Jianpi Qingchang Bushen decoction improves inflammatory response and metabolic bone disorder in inflammatory bowel disease-induced bone loss
Ya-Li Zhang, Qian Chen, Lie Zheng, Zi-Wei Zhang, Yu-Jun Chen, Yan-Cheng Dai, Zhi-Peng Tang
Abstract
Key Words: Inflammatory bowel disease; Osteoporosis; Jianpi Qingchang Bushen decoction; Inflammation;Bone metabolism
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic, relapsing immune-mediated inflammatory disease of the gastrointestinal tract, which includes Crohn's disease (CD) and ulcerative colitis (UC)[1 ,2 ]. Its main clinical manifestations are diarrhea, abdominal pain, bloody stool, and various degrees of systemic symptoms[3 ,4 ]. Nearly 50 % of IBD patients show at least one kind of extra-intestinal symptom during their lifetime, among which musculoskeletal damage is the most common[5 -7 ]. In recent years,increasing attention has been paid to the study of osteopenia and osteoporosis caused by IBD.Osteoporosis is a systemic bone disease characterized by low bone mass and the destruction of bone microstructure. It increases bone fragility, making it prone to fractures. Osteoporosis is one of the more common extraintestinal manifestations in IBD patients[8 ]. Studies have shown that the risk of osteoporosis is significantly higher in IBD patients than in healthy controls[9 ,10 ], exposing them to a higher risk of fracture. The risk of fracture in IBD patients is 40 % higher than that in healthy controls,seriously affecting their quality of life[11 ]. The search for a suitable treatment to reduce pain and the economic burden has become a priority of international medical and social establishments.
The pathogenesis of osteoporosis in IBD patients has not been fully elucidated. Many factors affect bone metabolism. Recent studies have shown that intestinal inflammation is one of the most important factors leading to osteoporosis in IBD patients. Inflammation is involved in the pathophysiological process of bone loss in patients with IBD[12 -14 ]. Therefore, controlling intestinal inflammation could help to prevent the occurrence and progression of osteoporosis. The receptor activator of nuclear factor kappa B (NF-κB; RANK), its ligand (receptor activator of NF-κB ligand, RANKL), and the soluble decoy receptor osteoprotegerin (OPG) orchestrate resorption of osteoclastic bone and play a key role in the common pathogenic pathway between gut inflammation and bone loss[15 ]. RANK/RANKL/OPG signaling is a key regulator of osteoclast biology and bone metabolism and an important pathway regulating osteoclast differentiation[16 -18 ]. Stanisławowski et al[19 ] reported that patients with UC have higher local expression of serum OPG mRNA and protein than healthy participants[19 ]. Krela-Kaź mierczaket al[20 ] reported an increase in the OPG/RANKL ratio in CD patients[20 ]. According to a recent study, elevated osteocyte tumor necrosis factor (TNF)-α, interleukin (IL)-6 , RANKL, and OPG correspond to higher osteoclast surfaces and a lower bone formation rate in an IBD model induced with dextran sodium sulfate[21 ]. No effective preventive or treatment modality exists for osteoporosis in IBD patients. A great deal of interest has been generated to evaluate the mechanism by which natural products exert their beneficial effects in the gastrointestinal tract[22 ]. Traditional Chinese medicine(TCM) has good effects on IBD patients with osteoporosis. Spleen and kidney insufficiencies and dampness-heat are important components of IBD-associated osteoporosis pathogenesis. Treatment is aimed at invigorating the spleen, clearing the intestine, and tonifying the kidney. Thus, Jianpi Qingchang Bushen decoction (JQBD) was established under the guidance of TCM theory. JQBD is a prescription developed by our team to treat IBD patients with osteoporosis. Our previous study reported that JQBD-medicated serum promotes osteoclast apoptosis by downregulating Bcl-2 and upregulating the expression of the Bax protein[23 ]. According to TCM theory, the kidney stores the essence and this can be transformed into the bone marrow to nourish the bones and strengthen the skeleton. Thus, a kidney deficiency can cause osteoporosis. Tonifying the kidneys regulates bone metabolism to alleviate osteoporosis. Modern pharmacological studies have confirmed thatPsoralea corylifoliaLinn,Alpinia oxyphyllaMiq, and their extracts improve bone metabolism to prevent bone loss and are commonly used to treat osteoporosis[24 -27 ].
In this study, the potential pharmacodynamic mechanism of JQBD for treating IBD-induced osteoporosis was studiedin vivofor the first time. An IBD-induced osteoporosis model was constructed by treating interleukin (IL)-10 -knockout mice with piroxicam. JQBD was given as an intervention, and its effects on the inflammatory response and bone metabolism were observed to provide a theoretical basis for the clinical prevention and treatment of osteoporosis in IBD patients.
MATERIALS AND METHODS
TCM preparation and identification of the phytochemicals
JQBD was prepared by the TCM pharmacy of Longhua Hospital, Shanghai University of TCM. It was composed of eight herbal species, includingAstragalus mongholicusBunge 30 g,Codonopsis pilosula(Franch.) Nannf. 15 g, Alpinia oxyphylla Miq. 12 g, Cullen corylifolium (L.) Medik. 9 g,Portulaca oleraceaL.30 g, Sanguisorba officinalis L. 15 g, Aucklandia costus Falc. 6 g, and Glycyrrhiza glabra L. 6 g. The components were soaked, boiled twice, filtered, concentrated, freeze-dried into a powder, and stored at−20 °C. Ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry(UPLC/Q-TOF/MS) was used to analyze the samples and identify 44 phytochemicals in JQBD. The MS chromatograms of the JQBD negative and positive ion modes are shown in Supplementary Figure 1 .The chemical information of the identified phytochemicals is shown in Supplementary Table 1 . Quality control measures were performed according to the guidelines of the Chinese State Food and Drug Administration.
Animal model and drug intervention
Six IL-10 -knockout C57 BL/6 mice were produced by the Shanghai Nanfang Research Center for Model Organisms (SNRCMO; Shanghai, China) and were maintained and bred in a specific-pathogen-free animal room at 23 ± 3 °C, 35 %–45 % relative humidity, and a 12 h/12 h light/dark regime at the Laboratory Animal Center of the Shanghai University of Traditional Chinese Medicine. Food and water were providedad libitum. The Animal Ethics Committee of the Shanghai University of Traditional Chinese Medicine approved this study (PZSHUTCM191108004 ).
The IL-10 -knockout mice were bred, and 70 F6 -generation mice underwent gene detection using tail tip samples. IL-10 knockout was detected in 30 of these mice. We used wild-type mice as controls, which were the progeny of IL-10 +/- mating and bred at the same facility. The gene detection results are shown in Figure 1 . The IBD-induced osteoporosis model was constructed by feeding the IL-10 -knockout mice 200 ppm piroxicam for 10 d[28 ]. The mice were randomly divided into IL-10 -knockout controls(model group) and IL-10 -knockout mice with JQBD intervention (JQBD group). Six mice were included in each group (three males and three females). The JQBD group was administered 16 .5 g/kg/d of the JQBD suspension by gavage, while the control and model groups were given the same volumes of normal saline at the corresponding time points. The intervention was conducted for 14 consecutive days. All mice were killed after the intervention, and blood samples and colon and bone tissues were harvested.

Figure 1 Gene detection results in F6 -generation mice. A: Interleukin (IL)-10 conditional knockout; B: IL-10 knockout; C: Dppa3 -cre.
Daily record of observational parameters
The mental state, coat color, activity, body weight, diet, fecal traits, occult blood, and gross bloody stool were observed and recorded daily from the beginning of modeling. The disease activity index (DAI)score was used to evaluate colitis severity in the mice daily following the grading scheme presented in Table 1 [29 ].
Measurement of colon length and colon histopathological examination
Dead mice were dissected to isolate the colon. The colons were placed on filter paper, and their length was measured with a ruler.
The colon samples were fixed in 4 % paraformaldehyde solution, dehydrated, paraffin-embedded,sliced, and stained with hematoxylin and eosin (HE).
Ultrastructural observation of the colon
Part of each colon was fixed in 2 .5 % glutaraldehyde within 1 min, dehydrated, embedded, sliced, and stained with 3 % uranium acetate and lead citrate. The colon ultrastructure was observed bytransmission electron microscopy (TEM).
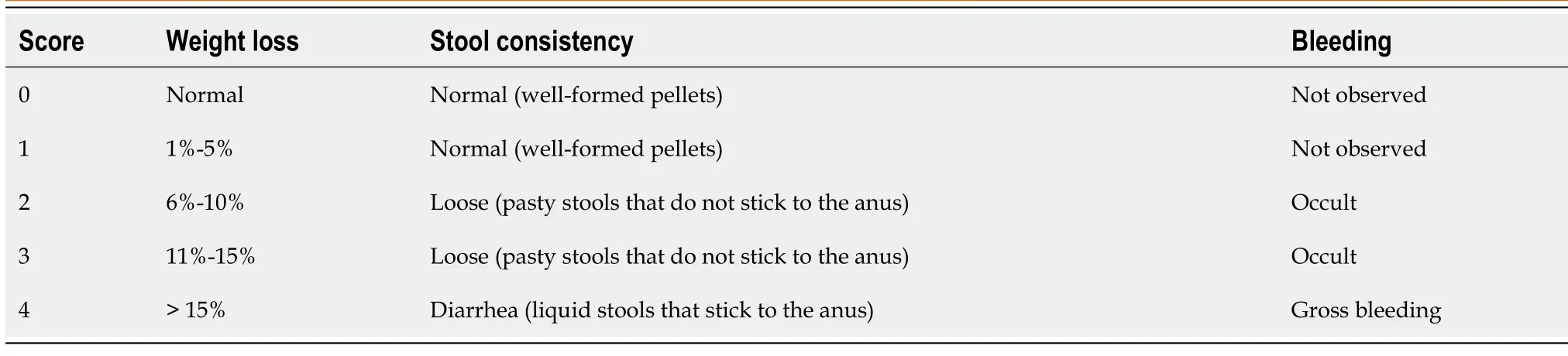
Table 1 Calculation of disease activity index score
Specimen preparation and micro-computed tomography
After the mice were killed, the intact lumbar vertebrae were separated, and the residual soft tissue was removed. The bone tissue was fixed in a 75 % ethanol solution pending the experiment. All specimen assessments were performed on the lumbar vertebra by μ-CT80 micro-computed tomography (CT)(SCANCO Medical AG, Bassersdorf, Switzerland). The scanning resolution was 18 μmperlayer,followed by three-dimensional (3 D) reconstruction. The morphometric analysis included bone volume/total volume (BV/TV) ratio, trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), connectivity-density (Conn-Dens), and bone mineral density (BMD).
Real-time polymerase chain reaction detection of NF-κB, TNF-α, IL-1 β, IL-6 , and IL-8 mRNA expression levels
Total RNA was extracted from the colon tissues with TRIzol, and the RNA concentration was determined. cDNA was reverse transcribed following the Takara kit instructions and was detected on the ABI StepOne Plus real-time fluorescence quantitative polymerase chain reaction (RT-qPCR)instrument. The primers were designed and synthesized by Shanghai ShineGene Molecular Biotechnology Co., Ltd. (Shanghai, China) (Table 2 ) and verified using the BLAST program. A 20 -μL RT-qPCR reaction system was configured, and the reaction amplification was carried out following the kit instructions. β-actin was used as the internal reference, fold-change was estimated by the 2-ΔΔCtmethod,and the mRNA expression level was compared between the groups. The assay was repeated three times.
Detecting the effect of JQBD on RANKL, OPG, RANK, and NF-κB protein expression in colon tissue by Western blot
We added 1 mL of RIPA Lysis Buffer and 10 μL of a protease inhibitor mixture to a homogenization tube and precooled it on ice; 50 μg of colon tissue was weighed and homogenized repeatedly in the tubes six times until no obvious tissue was seen in the homogenate. The homogenate was centrifuged(Eppendorf 5417 R, Eppendorf) at 4 °C and 15000 × g for 15 min, and the supernatant was placed in another centrifugation tube. A small volume of the supernatant was taken, and the protein concentration was determined by the bicinchoninic acid method. Samples of 30 μg were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a membrane, and sealed. TBSTdiluted RANKL, OPG, RANK, and NF-κB antibodies (1 :1000 ) were added, and the membrane was incubated overnight at 4 °C. After the incubation, the membrane was washed and incubated for 1 h with gentle shaking at room temperature with fluorescence-labeled secondary antibody diluted 1 :3000 . TBST was used to rinse the membranes. An enhanced chemiluminescence kit (reagents A and B were mixed at equal volumes) was placed on the surface of the polyvinylidene fluoride (PVDF) membrane and incubated in the dark at room temperature for 1 min. The PVDF membrane was placed on the operating table of a Western blot imager, and a strip image was acquired. The gray values of the bands were analyzed using ImageJ analysis software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism, version 8 .0 software (GraphPad Software Inc., La Jolla, CA, United States). Data are expressed as the mean ± SD. Differences between groups were assessed by one-way analysis of variance. APvalue < 0 .05 was considered significant.
RESULTS
General condition of the mice
The control mice showed a flexible response, agile movement, glossy fur, pink paws, active foraging,normal water intake, and mostly globular feces that were negative for occult blood. The mice in themodel group were lazy, with a reduced appetite, dirty and sparsely erected fur, white paws, soft feces,and perianal body hair stained with loose stools. Some had bloody stools, occasional anal peeling,perianal blood, feces strongly positive for occult blood, and decreased body weight. The JQBD group mice displayed a better mental status and they had glossy hair, only slightly white paws, feces not as soft, only occasional bloody stools, lower positive occult blood rate, and less lost weight than the model group (Figure 2 ).

Table 2 List of primers used in this study
Weight change and DAI scores
The weights of the mice in the model and JQBD groups on day 10 of the experiment were significantly lower(P< 0 .05 ) than that of the control group. The weight in the JQBD group was higher than that in the model group (P< 0 .05 ). The weight in all groups on day 14 was higher than that at the start of the experiment, but the weight increase in the model and JQBD groups was less evident than that in the control group (P< 0 .05 ). The weight of the model group was significantly lower than that of the other two groups (P< 0 .05 ). Higher DAI scores were observed in the model and JQBD groups. The DAI score of mice in the model group was higher than that of mice in the control group (P< 0 .05 ), which decreased significantly in response to JQBD treatment on day 14 (P < 0 .05 ) (Figure 3 ).
Morphological changes in the colonic mucosa
The colon wall in the control group was smooth, red, and resilient. In the model group, it was thickened,stiff, hyperemic, edematous, and erosive, with some irregular ulcers and a brittle texture. The colonic mucosa hyperemia and edema in the JQBD group were less severe than those in the model group with significantly reduced ulcer formation. The colon in the model group was significantly shorter than that in the control (P< 0 .001 ) and the JQBD (P < 0 .05 ) groups.
Histological evaluation of the colonic mucosa
The colonic mucosal epithelium in the control group was intact and continuous, the glands were orderly arranged, the goblet cells were visible, the blood vessels and fibrous interstitium in the lamina propria and muscle layer were normal, and inflammatory cell infiltration was rarely observed. Necrosis,erosion, disordered gland arrangement, different degrees of inflammatory cell infiltration, a reduced number of goblet cells, and ulcer formation were observed in the colonic mucosa of mice in the model group, suggesting successful IBD modeling in these mice. JQBD provided partial protection to the mucosa, so it was less damaged, showing mild to moderate hyperemia and edema. Lower inflammatory cell infiltration and ulcer formation rates were noted, and the glands were arranged in an orderly manner (Figure 4 ).
Bone metabolic parameters on micro-computed tomography
The 3 D imaging of the vertebral centrum in the model group revealed a lower bone mass, loose trabeculae, and “rod-shaped” changes in the structure compared to the control group. Treatment with JQBD resulted in a higher bone mass, tighter trabecular bone gaps, and higher connectivity than those in the model group. The quantitative micro-CT results showed that the BV/TV ratio and BMD decreased in the model group. The Conn-Dens and Tb.Th were similar in the normal and model groups. Although BV/TV and BMD were significantly lower in the model group than in the control group, these values were similar in the JQBD, control, and model groups (Figure 5 ).
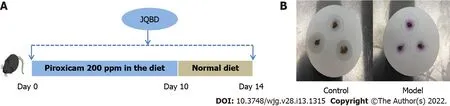
Figure 2 Animal experimental flow and fecal occult blood test. A: An experimental bone loss inflammatory bowel disease model was induced by peroral administration of piroxicam for 10 d in interleukin-10 -/- mice. Normal saline or Jianpi Qingchang Bushen decoction (JQBD; 16 .5 g/kg/d) was given intragastrically to the control/model groups and JQBD group, respectively (n = 6 , each); B: Fecal occult blood test of control and model groups: The control group was negative, and the model group was strongly positive for occult blood. JQBD: Jianpi Qingchang Bushen decoction Group.

Figure 3 General condition of the mice. A: Disease activity index scores gauged daily (n = 6 per group); B: Body weight measured daily (n = 6 per group); C and D: Colon length measurement and graph presenting the statistical analysis results. Data are presented as the mean ± SD. aP < 0 .05 ; bP < 0 .01 ; cP < 0 .001 (n = 6 per group).
Structural changes in the colonic epithelial cells as observed by TEM
The TEM images illustrated intact colon cells in the control group, with no increase in intercellular space. The endoplasmic reticulum had a reticular structure and was bundled, the cavity was not expanded, many ribosomes were attached to it, and numerous mitochondria were observed. In the model group, the organelles were significantly swollen and the endoplasmic reticulum cavity was significantly expanded, attaining different shapes and sizes, with many vacuoles. Some of the organelles fused into clusters. A microvillar structure was partially observed in the JQBD group. The mitochondria were slightly swollen, the number of epithelial cells increased, and the cell structure was more intact than in the model group. The endoplasmic reticulum cavity was slightly expanded (Figure 4 ).
Effect of JQBD on NF-κB, TNF-α, IL-1 β, IL-6 , and IL-8 gene expression
The expression of the five genes was verified by RT-qPCR. The mRNA expression levels of NF-κB, TNFα, IL-1 β, IL-6 , and IL-8 were significantly upregulated in the colon of the model group compared to the control group. JQBD intervention downregulated the mRNA expression levels of these genes,suggesting that JQBD improved the mucosal inflammatory response (Figure 6 ).

Figure 4 Histological evaluation of the colonic mucosa following hematoxylin and eosin staining (× 100 ) and ultrastructure of the colonic epithelium by transmission electron microscopy (× 6000 ). Arrows indicate goblet cells (a), crypts (b), inflammatory cells infiltration (c), epithelium surface erosion (d), and submucosal oedema (e). Control: Control group; Model: Model group; JQBD: Jianpi Qingchang Bushen decoction Group; Nu: Nucleus; Mi:Mitochondrial; ER: Endoplasmic Reticulum; rER: Rough endoplasmic reticulum; Mv: Microvillus.
Effect of JQBD on RANKL, OPG, RANK, and NF-κB protein expression
The RANKL, OPG, RANK, and NF-κB protein levels were measured by Western blot. RANKL and OPG protein expression was significantly upregulated in the model group colon and was markedly downregulated following JQBD intervention. Although the RANK and NF-κB protein expression levels were significantly higher in the model group than in the control group, they were similar between the model and JQBD groups. Taken together, these data indicate that JQBD improved the mucosal inflammatory response and restrained the RANK/RANKL/OPG signaling pathway during the development of bone loss in IBD (Figure 7 ).
DISCUSSION
IBD is a chronic, non-specific, intestinal inflammatory disease with unclear etiology and common digestive system clinical presentation. The disease is protracted and difficult to cure, and the symptoms easily recur. IBD affects multiple systems, including the bones and calcium deposits. Studies have shown that IBD patients have a lower bone mass than healthy people[30 -33 ]. The risk of osteoporosis and subsequent fractures is significantly higher in IBD patients[34 ,35 ], and it is the main cause of mobility problems and decreased quality of life in these patients[36 ]. Osteoporosis is a common and easily overlooked extra-intestinal manifestation in IBD patients. Early diagnosis and prevention of osteoporosis in IBD patients are very important. Therefore, we explored the possible pathogenesis of IBD-induced osteoporosis and evaluated the effectiveness of a promising drug in the present study. We propose that JQBD may have a protective effect against IBD-induced osteoporosis through the RANK/RANKL/OPG signaling pathway.
We established a bone loss IBD model in IL-10 -knockout mice by peroral administration of piroxicam.IL-10 -knockout mice are commonly used as an animal model to study IBD[37 ,38 ]. When exposed to nonsteroidal anti-inflammatory drugs, such as piroxicam, these animals rapidly develop colitis that persists for a long time[39 ,40 ]. Holgersen et al[15 ] reported that they orally fed IL-10 knockout mice piroxicam for 12 d before the bone loss and trabecular bone structural changes occurred[15 ]. We report similar results in IL-10 -knockout mice following 10 d of oral piroxicam administration. Our micro-CT data show that the BV/TV ratio and BMD were significantly lower in the model mice than in the controls.The 3 D simulation map suggested that bone mass was significantly lower in the model group than in the control group and that the trabecular bone structure was lost, with visible fractures and destruction of the bone microstructure. The model group exhibited bone loss and significant colitis, similar to another report[41 ]. Our results preliminarily confirm the abnormal bone metabolism in the model group, suggesting successful modeling.

Figure 5 Three-dimensional reconstruction of the lumbar spine trabecular structure in mice and micro-computed tomographic analyses of the lumbar vertebral metaphysis. Bone volume to total volume (BV/TV) ratio, Conn-Dens, trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and bone mineral density (BMD) were obtained for all mice. A: Control; B: Model; C: JQBD; D: BV/TV; E: Conn-Dens; F: Trabecular number; G:Trabecular thickness; H: Trabecular separation; I: Bone mineral density. Control: Control group; Model: Model group; JQBD: Jianpi Qingchang Bushen decoction Group; BV/TV: Bone volume to total volume ratio; Conn-Dens: Connectivity-density; Tb.N: Trabecular number; Tb.Th: Trabecular thickness; Tb.Sp: Trabecular separation; BMD: Bone mineral density. Data are presented as the mean ± SD. aP < 0 .05 . (n = 6 per group).
Our data show that the JQBD was protective against IBD-induced bone loss by inhibiting inflammation. Evidence shows that intestinal inflammation is involved in the pathophysiology of osteoporosis in IBD[42 -44 ]. Expression of NF-κB, TNF-α, IL-1 β, IL-6 , and IL-8 mRNA in colonic tissue from the model group increased significantly, confirming that pro-inflammatory cytokines are involved in the occurrence of experimental colitis in piroxicam-induced IL-10 -knockout mice with IBD. The administration of the JQBD reduced the levels of pro-inflammatory cytokines and decreased colonic inflammation. Several studies have evaluated the pathophysiology of IBD and its association with osteoporosis. Bone loss was initially attributed to the use of drugs, such as corticosteroids. However, it was later found that BMD decreases in IBD patients even without drug treatment, suggesting that IBD might be involved in the pathophysiology of bone loss, and chronic inflammation is the primary determinant[45 -47 ].
We evaluated the curative effects of JQBD on the bone-loss IBD model mice and confirmed that the RANK/RANKL/OPG signaling pathway was involved in the process. The RANKL, OPG, RANK, and NF-κB protein levels increased significantly in colon tissue from the model group. Their expression levels were downregulated after JQBD intervention, suggesting that JQBD inhibits activation of the RANK/RANKL/OPG signaling pathway. Several pro-inflammatory cytokines, including IL-1 β, TNF-α,IL-6 , and IL-8 increase in IBD and have been shown to stimulate osteoclast differentiation[48 ]. Studies have suggested that the abnormal intestinal tract immune response in IBD leads to injury and inflammation of the intestinal mucosa, activation of the RANK /RANKL/OPG signaling pathway, changes in the bone conversion rate, osteoclast activation, and bone loss, leading to IBD-induced osteoporosis[21 ,49 ,50 ]. These results highlight the interactions between inflammation and the RANK/RANKL/OPG signaling pathway during the development of osteoporosis in IBD. JQBD reduced inflammation of the colonic mucosa and inhibited activation of the RANK/RANKL/OPG signaling pathway (Figure 8 ).Taken together, our present study data demonstrate that the JQBD improved IBD-induced osteoporosisviathe RANK/RANKL/OPG signaling pathway.
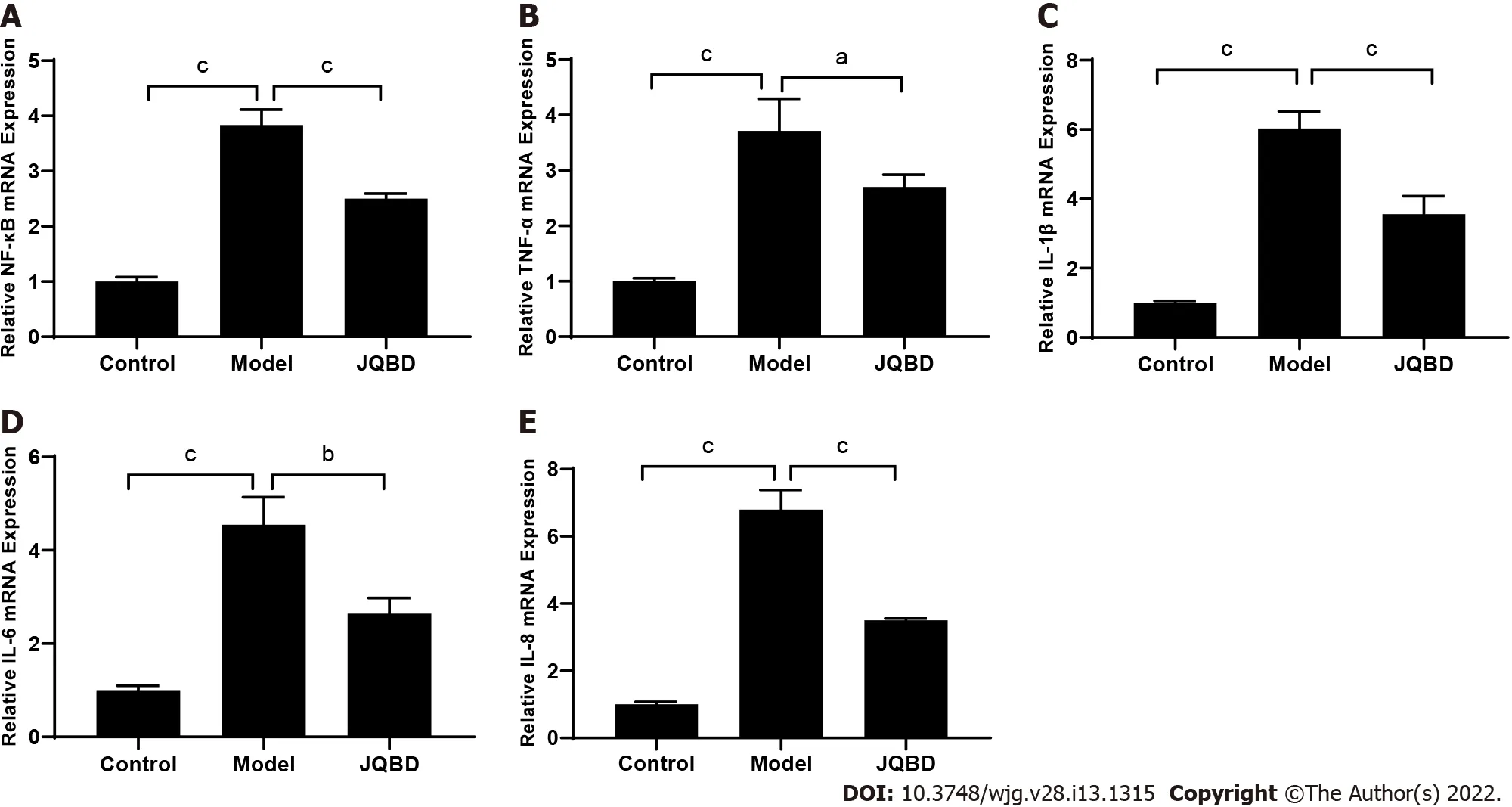
Figure 6 Effect of Jianpi Qingchang Bushen decoction on nuclear factor-kappaB, tumor necrosis factor-α, interleukin-1 β, interleukin-6 ,and interleukin-8 gene expression. The relative expression of these genes was quantified by real-time fluorescence quantitative polymerase chain reaction. A:NF-κB; B: TNF-α; C: IL-1 β; D: IL-6 ; E: IL-8 . Control: Control group; Model: Model group; JQBD: Jianpi Qingchang Bushen decoction Group; NF-κB: Nuclear factorkappaB; TNF-α: Tumor necrosis factor-α; IL: Interleukin. Data are presented as the mean ± SD. aP < 0 .05 ; bP < 0 .01 ; cP < 0 .001 (n = 3 per group).

Figure 7 Effect of Jianpi Qingchang Bushen decoction on receptor activator of nuclear factor κB ligand, osteoprotegerin, receptor activator of nuclear factor kappa B, and nuclear factor-kappaB protein expression. A: Protein expression quantified by Western blot; B: RANKL; C:OPG; D: RANK; E: NF-κB. Control: Control group; Model: Model group; RANK: Receptor activator of nuclear factor kappa B; RANKL: Receptor activator of nuclear factor κB ligand; JQBD: Jianpi Qingchang Bushen decoction Group. Data are presented as the mean ± SD. aP < 0 .05 ; bP < 0 .01 ; cP < 0 .001 (n = 3 per group).
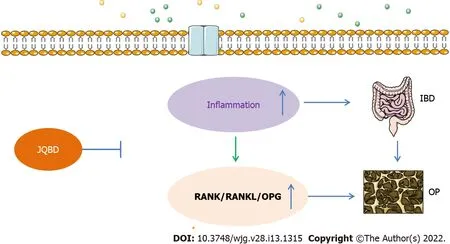
Figure 8 Possible mechanisms of bone loss in inflammatory bowel disease. IBD: Inflammatory bowel disease; OPG: Osteoprotegerin; RANK:Receptor activator of nuclear factor kappa B; RANKL: Receptor activator of nuclear factor κB ligand; JQBD: Jianpi Qingchang Bushen decoction Group.
This study primarily focused on the mechanism by which JQBD regulates the RANK/RANKL/OPG signaling pathway and improves IBD-induced osteoporosis. Our investigation has only preliminarily discussed the topic at the animal level and had several limitations. First, we considered using future emerging methods, such as systems pharmacology, to systematically explore the potential efficacy of JQBD in the treatment of IBD-induced osteoporosis. Such a study would include related experimental verification at the cell and animal levels. Second, the basic mechanism behind IBD-induced osteoporosis needs to be further explored. We will perform long-term observations in future experiments by extending the experimental period to 1 or 2 mo. We will also investigate the activation of the RANK/RANKL/OPG signaling pathway in bone tissues. Third, changes in intestinal microecology play an important role in the healing of the mucosa. Some studies have reported that an increase in the intestinal microbial bifidobacteria promotes healing of the colonic mucosa, increases the IL-10 level, and reduces the IL-6 and TNF-α levels[51 ,52 ]. The intestinal flora was not determined in this study. Thus,whether JQBD regulates intestinal microecology is worthy of further study.
CONCLUSION
JQBD plays a role in treating IBD-related bone metabolic abnormalities by inhibiting colonic mucosal inflammation, promoting mucosal healing, and inhibiting activation of the RANK/RANKL/OPG signaling pathway, osteoclast formation, and bone resorption.
ARTICLE HIGHLIGHTS

Research results
Our data show that JQBD was protective against IBD-induced bone loss by inhibiting inflammation. The receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL), osteoprotegerin (OPG), receptor activator of NF-κB (RANK), and NF-κB protein levels increased significantly in colon tissue from the model group. Their expression levels were downregulated after JQBD intervention.
Research conclusions
We evaluated the curative effects of JQBD on the bone-loss IBD model mice and confirmed that the RANK/RANKL/OPG signaling pathway is involved in the process.
Research perspectives
This study primarily focused on the mechanism by which JQBD regulates the RANK/RANKL/OPG signaling pathway and improves IBD-induced osteoporosis.
FOOTNOTES
Author contributions:Zhang YL and Chen Q contributed equally to this work, and both performed the majority of research; Dai YC, Zheng L, Zhang ZW, and Chen YJ performed the research and analyzed the data; Tang ZP designed and coordinated the research; Zhang YL and Chen Q wrote and revised the paper; all authors read and approved the final manuscript.
Supported byNational Natural Science Foundation of China, No. 81704009 and No. 81873253 ; the Key Clinical Specialty Construction Project supported by Hongkou District Health Committee, No. HKZK2020 A01 ; and the Sixth Round of Academic Experience Successors Training Project for Veteran Practitioner of Traditional Chinese Medicine(The Document of the State Administration of Traditional Chinese Medicine 2017 ), No. 29 .
Institutional review board statement:The study was reviewed and approved by the Animal Ethics Committee of the Shanghai University of Traditional Chinese Medicine, No. PZSHUTCM191108004 .
Conflict-of-interest statement:The authors declare that there are no conflicts of interest related to this study.
Data sharing statement:The datasets generated during and/or analyzed during the current study will be available upon request from the principle investigator. The shared data will only be allowed to be used by the applicant for scientific studies. No commercial activities are allowed.
ARRIVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:China
ORCID number:Ya-Li Zhang 0000 -0002 -8987 -3558 ; Qian Chen 0000 -0002 -1598 -2735 ; Lie Zheng 0000 -0002 -0918 -0728 ; Zi-Wei Zhang 0000 -0001 -6557 -4062 ; Yu-Jun Chen 0000 -0001 -9731 -1400 ; Yan-Cheng Dai 0000 -0002 -3571 -077 X; Zhi-Peng Tang 0000 -0001 -5695 -8072 .
S-Editor:Fan JR
L-Editor:Wang TQ
P-Editor:Yuan YY
 World Journal of Gastroenterology2022年13期
World Journal of Gastroenterology2022年13期
- World Journal of Gastroenterology的其它文章
- c-MET immunohistochemical expression in sporadic and inflammatory bowel disease associated lesions
- Management of incidentally discovered appendiceal neuroendocrine tumors after an appendicectomy
- Comparison of the performance of MS enteroscope series and Japanese double- and single-balloon enteroscopes
- Increased prognostic value of clinical–reproductive model in Chinese female patients with esophageal squamous cell carcinoma
- Locoregional therapies and their effects on the tumoral microenvironment of pancreatic ductal adenocarcinoma
- Generic and disease-specific health-related quality of life in patients with Hirschsprung disease: A systematic review and meta-analysis
