biopriming, nanopriming or some plant- based natural extracts (Farooq et al, 2019). Different parameters for seed priming with the consequesnes for rice are presented in Table 2.
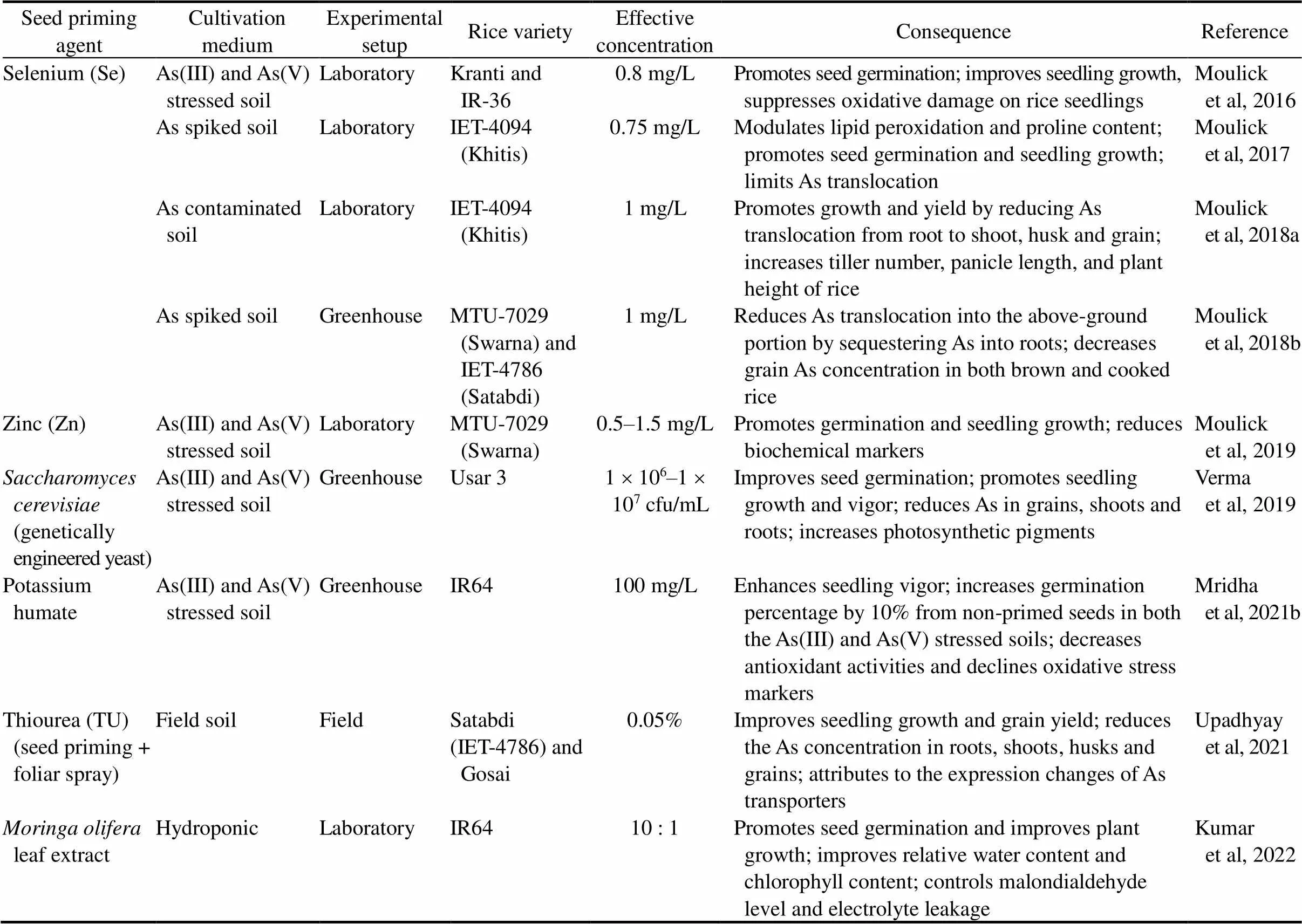
Table 2. Effect of seed priming on arsenic (As) accumulation and toxicity in rice plant.
Mridha et al (2021b) suggested that the overall impact of potassium humate (K-humate) on As stress is the gibberellin and abscisic acid regulating capacity of both K and humic acid and the metal chelation characteristics of humate. Moulick et al (2016, 2017, 2018a, b, 2019) proposed that rice seed priming with selenium (Se) prior to sowing may stimulate seed germination, and increase seedling growth by quenching the harmful effects of reactive oxygen species, and reduce As induced toxicity by building Sepool around the seed coat and enhancing detoxification, and defend the damage of cell membrane by maintaining total phenolics and proline and decrease As uptake by As-induced redox imbalance modulation in both soil-less and soil-based environments.
Upadhyay et al (2021) reported a decline in As uptake by rice roots in Thiourea (TU) amendment might be attributed to the expression changes of As transporters such as(). Further, nodes and shoot tissues play a potential role in As sequestering to cell walls and vacuoles to limit its upward transportation. TU application works at the molecular level and controls redox which acts critically to maintain the reduced As(III) to facilitate its sequestration. Yadav and Srivastava (2021) also reported a similar finding. Verma et al (2019) suggested the seedling improvement and significant As reduction in rice plantsmight due to the methylation and volatilization process imparted by genetically engineered (GE) yeast. This finding is comparable with the result of Chen et al (2014) using GE microorganisms. Enhanced seed germination and improved seedling growth parameters reported by Sivakumar et al (2017) with phosphobacteria are due to its phosphorus solubilizing capacity. Apart from the above, Kumar et al (2022) foundleaf extract ameliorates As toxicity through limiting malondialdehyde level and electrolyte leakage.
The prime benefit of priming rice seeds in respect of impacting the agro-environmental factors is that this technology bypasses soil texture, presence or absence of cationic and anionic species, pH for irrigation water and soil, redox potential (Eh), varietal differences, agronomic practices and seasonal aspects that can influence As loading in the plant (Moulick et al, 2021). Nanomaterial utilization in seed priming supplements nutrients, and enhances nitrogen use efficiency and consequently reduces chemical fertilizer application and protects the agro-environmental (Upadhyaya et al, 2017; Priya et al, 2018). Despite substantial improvements in the utilization of seed priming nanotechnology in rice cultivation, of concern, there is still no general rule regarding the nanopriming of rice seeds and no distinct trend concerning the responses of priming based on the species taxonomy (Shelar et al, 2021). Such misdirections can facilitate bacterial and fungal contamination, significantly impacting the rice agro-environment and subsequently hindering seed germination (do Espirito Santo Pereira et al, 2021). Extensive research is still required in applying nanomaterials and nanoparticles used for priming rice seeds, particularly for shaping the comprehensive impact of those techniques on rice agro-environment and human health (Moulick et al, 2021; Shelar et al, 2021).
Application of technical agronomic innovations
Application of nanotechnology
In recent years, the rapid advancement and utilization of nanotechnology hastens the invention of engineered nanoparticles and their practical applications in crop production (Wang et al, 2019). The modification of the synthesized nanomaterials into nano-adsorbents, nanofertilizers and nanopesticides makes it suitable for need-based nano-remediation of heavy metals (Khan et al, 2020; Ma et al, 2020; Maity et al, 2021). Different parameters and consequences of nanoparticlescurrently being used predominantly for As reduction in rice production are presented in Table 3.ZnO and zinc ion (Zn2+) significantly lowers the As load in rice tissues (Ma et al, 2020), suggesting thattransporter might have been affected by the Zn2+treatment. The significant decrease of As(V) in Zn2+amended roots may be because of enhanced reduction of As(V) into As(III) by As reductase within the root cells. Wu F et al (2020) and Yan et al (2021) suggested that the significant improvement in germination, promoted biomass, and reduced As accumulation in rice are due to the supplement of Zn nutrients upon ZnO application. On the other hand, Wang et al (2018) claimed a possible negative phyto-effect of co-occurring As(V) or As(III) and ZnO NPs (nanoparticles), leading to a significantly weak performance of As transporters for less As content in rice plants.
Wu et al (2021) demonstrated that nano-titanium oxide (TiO2) amendment notably alleviates oxidative stress resulting from As exposure and significantly reduces As bioaccumulation in rice seedlings. According to Li et al (2019), the addition of α-MnO2nanorods can effectively control the soil-to-solution partitioning of As under anaerobic conditions, which dramatically reduces the mobilization and transportation of As in soil-rice systems. Zhou et al (2015) reported a significant negative relation between As and Mn in rice, leading to less As loading in rice. Liu et al (2014) showed that reduction of As content in brown rice is possibly by As sequestration into shoot cell walls facilitated by nanoscale silica (Si) of rice shoot. Cui et al (2020) found that the minimized As uptake in rice is due to the loweredand2 gene expression.
Hu et al (2020) revealed iAs reduction in rice is due to Fe plaque formation upon zerovaleation (Fe0)application. On the other hand, Huang et al (2018) found nano-Fe3O4and nano-Fe0lower the effect of antioxidants to the aboveground portion of rice plants.
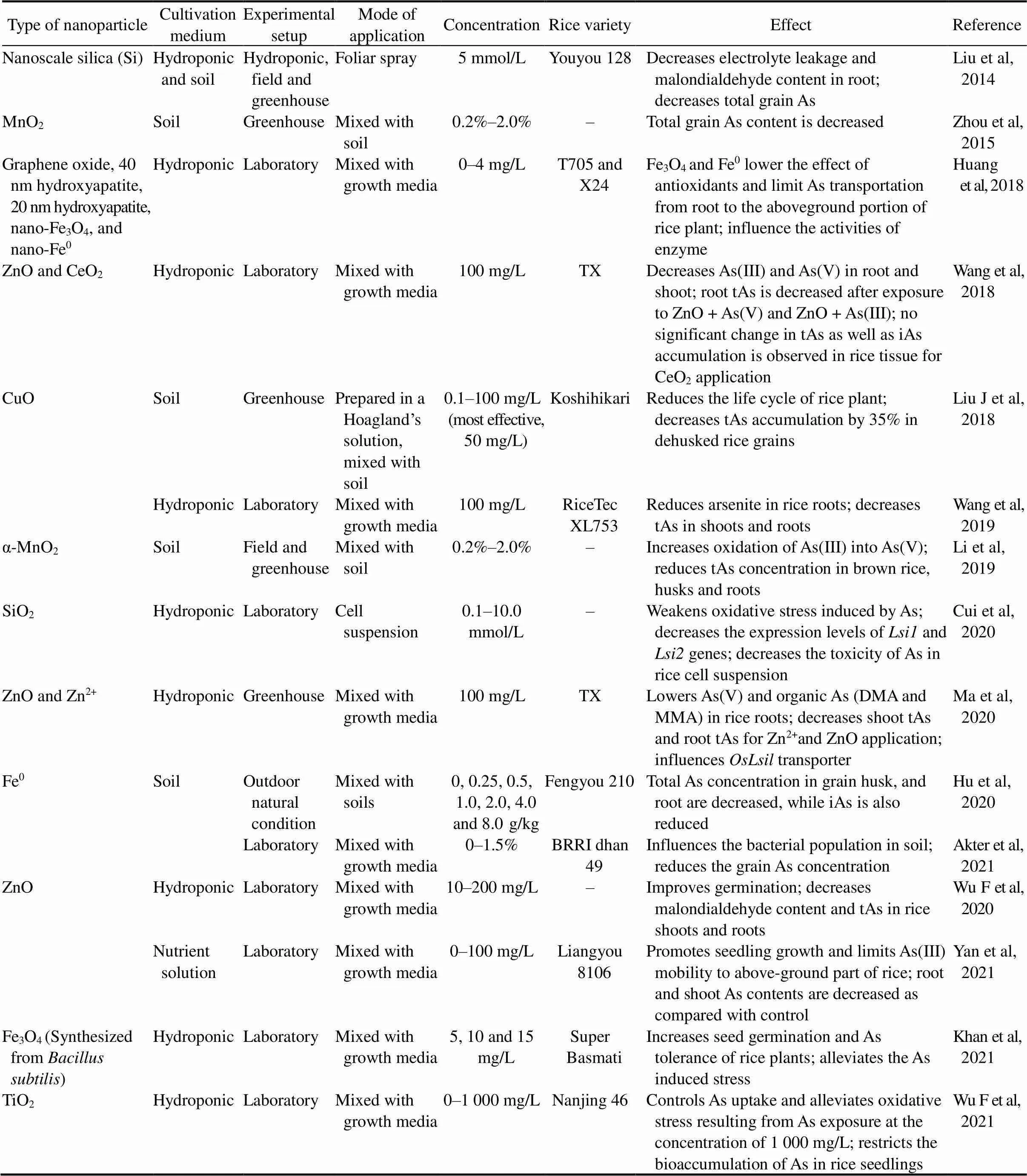
Table 3. Effect of nanotechnology on arsenic (As) accumulation and toxicity in rice plant.
Fe0, Fe plaque formation upon zerovaleation; DMA, Dimethylarsinic acid; MMA, Monomethylarsonic acid; iAs, Inorganic As; tAs, Total As.
Khan et al (2020) showed Fe3O4attracts negatively charges As on its surface and reduces As entry. Wang et al (2019) addressed CuO’s unique ‘nano-effect’ for impacting As loading in rice. Liu et al (2018a) reported that CuO decreases the As concentration below the ‘no observed adverse effect level’ (0.80 μg/kg per day) by reducing the life cycle of rice plants.
Biochar application
Biochar is prepared through the thermal decomposition of organic biomass under low levels of O2(pyrolysis) (Kumarathilaka et al, 2020). Different parameters of biochar including its preparation, using media and effect on rice are presented in Table 4. According to Khan et al (2014), sewage sludge- derived biochar increases the phosphate content which protects As to be transferred together with the same transporter. Seyfferth et al (2016) observed the formation of As-DOC (dissolved organic carbon) complexes speeds up As immobilization due to the application of rice husk-derived biochar (1%) enriched with Si. According to Wu et al (2017), rice straw biochar modified with the red mud develops special features by combining metal oxides of red mud with the large surface area and porous structure of biochar. Wu J Z et al (2020) revealed that calcium-based magnetic biochar (Ca-MBC) facilitates the adsorption of pore water As and limits rice As. Yu et al (2017) suggested that manganese oxide reduces As content because it can strongly adsorb As(V) and promote arsenite to arsenate oxidation.
As reduction in rice tissue uponiron-manganese oxide modified corn straw-based biochar (FMBC1) application reported by Lin et al (2017) is due to the oxidation of As(III) to As(V) which might be facilitated by Fe-Mn oxide and the formation of Mn and Fe plaque on the root surface. Qiao et al (2018, 2019) explored that the palm fiber and Fe0application might impose a synergistic effect and reduce As bioavailability by forming Fe plaque. Pan et al (2019) observed that the grain As reduction is facilitated by the increase in soil pH by single or combined application of biochar/FeSO4/silica sol. On the other hand, Leksungnoen et al (2019) and Linam et al (2021) suggested that Si facilitates the methylation of As and reduces As uptake by rice plant.
According to Rong et al (2020), biochar +Fe(II) facilitates Fe plaque formation and limits As content in rice tissues. The As immobilization for modified rice husk-based biochar application is facilitated by (i) oxidation of As(III) to As(V) bygene, (ii) lowering of microbe mediated As release from iron minerals, and (iii) adsorption on a Si-ferrihydrite complex(Herath et al, 2020). Liu et al (2020) postulated that carbide slag amendments change the forms of As to less-available and form Fe-Mn plaque to restrict As transfer in rice.
Islam et al (2021a) recorded substantial grain As reduction due to the formation of Fe-plaque in roots. Similarly, Yao Y et al (2021) revealed As reduction in rice since As gets bound with the crystalline hydrous oxide and Fe-plaque. According to Kumarathilaka et al (2021a), As reduction in RBC (rice biochar) is due to the introduction of Si and sulphate in rice water. Kumarathilaka et al (2021b) suggested that grain As reduction in birnessite-modified rice hull biochar (Mn-RBC)-intermittent water management facilitates through oxidation of As(III) by Mn. Wen et al (2021) revealed a significant grain As reduction might be due to the reduction of soil urease and catalase activities with Fe+ biochar application.
According to Lin et al (2021), the application of corn stem-based biochar amended with Fe-Mn-La can control As volatilization, and reduce methylation, crystallization and dissolution of As. Yang et al (2020) revealed that-gene carried byand-gene carried byandpotentially mediate As(V) decrement under straw and straw biochar amendments, respectively. On the contrary, Wang et al (2017) showed that the supplementation of biochar augments the richness ofandgenes, which is associated with the reduction of As(V) into As(III). Zhang et al (2020) showed that the corn stalks derived biochar amended with iron-manganese-cerium oxide decreases As bioavailability, and improves soil pH and potentially enhances soil redox capacity. With similar biochar and amending materials, Lian et al (2020) showed grain As reduction due to the formation of Fe-Mn plaques, which restricts the As uptake by the rice plants.
Impact of cooking methods on iAs load in rice
The prime causes for higher As content in cooked rice in As burdened regions are the As content in raw rice derived from soils and irrigating As contaminated groundwater and cooking with As-contaminated water (Mridha et al, 2021a). Again, since changes in As speciation are not evident due to cooking, the As species present in the cooked rice depends on its source orientation, which means cooking water or raw rice. Laparra et al (2005) revealed no significant modifications in iAs and tAs contents in cooked rice boiled with uncontaminated water while iAs concentrationis increased found after adding As(V) in the cooking water. O’Neill et al (2013) measured 24 times higher iAs ingestion through cooked rice whencooking water containing As above 50 μg/L. Similarincreasing trend is also evident from the studies of Roychowdhury (2008) and Mandal et al (2019). Sengupta et al (2006) revealed that the traditional method of cooking (at rice water ratio of 1:6) with less As-contaminated water and discarding gruel removes approximately 57% of As in the cooked rice while cooking rice with As contaminated water of 50 μg/L enhances up to 35%–40% of As in cooked rice. According to Mridha et al (2021a), cooking rice with As-safe water significantly releases As into the gruels. The result of Chowdhury et al (2020) supports this finding. Bae et al (2002) measured the retained As content in cooked rice as the impact of boiling and observed a higher As content in cooked rice than the raw rice, which might be due to the induced chelating effect. Laparra et al (2005) explored the iAs and tAs in cooked rice simulated gastrointestinal digestion.

Table 4. Biochar type to mitigate arsenic (As) accumulation and toxicity in rice.
Fe0, Fe plaque formation upon zerovaleation; Fe-BC, Iron-modified biochar; MMT, Montmorillonite; iAs, Inorganic As; tAs, Total As.
Gray et al (2016) demonstrated a decreasing trend of grain iAs accumulation while cooking As contaminated brown longgrain, white medium grain, and parboiled rice with deionized water at a ratio of 2:1, 6:1 and 10:1 (water:grain) with increasing the volume of water. Cooking with a low amount of water does not eliminate As content, however, increasing water volume reduces up to 45% of iAs. A similar trend has been reported with As water by Jitaru et al (2016). Liu K L et al (2018) and Liao et al (2019) revealed an insignificant change in As concentration in cooked rice cooking in pressure cooker and stainless steel pot with deionized water. Menon et al (2021) tested a revised absorption method combining some cooking techniques. They suggested that parboiled and absorbed treatmentsdecrease 73% and 54% of iAs in white and brown rice, respectively.
PROSPECTS
The future research should consider integrating water management with other As mitigating strategies such as the application of nanotechnology, biochar and/or seed priming to avail the dual effect for bioaccumulation of As in rice.
ACKNOWLEDGEMENTS
This study was supported by the Seed Funding Grant (Grant No. RG53/19-20R), General Research Fund Proposal (Grant No. RG21/2020-2021R), Dean’s Research Fund (Grant No. IRS-10-2020) and Department of Science and Environmental Studies Grant for Collaborative Research Project of the Education University of Hong Kong, China (Grant No. 04487).
Acharjee P U, Bhattacharyya K, Poddar R, Pari A, Ray K, Patra S K, Halder S. 2021. Water management and varietal selection approach in mitigation of arsenic inof West Bengal, India.,52(9): 1008–1022.
Ahmed Z U, Panaullah G M, Gauch H, McCouch S R, Tyagi W, Kabir M S, Duxbury J M. 2011. Genotype and environment effects on rice (L.) grain arsenic concentration in Bangladesh., 338: 367–382.
Aide M, Beighley D, Dunn D. 2016. Arsenic uptake by rice (L.) having different irrigation regimes involving two southeastern Missouri soils.,11(1): 71–81.
Akter S, Rahman G K M M, Hasanuzzaman M, Alam Z, Watanabe T, Islam T. 2021. Zerovalent iron modulates the influence of arsenic-contaminated soil on growth, yield and grain quality of rice., 1(2): 90–104.
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S. 2009. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice., 43(24): 9361–9367.
Aziz A, Mohammad Ullah S, Ullah M R. 2015. Arsenic in rice grains at Sonargaon, Bangladesh., 44(1): 85– 89.
Bae M, Watanabe C, Inaoka T, Sekiyama M, Sudo N, Bokul M H, Ohtsuka R. 2002. Arsenic in cooked rice in Bangladesh., 360: 1839–1840.
Baig J A, Kazi T G, Shah A Q, Kandhro G A, Afridi H I, Khan S, Kolachi N F. 2010. Biosorption studies on powder of stem of: Removal of arsenic from surface water., 178: 941–948.
Batista B L, Souza J M O, de Souza S S, Barbosa Jr F. 2011. Speciation of arsenic in rice and estimation of daily intake of different arsenic species by Brazilians through rice consumption., 191: 342–348.
Bhattacharya P, Samal A C, Majumdar J, Santra S C. 2010a. Arsenic contamination in rice, wheat, pulses, and vegetables: A study in an arsenic affected area of West Bengal, India., 213: 3–13.
Bhattacharya P, Samal A C, Majumdar J, Santra S C. 2010b. Uptake of arsenic in rice plant varieties cultivated with arsenic rich groundwater., 3(2): 34–37.
Bogdan K, Schenk M K. 2009. Evaluation of soil characteristics potentially affecting arsenic concentration in paddy rice (L.)., 157(10): 2617–2621.
Carrijo D R, Akbar N, Reis A F B, Li C Y, Gaudin A C M, Parikh S J, Green P G,Linquist B A. 2018. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics., 222: 101–110.
Chakraborti D, Singh S K, Rahman M M, Dutta R N, Mukherjee S C, Pati S, Kar P B. 2018. Groundwater arsenic contamination in the Ganga River basin: A future health danger.,15(2): 180.
Chauhan V S, Nickson R T, Chauhan D, Iyengar L, Sankararamakrishnan N. 2009. Ground water geochemistry of Ballia district, Uttar Pradesh, India and mechanism of arsenic release.,75(1): 83–91.
Chen J, Sun G X, Wang X X, Lorenzo V D, Rosen B P, Zhu Y G. 2014. Volatilization of arsenic from polluted soil byengineered for expression of the arsM Arsenic(III) S-adenosine methyltransferase gene., 48(17): 10337–10344.
Chen X P, Zhu Y G, Hong M N, Kappler A, Xu Y X. 2008. Effects of different forms of nitrogen fertilizers on arsenic uptake by rice plants., 27(4): 881–887.
Cheng Z, van Geen A, Seddique A A, Ahmed K M. 2005. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh., 39(13): 4759–4766.
Chohan M, Memon M, Rajpar I, Akhtar M S. 2020. Association of arsenic fractions to arsenic in rice plants., 52(6): 2011–2016.
Chou M L, Jean J S, Sun G X, Yang C M, Hseu Z Y, Kuo S F, Tseng H Y, Yang Y J. 2016. Irrigation practices on rice crop production in arsenic-rich paddy soil., 56(1): 422–431.
Chowdhury N R, Das A, Joardar M, De A, Mridha D, Das R, Rahman M M, Roychowdhury T. 2020. Flow of arsenic between rice grain and water: Its interaction, accumulation and distribution in different fractions of cooked rice., 731: 138937.
Cui J H, Li Y D, Jin Q, Li F B. 2020. Silica nanoparticles inhibit arsenic uptake into rice suspension cellsimproving pectin synthesis and the mechanical force of the cell wall.:, 7(1): 162–171.
Das S, Chou M L, Jean J S, Liu C C, Yang H J. 2016. Water management impacts on arsenic behavior and rhizosphere bacterial communities and activities in a rice agro-ecosystem., 542: 642–652.
de la Calle M B, Emteborg H, Linsinger T P J, Montoro R, Sloth J J, Rubio R, Baxter M J, Feldmann J, Vermaercke P, Raber G. 2011. Does the determination of inorganic arsenic in rice depend on the method?, 30(4): 641–651.
del Razo L M, Garcia-Vargas G G, Garcia-Salcedo J, Sanmiguel M F, Rivera M, Hernandez M C, Cebrian M E. 2002. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico., 40(10): 1423–1431.
Delgado-Andrade C, Navarro M, López H, López M C. 2003. Determination of total arsenic levels by hydride generation atomic absorption spectrometry in foods from south-east Spain: Estimation of daily dietary intake., 20(10): 923–932.
Díaz O P, Leyton I, Muñoz O, Núñez N, Devesa V, Súñer M A, Vélez D, Montoro R. 2004. Contribution of water, bread, and vegetables (raw and cooked) to dietary intake of inorganic arsenic in a rural village of Northern Chile.52(6): 1773–1779.
do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. 2021. Nanotechnology potential in seed priming for sustainable agriculture., 11(2): 267.
Duxbury J M, Panaullah G. 2007. Remediation of arsenic for agriculture sustainability, food security and health in Bangladesh. FAO, Rome: Cornell University and Bangladesh Joint Publication: 1–30.
FAO (Food and Agriculture Organization of the United Nations). 2004. http://faostat.fao.org/site/336/DesktopDefault.aspx?PageID=336.
EFSA (European Food Safety Authority). 2014. Dietary exposure to inorganic arsenic in the European population., 12(3): 3597.
Farooq M, Usman M, Nadeem F, ur Rehman H, Wahid A, Basra S M A, Siddique K H M. 2019. Seed priming in field crops: Potential benefits, adoption and challenges., 70(9): 731–771.
Fernández-Baca C P, McClung A M, Edwards J D, Codling E E, Reddy V R, Barnaby J Y. 2021. Grain inorganic arsenic content in rice managed through targeted introgressions and irrigation management., 11: 612054.
Gray P J, Conklin S D, Todorov T I, Kasko S M. 2016. Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain., 33(1): 78–85.
Guillod-Magnin R, Brüschweiler B J, Aubert R, Haldimann M. 2018. Arsenic species in rice and rice-based products consumed by toddlers in Switzerland., 35(6): 1164– 1178.
Gunderson E L. 1995. Dietary intakes of pesticides, selected elements, and other chemicals: FDA total diet study, June 1984–April 1986., 78(4): 9110–9120.
Guo H M, Zhang Y, Jia Y F, Zhao K, Kim K. 2013. Spatial and temporal evolutions of groundwater arsenic approximately along the flow path in the Hetao Basin, Inner Mongolia., 58(25): 3070–3079.
Habib M A, Miono S, Sera K, Futatsugawa S. 2002. Pixe analysis of hair in arsenic pollution, Bangladesh., 12: 19–34.
Herath I, Zhao F J, Bundschuh J, Wang P, Wang J, Ok Y S, Palansooriya K N, Vithanage M. 2020. Microbe mediated immobilization of arsenic in the rice rhizosphere after incorporation of silica impregnated biochar composites., 398: 123096.
Hojsak I, Braegger C, Bronsky J, Campoy C, Colomb V, Decsi T, Domellöf M, Fewtrell M, Mis N F, Mihatsch W, Molgaard C, van Goudoever J, Nutrition E C O. 2015. Arsenic in rice: A cause for concern., 60(1): 142– 145.
Hollibaugh J T, Carini S, Gürleyük H, Jellison R, Joye S B, LeCleir G, Meile C, Vasquez L, Wallschläger D. 2005. Arsenic speciation in Mono Lake, California: Response to seasonal stratification and anoxia., 69(8): 1925–1937.
Hollibaugh J T, Budinoff C, Hollibaugh R A, Ransom B, Bano N. 2006. Sulfide oxidation coupled to arsenate reduction by a diverse microbial community in a soda lake., 72(3): 2043–2049.
Hossain M B, Jahiruddin M, Panaullah G M, Loeppert R H, Islam M R, Duxbury J M. 2008. Spatial variability of arsenic concentration in soils and plants, and its relationship with iron, manganese and phosphorus., 156(3): 739–744.
Hu L Q, Zeng M, Lei M, Liao B H, Zhou H. 2020. Effect of zero-valent iron on arsenic uptake by rice (L.) and its relationship with iron, arsenic, and phosphorus in soil and iron plaque., 231(9): 481.
Hua B, Yan W G, Wang J M, Deng B L, Yang J. 2011. Arsenic accumulation in rice grains: Effects of cultivars and water management practices., 28(8): 591–596.
Huang Q, Liu Q, Lin L N, Li F J, Han Y F, Song Z G. 2018. Reduction of arsenic toxicity in two rice cultivar seedlings by different nanoparticles., 159: 261–271.
Imamul Huq S M, Sultana S, Chakraborty G, Chowdhury M T A. 2011. A mitigation approach to alleviate arsenic accumulation in rice through balanced fertilization., 2011: 835627.
Islam S, Rahman M M, Islam M R, Naidu R. 2016. Arsenic accumulation in rice: Consequences of rice genotypes and management practices to reduce human health risk., 96: 139–155.
Islam S, Rahman M M, Islam M R, Naidu R. 2017. Effect of irrigation and genotypes towards reduction in arsenic load in rice., 609: 311–318.
Islam S, Rahman M M, Naidu R. 2019. Impact of water and fertilizer management on arsenic bioaccumulation and speciation in rice plants grown under greenhouse conditions., 214: 606–613.
Islam S F U, Sander B O, Quilty J R, de Neergaard A, van Groenigen J W, Jensen L S. 2020a. Mitigation of greenhouse gas emissions and reduced irrigation water use in rice production through water-saving irrigation scheduling, reduced tillage and fertiliser application strategies., 739: 140215.
Islam S F U, de Neergaard A, Sander B O, Jensen L S, Wassmann R, van Groenigen J W. 2020b. Reducing greenhouse gas emissions and grain arsenic and lead levels without compromising yield in organically produced rice., 295: 106922.
Islam M S, Magid A S I A, Chen Y L, Weng L P, Ma J, Arafat M Y, Khan Z H, Li Y T. 2021a. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues., 785: 147163.
Islam M S, Chen Y L, Weng L P, Ma J, Khan Z H, Liao Z B, Magid A S I A, Li Y T. 2021b. Watering techniques and zero- valent iron biochar pH effects on As and Cd concentrations in rice rhizosphere soils, tissues and yield., 100: 144–157.
Jitaru P, Millour S, Roman M, El Koulali K, Noël L, Guérin T. 2016. Exposure assessment of arsenic speciation in different rice types depending on the cooking mode., 54: 37–47.
Jorhem L, Becker W, Slorach S. 1998. Intake of 17 elements by Swedish women, determined by a 24-h duplicate portion study., 11(1): 32–46.
Kar S, Das S, Jean J S, Chakraborty S, Liu C C. 2013. Arsenic in the water-soil-plant system and the potential health risks in the coastal part of Chianan Plain, Southwestern Taiwan., 77: 295–302.
Khan K A, Stroud J L, Zhu Y G, McGrath S P, Zhao F J. 2010. Arsenic bioavailability to rice is elevated in Bangladeshi paddy soils., 44(22): 8515–8521.
Khan S, Reid B J, Li G, Zhu Y G. 2014. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China., 68: 154–161.
Khan S, Akhtar N, Rehman S U, Shujah S, Rha E S, Jamil M. 2020. Biosynthesized iron oxide nanoparticles (Fe3O4NPs) mitigate arsenic toxicity in rice seedlings., 9(1): 2.
Kumar A, Basu S, Kumar G. 2022. Evaluating the effect of seed-priming for improving arsenic tolerance in rice., 31: 197–201.
Kumarathilaka P, Seneweera S, Ok Y S, Meharg A A, Bundschuh J. 2020. Mitigation of arsenic accumulation in rice: An agronomical, physico-chemical, and biological approach: A critical review., 50(1): 31–71.
Kumarathilaka P, Bundschuh J, Seneweera S, Ok Y S. 2021a. An integrated approach of rice hull biochar-alternative water management as a promising tool to decrease inorganic arsenic levels and to sustain essential element contents in rice., 405: 124188.
Kumarathilaka P, Bundschuh J, Seneweera S, Ok Y S. 2021b. Rice genotype’s responses to arsenic stress and cancer risk: The effects of integrated birnessite-modified rice hull biochar-water management applications., 768: 144531.
Laparra J M, Vélez D, Barberá R, Farré R, Montoro R. 2005. Bioavailability of inorganic arsenic in cooked rice: Practical aspects for human health risk assessments., 53(22): 8829–8833.
Larsen E H. 1993. Arsenic speciation: Development of analytical methods and their application to biological samples and food. [PhD Thesis].Lyngby, Denmark: Technical University of Denmark.
Lee H S, Cho Y H, Park S O, Kye S H, Kim B H, Hahm T S, Kim M, Ok Lee J, Kim C I. 2006. Dietary exposure of the Korean population to arsenic, cadmium, lead and mercury., 19: S31–S37.
Leksungnoen P, Wisawapipat W, Ketrot D, Aramrak S, Nookabkaew S, Rangkadilok N, Satayavivad J. 2019. Biochar and ash derived from silicon-rich rice husk decrease inorganic arsenic species in rice grain., 684: 360–370.
Li B Y, Zhou S, Wei D N, Long J M, Peng L, Tie B Q, Williams P N, Lei M. 2019. Mitigating arsenic accumulation in rice (L.) from typical arsenic contaminated paddy soil of Southern China using nanostructured α-MnO2: Pot experiment and field application., 650: 546–556.
Li R Y, Stroud J L, Ma J F, McGrath S P, Zhao F J. 2009. Mitigation of arsenic accumulation in rice with water management and silicon fertilization., 43(10): 3778– 3783.
Lian F, Liu X W, Gao M L, Li H Z, Qiu W W, Song Z G. 2020. Effects of Fe-Mn-Ce oxide-modified biochar on As accumulation, morphology, and quality of rice (L.)., 27: 18196–18207.
Liao W, Wang G, Li K M, Zhao W B, Wu Y. 2019. Effect of cooking on speciation andbioaccessibility of Hg and as from rice, using ordinary and pressure cookers., 187(1): 329–339.
Lin L N, Gao M L, Qiu W W, Wang D, Huang Q, Song Z G. 2017. Reduced arsenic accumulation inrice (L.) cultivar with ferromanganese oxide impregnated biochar composites amendments., 231: 479–486.
Lin L N, Gao M L, Liu X W, Qiu W W, Song Z G. 2021. Effect of Fe-Mn-La-modified biochar composites on arsenic volatilization in flooded paddy soil., 28: 49889–49898.
Lin S C, Chang T K, Huang W D, Lur H S, Shyu G S. 2015. Accumulation of arsenic in rice plant: A study of an arsenic- contaminated site in Taiwan., 13(1): 11–18.
Linam F, McCoach K, Limmer M A, Seyfferth A L. 2021. Contrasting effects of rice husk pyrolysis temperature on silicon dissolution and retention of cadmium (Cd) and dimethylarsinic acid (DMA)., 765: 144428.
Linquist B A, Anders M M, Adviento-Borbe M A A, Chaney R L, Nalley L L, da Rosa E F F, van Kessel C. 2015. Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems., 21(1): 407–417.
Liu C P, Wei L, Zhang S R, Xu X H, Li F B. 2014. Effects of nanoscale silica sol foliar application on arsenic uptake, distribution and oxidative damage defense in rice (L.) under arsenic stress., 4: 57227–57234.
Liu G F, Meng J, Huang Y L, Dai Z M, Tang C X, Xu J M. 2020. Effects of carbide slag, lodestone and biochar on the immobilization, plant uptake and translocation of As and Cd in a contaminated paddy soil., 266: 115194.
Liu J, Simms M, Song S, King R S, Cobb G P. 2018. Physiological effects of copper oxide nanoparticles and arsenic on the growth and life cycle of rice (‘Koshihikari’)., 52(23): 13728–13737.
Liu K L, Zheng J B, Chen F S. 2018. Effects of washing, soaking and domestic cooking on cadmium, arsenic and lead bioaccessibilities in rice., 98(10): 3829–3835.
Llobet J M, Falcó G, Casas C, Teixidó A, Domingo J L. 2003. Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain., 51(3): 838– 842.
Lu Y, Adomako E E, Solaiman A R M, Islam M R, Deacon C, Williams P N, Rahman G K M M, Meharg A A. 2009. Baseline soil variation is a major factor in arsenic accumulation in Bengal Delta paddy rice., 43(6): 1724–1729.
Luvonga C, Rimmer C A, Yu L L, Lee S B. 2020. Organoarsenicals in seafood: Occurrence, dietary exposure, toxicity, and risk assessment considerations: A review., 68(4): 943–960.
Ma J F, Yamaji N, Mitani N, Xu X Y, Su Y H, McGrath S P, Zhao F J. 2008. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain., 105(29): 9931–9935.
Ma L, Wang L, Jia Y Y, Yang Z G. 2017. Accumulation, translocation and conversion of six arsenic species in rice plants grown near a mine impacted city., 183: 44–52.
Ma X M, Sharifan H, Dou F G, Sun W J. 2020. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles., 384: 123802.
MAFF (Ministry of Agriculture, Fisheries and Food). 1999. National Food Survey 1998. London, UK: HMSO.
Mainuddin M, Maniruzzaman M, Alam M M, Mojid M A, Schmidt E J, Islam M T, Scobie M. 2020. Water usage and productivity of Boro rice at the field level and their impacts on the sustainable groundwater irrigation in the North-West Bangladesh., 240: 106294.
Maity J P, Chen C Y, Bhattacharya P, Sharma R K, Ahmad A, Patnaik S, Bundschuh J. 2021. Advanced application of nano- technological and biological processes as well as mitigation options for arsenic removal., 405: 123885.
Mandal U, Singh P, Kundu A K, Chatterjee D, Nriagu J, Bhowmick S. 2019. Arsenic retention in cooked rice: Effects of rice type, cooking water, and indigenous cooking methods in West Bengal, India., 648: 720–727.
Mazumder D N G, Haque R, Ghosh N, De B K, Santra A, Chakraborti D, Smith A H. 2000. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India., 29(6): 1047–1052.
Medhi B K, Hazarika I H, Hazarika P P, Thakuria R K. 2021. Assessment of groundwater arsenic vulnerable zones using Geographic Information System for employing biochar as soil amendment in irrigated rice ecosystem: A case study from Central Assam., 42: 462–470.
Meharg A A, Zhao F J. 2012. Strategies for producing low arsenic rice.: Arsenic and Rice. Dordrecht:Springer: 139–151.
Meharg A A, Williams P N, Adomako E, Lawgali Y Y, Deacon C, Villada A, Cambell R C J, Sun G X, Zhu Y G, Feldmann J, Raab A, Zhao F J, Islam R, Hossain S, Yanai J T. 2009. Geographical variation in total and inorganic arsenic content of polished (white) rice., 43(5): 1612–1617.
Menon M, Dong W R, Chen X M, Hufton J, Rhodes E J. 2021. Improved rice cooking approach to maximise arsenic removal while preserving nutrient elements., 755: 143341.
Mohri T, Hisanaga A, Ishinishi N. 1990. Arsenic intake and excretion by Japanese adults: A7-day duplicate diet study.,28(7): 521–529.
Moreno-Jiménez E, Meharg A A, Smolders E, Manzano R, Becerra D, Sánchez-Llerena J, Albarrán Á, López-Piñero A. 2014. Sprinkler irrigation of rice fields reduces grain arsenic but enhances cadmium., 485/486: 468–473.
Mossop R T. 1989. On living in an arsenical atmosphere: Part 2.Clinical observations, animal experiments and ecologial problems., 35(12): 546–551.
Moulick D, Ghosh D, Chandra Santra S. 2016. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress., 109: 571–578.
Moulick D, Santra S C, Ghosh D. 2017. Seed priming with Se alleviate As induced phytotoxicity during germination and seedling growth by restricting As translocation in rice (L. c.v. IET-4094)., 145: 449–456.
Moulick D, Santra S C, Ghosh D. 2018a. Effect of selenium induced seed priming on arsenic accumulation in rice plant and subsequent transmission in human food chain., 152: 67–77.
Moulick D, Santra S C, Ghosh D. 2018b. Rice seed priming with Se: A novel approach to mitigate As induced adverse consequences on growth, yield and As load in brown rice., 355: 187–196.
Moulick D, Santra S C, Ghosh D, Panda S K. 2019. An assessment of efficiency of zinc priming in rice (cv. MTU-7029) during germination and early seedling growth.: Hasanuzzaman M, Fotopoulos V.Priming and Pretreatment of Seeds and Seedlings. Singapore:Springer: 495–507.
Moulick D, Samanta S, Sarkar S, Mukherjee A, Pattnaik B K, Saha S, Awasthi J P, Bhowmick S, Ghosh D, Samal A C, Mahanta S, Mazumder M K, Choudhury S, Bramhachari K, Biswas J K, Santra S C. 2021. Arsenic contamination, impact and mitigation strategies in rice agro-environment: An inclusive insight., 800: 149477.
Mridha D, Ray I, Sarkar J, De A Y, Joardar M, Das A, Chowdhury N R, Acharya K, Roychowdhury T. 2021a. Effect of sulfate application on inhibition of arsenic bioaccumulation in rice (L.) with consequent health risk assessment of cooked rice arsenic on human: A pot to plate study., 293: 118561.
Mridha D, Paul I, De A Y, Ray I, Das A, Joardar M, Chowdhury N R, Bhadoria P B S, Roychowdhury T. 2021b. Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress., 219: 112313.
Mukherjee A, Kundu M, Basu B, Sinha B, Chatterjee M, Bairagya M D, Singh U K, Sarkar S. 2017. Arsenic load in rice ecosystem and its mitigation through deficit irrigation., 197: 89–95.
Nookabkaew S, Rangkadilok N, Mahidol C, Promsuk G, Satayavivad J. 2013. Determination of arsenic species in rice from Thailand and other Asian countries using simple extraction and HPLC-ICP-MS analysis., 61(28): 6991–6998.
Norton G J, Duan G L, Dasgupta T, Islam M R, Lei M, Zhu Y G, Deacon C M, Moran A C, Islam S, Zhao F J, Stroud J L, McGrath S P, Feldmann J, Price A H, Meharg A A. 2009. Environmental and genetic control of arsenic accumulation and speciation in rice grain: Comparing a range of common cultivars grown in contaminated sites across Bangladesh, China, and India., 43(21): 8381–8386.
Norton G J, Dasgupta T, Islam M R, Islam S, Deacon C M, Zhao F J, Stroud J L, McGrath S P, Feldmann J, Price A H, Meharg A A. 2010. Arsenic influence on genetic variation in grain trace- element nutrient content in Bengal delta grown rice., 44(21): 8284–8288.
Norton G J, Travis A J, Talukdar P, Hossain M, Islam M R, Douglas A, Price A H. 2019. Genetic loci regulating arsenic content in rice grains when grown flooded or under alternative wetting and drying irrigation., 12(1): 54.
O’Neill A, Phillips D H, Kok S, Chea E, Seng B, Gupta B S. 2013. Arsenic in groundwater and its influence on exposure risks through traditionally cooked rice in Prey Vêng Province, Cambodia., 262: 1072–1079.
Oremland R S, Stolz J F. 2003. The ecology of arsenic., 300: 939–944.
Pan D D, Liu C P, Yu H Y, Li F B. 2019. A paddy field study of arsenic and cadmium pollution control by using iron-modified biochar and silica sol together., 26(24): 24979–24987.
Priya P, Bisen K, Rakshit A, Singh H B. 2018. Seedling bio-priming withspp. enhances nitrogen use efficiency in rice.: Rakshit A, Singh H. Advances in Seed Priming. Singapore:Springer: 297–307.
Qian Y Z, Chen C, Zhang Q, Li Y, Chen Z J, Li M. 2010. Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk., 21(12): 1757–1763.
Qiao J T, Liu T X, Wang X Q, Li F B, Lv Y H, Cui J H, Zeng X D, Yuan Y Z, Liu C P. 2018. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils., 195: 260–271.
Qiao J T, Yu H Y, Wang X Q, Li F B, Wang Q, Yuan Y Z, Liu C P. 2019. The applicability of biochar and zero-valent iron for the mitigation of arsenic and cadmium contamination in an alkaline paddy soil., 1(2): 203–212.
Qin Y M, Liu S W, Guo Y Q, Liu Q H, Zou J W. 2010. Methane and nitrous oxide emissions from organic and conventional rice cropping systems in Southeast China., 46(8): 825–834.
Rahman M A, Rahman M M, Reichman S M, Lim R P, Naidu R. 2014. Arsenic speciation in Australian-grown and imported rice on sale in Australia: Implications for human health risk., 62: 6016–6024.
Rahman M S, Islam M N, Hassan M Z, Islam S A, Zaman S K. 2015. Impact of water management on the arsenic content of rice grain and cultivated soil in an arsenic contaminated area of Bangladesh., 7(2): 43–46.
Rasmussen R R, Qian Y T, Sloth J J. 2013. SPE HG-AAS method for the determination of inorganic arsenic in rice: Results from method validation studies and a survey on rice products., 405(24): 7851–7857.
Roberts L C, Hug S J, Dittmar J, Voegelin A, Kretzschmar R, Wehrli B, Cirpka O A, Saha G C, Ashraf Ali M, Badruzzaman A B M. 2010. Arsenic release from paddy soils during monsoon flooding., 3(1): 53–59.
Rong Q, Zhong K, Li F Y, Huang H, Li C Z, Nong X Y, Zhang C L. 2020. Combined effect of ferrous ion and biochar on cadmium and arsenic accumulation in rice., 10(1): 300.
Roychowdhury T. 2008. Impact of sedimentary arsenic through irrigated groundwater on soil, plant, crops and human continuum from Bengal delta: Special reference to raw and cooked rice., 46(8): 2856–2864.
Roychowdhury T, Tokunaga H, Ando M. 2003. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India., 308: 15–35.
Ruangwises S, Saipan P. 2009. Dietary intake of total and inorganic arsenic by adults in arsenic-contaminated area of Ron Phibun district, Thailand., 84(3): 274–277.
Sarkar S, Basu B, Kundu C K, Patra P K. 2012. Deficit irrigation: An option to mitigate arsenic load of rice grain in West Bengal, India., 146(1): 147–152.
Sarwar T, Khan S, Yu X W, Amin S, Khan M A, Sarwar A, Muhammad J, Nazneen S. 2021. Analysis of arsenic concentration and its speciation in rice of different markets of Pakistan and its associated health risk., 21: 101252.
Sengupta M K, Hossain M A, Mukherjee A, Ahamed S, Das B, Nayak B, Pal A, Chakraborti D. 2006. Arsenic burden of cooked rice: Traditional and modern methods., 44(11): 1823–1829.
Seyfferth A L, Morris A H, Gill R, Kearns K A, Mann J N, Paukett M, Leskanic C. 2016. Soil incorporation of silica-rich rice husk decreases inorganic arsenic in rice grain., 64(19): 3760–3766.
Shah A L, Naher U A, Hasan Z, Islam S M M, Rahman M S, Panhwar Q A, Shamshuddin J. 2016. Arsenic management in contaminated irrigation water for rice cultivation., 39: 155–166.
Shelar A, Singh A V, Maharjan R S, Laux P, Luch A, Gemmati D, Tisato V, Singh S P, Santilli M F, Shelar A, Chaskar M, Patil R. 2021. Sustainable agriculture through multidisciplinary seed nanopriming: Prospects of opportunities and challenges., 10(9): 2428.
Shrivastava A, Barla A, Majumdar A, Singh S, Bose S. 2020. Arsenic mitigation in rice grain loading via alternative irrigation by proposed water management practices., 238: 124988.
Singh R, Singh S, Parihar P, Singh V P, Prasad S M. 2015. Arsenic contamination, consequences and remediation techniques: A review., 112: 247–270.
Sivakumar T, Ambika S, Balakrishnan K. 2017. Biopriming of rice seed with phosphobacteria for enhanced germination and vigour., 54(3): 346–349.
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider U, Towprayoon S, Wattenbach M, Smith J. 2008. Greenhouse gas mitigation in agriculture., 363: 789–813.
Spanu A, Daga L, Orlandoni A M, Sanna G. 2012. The role of irrigation techniques in arsenic bioaccumulation in rice (L.)., 46(15): 8333–8340.
Somenahally A C, Hollister E B, Yan W G, Gentry T J, Loeppert R H. 2011. Water management impacts on arsenic speciation and iron-reducing bacteria in contrasting rice-rhizosphere compartments., 45(19): 8328–8335.
Sobel M H, Sanchez T R, Jones M R, Kaufman J D, Francesconi K A, Blaha M J, Vaidya D, Shimbo D, Gossler W, Gamble M V, Genkinger J M, Navas-Acien A. 2020. Rice intake, arsenic exposure, and subclinical cardiovascular disease among US adults in MESA., 9(4): e015658.
Suriyagoda L D B, Dittert K, Lambers H. 2018. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains., 253: 23–37.
Talukder A S M H M, Meisner C A, Sarkar M A R, Islam M S. 2011. Effect of water management, tillage options and phosphorus status on arsenic uptake in rice., 74(4): 834–839.
Talukder A S M H M, Meisner C A, Sarkar M A R, Islam M S, Sayre K D, Duxbury J M, Lauren J G. 2012. Effect of water management, arsenic and phosphorus levels on rice in a high-arsenic soil-water system: II. Arsenic uptake., 80: 145–151.
Torres-Escribano S, Leal M, Vélez D, Montoro R. 2008. Total and inorganic arsenic concentrations in rice sold in Spain, effect of cooking, and risk assessments., 42(10): 3867–3872.
Tsuda T, Inoue T, Kojima M, Aoki S. 1995. Market basket and duplicate portion estimation of dietary intakes of cadmium, mercury, arsenic, copper, manganese, and zinc by Japanese adults., 78(6): 1363–1368.
Tsuji J S, Garry M R, Perez V, Chang E T. 2015. Low-level arsenic exposure and developmental neurotoxicity in children: A systematic review and risk assessment., 337: 91–107.
Tyson J. 2013. The determination of arsenic compounds: A critical review., 2013: 835371.
Upadhyaya H, Begum L, Dey B, Nath P K, Panda S K. 2017. Impact of calcium phosphate nanoparticles on rice plant.,1: 1–10.
Upadhyay M K, Shukla A, Yadav P, Srivastava S. 2019. A review of arsenic in crops, vegetables, animals and food products., 276: 608–618.
Upadhyay M K, Majumdar A, Barla A, Bose S, Srivastava S. 2021. Thiourea supplementation mediated reduction of grain arsenic in rice (L.) cultivars: A two year field study., 407: 124368.
Vahter M. 2002. Mechanisms of arsenic biotransformation., 181/182: 211–217.
Verma S, Verma P K, Chakrabarty D. 2019. Arsenic bio- volatilization by engineered yeast promotes rice growth and reduces arsenic accumulation in grains., 13(3): 475–485.
Wang N, Xue X M, Juhasz A L, Chang Z Z, Li H B. 2017. Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic., 220: 514–522.
Wang P T, Zhang W W, Mao C Z, Xu G H, Zhao F J. 2016. The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice., 67(21): 6051–6059.
Wang X X, Sun W J, Zhang S, Sharifan H, Ma X M. 2018. Elucidating the effects of cerium oxide nanoparticles and zinc oxide nanoparticles on arsenic uptake and speciation in rice () in a hydroponic system., 52(17): 10040–10047.
Wang X X, Sun W J, Ma X M. 2019. Differential impacts of copper oxide nanoparticles and copper(II) ions on the uptake and accumulation of arsenic in rice ()., 252: 967–973.
Watanabe C, Kawata A, Sudo N, Sekiyama M, Inaoka T, Bae M, Ohtsuka R. 2004. Water intake in an Asian population living in arsenic-contaminated area., 198(3): 272–282.
Wen E G, Yang X, Chen H B, Shaheen S M, Sarkar B, Xu S, Song H, Liang Y, Rinklebe J, Hou D Y, Li Y, Wu F C, Pohořelý M, Wong J W C, Wang H L. 2021. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil., 407: 124344.
Wilhelm M, Wittsiepe J, Schrey P, Lajoie-Junge L, Busch V. 2003. Dietary intake of arsenic, mercury and selenium by children from a German North Sea Island using duplicate portion sampling., 17(2): 123–132.
Williams P N, Price A H, Raab A, Hossain S A, Feldmann J, Meharg A A. 2005. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure., 39(15): 5531–5540.
Williams P N, Islam M R, Adomako E E, Raab A, Hossain S A, Zhu Y G, Feldmann J, Meharg A A. 2006. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters., 40(16): 4903–4908.
Williams P N, Villada A, Deacon C, Raab A, Figuerola J, Green A J, Feldmann J, Meharg A A. 2007. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley., 41(19): 6854–6859.
Williams P N, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman A R M, Rahman G K M M, Lu Y, Deacon C, Zhu Y G, Meharg A A. 2009. Arsenic limits trace mineral nutrition (selenium, zinc, and nickel) in Bangladesh rice grain., 43(21): 8430–8436.
Wu C, Ye Z H, Shu W S, Zhu Y G, Wong M. 2011. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes., 62(8): 2889–2898.
Wu C, Huang L, Xue S G, Huang Y Y, Hartley W, Cui M Q, Wong M H. 2017. Arsenic sorption by red mud-modified biochar produced from rice straw., 24(22): 18168–18178.
Wu F, Fang Q, Yan S W, Pan L, Tang X J, Ye W L. 2020. Effects of zinc oxide nanoparticles on arsenic stress in rice (L.): Germination, early growth, and arsenic uptake., 27(21): 26974–26981.
Wu J Z, Li Z T, Wang L, Liu X M, Tang C X, Xu J M. 2020. A novel calcium-based magnetic biochar reduces the accumulation of As in grains of rice (L.) in As-contaminated paddy soils., 394: 122507.
Wu X Y, Hu J, Wu F, Zhang X Y, Wang B, Yang Y, Shen G F, Liu J F, Tao S, Wang X L. 2021. Application of TiO2nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (L.): A mechanistic study., 405: 124047.
Xu X Y, McGrath S P, Meharg A A, Zhao F J. 2008. Growing rice aerobically markedly decreases arsenic accumulation., 42(15): 5574–5579.
Yadav P, Srivastava S. 2021. Effect of thiourea application on root, old leaf and young leaf of two contrasting rice varieties (L.) grown in arsenic contaminated soil., 21: 101368.
Yan S W, Wu F, Zhou S, Yang J H, Tang X J, Ye W L. 2021. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (L.)., 21(1): 150.
Yang Y Q, Hu H Q, Fu Q L, Zhu J, Huang G Y. 2019. Water management of alternate wetting and drying reduces the accumulation of arsenic in brown rice: As dynamic study from rhizosphere soil to rice., 185: 109711.
Yang Y P, Tang X J, Zhang H M, Cheng W D, Duan G L, Zhu Y G. 2020. The characterization of arsenic biotransformation microbes in paddy soil after straw biochar and straw amendments., 391: 122200.
Yao B M, Chen P, Zhang H M, Sun G X. 2021. A predictive model for arsenic accumulation in rice grains based on bioavailable arsenic and soil characteristics., 412: 125131.
Yao Y, Zhou H, Yan X L, Yang X, Huang K W, Liu J, Li L J, Zhang J Y, Gu J F, Zhou Y Y, Liao B H. 2021. The Fe3O4-modified biochar reduces arsenic availability in soil and arsenic accumulation inrice (L.)., 28(14): 18050–18061.
Yost L J, Tao S H, Egan S K, Barraj L M, Smith K M, Tsuji J S, Lowney Y W, Schoof R A, Rachman N J. 2004. Estimation of dietary intake of inorganic arsenic in US children., 10(3): 473–483.
Yu S B, Ali J, Zhang C P, Li Z K, Zhang Q F. 2020. Genomic breeding of green super rice varieties and their deployment in Asia and Africa., 133(5): 1427–1442.
Yu Z H, Qiu W W, Wang F, Lei M, Wang D, Song Z G. 2017. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in anrice (L.) cultivar., 168: 341–349.
Zhang G G, Liu X W, Gao M L, Song Z G. 2020. Effect of Fe-Mn-Ce modified biochar composite on microbial diversity and properties of arsenic-contaminated paddy soils., 250: 126249.
Zhao F J, McGrath S P, Meharg A A. 2010. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies., 61: 535–559.
Zhao F J, Zhu Y G, Meharg A A. 2013. Methylated arsenic species in rice: Geographical variation, origin, and uptake mechanisms., 47(9): 3957–3966.
Zheng R L, Cai C, Liang J H, Huang Q, Chen Z, Huang Y Z, Arp H P H, Sun G X. 2012. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (L.) seedlings., 89(7): 856–862.
Zheng R L, Chen Z, Cai C, Tie B Q, Liu X L, Reid B J, Huang Q, Lei M, Sun G X, Baltrėnaitė E. 2015. Mitigating heavy metal accumulation into rice (L.) using biochar amendment: A field experiment in Hunan, China., 22(14): 11097–11108.
Zhou S, Peng L, Lei M, Pan Y Q, Lan D Z. 2015. Control of As soil to rice transfer (L.) with nano-manganese dioxide.,35: 855–861.
Zhu Y G, Sun G X, Lei M, Teng M, Liu Y X, Chen N C, Wang L H, Carey A M, Deacon C, Raab A, Meharg A A, Williams P N. 2008. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice., 42(13): 5008–5013.
28 October 2021;
11 February 2022
Li Wai Chin (waichin@eduhk.hk)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.02.002
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- RPA-Assisted Cas12a System for Detecting Pathogenic Xanthomonas oryzae, a Causative Agent for Bacterial Leaf Blight Disease in Rice
- Polycomb Repressive Complex 2-Mediated H3K27 Trimethylation Is Required for Pathogenicity in Magnaporthe oryzae
- Genome-Wide Analysis of von Willebrand Factor A Gene Family in Rice for Its Role in Imparting Biotic Stress Resistance with Emphasis on Rice Blast Disease
- Selenium Alleviates Carbohydrate Metabolism and Nutrient Composition in Arsenic Stressed Rice Plants
- Feasibility of Improving Unmanned Aerial Vehicle-Based Seeding Efficiency by Using Rice Varieties with Low Seed Weight
- Genetic Variation for Anaerobic Germination and Emergence from Deeper Soil Depth in Oryza nivara Accessions

