Genetic Variation for Anaerobic Germination and Emergence from Deeper Soil Depth in Oryza nivara Accessions
Revanayya M. Gothe, Dharminder Bhatia, Akashdeep Kamboj, Nitika Sandhu, Buta Singh Dhillon
Letter
Genetic Variation for Anaerobic Germination and Emergence from Deeper Soil Depth inAccessions
Revanayya M. Gothe1, Dharminder Bhatia1, Akashdeep Kamboj1, Nitika Sandhu2, Buta Singh Dhillon1
()
Anaerobic germination and emergence from deeper soil depth are two important traits for breeding rice suitable for direct seeded conditions. In order to evaluate genetic variation for these traits, a total of 300accessions were evaluated along with checks, of which, 159 accessions germinated under anaerobic conditions indicating presence of immense variability in. Of 159 accessions, 69 germinated in submerged conditions and 90 germinated after removing water with availability of aerobic conditions, indicating two different mechanisms operating in. Similarly, out of 300accessions, 218 germinated from 6 cm and 95 germinated from 8 cm soil depth. The accessions that germinated from deeper soil depth had longer mesocotyl and coleoptile lengths. For both the traits, the bestaccessions that should be used in breeding programme were selected. Further, genome-wide association study (GWAS)identified 10 significant QTLs for anaerobic germination. Similarly, 8 QTLs for mesocotyl length and 12 QTLs for coleoptile length were identified. These donors and QTLs for anaerobic germination and emergence from deeper soil depth will serve as a platform for developing rice varieties suitable for direct seeded conditions.
Rice plays a pivotal role in the food security of the world’s population.It is an important crop in Punjab, a north-western state of India due to cultivation of high-yielding varieties, favourable policy regime of free energy and open-ended purchase of this crop at an assured price, thus promising higher returns to farmers. In general, puddled transplanted rice is the cultivation method of rice which requires a lot of standing water in the field for irrigation besides using a lot of labour for transplanting. But, about 73% of water requirement for transplanted rice crop is met from ground water in the state (Sidhu et al, 2021). The practice has led to rapid decline in the water table of soil, which is threatening environmental sustainability (Custodio, 2002). Looming water scarcity, water-intensive nature of this rice cultivation practice and escalating labour shortage propel to adopt alternative methods of rice cultivation in the state. Direct seeded rice is emerging as an alternative method due to low input demand. Punjab Agricultural University, India has recently established the agronomic practices of sowing rice under direct seeded conditions (Anonymous, 2022). However, there is a need to develop varieties suitable fordirect seeded conditions.
The development of rice varieties suitable for direct-seeded conditions requires breeding for several component traits such as germination under anaerobic conditions, emergence from deeper soil depth, uniform emergence, tolerance to Fe deficiency, resistance to soil nematodes and many plant architectural and yield traits. Among them, germination under anaerobic conditions and emergence from deeper soil depth are two important components for breeding direct seeded rice. In addition, germination under anaerobic conditions is also a feasible way to suppress weeds economically.
Flooding in farmer field after seeding is a common problem in flood prone areas and in some areas, it may occur due to unexpected rain, unlevelled fields and poor drainage that results in uneven crop establishment. Under direct seeded conditions, the varieties having ability to germinate under anaerobic conditions will show better performance (Doley et al, 2018). On the other hand, rice has a narrow range of optimal sowing depth and deep sowing often causes poor seedling emergence. Germination from upper soil layer may suffer due to more transpiration losses under direct seeded conditions, leading to the lack of moisture required for germination and seedling growth. Nevertheless, deep sowing will enhance seedling emergence and establishment because of the high soil moisture in the seed zone quickens germination. Elongation of both mesocotyl and coleoptile, however, can facilitate the emergence of rice seed when sown deep in the soil under direct seeded conditions (Chung, 2010).
along withis considered as the wild progenitor of(Lu et al, 2001). Itis a reservoir of an abundant genetic diversity which has contributed genes for resistance to pests, diseases, tolerance to abiotic stress, and yield related traits (Cheema et al, 2008; Gaikwad et al, 2014; Bhatia et al, 2017; Kumar et al, 2018). In addition,with ‘AA’ genome can easily hybridize with cultivatedand stable introgressions can be developed. Few scattered studies have been conducted to identify rice germplasm that can germinate under anaerobic conditions (Angaji et al, 2010; Adigbo et al, 2018) and from deeper soil depths (Wu et al, 2005; Alibu et al, 2012). Efforts have also been made to map QTLs associated with these traits in rice (Angaji et al, 2010; Baltazar et al, 2014; Lee and Kwon, 2015). However, many efforts are still needed to identify donors and QTLs for these traits.
In this study, we screened 300accessions along with positive and negative checks for anaerobic germination and for emergence from 6 cm and 8 cm soil depths. During screening for anaerobic germination, seeds were submerged for 21 d and thereafter the water was drained out. All the accessions were allowed to germinate for another 10 d, and days to germination and the number of plants germinated were recorded. Wide range of variation was recorded inaccessions for survival under anaerobic conditions. Positive checks started germination from 9 d whereasaccessions started germination from 7 d of submergence. Out of 300accessions, 159 accessions germinated under anaerobic conditions (Table S1). Of these, 69 accessions germinated before removing water or within submerged conditions, whereas 90 accessions germinated after removing water (after 21 d of seeding)(Fig.S1). Of 90 accessions, more than half germinated after 4 d of removing water (21–25 d after seeding). Negative checks didn’t germinate before and after removing water. After removing water and allowing it to germinate for a few more days, we examined seeds of negative checks andaccessions that didn’t germinate, and found thatthese seeds had softened and started decaying, while the seeds ofaccessions that got germinated after some days of removing water were as hard as it was at the time of sowing. The 159 germinated accessions were further evaluated, and the accessions germinated within 7 to 9 d after seeding or within 4 d of removing water were selected (Table 1).

Table 1. Oryza nivara accessions selected for anaerobic conditions and deeper soil depth (8 cm) with germination in minimum number of days as compared to 2 cm soil depth.
Numbers in parenthesis are mesocotyl length (cm) and coleoptilelength (cm) obtained at 8 cm soil depth.‘–’ indicates no data for the trait.
CR, Cuttack rice; IRGC, International rice germplasm collection.
For anaerobic germination, two types of mechanisms seemed to be operating inaccessions. In one mechanism,accessions started germinating under submerged conditions after 7–8 d of seeding, showing early vigour and elongated coleoptiles. This mechanism seems similar togene mechanism, which helps the plant to survive from flood-like situations by elongating the stem internode and keeping the leaf above water (Hattori et al, 2009). In the other mechanism, germination ofaccessions remained suppressed under anaerobic conditions and as soon as the aerobic conditions prevailed, germination started. However, it will be interesting to get deep insight into molecular mechanisms in both cases inaccessions.
Similarly, under control conditions at 2 cm soil depth, emergence ofaccessions started as early as the 5th day and by the end of the 9th day, all the accessions germinated. Under the 6 cm soil depth, seed emergence started as early as the 7th day and ended on the 13th day. Most of the accessions germinated between 8 to 12 d under the 6 cm soil depth. Under the 8 cm soil depth, seed emergence started as early as the 8th day and ended on the 14th day and most of the accessions germinated between 10 to 13 d. All the negative checks germinated under the 2 cm soil depth but didn’t germinate under the 6 and 8 cm soil depths except NPT1 and LIL427, which also germinated at the 6 cm soil depth. Out of 300accessions, 218 accessions germinated at the 6 cm soil depth and 95 lines germinated at the 8 cm soil depth. At 15 d after seeding, mesocotyl length and coleoptile length were measured by carefully uprooting the seedlings. Significant variations were observed amongaccessions for mesocotyl and coleoptile lengths under the 6 and 8 cm soil depths (Fig. S2). In the control conditions, as seeds were sown on the upper surface, mesocotyl didn’t get elongated but only coleoptile elongated and it varied from 0.40 to 0.90 cm. Under the 6 cm soil depth, mesocotyl length varied from 1.72 to 5.12 cm and coleoptile length varied from 0.41 to 3.91 cm. Under the 8 cm depth, mesocotyl length varied in the range of 2.90 to 7.70 cm and coleoptile length varied from 0.30 to 4.15 cm.
Among the 95 accessions germinated at the 8 cm soil depth, the accessions CR100113A, IRGC92745, IRGC92910 and IRGC100916 showed the longest mesocotyl and coleoptile lengths and higher germination rate. At 15 d after seeding, we examined the seeds of negative checks andaccessions which didn’t emerge from the 8 cm depth. Seeds started germinating but they were unable to reach the soil surface due to shorter mesocotyl and coleoptile lengths, and hence the coleoptile leaves unfurled underground which finally terminated. Rice seedlings with longer mesocotyls and coleoptiles can emerge better under deeper soil depths.Highly significant variationwas observed among 95 accessions for mesocotyl and coleoptile lengths (< 0.0001). Based on replicated evaluation, the accessions which germinated within 10 d of seeding showed higher germination rate and possessed longer mesocotyl and coleoptile lengths for emergence from the 8 cm soil depth (Table 1).
Eizenga et al (2016) indicated thatintrogressions show higher seedling vigour by increasing both coleoptile and shoot lengthsusing backcross inbred lines derived from M-202 and.hides genetic variation for emergence from deeper soil depth. Here, a set of 300accessions was tested for emergence from deeper soil depths, of which, 218 accessions germinated from the 6 cm soil depth and 95 accessions germinated from the 8 cm soil depth, indicating the presence of huge variation for this trait in annual wild relative of rice. Shift from transplanting to direct seeding for rice crop establishment has been evident in Punjab, India due to scarcity of labour required for transplanting, simplicity and additional advantages associated with direct seeded rice. Most of the present cultivated varieties might lack these traits due to breeding efforts directed towards development of cultivars suitable under transplanted conditions for past many decades. However, large number ofaccessions possess these traits, indicating large amount of variations of such traits might be present in wild relatives of rice. Based on thorough screening, theaccessions showing germination under anaerobic conditions with less days to germination could be used further in breeding programme.
Rice varieties having the ability to elongate its mesocotyl can emerge from deeper soil depths (Luo et al, 2007; Chung, 2010). The mesocotyl elongation ability varied inaccessions. Thus, failure of seedlings to reach soil surface in deep seed placement is due to inability of the mesocotyl to elongate. The mesocotyl and coleoptile lengths increase with changing soil depths and genetic ability present in the accessions.accessions that germinated from the 8 cm soil depth had the ability to elongate its mesocotyl and emerge from the soil surface.
GWAS was used to identify QTLs governing anaerobic germination and emergence from deeper soil depths. The method uses historic recombination events to identify markers located much closer to the genes of interest (Zhu et al, 2008). In addition, GWAS is an important strategy to identify founder lines that can be used further in breeding programme. A total of 21912 single nucleotide polymorphism (SNPs) were obtained from ddRADseq ofaccessions after data analysis and filtering based on missing data point < 10%, MAF (minor allele frequency) of 0.05 and read depth > 2 (Table S2). Of 21912 SNPs, the highest number of SNPs was obtained on chromosome 1 and the lowest on chromosome 12. SNP data along with phenotypic data of anaerobic germination, mesocotyl and coleoptile lengths for emergence from deeper soil depth were used for GWAS. Principal component analysis (PCA) ofaccessions based on 21912SNPs divided the whole population into two major sub-clusters while few accessions in one major sub-cluster seemed to bear differences with others(Fig. S3).
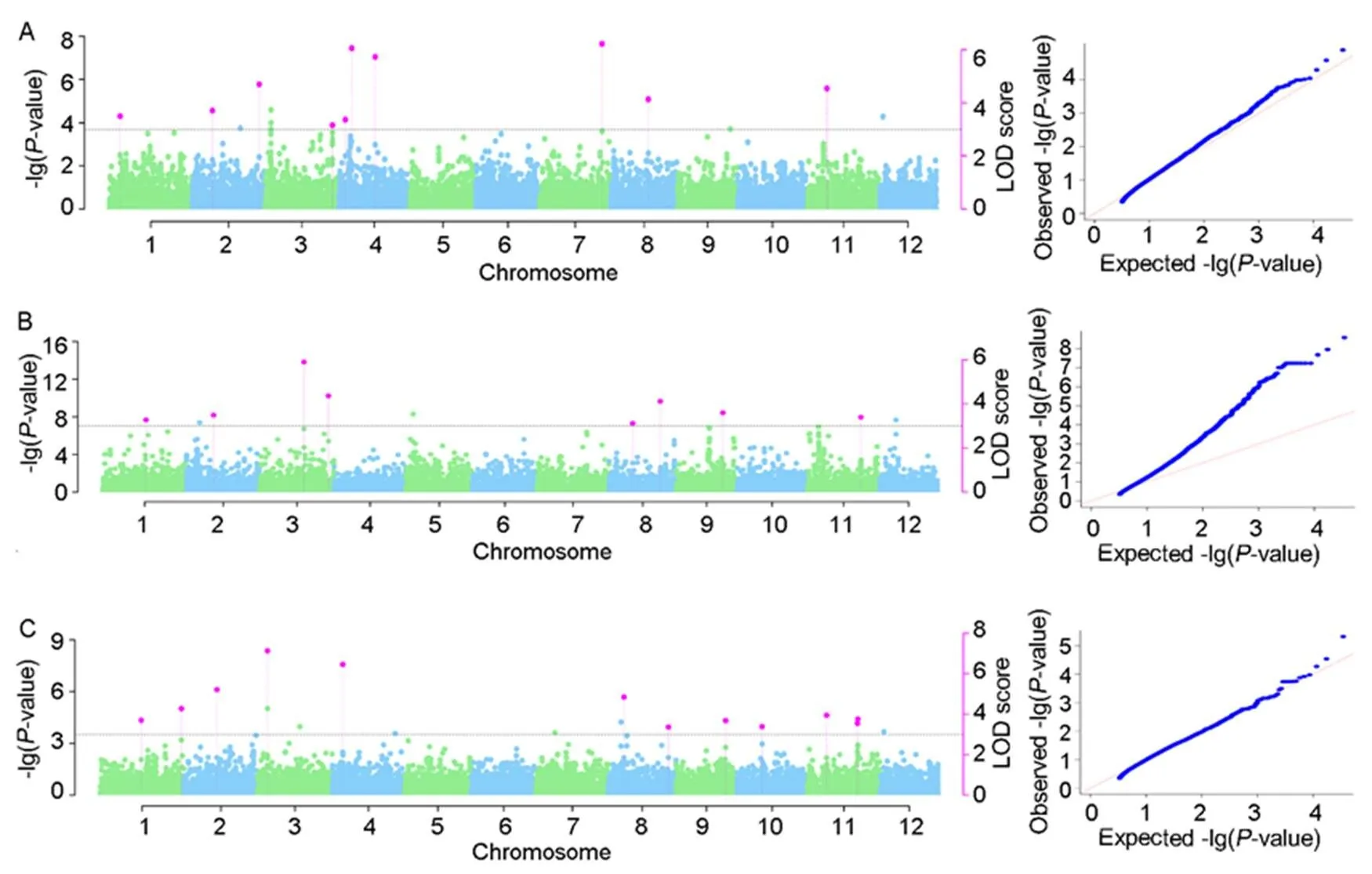
Fig. 1. Manhattan plot and quantile-quantile plot for anaerobic germination (A), mesocotyl length (B) and coleoptile length (C).
Horizontal dotted line is the threshold plotted at LOD = 3 and correspondingvalue. The vertical bars show the QTL region identified based on genome-wide association study using multi-locus mrMLM approach.

Table 2. Single nucleotide polymorphisms associated with anaerobic germination (AG), mesocotyl length (ML) and coleoptile length (CL) in O. nivara accessions.
Chr, Chromosome;2, Contribution to the total phenotype; MAF,Minor allele frequency.
For GWAS,accessions were scored for anaerobic germination as ‘1’ which germinated under anaerobic conditionsand the rest as ‘0’. GWAS with anaerobic germination identified10 SNPs present on chromosomes 1, 2, 3, 4, 7, 8 and 11 (Fig. 1-A and Table 2). Similarly, GWAS was conducted to identify QTLs governing emergence from deeper soil depths using associated traits, mesocotyl length and coleoptile length. Mesocotyl and coleoptile lengths of all the accessions were obtained by combining data from different soil depths(2, 6 and 8 cm). GWAS with mesocotyl length identified 7 SNPs present on chromosomes 1, 2, 3, 8, 9 and 11 (Fig. 1-B and Table 2). GWAS with coleoptile length identified 10 SNPs on chromosomes 1, 2, 3, 4, 8, 9, 10 and 11 (Fig. 1-C and Table 2).
QTLs for anaerobic germination have been reported on chromosomes 1, 2, 3, 7, 9, 11 and 12 (Angaji et al, 2010; Baltazar et al, 2014). Of these QTLs, trehalose-6-phosphate phosphatase genehas been identified as the genetic determinant in, a major QTL responsible for anaerobic germination.is involved in starch mobilization to the germinating embryo and elongating coleoptile, which consequentlyfacilitates germination under anaerobic conditions (Kretzschmaret al, 2015). This mechanism seems to be operating inaccessions which are germinating under submerged conditions, though further elucidation is required for validation. Ten QTLs for anaerobic germination inaccessions could be further explored by generating bi-parental population, however, no QTL was observed inregion. Wu et al (2015) and Lu et al (2016) have identified QTLs for emergence from deeper soil depth using associated traits such as mesocotyl and coleoptile lengths. This study identified 7 QTLs for mesocotyl length and 10 QTLs for coleoptile length inaccessions.These QTLs are being validated and introgressed into elite cultivars by making bi-parental populations and converting associated SNP to KASP (kompetitive allele specificPCR) markers. At this stage, it is also difficult to predict the candidate genes responsible for anaerobic germination and emergence under deeper soil depth present in QTL regions. However, the donors for anaerobic germination and emergence under deeper soil depth identified in this study can be used in the breeding for direct seeded rice. Further identification of genes underlying these QTL regions will unfold the mechanism that is responsible for anaerobic germination tolerance and emergence under deeper soil depth.
ACKNOWLEDGEMENT
This study was funded by the Department of Science and Technology, India (Grant No. EMR/2017/003069).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Frequency distribution for days to germination ofaccessions under anaerobic conditions.
Fig. S2. Germination status under anaerobic conditions (submerged conditions) and emergence from deeper soil depths.
Fig. S3. Principal component analysis (PCA) plot showing clustering of 294accessions into different groups.
Table S1. Information of 300accessions and checks being evaluated under anaerobic conditions and emergence from deeper soil depths at 6 and 8 cm.
Table S2.ddRADseq based SNPs inaccessions spanning all the 12 chromosomes.
Adigbo S O, Osadebay P J, Iseghohi I, Alarima C I, Agbenin N O, Odedina J N, Fabunmi T O. 2018. Screening and evaluation of upland rice (L.) varieties in inundated soil., 51(2): 63–69.
Alibu S, Saito Y, Shiwachi H, Irie K. 2012. Genotypic variation in coleoptile or mesocotyl lengths of upland rice (L.) and seedling emergence in deep sowing., 7:6239–6348.
Angaji S A, Septiningsih E M, Mackill D J, Ismail A M. 2010. QTLs associated with tolerance of flooding during germination in rice (L.)., 172(2): 159–168.
Anonymous. 2022. Package of Practices ofCrops. Ludhiana, India: Punjab Agricultural University: 21–24.
Baltazar M D, Ignacio J C I, Thomson M J, Ismail A M, Mendioro M S, Septiningsih E M. 2014. QTL mapping for tolerance of anaerobic germination from IR64 and thelandrace Nanhi using SNP genotyping., 197: 251–260.
Bhatia D, Joshi S, Das A, Vikal Y, Sahi G K, Neelam K, Kaur K, Singh K. 2017. Introgression of yield component traits in rice (ssp.) through interspecific hybridization., 57(3): 1557–1573.
Cheema K K, Grewal N K, Vikal Y, Sharma R, Lore J S, Das A, Bhatia D, Mahajan R, Gupta V, Bharaj T S, Singh K. 2008. A novel bacterial blight resistance gene frommapped to 38 kb region on chromosome 4L and transferred toL., 90(5): 397–407.
Chung N J. 2010. Elongation habit of mesocotyls and coleoptiles in weedy rice with high emergence ability in direct-seeding on dry paddy fields., 61(11): 911.
Custodio E. 2002. Aquifer overexploitation: What does it mean?, 10(2): 254–277.
Doley D, Barua M, Sarma D, Barua P K. 2018. Screening and enhancement of anaerobic germination of rice genotypes by pre-sowing seed treatments., 115:1185–1190.
Eizenga G C, Neves P C F, Bryant R J, Agrama H A, Mackill D J. 2016. Evaluation of a M-202 ×advanced backcross mapping population for seedling vigor, yield components and quality., 208(1): 157–171.
Gaikwad K B, Singh N, Bhatia D, Kaur R, Bains N S, Bharaj T S, Singh K. 2014. Yield-enhancing heterotic QTL transferred from wild species to cultivated riceL., 9(6): e96939.
Hattori Y, Nagai K, Furukawa S, Song X J, Kawano R, Sakakibara H, Wu J Z, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. 2009. The ethylene response factorsandallow rice to adapt to deep water., 460: 1026–1030.
Kretzschmar T, Pelayo M A F, Trijatmiko K R, Gabunada L F M, Alam R, Jimenez R,Mendioro M S, Slamet-Loedin I H, Sreenivasulu N, Bailey-Serres J, Ismail A M, Mackill D J, Septiningsih E M. 2015. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice., 1:15124.
Kumar K, Sarao P S, Bhatia D, Meelam K, Kaur A, Mangat G S, Brar D S, Singh K. 2018. High-resolution genetic mapping of novel brown planthopper resistance locus,inL.×(Sharma & Shastry) derived interspecific F2population., 131(5):1163–1171.
Lee J, Kwon S W. 2015. Analysis of quantitative trait loci associated with seed germination and coleoptile length under low temperature condition., 18(4): 273–278.
Lu B R, Ge S, Sang T, Chen J K, Hong D Y. 2001. The current taxonomy and perplexity of the genus(Poaceae)., 39(4):373–388.
Lu Q, Zhang M C, Niu X J, Wang C H, Xu Q, Feng Y, Wang S, Yuan X P, Yu H Y, Wang Y P, Wei X H. 2016. Uncovering novel loci for mesocotyl elongation and shoot length inrice through genome-wide association mapping., 243(3): 645–657.
Luo J, Tang S Q, Hu P S, Louis A, Jiao G A, Tang J. 2007. Analysis on factors affecting seedling establishment in rice., 14(1): 27–32.
Sidhu B S, Sharda R, Singh S. 2021. Spatio-temporal assessment of groundwater depletion in Punjab, India., 12: 100498.
Wu J H, Feng F J, Lian X M, Teng X Y, Wei H B, Yu H H, Xie W B, Yan M, Fan P Q, Li Y, Ma X S, Liu H Y, Yu S B, Wang G W, Zhou F S, Luo L J, Mei H W. 2015. Genome-wide association study (GWAS) of mesocotyl elongation based on re-sequencing approach in rice., 15: 218.
Wu M G, Zhang G H, Lin J R, Cheng S H. 2005. Screening for rice germplasms with specially-elongated mesocotyl., 12(3):226–228.
Zhu C S, Gore M, Buckler E S, Yu J M. 2008. Status and prospects of association mapping in plants., 1(1):5–20.
30 October 2021;
18 February 2022
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.02.001
Dharminder Bhatia (d.bhatia@pau.edu)
- Rice Science的其它文章
- RPA-Assisted Cas12a System for Detecting Pathogenic Xanthomonas oryzae, a Causative Agent for Bacterial Leaf Blight Disease in Rice
- Polycomb Repressive Complex 2-Mediated H3K27 Trimethylation Is Required for Pathogenicity in Magnaporthe oryzae
- Genome-Wide Analysis of von Willebrand Factor A Gene Family in Rice for Its Role in Imparting Biotic Stress Resistance with Emphasis on Rice Blast Disease
- Selenium Alleviates Carbohydrate Metabolism and Nutrient Composition in Arsenic Stressed Rice Plants
- Feasibility of Improving Unmanned Aerial Vehicle-Based Seeding Efficiency by Using Rice Varieties with Low Seed Weight
- Arsenic Accumulation in Rice:Sources, Human Health Impact and Probable Mitigation Approaches

