The role of zooplankton as live feeds on the thyroid hormone profile related to metamorphosis of marine fish larvae coral trout Plectropomus leopardus(Lacep`ede, 1802)
Regina Melianawati, Rarastoeti Pratiwi, Nyoman Puniawati, Pudji Astuti
aInstitute for Mariculture Research and Fisheries Extension, Jl. Singaraja-Gilimanuk, Buleleng, Bali, 81119, Indonesia
bFaculty of Biology, Universitas Gadjah Mada, Jl. Teknika Selatan Sekip Utara, Yogyakarta, 55164, Indonesia
cFaculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna 2, Karangmalang, Yogyakarta, 55164, Indonesia
Keywords:
Coral trout larvae
Copepods
Rotifers
Feeding effect
Thyroid hormone
Metamorphosis
A B S T R A C T
Coral trout Plectropomus leopardus is a high-economical value of marine fish commodity. So, the larviculture for this species should be performed in hatchery scale for their sustainability. One of the important thing in larval stage is hormonal mechanisms. Thyroid hormone is known plays an important role in the metamorphosis of marine fish larvae. The source of that hormone is assumed derived from zooplankton as their live feeds. The live feeds commonly used for marine fish larvae in hatchery are zooplankton copepods and rotifers. The purpose of this research to determine the influence of feeding copepods and rotifers on thyroid hormone profile activity,consisted of triiodothyronine (T3) and thyroxine (T4), related to the metamorphoses of coral trout larvae. The research was conducted using three feeding schemes: copepods (A), copepods-rotifers (B), and rotifers (C). It was done for 50 Days After Hatching (DAH). The results showed that larvae from treatment (A) and (B) achieved the highest level of T3 and T4 earlier at 30 DAH, which indicated that both of those larvae have entered the metamorphosis stage earlier. On the other hand, larvae from treatment (C) achieved it and entered the metamorphosis stage later at 40 DAH. In addition, iodine levels in the copepods were significantly higher than those of in rotifers (P <0.05). Based on this research, live feeds copepods and rotifers have effect on thyroid hormone activity. Copepods have better potential in accelerating the metamorphosis achievement of coral trout larvae with the fact that they have higher iodine content.
1.Introduction
Coral trout is a high-value grouper in live fish markets in Hong Kong and Southern China (Baker, 2013), which is also a mariculture priority commodity in Indonesia (Koeshendrajana & Hartono, 2006). There are some efforts on coral trout hatchery production in Indonesia (Suwirya,2005), but the seed production volume is unstable (Aslianti et al., 2008;Suwirya & Giri, 2010; Melianawati et al., 2012) due to high mortality which occurs in the early stage of larvae and metamorphoses stage(Melianawati et al., 2007; Andamari, 2009).
Metamorphosis is a critical stage at the end of larval rearing period to become juveniles. That process is influenced by several factors,including hormonal. Regulation of hormonal mechanism influences every physiological change that occurs in the growth process at every stage in the life cycle of fish (Tanaka et al., 1995). One of the major hormones involved in fish development, especially in larval stages, is thyroid hormone. Thyroid hormone plays an important role in the growth process during the larval stage (Power et al., 2001), particularly when the larvae metamorphose is towards juvenile (Inui et al., 1995;Einarsdóttir et al., 2006; Taillebois et al., 2011). Thyroid hormone levels usually increase in line with the development of larval growth and will reach the highest level when the larvae enter the metamorphosis stage(Yamano, 2005; Chang et al., 2012; Sudo et al., 2014). Therefore, the achievement of the highest level of thyroid hormone could indicate that larval fish have entered into the metamorphosis stage to be juveniles.
Thyroid hormones secreted by the thyroid gland are triiodothyronine(T3) and thyroxine (T4), and their activities can be observed through hormone levels (Moren et al., 2006). Commonly, T3 has a higher level of activity but is usually available in low concentration, whereas T4 has a lower activity level but it is available in higher concentration (Silbernagl and Despopoulos, 2009). Some fishes have a higher level of T3 (de Jesus et al., 1991; Plohman et al., 2002). On the other hand, some fishes such as described by Hotta et al. (2001), Szisch et al. (2005), Kawakami et al.(2008) and Chang et al. (2012) had higher level of T4 compared than T3 level. Hence, the level of thyroid hormones, both T3 and T4, is different among species, as also described by Yamano (2005).
In some cases, hormonal processes was also influenced by feed(Messina et al., 2014). The ingredients contained in feed may influence thyroid hormone levels (Deng et al., 2011). One of those ingredients is iodine because higher level of iodine contained in zooplankton had affect higher level of thyroid hormone in larvae which consumed them(Solbakken et al., 2002; Moren et al., 2006).
Zooplankton rotifersBrachionusspp. are commonly used widely as live feed for marine fish larvae in mariculture, including groupers(Rimmer & Glamuzina, 2019). In addition, copepods are another kind of zooplankton that are also used as the live feed for marine fish larvae(Ribeiro & Souza-Santos, 2011). However, there is still limited information on the links between various kinds of live feed and thyroid hormones in marine fin fish larvae. Therefore, the purpose of this research was to determine the influence of different kinds of zooplankton as live feeds, i.e. copepods and rotifers, on the thyroid hormone profile of coral trout (P. leopardus) larvae, particularly in metamorphosis achievement. As supporting information, the iodine levels contained in copepods and rotifers are also determined.
2.Materials and methods
2.1.Research design
The research was conducted as an experimental design consisting of three larval feed treatments: (A) copepods ; (B) copepods-rotifers; and(C) rotifers. Each treatment consisted of three replications. The research was done until 50 Days After Hatching (DAH), when mostly larvae have metamorphosed become juveniles.
2.2.Larval rearing
This research performed in Institute for Mariculture Research and Fisheries Extension, which located in the Northern part of Bali Province,Indonesia. Larvae rearing was conducted in hatchery room by using nine concrete tanks. The tanks were rectangular shape and the size in length,width and height was 2.5 m, 2 m and 1.2 m, respectively. The volume of each tank was 6.000 L, but only filled with seawater as much as 4.000 L.The tanks were also completed with aeration system to supply the dissolved oxygen for larvae.
The eggs used in this research came from naturally spawned of domesticated coral trout broodstocks in institute. Selected fertile eggs then were transferred to larvae rearing tanks. The number of eggs stocked in each tank was 70,000. Gentle aeration was set during the incubation of the eggs. The larvae hatched after 24 h incubation. Newly hatched larvae had yolk sac as the source of endogenous feeding, so there was no additional exogenous feeding until 2 DAH.
PhytoplanktonNannochloropsis oculatawas added to larval rearing tanks started at 2 DAH morning. The green color of this phytoplankton working to reduce sunlight intensity fall into the surface of larval rearing tanks. The addition of phytoplankton was done every day in the morning by flowing it through aeration hose which enter the larval tank. This was done until 30 DAH.
2.3.Larval feeding
Two kinds of zooplanktons used as live feeds for larval rearing consisted of copepods and rotifers. The feeding schedule by using those two live feeds based on treatments tested in this research. Feeding times were done twice a day, in the morning at 08:00–09:00 a.m. and afternoon at 02:00–03:00 p.m.
The copepods used in this research came from the wild. The copepods were collected at night time from a brackish water pond that belongs to institute and is located approximately 20 km from the institute. The copepods were filtered by plankton net mesh sized 45 μm; the smaller copepods, nauplius, would passed through the filter, while the bigger copepods, consisted of copepodit and adult, were collected in the plankton net. Nauplius used for 2–13 DAH. Furthermore, all of the stages of copepods given to larvae every 2 days until the end of the research.The number of copepods given to larvae depended on the availability that can be collected from the wild. Copepods was given once a day in the morning.
RotifersBrachionus rotundiformiscame from mass culture in institute.The rotifers were harvested by using plankton net mesh size 60 μm.Rotifers were given every day from 2 until 40 DAH larvae. The number of rotifers given to larvae also depended on the availability in mass culture. Rotifers were given twice a day in the morning and afternoon.
Larvae was also fed by micro pellet,Artemianauplius and mysid.Micro pellet was given twice a day in the morning and afternoon as much as one teaspoon each for 8–10 DAH larvae. Then, the amount increased became two teaspoons for 11–25 DAH larvae and afterwards increased again as much as three teaspoons.Artemiawas 1–2 individuals/L given once a day in the afternoon for 25–35 DAH larvae and after that twice a day in the morning and afternoon. Mysid was approximately one individual/ml started given to 35 days old larvae once a day at 4 p.m.
2.4.Thyroid hormone analysis in larvae
Samples for this analysis were taken from 1, 10, 20, 30, 40, and 50 DAH larvae. The number of samples from those each age varied from 1 to 350 larvae, depending on the size of the larvae. The whole body of larvae was used as the sample.The number of larval sample was calculated by sampling method. Samples were kept in −80 ℃ freezer until they were analyzed.
Analysis was performed at the Integrated Research and Testing Laboratory, Universitas Gadjah Mada, Yogyakarta, Indonesia. Firstly,samples were extracted in phosphate buffer saline pH 7.4 by dilution 100:1, and then were centrifuged using a refrigerated centrifuge (Sorvall Biofuge Primo R) at 5000 g for 5 min at temperature 4 ℃. Afterwards,the supernatant was transferred to new micro tube and was kept in−80 ℃ freezer until further analysis.
The level of T3 hormone was measured by enzyme-linked immunosorbent assay DRG EIA-1780. The analysis was carried out based on instructions. Brie fly, into each determined well, 50 μl standard, 50 μl sample, and 100 μl working conjugate reagent added, gently mixed, and then incubated for 60 min in room temperature. After incubation, the solution was removed and was rinsed 5 times with distilled water, then 100 μl substrate solution tetramethylbenzidine was added to each well.Samples was incubated in the dark for 20 min at room temperature. The reaction stopped by adding 100 μl stop solution. Samples’ absorbance was measured within 15 min using a micro titer plate reader BioRad model 680 XR at wavelength of 450 nm.
The level of T4 hormone was measured using enzyme-linked immunosorbent DRG EIA-1781. Into each determined well, 25 μl standard, 25 μl sample, and 100 μl working conjugate reagent added, mixed gently, and then incubated for 60 min at room temperature. After that,the solution was removed and rinsed 5 times with distilled water followed by the addition of 100 μl substrate solution tetramethylbenzidine into each well. The Samples were incubated in the dark for 20 min at room temperature. The reaction stopped by adding 100 μl stop solution.Finally, the samples’ absorbance was measured within 15 min by using a micro titer plate reader Bio-Rad model 680 XR at wavelength of 450 nm.
2.5.Iodine level of zooplankton
Samples were obtained by filtering each kind of zooplankton using plankton net with mesh size 40 μm for copepods and 60 μm for rotifers.The water content in sample was reduced as much as possible. The samples were then dried using Labconco Japan freeze dryer at −40 ℃ for 24 h.
Iodine analysis was performed using the spectrophotometric method at the Center of Food and Nutrition Study Laboratory, Universitas Gadjah Mada. The Solution I was made by adding 1 g sample with H2SO4at 50 ℃. Solution II was made by taking 1 ml of the first sample solution and adding 9 ml of a solution consisting of H2SO4, HClO4, and HnO3,heated using a hot plate, then was diluted with distilled water until 25 ml. Further, 0.5 ml of solution I, 2 ml of solution II were taken and added by 2 ml of distilled water, then heated in a water bath 50 ℃ for 3 min.The next step was to add those solutions by 3.5 ml CeSO4and was diluted until 10 ml, heated again at 50 ℃ for 30 min. Finally, read the samples’ absorbance in the spectrophotometer at wavelength of 410 nm.
2.6.Statistical analysis
Results were expressed as means ±standard deviation. Statistical analyses were carried out using SPSS software version 21. Analysis was performed on evaluated thyroid hormone level among treatments groups and iodine content among live feeds tested. Data were tested for normality by Kolmogorov smirnov and homogeneity by test of homogeneity of variance. Then, the homogeneous data were tested significant differences by one-way analysis of variance and continued by using the Duncan’s test to discover differences between means. In condition where data were not ful filled the homogeneity, Kruskal-Wallis and Mann-Whitney analysis were used to test the differences. The differences were considered statistically significant at 95% confidence level (P<0.05).
3.Results
3.1.Level of T3
The profile of T3 level in coral trout larvae was shown in Fig. 1. The T3 level was still very low at 1 DAH larvae. However, the level started to increase from 10 to 20 DAH larvae. Larvae from treatment (A) and (B)achieved the highest level of T3 at 30 DAH, while larvae from treatment(C) occurred later at 40 DAH. The level of T3 in all of treatments decreased after reaching the highest level. The result also indicated there was different profile of T3 level and significant differences in certain days among the time.
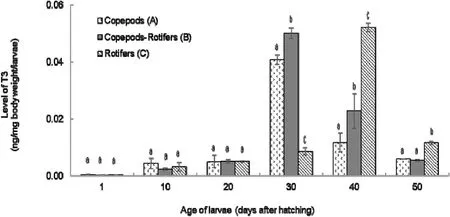
Fig. 1.Level of T3 in coral trout larvae from 1 to 50 DAH fed different live feeds.
3.2.Level of T4
The profile of T4 level was displayed in Fig. 2. It looked almost the same as T3 profile. Low level of T4 occurred at 1 DAH larvae and started to increase started from 10 DAH larvae. Larvae (A) and (B) achieved the highest level of T4 at 30 DAH. In contrast, larvae (C) achieved the highest level of T4 later at 40 DAH. The level of T4 in all of treatments then decreased after reaching the highest level. The result also indicated there was different pattern of T4 level and significant differences in certain days among the time.
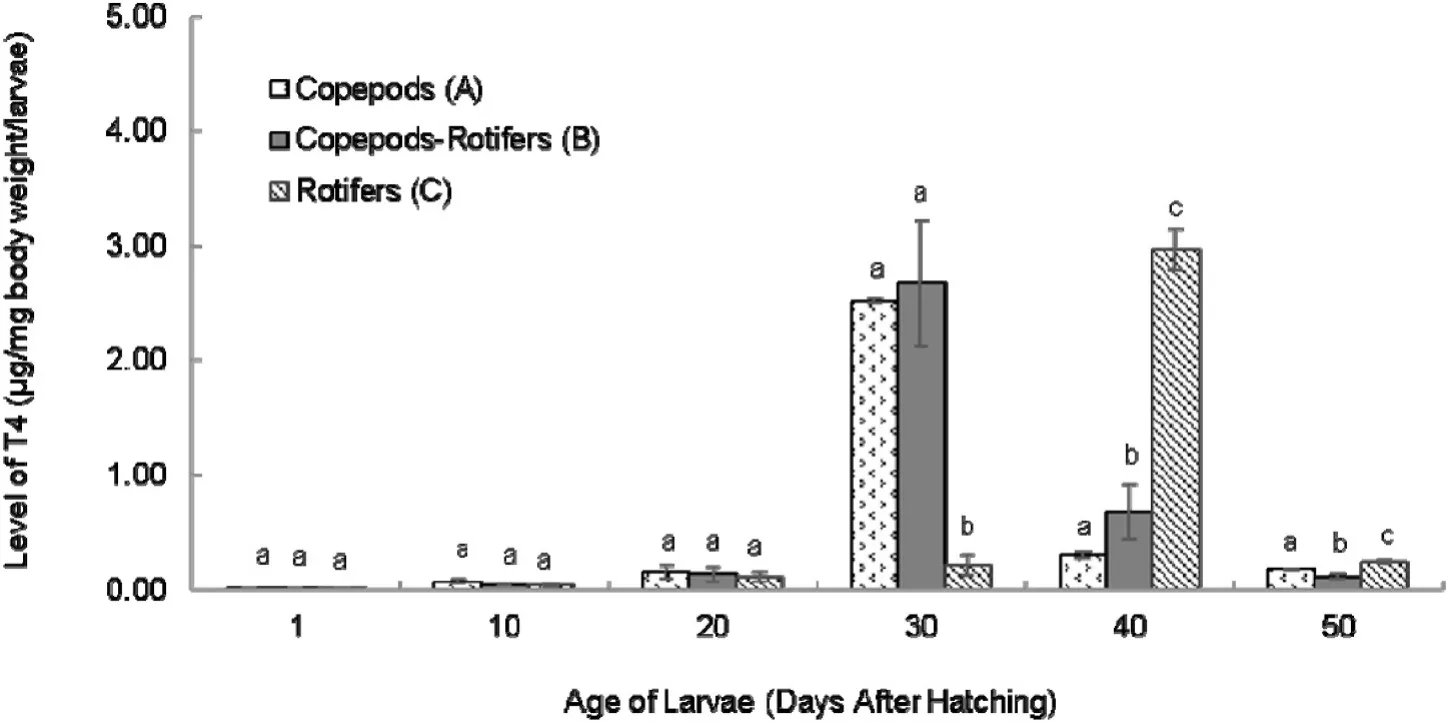
Fig. 2.Level of T4 in coral trout larvae from 1 to 50 DAH fed different live feeds.
3.3.Iodine level of zooplankton
The two kinds of zooplankton used in this research contained iodine(Fig. 3). Copepods contained higher iodine levels (128.60 ±4.92 ppm)compared to rotifers (90.16 ±0.97 ppm) and they are significantly different (P<0.05).
4.Discussion
Coral trout larvae that were fed with copepods (A), copepods and rotifers (B), and rotifers (C), demonstrated measurable levels of both T3 and T4 thyroid hormones, however the level of T3 was lower (Fig. 1)compared than T4 levels (Fig. 2). Low level of T3 in this research evidently, because indeed the basic principle of T3 is always available in less quantity, while T4 is greater in quantity. Some other fish’s larvae was also reported to have higher T4 levels compared than T3 levels, such as chum salmonOncorhynchus keta(Tagawa & Hirano, 1990), conger eelConger myriaster(Yamano et al., 1991), Japanese flounderP. Olivaceus(de Jesus et al., 1991), and Senegalese soleSolea senegalensis(Klaren et al., 2008).
The result of this research also indicated that the T3 and T4 levels in the early stage of coral trout larvae were relatively low. Black rock fishSebastes schlegeliilarvae also reported have low T3 and T4 levels immediately after hatching. It was assumed that this is due to the lack of hormones derived from maternal broodstock because they have been used during the embryonic stage before hatching (Chin et al., 2010).
In the developmental process from larvae to become juvenile, larvae will undergo metamorphosis stage that is characterized by changes in anatomy, physiology, behavior, and ecology (Hensel, 1999), and this process is mediated by thyroid hormones (Inui et al., 1995; Schreiber et al., 2010). Thyroid hormones reached the highest level at the metamorphosis stage in several fish’s larvae such as olive flounderP. olivaceus(Miwa et al., 1988) and spotted halibutVerasper variegatus(Hotta et al., 2001). This pattern indicates the highest level of thyroid hormone level is a biological indicator that signifies that the larvae has entered the metamorphosis stage. In this research, the increasing level of thyroid hormones at 30 DAH larvae from (A) and (B) indicated that larvae from these two treatments have achieved metamorphosis earlier compared to larvae from (C). Therefore, the metamorphosis in larvae,which was fed with copepods in treatments (A) and (B) was occurred earlier compared to larvae fed with rotifers only in treatment (C).
Our previous study proved that copepods also have effect on the development of larval thyroid structure. Brie fly, coral trout larvae which were fed copepods had greater in number, bigger in size and showed more active thyroid follicles compared to larvae fed with rotifers(Melianawati et al., 2016). The result of this research indicated there was close relationship between thyroid hormones level and the development of its structure as described by Tanaka et al. (1995). The thyroid structure of larvae fed with copepods resulting in the enhancement of thyroid hormones secretion to the highest level, so those larvae achieved metamorphosis stage earlier compared to larvae that was fed by rotifers only.
Metamorphosis is an important stage in the life cycle of larvae, and thyroid hormones are an important biological indicator in this process.Thyroid hormone plays an important role in the metamorphosis of some fishes larvae such as tarponMegalops cyprinoidesleptocephali (Shiao &Hwang, 2006), Senegalese soleSolea senegalensis(Manchado et al.,2008), and the gobySicyopterus lagocephalus(Teleostei:Gobioidei)(Taillebois et al., 2011).
Thyroid hormone levels commonly increase with the development of larval growth and will reach the highest level when the larvae enter the metamorphosis stage (Yamano, 2005; Sudo et al., 2014), as reported for spotted halibutVerasper variegatus(Hotta et al., 2001), fathead minnowPimephales promelas(Crane et al., 2004), tarponMegalops cyprinoidesleptocephali (Shiao & Hwang, 2006), and zebra fishDanio rerio(Chang et al., 2012). On the other hand, thyroid hormone de ficiency will have negative impacts on the metamorphosis process (Okada et al., 2005).
As mentioned before, larvae from (A) and (B) have higher thyroid hormone levels than larvae from (C) at the same age. According to study conducted by Solbakken et al. (2002), larvae that consume feed which contain high levels of iodine will have high thyroid hormone levels as well. Copepods contain iodine as a basic component in thyroid hormone synthesis (Penglase et al., 2013), whereas rotifers also contain iodine,but the level is less than in copepods (Hamre et al., 2008). In this research, the higher iodine levels in copepods (Fig. 3) were associated with the faster achievement of the highest level of thyroid hormone in coral trout larvae fed copepods.
Iodine, besides being derived from the feed, also can be obtained from the aquatic environment. A previous study showed that the metamorphosis of Pacific thread finPolydactylus sex filislarvae was influenced by the iodine level in seawater (Witt et al., 2009). Iodide (I−)is another form of iodine in seawater, besides iodate (IO3) (Crompton,2006). Iodide is derived from the reduction of iodate (Bluhm et al.,2010), and marine phytoplankton is able to convert iodate to iodide(Gall et al., 2005). Fish larvae could accumulate iodide from water, but in part of the processing, it is blocked by an inhibitor (Moren et al.,2008).
The addition of iodine from some sources increases the iodine level inside rotifers, and the results showed that rotifers could keep iodine(Srivastava et al., 2012). This result can be used as an iodine enrichment protocol for rotifers in order to larval feed manipulation. Nevertheless,an elevated level of iodine in rotifers, to the same level as that found in copepods, may be toxic to rotifers themselves (Nordgreen et al., 2013)and the larvae that consume those rotifers (Penglase et al., 2013).Therefore, copepods act as natural source of iodine in the process of thyroid hormone synthesis in larvae.
5.Conclusions
Thyroid hormone activity of coral trout larvae is influenced by the different live feeds given, either copepods or rotifers. Copepods which function both as single live feed or when they are combined with rotifers, have better potency in accelerating metamorphosis in coral trout larvae with the fact that they have higher iodine content.
CRediT authorship contribution statement
Regina Melianawati: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Rarastoeti Pratiwi: Writing – review & editing. Nyoman Puniawati: Writing –review & editing. Pudji Astuti: Conceptualization, Methodology,Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that there is no conflicts of interest.
Acknowledgements
This research was part of postgraduate study supported by Human Resources Development Agency of Marine and Fisheries, Ministry of Marine Affairs and Fisheries, Indonesia. The authors would like to thank to the editors and reviewers of Aquaculture and Fisheries for all of their useful comments and suggestions to improve this manuscript to be better. We also thank to laboratory technician at the Universitas Gadjah Mada, for technical assistance on hormone analysis. Grateful appreciation also to all of the first author colleagues for their hatchery works in larvae rearing.
 Aquaculture and Fisheries2022年2期
Aquaculture and Fisheries2022年2期
- Aquaculture and Fisheries的其它文章
- An overview of disruptive technologies for aquaculture
- CRISPR-Cas9 sgRNA design and outcome assessment: Bioinformatics tools and aquaculture applications
- The integrated analyses of metabolomics and transcriptomics in gill of GIFT tilapia in response to long term salinity challenge
- Phenotyping and phenomics in aquaculture breeding
- VNN disease and status of breeding for resistance to NNV in aquaculture
- LAMP for the rapid diagnosis of iridovirus in aquaculture
