Increased prognostic value of clinical–reproductive model in Chinese female patients with esophageal squamous cell carcinoma
Dong-Yun Zhang, Jian-Wei Ku, Xue-Ke Zhao, Hai-Yan Zhang, Xin Song, Hong-Fang Wu, Zong-Min Fan,Rui-Hua Xu,Duo You, Ran Wang,Ruo-Xi Zhou,Li-Dong Wang
Abstract
Key Words: Esophageal squamous cell carcinoma; Female; Nomogram; Prognosis; Estrogen receptor
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is highly invasive malignancy in China with a 5 -year overall survival (OS) rate of only 10 %-20 % in patients with advanced disease[1 ]. In addition to pathological features, reproductive factors such as menopausal status, estrogen receptors, and pregnancy number are also correlated with clinical outcome in female ESCC patients[2 ,3 ]. The increased risk of esophageal cancer is associated with a decrease in estrogen level[4 ]. An epidemiological study of menopausal hormone therapy (MHT) confirmed that patients who have been using MHT have a reduced risk of ESCC[5 ]. Previous research also supports the protective effects of female hormones on the risk of ESCC[6 ].
The most common prognostic evaluation system for ESCC mainly depends on the tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer, which has been adopted in the United States since 1959 [7 ,8 ]. Recently, the prognostic value of the TNM system has been challenged due to its unsatisfactory discriminative ability[9 ]. Relevant studies have shown significant differences in OS in female ESCC patients even with the same TNM stage[3 ]. Combined with other clinical prognostic factors such as age and tumor differentiation, individual prognosis can be moreaccurately predicted[10 ,11 ]. An effective predictive model is needed for female ESCC to precisely assess the clinical outcome.

Table 1 Distribution of the clinical and reproductive factors for female patients with esophageal squamous cell carcinoma in primary training, internal and external validation cohorts (mean ± SD)

T: Tumor invasion depth; N: Lymph node metastasis; ESR1 : Estrogen receptor alpha; ESR2 : Estrogen receptor beta.
A nomogram is a graphical representation tool based on statistical predictive modeling[12 ]. So far, no nomogram including reproductive factors has been constructed to predict OS in female ESCC. The current study was designed to identify independent prognostic factors based on univariatie and Cox proportional hazards survival analysis, and then develop and validate a new prognostic nomogram incorporating clinical and reproductive characteristics to predict 1 -, 3 -, and 5 -years OS in female ESCC.We also aimed to establish whether the nomogram model could provide more accurate prognostic prediction than the clinical model and TNM stage.
MATERIALS AND METHODS
Study population
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and Institutional Review Board of the First Affiliated Hospital of Nanyang Medical College. The documentation of informed consent was waived due to anonymity of the participants. In the multicenter study, the analysis was performed on the two independent cohorts of female patients with ESCC (Cohort 1 , 500000 esophageal and gastric cardiac carcinoma database of First Affiliated Hospital of Zhengzhou University[3 ]; Cohort 2 , First Affiliated Hospital of Nanyang Medical College). Patients who were pathologically diagnosed with ESCC, underwent surgery, and had detailed follow-up information where eligible. Patients who received preoperative therapy (radiotherapy and/or chemotherapy) and lost clinical and reproductive information were excluded.
Baseline characteristics and clinical outcome
Clinicopathological factors, including age at diagnosis, incidence area, tumor location, tumor differentiation, tumor invasion depth (T), lymph node metastasis (N), metastasis, and therapy methods were collected from the medical records. Reproductive factors such as menarchal age, menopausal status,menopausal age, pregnancy number, and biomarker levels [estrogen receptor alpha (ESR1 ) and estrogen receptor beta (ESR2 )] were also recorded. All eligible patients were followed-up every 3 moviatelephone interview or outpatient review. Patients were followed to the latest lost-to-follow-up time(December 31 , 2020 ) or time of death. In our study, the primary clinical outcome was OS which was defined with reference to our previous study[3 ].
Construction and validation of the nomogram
Factors affecting OS in univariate analysis (P< 0 .20 ) were included in a Cox proportional hazards regression for multivariable analysis[13 ]. Hazard ratio and 95 % confidence interval were assessed. After testing for collinearity, a prognostic nomogram was constructed to predict 1 -, 3 -, and 5 -years OS for female ESCC. The discrimination was evaluated using the time-dependent receiver operating characteristic (ROC) and concordance index (C-index) calculated by bootstrapping with 1000 resamples[14 ].Calibration was examinedviacalibration plots. Decision curve analysis (DCA) was conducted to evaluate the clinical application and net benefit at different threshold probabilities[15 ]. Patients in the study were separated into high- or low-risk groups based on the best cut-off point of total prognostic score (TPS) that was decided by X-Tile software[16 ]. Survival curves were plotted using the Kaplan–Meier analysis. Sub-analysis was conducted to confirm the potential correlations between risk score and OS among different subgroups in the primary training cohort.
Correlations with immune-related pathways and gene markers of immune cells
We analyzed the correlations between estrogen response and immune-related pathways such as p53pathway and apoptosis pathway in esophageal cancer from The Cancer Genome Atlas (TCGA) by R packages. We also assessed the associations of ESR1 and ESR2 levels with gene markers of tumor-infiltrating immune cells, including B cells, tumor-associated macrophages (TAMs), M1 macrophages, M2 macrophages, and neutrophils by TIMER 2 .0 (http://timer.comp-genomics.org/).
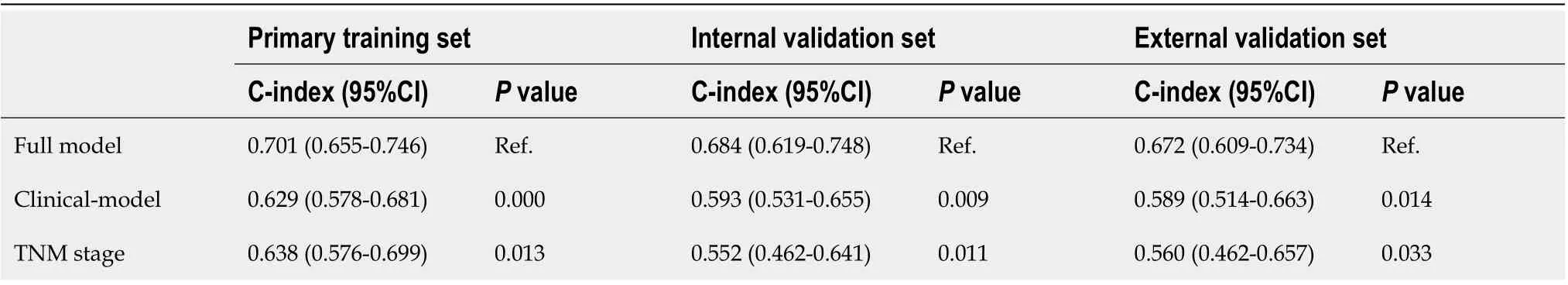
Table 2 Comparison of three models in primary training, internal validation, and external validation sets
Statistical analysis
All statistical analyses were conducted using R language (version 4 .1 .0 ). Baseline characteristics of female ESCC patients were summarized. Student’sttest was used to analyze the continuous variables,and categorical variables were compared with theχ2test and Fisher’s exact bilateral test. Survival curves were conducted by using the Kaplan–Meier method, and OS was analyzed with a log-rank test. The rms, Hmisc, survival, survcomp, and ggplot2 packages were used for analysis. P < 0 .05 (two-sided) was considered statistically significant.
RESULTS
Patient characteristics
In Cohort 1 , all 286 eligible patients were randomly divided into two sets according by computergenerated random numbers: 175 (60 %) (mean age, 61 .35 years; range, 41 –78 years) were randomly assigned to a primary training set; 111 (40 %) (mean age, 60 .25 years; range, 42 –81 years) to an internal validation set. An independent dataset (n= 85 ) from Cohort 2 was established to be an external validation cohort. The mean age was 60 .15 years with a range of 42 –80 years. The median survival time in the primary training cohort was 47 .59 mo and the 1 -, 3 -, and 5 -years OS rates were 84 .0 %, 58 .9 % and 31 .4 %, respectively. In the internal validation cohort, the median survival time was 45 .63 mo, and the 1 -,3 -, and 5 -years OS rates were 85 .6 %, 64 .0 % and 31 .5 %, respectively. The median survival time of the external validation cohort was 39 .94 mo, and the 1 -, 3 -, and 5 -years OS rates were 84 .7 %, 56 .5 % and 23 .5 %, respectively. The detailed distribution of baseline characteristics of the primary training, internal validation, and external validation datasets are shown in Table 1 .
Prognostic roles of clinical and reproductive variables
The prognostic role of each variable in OS was tested in the training cohort (Figure 1 ). Incidence area (P= 0 .014 ; high vs low area), N stage (P < 0 .001 ; N0 vs N1 –N3 ), therapy methods (P = 0 .02 ; operationvsother methods), ESR1 expression level (P = 0 .006 ; negative vs positive), ESR2 level (P < 0 .001 ; negativevspositive), menarche age (P= 0 .005 ; > 16 years vs < 13 years), and pregnancy number (P = 0 .001 ; > 6vs<4 –6 ) were significantly associated with OS in the univariate analysis (Figure 1 A). Data were also represented using Kaplan–Meier curves (Figure 1 C-I). Parameters associated with P < 0 .20 based on univariate analysis and relevant clinical factors were entered into a Cox proportional hazards regression model, which included age, incidence area, tumor differentiation, N stage, therapy, ESR1 , ESR2 ,menarche age, menopausal age, and pregnancy number (Figure 1 B). No evidence of problematic multicollinearity was found.
Building and performance of prognostic nomogram
To address patient prognosis, we identified a nomogram model for the prognosis prediction of female ESCC patients at 1 -, 3 -, and 5 -years OS in the primary training cohort. Incidence area, diagnose age,differentiation, N stage, ESR1 expression, ESR2 expression, menopausal age, and pregnancy number were finally included in the full model (Figure 2 ). Pregnancy number contributed to the highest points in the model, followed by menopausal age, N stage, differentiation, and ESR2 status. Incidence area, age and ESR1 Level had a minor impact on OS. To investigate whether the clinical–reproductive model had incremental prognostic value for individualized OS prediction, a clinical model was also constructed by only including incidence area, age, differentiation, and N stage.

Figure 1 Survival analysis for female patients with esophageal squamous cell carcinoma in primary training cohort. A: Univariate analysis; B:Multivariate analysis; C-I: Survival curves for patients with incidence area, lymph node metastasis, surgery methods, estrogen receptor alpha, estrogen receptor beta,menarche age, and pregnancy number, respectively. T: Tumor invasion depth; ESR1 : Estrogen receptor alpha; ESR2 : Estrogen receptor beta; N: Lymph node metastasis.
To further reveal the prognostic prediction of the full model, patients were categorized into high- and low-risk groups based on the optimal cut-off value of TPS in the primary training set. The relationships among risk scores and survival status of patients are shown in Figure 3 A. Kaplan–Meier survival curves revealed that patients with higher risk scores had significantly poorer OS (0 .28 , 95 %CI: 0 .20 –0 .40 ,P<0 .001 , Figure 3 B). Using the same cut-off values, we identified the same risk groups in both internal and external validation sets with OS represented by Kaplan–Meier curves (0 .47 , 95 %CI: 0 .30 –0 .73 , P = 0 .001 ;0 .54 , 95 %CI: 0 .34 –0 .85 , P = 0 .009 , respectively, Figure 3 C–F). In addition to highly differentiated patients and patients who received other treatments (surgery plus postoperative radiotherapy, surgery plus postoperative chemotherapy), the clinical–reproductive model also maintained a good and stable prediction performance among other subgroups (Figure 4 ).
Calibration and validation of clinical–reproductive model
The Harrell C-indexes are summarized in Table 2 for the full model, clinical model, and TNM stage in the training, internal and external validation sets. For OS prediction, the clinical model achieved a Cindex of 0 .629 (95 %CI: 0 .578 –0 .681 ) in the training set, with internal and external validation C-index of(0 .593 , 95 %CI: 0 .531 –0 .655 , and 0 .589 , 95 %CI: 0 .514 –0 .633 , respectively). After integrating the clinical with reproductive risk factors, the C-index of the full model increased to 0 .701 (95 %CI: 0 .655 –0 .746 ,P=0 .000 ) in the training set, 0 .684 (95 %CI: 0 .619 –0 .748 , P = 0 .009 ) in the internal validation, and 0 .672 (95 %CI: 0 .609 –0 .734 , P = 0 .014 ) in the external validation set. The C-indexes for OS were also higher than those for TNM stage in all of the three cohorts (P= 0 .013 , 0 .011 and 0 .033 , respectively) (Table 2 ).

Figure 2 The Clinical-reproductive model for predicting 1 -, 3 -, and 5 -yr overall survival probability in female patients with esophageal squamous cell carcinoma. ESR1 : Estrogen receptor alpha; ESR2 : Estrogen receptor beta; N: Lymph node metastasis.
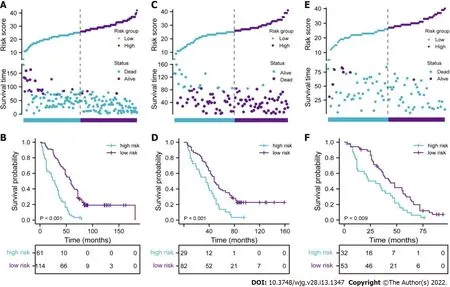
Figure 3 Evaluation of full model performance in the primary training set and confirmation based on both internal and external validation sets. A, C, and E: Distribution of risk scores and survival status in primary training, internal and external validation sets, respectively; B, D, and F: Kaplan-Meier survival curves in primary training, internal and external validation sets, respectively.
The calibration suggested that the OS prediction at 1 -, 3 -, and 5 -years was well matched to the actual outcomes in the primary training cohort (Figure 5 A). The time-dependent ROC curve also demonstrated that the full model showed a good performance in predicting OS in the training set (Figure 5 B). The AUC at 1 -, 3 -, and 5 -years was 0 .792 , 0 .738 and 0 .789 , respectively. These discoveries were verified in two validation sets (Figure 5 C–F). Compared with the clinical model and TNM stage, the predicted value of the full model was in good agreement with the OS at 1 year (Figure 6 A), 3 years (Figure 6 B),and 5 years (Figure 6 C). The full model also confirmed the better discrimination for OS at 1 year (AUC:0 .792 vs 0 .719 vs 0 .744 ; Figure 6 D), 3 years (AUC: 0 .738 vs 0 .631 vs 0 .635 ; Figure 6 E), and 5 years (AUC:0 .789 vs 0 .629 vs 0 .640 ; Figure 6 F) OS in the primary training cohort. DCA for 18 mo OS prediction showed that the clinical-reproductive model yielded a larger net benefit than either clinical model or TNM stage when the threshold probability was > 0 .10 (Figure 7 ).

Figure 4 Stratified analysis of the clinical-reproductive model in different subgroups. T: Tumor invasion depth, N: Lymph node metastasis; ESR1 :Estrogen receptor alpha; ESR2 : Estrogen receptor beta.
Correlations with immune-related pathways and gene markers of immune cells
The estrogen response was positively correlated with p53 pathway (r = 0 .436 , P < 0 .001 ), and apoptosis pathway (r= 0 .245 , P < 0 .001 ) (Supplementary Figure 1 ). ESR1 and ESR2 Levels were positively correlated with some biomarkers of immune cells or their subsets (Supplementary Table 1 ).
Clinical relevance of clinical–reproductive model
The relationships between the full model and clinical parameters are shown in Figure 8 . In terms of TNM stage, female patients with III/IV stage ESCC had significantly higher risk scores than patients with I/II stage disease had (P< 0 .001 , Figure 8 A). The risk scores were also higher for patients with than those without lymph node metastasis (P< 0 .001 , Figure 8 B). No significant difference was found in patients with different T stages (P= 0 .152 , Figure 8 C).
To improve the predictive performance and clinical utility of the full model, we transferred the data and formulae to a user-friendly website. Figure 9 shows a snapshot of web-based nomogram that is available on predictbcos.shaws.cn: https://female-escc-predictor.shinyapps.io/DynNomapp/. Visitors can select values from the drop-down list according to the circumstance of clinical and reproductive factors, and then click the “predict” button to predict the OS rates in Chinese female ESCC.

Figure 5 Calibration curves and time-dependent receiver operating characteristic curves for validation of clinical-reproductive model in the primary training, internal and external validation cohorts. A and B: Calibration curves for predicting 1 -, 3 -, and 5 -yr overall survival and timedependent receiver operating characteristic (ROC) curves in primary training cohort; C and D: Calibration curves and time-dependent ROC curves in internal validation cohort; E and F: Calibration curves and time-dependent ROC curves in external validation cohort. OS: Overall survival.
DISCUSSION
Although advances have been achieved in the prevention and treatment of ESCC in the past few decades, the prognosis of ESCC is still poor with high mortality rates[17 ,18 ]. Traditionally, the TNM staging system has been used to predict the prognosis of many cancers. However, accumulated studies have reported that the prognostic predictive probability of clinical nomograms is more accurate than that of TNM stage due to the incorporation of all known significant prognostic factors of individual patients[19 -21 ]. To date, nomograms have been widely applied in clinical prognostic evaluation for several cancers[22 -24 ].
We constructed and validated a clinical–reproductive prognostic model for assessing the added value of reproductive factors over existing risk factors in female ESCC. Our results revealed that reproductive factors have independent prognostic value with respect to clinical parameters for individualized OS prediction in female ESCC. Subgroup analysis showed that the prognosis of female patients in the lowrisk group was significantly better than that in the high-risk group. Our results of discrimination,calibration and DCA curves also showed that the clinical–reproductive model had enhanced prognostic value compared with clinical model and TNM stage.

Figure 6 Calibration curves and time-dependent receiver operating characteristic curves of 1 -, 3 -, and 5 -yr overall survival prediction for the full model, clinical model and tumor-node-metastasis stage in the primary training set. A-C: Calibration curves for predicting 1 -, 3 -, and 5 -yr overall survival (OS) in primary training set, respectively; D-F: Time-dependent receiver operating characteristic curves of 1 -, 3 -, and 5 yr OS prediction in primary training cohort, respectively. Full model: Pregnancy number + menopausal age + estrogen receptor alpha + estrogen receptor beta + N stage + differentiation +diagnose age + incidence area; Clinical model: N stage + differentiation + diagnose age + incidence area; TNM stage: Tumor-node-metastasis stage.
To further explore the potential molecular mechanism of increased prognostic value of our clinical–reproductive model in female ESCC, we analyzed the correlations of estrogen response with immune-related pathways and biomarker genes of immune cells in esophageal cancer. An in vitro study has found that estrogen mediates the apoptosis of esophageal cancer cells by interacting with ERs[25 ].The p53 signaling pathway serves as a major barrier to prevent the occurrence and progression of cancer[26 ,27 ]. Our results revealed that estrogen response could prolong clinical outcome by promoting the p53 pathway and inducing apoptosis. ESCC expresses ERs and estrogen plays a protective role through ERs[28 ]. Overexpression of ERs is associated with prognosis and female ESCC patients with higher ESR2 expression have a better prognosis and those with higher ESR1 seem to have shorter survival[3 ].In the present study, the expression levels of some biomarker genes in immune cells were positively correlated with ESR1 and ESR2 levels. Significant correlations were found between ESR2 and PTGS2 (Prostaglandin-Endoperoxide Synthase 2 , biomarker of M1 macrophages), ESR1 and CD163 , VSIG4 (VSet And Immunoglobulin Domain Containing 4 ), MS4 A4 A (Membrane Spanning 4 -Domains A4 A,biomarker of M2 macrophages). It is well known that different cell subtypes may have distinct roles in the immune microenvironment. M1 macrophages showed an antitumor effect after being activated by Th1 cytokines, while M2 macrophages showed protumor activity[29 ]. This may partly explain the survival difference in female ESCC patients with different expression levels of ESR1 and ESR2 .

Figure 7 Decision curve analysis for full model, clinical model, and tumor node metastasis stage. Black line: All patients were dead. Gray line:None of patients was dead. Full model: Pregnancy number + menopausal age + estrogen receptor alpha + estrogen receptor beta + N stage + differentiation +diagnose age + incidence area; Clinical model: N stage + differentiation + diagnose age+ incidence area; TNM stage: Tumor-node-metastasis stage.

Figure 8 Clinical relevance of full model. Distribution of the risk score based on different tumor-node-metastasis (TNM) stage, lymph node metastasis (N)stage, and tumor invasion depth (T) stage in the primary training cohort. A: TNM stage; B: N stage; C: T stage. aP < 0 .001 . T: Tumor invasion depth, N: Lymph node metastasis; TNM stage: Tumor-node-metastasis stage.
Several studies have developed some prognostic nomograms to predict prognosis in ESCC. These models provide useful tools to stratify the risk and predict survival probability in ESCC. However, the patients in these studies were mainly extracted from the Surveillance, Epidemiology, and Final Results(SEER) database in the United States, and these models were not entirely suitable for predicting OS for Chinese female ESCC due to the ethnic difference. In our study, the internal C-index of our model was 0 .701 , which is higher than two previously released models (0 .67 and 0 .66 for both nomograms)[30 ,31 ].Although the C-index of the Yuet al[32 ] model was higher (0 .749 ), the nomogram can only be used to predict the clinical outcome of T1 esophageal cancer patients with positive lymph nodes. A recent study on breast cancer showed that user-friendly online prognostic tools could greatly improve patient care[33 ]. There is no such online tool available for female ESCC. We implemented our nomogram in an online webserver, which means that clinicians can easily use it to predict prognosis. For example, a 46 -year-old menopausal ESCC patient with five pregnancies showed a poorly differentiated tumor with positive lymph node metastasis, and the tumor was ESR1 negative and ESR2 positive. The predicted results by the website show that the OS rates at 1 -, 3 -, and 5 -years were 0 .94 , 0 .81 and 0 .58 , respectively.
There were some limitations to our research. First, our analysis may have been affected by bias and loss of follow-up due to the retrospective nature of the study. Second, we must be careful to extrapolate our findings to patients of other races because most of our patients were of Han nationality. Finally, the in-depth molecular mechanisms of reproductive factors in progression and prognosis of female ESCC depend on further experimental studies to elucidate.
CONCLUSION

Figure 9 Screenshot from the web-based nomogram for predicting 1 -, 3 -, and 5 -yr overall survival for female patients with esophageal squamous cell carcinoma. N: Lymph node metastasis; ESR1 : Estrogen receptor alpha; ESR2 : Estrogen receptor beta.
We developed and verified a prognostic nomogram with clinical and reproductive factors to improve the accuracy of prognostic prediction in Chinese female patients with ESCC. Compared with the clinical model and TNM stage, the full model has increased prognostic value and can help clinicians to make individual treatment and medical decisions.
ARTICLE HIGHLIGHTS

Research results
The clinical-reproductive model incorporated incidence area, age, differentiation, N stage, estrogen receptor alpha expression, estrogen receptor beta expression, menopausal age, and pregnancy number.Compared to the clinical model and TNM stage, the ROC curve and C-index indicated good discriminative ability of the full model for predicting 1 -, 3 -, and 5 -years OS in the primary, internal, and external validation sets.
Research conclusions
A clinical-reproductive nomogram for OS prediction in Chinese female ESCC was developed and validated in the present study, which showed superior survival prediction than the clinical model and TNM stage.
Research perspectives
The clinical–reproductive model has incremental prognostic predictive value in Chinese female ESCC,which may be beneficial to individualized treatment and medical decision-making.
ACKNOWLEDGEMENTS
We thank Professor Xue-Zhong Shi (Department of Epidemiology and Biostatistics, College of Public Health in Zhengzhou University) for help in statistical analysis.
FOOTNOTES
Author contributions:Wang LD and Zhang DY designed and wrote the paper; Ku JW, Xu RH, Wang R, Wu HF, and Fan ZM performed the data collection, interpretation and follow-up; Zhang DY, Zhao XK, Zhou RX, and Song X contributed to the data analysis; all authors performed the final approvlement.
Supported byNational Natural Science Foundation of China, No. 81872032 and No. U1804262 ; National Key R&D Program of China, No. 2016 YFC0901403 ; High-Tech Key Projects of High School of Henan Province, No. 20 B320011 ;and High-Tech Key Projects of Science and Technology of Henan Province Government, No. 202102310366 .
Institutional review board statement:This research content and process of the project followed the international and national ethical requirements for biomedical research and agreed to publish. The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and Institutional Review Board of the First Affiliated Hospital of Nanyang Medical College.
Conflict-of-interest statement:We have no potential conflicts of interest to disclose.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:China
ORCID number:Dong-Yun Zhang 0000 -0001 -5885 -9238 ; Jian-Wei Ku 0000 -0002 -0015 -4662 ; Xue-Ke Zhao 0000 -0002 -9036 -6342 ; Hai-Yan Zhang 0000 -0001 -5611 -2034 ; Xin Song 0000 -0002 -7680 -4908 ; Hong-Fang Wu 0000 -0001 -7713 -4523 ; Zong-Min Fan 0000 -0003 -0087 -3410 ; Rui-Hua Xu 0000 -0002 -6914 -2168 ; Duo You 0000 -0003 -1035 -8375 ; Ran Wang 0000 -0002 -2588 -5760 ; Ruo-Xi Zhou 0000 -0001 -5461 -0400 ; Li-Dong Wang 0000 -0001 -5103 -8226 .
S-Editor:Fan JR
L-Editor:A
P-Editor:Yuan YY
 World Journal of Gastroenterology2022年13期
World Journal of Gastroenterology2022年13期
- World Journal of Gastroenterology的其它文章
- Therapeutic drug monitoring in inflammatory bowel disease: At the right time in the right place
- Endoscopic resection for early gastric cancer: Towards a global understanding
- Generic and disease-specific health-related quality of life in patients with Hirschsprung disease: A systematic review and meta-analysis
- Locoregional therapies and their effects on the tumoral microenvironment of pancreatic ductal adenocarcinoma
- Comparison of the performance of MS enteroscope series and Japanese double- and single-balloon enteroscopes
- Management of incidentally discovered appendiceal neuroendocrine tumors after an appendicectomy
