VNN disease and status of breeding for resistance to NNV in aquaculture
Zituo Yang, Gen Hua Yue,*, Sek-Man Wong,**
aDepartment of Biological Sciences, National University of Singapore, 14 Science Drive 4, 117543, Singapore
bTemasek Life Sciences Laboratory, 1 Research Link, National University of Singapore, 117604, Singapore
Keywords:
Aquaculture
Disease
NNV
Breeding
Genome editing
A B S T R A C T
Viral nervous necrosis (VNN) disease, caused by the nervous necrosis virus (NNV), is a devastating disease,leading to high mortality rate and huge economical loss in aquaculture. In the past 30 years, many studies on the virus, host responses to the virus infection and diagnostics have yielded a lot of knowledge on developing measures to prevent the VNN disease. Although genetic improvement of disease resistance through breeding is inheritable and has long-lasting positive effect on aquaculture, it is a very challenging task in conventional selective breeding for improving disease resistance. With the advances in mapping quantitative trait loci (QTL) and genome wide association studies (GWAS) for NNV resistance in aquaculture species, DNA markers and genes associated with NNV resistance have been identified, making the application of marker-assisted selection (MAS)and genomic selection (GS) for NNV resistance possible. A few genes for NNV resistance are identified and are being used for genome editing to rapidly improve NNV resistance. In this review, we present the current knowledge on the NNV, host responses to NNV infection, diagnostic methods and vaccines available for NNV disease. In addition, we summarize the current status of conventional and molecular breeding for NNV resistance and highlight future directions, including genome editing for improving NNV resistance in aquaculture.
1.Introduction
It is anticipated that 10 billion people will inhabit this planet by 2050. The demand for high quality proteins from animals will increase by over 50%. Sustainable and healthy production of foods to feed the world is becoming critically important. Fish is an important source of high-quality proteins (FAO, 2020). Due to over fishing of our ocean, wild fish populations have been decreasing for generations. According to recent estimation, with the current rate of wild catch, fish in the ocean will be depleted in less than 50 years (FAO, 2020). Aquaculture is likely to become the only way to fill in the gap of seafood supply. Aquaculture fish species have the highest protein retention in comparison with livestock (Béné et al., 2015). In terms of greenhouse gas emissions,aquaculture is much lower than other types of farming. Aquaculture has become the fastest growing sector in agriculture for several decades(FAO, 2020).
Fish diseases are the largest single cause of economic losses in aquaculture (Jeney, 2017). So far, intensification and commercialization of aquaculture have become the mainstream trend for increasing the production and decreasing the production cost. In that case, with the expanding of scale and several unknown chemical or biological reaction,increase in disease infection becomes a serious issue in the aquaculture industry. Viral nervous necrosis (VNN) disease, caused by nervous necrosis virus (NNV), is a serious disease in aquaculture. It has led to huge economic loss in the aquaculture industry (Liu, Wang, Wan, et al., 2016;Munday et al., 2002). A lot of knowledge about NNV and VNN disease has been accumulated (Arimoto et al., 1992; Costa & Thompson, 2016;Liu et al., 2012; Lopez-Jimena et al., 2011). Many diagnostic methods have been developed and used in aquaculture (Arimoto et al., 1992;Dalla Valle et al., 2005; Jaramillo et al., 2016; Liu et al., 2020; Su et al.,2015). However, most papers on NNV and VNN are published in different journals, and the information on NNV and VNN in aquaculture is fragmented. Although there are some very good reviews on NNV and VNN in aquaculture (Bandín & Souto, 2020; Doan et al., 2017; Munday et al., 2002), there is no review on breeding for NNV resistance in aquaculture.
This review synthesises the current knowledge on NNV and VNN disease in aquaculture, lists the various methods for diagnosis of VNN disease and summarises recent vaccines research and effects in preventing NNV in aquaculture. Importantly, this review highlights the progress of selective breeding programmes with conventional and molecular methods for genetic improvement against VNN disease in different fish species. Among them, we discuss the marker-assistedselection (MAS) and genomic selection (GS) as the critical tools in breeding for NNV resistance. Also, the potentiality of genome editing employed in NNV resistance and future directions are discussed.
2.The nervous necrosis virus (NNV)
This nervous necrosis virus (NNV) is the causative agent of viral nervous necrosis (VNN) disease in fish (Awang, 1987; MacKinnon, 1987;Yoshikoshi & Inoue, 1990; Bloch et al., 1991; Breuil et al., 1991; Glazebrook et al., 1990). The NNV is a betanodavirus and belongs to the family Nodaviridae. In the Nodaviridae family, betanodaviruses commonly destroy the nervous system of fish. According to sequencing analysis, the genome of betanodavirus consists of two single-strand positive RNAs without poly (A) (polyadenylated) at the 3′- ends (Mori et al., 1992). The genome size of betanodavirus is 4500 nt (nucleotide)with larger RNA1 (3103 nt) encoding a RNA-dependent RNA polymerase (RdRp) with a mitochondrial localization targeting signal (Guo et al.,2004; Mézeth et al., 2007) which is employed for replication of virus genome in mitochondria. Another segment, RNA2 (1433 nt) encodes a 37 kDa capsid protein to form virus particles (Chérif et al., 2010; Liu et al., 2012). Also, the capsid protein will induce cell apoptosis through mitochondria-mediated cell death pathway leading to secondary necrosis (Wu et al., 2008). In addition, RNA1 also encodes a sub-genomic RNA3 (371 nt), which encodes protein B1 (11 kDa) and B2 (8.5 kDa)(Johnson et al., 2001). Protein B1 inhibits cell death after infection by virus (Chen et al., 2009). Protein B2 suppresses host short interfering RNA (siRNA) to avoid cleavage and thus allow replication (Fenner et al.,2007).
3.Viral nervous necrosis (VNN) disease
VNN disease caused by NNV is also known as viral encephalopathy and retinopathy (VER) and encephalomyelitis (Bloch, 1991; Munday et al., 1992). It is a devastating disease that destroys the central nervous system of infected fish, causing huge economic losses in cultured marine species. Except in South America, VNN disease occurs all over the world.In Europe, this disease was reported in various fish species: turbot in Norway (Bloch, 1991), Atlantic halibut in Norway and Scotland (Grotmol et al., 1997; Starkey et al., 2000), European sea bass in France(Breuil et al., 1991), Greece (Le Breton et al., 1997) and Italy (Bovo et al., 1999), as well asSolea senegalensisin Spain (Thiery et al., 2004).Also, in Asia, mass mortalities was recorded at hatchery-reared stage in grouper in Singapore (Hegde et al., 2002), red-spotted grouper (Epinephelus akaara) (Mori et al., 1991) and striped jack (Pseudocaranx dentex) in Japan (Mori et al., 1992), flathead grey mullet (Mugil cephalus)in China (Liu et al., 2015), as well as red drum (Sciaenops Ocellatus) in Israel (Ucko et al., 2004). In North America, VNN disease was detected in white seabass (Atractoscion nobilisthe) (Curtis et al., 2001) and Atlantic cod (Johnson et al., 2002). So far, there are more than 40 species of marine fish that have been infected with VNN disease and have betanodavirus isolated from them (Chi et al., 2003; Hegde et al.,2003; Lu et al., 2008). In addition, VNN disease is not only infecting the marine fish but also in a variety of freshwater aquatic animals. Since 2002, more than 60 freshwater species have been verified as the host of betanodavirus. VNN disease induced high mortality rate in the infected populations, including Australian cat fish (Tandanus tandanus) in 2002(Munday et al., 2002), guppy (Poecilia reticulate) in 2003 (Hegde et al.,2003), sturgeon (Acipenser gueldenstaedtii) in 2004 (Athanassopoulou et al., 2004), tilapia (Oreochromis niloticus) in 2009 (Bigarré et al., 2009),Zebra fish (Danio rerio) in 2013 (Binesh, 2013) and mosquito fish(Gambusia affinis) in 2018 (Praveenraj et al., 2018).
VNN disease is transmitted from virus carrier fish to healthy fish by co-habitation and immersion in same water or direct infection of virus(Munday et al., 2002). Also, the optimal virus growth water temperature, unsuitable husbandry practices, low nutritional intake, poor habitat conditions, high breeding density and injury during transport,will lower immunity of fish and increase its susceptibility to be infected by disease (Johansen et al., 2004; Mushiake et al., 1994; Tanaka et al.,1998; Yuasa et al., 2007). In the water, VNN disease has two principal ways to transmit virus between aquatic animals: horizontal and vertical transmission.
Horizontal transmission of NNV commonly spreads over the entire life span of infected fish in water. In this situation, the virus is transmitted from diseased fish, virus-carrier animals, contaminated water and even feed to healthy fish (Chérif et al., 2009), especially in Asian seabass and grouper, which have cannibalistic nature and thus more chances of transmission of VNN disease (Manin & Ransangan, 2011).NNV can survive more than one month without host and then transmit to fish from water or other animals like crabs, mussels and brine shrimp(Gomez et al., 2010).
Another source of VNN disease is vertical transmission from broodstock gonads and sperm where the betanodavitus can be detected.Similarly, the virus also presents in the fertilized eggs and passes to the next generation (Kuo et al., 2012). The VNN disease spreads from broodstock to larvae through vertical transmission pathway in various fish species, including striped jack, European sea bass and Asian sea bass(Azad et al., 2005; Dalla Valle et al., 2005; Mushiake et al., 1994). Due to the vertical transmission pathway, it is critical to select virus-free fish as broodstock in breeding programs.
4.Diagnosis of VNN disease
Since 1990s, in order to control the VNN disease and protect the culture environment from infected fish, several methods and tools have been established for VNN disease diagnosis, including microscopies,molecular methods and cell culture in cells.
4.1.Optical observation and microscopy
Clinical signs are the most important way to diagnose VNN disease.After VNN infection, the fish is characterized by rapid swimming,spiralling, whirling, lying down at bottom and becoming dark skinned(Yoshikoshi & Inoue, 1990). In diseased juveniles, the swim bladder contains a severe hyperinflation. Haemorrhages is found in the brain tissue. Under a light microscope, the most typical histopathology is vacuolation exhibited in cells of the spinal cord, brain and retina. The nervous cells show the most necrosis, especially in the larvae and juveniles stages (Munday et al., 2002). Also, lesions can be detected in liver and spleen tissues. Light microscopy is unable to provide a full confirmation of VNN infection (Munday et al., 2002). With immunof l uorescence microscopy, betanodavirus can be stained through antibody and detected in a laboratory (Qin et al., 2006). In addition,transmission electron microscopy can be employed to observe virus particles and cell morphology in infected cells of different tissues and organs.
4.2.Molecular methods
With the development of molecular technology, numerous molecular tools are used for detection of VNN disease. It has become an effective and precise method for virus detection. The major molecular method for diagnosis of VNN is the reverse transcription polymerase chain reaction(RT-PCR) in the laboratory (Grotmol et al., 2000). The World Organization for Animal Health (OIE) consents the use of amplification of RNA2 fragment as a routine diagnostic of VNN disease. However, due to low sensitivity, the nested RT-PCR could only detect the virus if there is high viral load in the sample. Since 2005, real-time quantitative RT-PCR assay (qRT-PCR) has been used as a precise and powerful tool for detection and quantification of betanodavirus (Dalla Valle et al., 2005).The qPCR assay could detect concentrations of viral titer of about 10 TCID50/mL (Panzarin et al., 2010). In addition, there are several other molecular methods for detection of VNN disease, including loop-mediated isothermal amplification (LAMP) method (Notomi et al.,2015), nucleic acid sequence base amplification (NASBA) (Starkey et al.,2004), lateral flow paper biosensors (LFB) (Toubanaki et al., 2015),cross-priming isothermal amplification coupled with lateral flow dipstick (CPA-LFD) (Su et al., 2015). These molecular methods are novel tools for sensitive and specific detection of VNN disease as future biological applications even though each method has its own limitations.
4.3.Immunoassays
Protein-based molecular diagnostic methods have been applied early on to detect VNN disease. The most common detection method is enzyme-linked immuno-sorbent assay (ELISA) through antigen capture of betanodavirus coat proteins in infected samples (Arimoto et al.,1992), which can determine the critical point between health condition and viral infection status in fish. However, when it comes to persistence and latency in viral-carrier fish, the sensitivity of ELISA is insufficient to detect the virus (Shetty et al., 2012). In addition, two other methods, the indirect fluorescent antibody technique (IFAT) and immuno-histochemistry (IHC) (Munday & Nakai, 1997), are also effective immunological diagnostic tools for verification of VNN disease.
4.4.Lateral flow biosensor
Recently, an aptamer-based lateral flow biosensor (LFB) was developed for rapidly detecting NNV protein in red-spotted grouper (Liu et al.,2020). In this method, based on a previously selected aptamer specifically bound to the coat protein of the grouper NNV, two modified aptamers are designed and applied. One aptamer is for magnetic bead enrichment and another is for isothermal strand displacement ampli fication. The LFB is sensitive and able to detect very low coat protein amount of NNV (i.e. 5 ng/mL). The LFB provides a sensitive and rapid way for detecting NNV in aquaculture. However, it is not known how much each test would cost.
Although many diagnostic methods for detecting NNV are available and applicable to the aquaculture industry, most of these methods are still laborious, time-consuming and costly for fish farmers. Therefore, it is essential to develop paper-based, fast, on-site and cost-effective methods for NNV detection in the aquaculture industry (Zorriehzahra et al., 2019). Aquaculture scientists can learn from human virus disease diagnostics (Zhang, Wang, et al., 2020) in developing these novel diagnostic methods in aquaculture industry.
5.Vaccines for NNV
Vaccines are an effective and essential way to control viral diseases.To date, numerous studies on vaccination have been undertaken to prevent the VNN disease in aquaculture.
In 1995, a first recombinant capsid protein synthesized from striped jack nervous necrosis virus (SJNNV) was produced to generate a virusneutralising antibody (Nakai et al., 1995). Nowadays, more recombinant coat proteins are employed against the VNN disease for several fish species. In turbot (Scophthalmus maximus), injection of recombinant coat proteins expressed inE. colifrom SJNNV resulted in expected protection after infection by the VNN disease (Húsgar et al., 2001). In addition, the recombinant protein can induce specific antibodies production in adult fish (Sommerset et al., 2005). In sevenband grouper (Epinephelus septemfasciatus) (Tanaka et al., 2001), humpback grouper (Cromileptes altivelis) (Yuasa et al., 2002), orange-spotted grouperEpinephelus coioides(Lin et al., 2007) and Asian seabass (Vimal et al., 2014), recombinant protein vaccines can increase the fish survival rate and vaccination eff i cacy ranged from 67% to 82%. However, the performance of viral recombinant protein varies between different hosts and it needs more study for commercial use in the future.
So far, there are several types of inactivated virus regarded as candidate vaccines against VNN disease, such as: inactivated hump-back grouper (Cromileptes altivelis) nervous necrosis virus vaccine in orangespotted grouper (Kai & Chi, 2008), as well as formalin-inactivated vaccine red-spotted grouper nervous necrosis virus (RGNNV) in Sevenband grouper (Yamashita et al., 2009) and Brown-marbled grouper(Pakingking et al., 2010). The vaccination efficacy of these vaccines ranged from 86% to 100%. Although inactivated-virus vaccination is more specific and efficient than viral recombinant protein against VNN disease, the viral diversity of NNV strains and the high cost of vaccines remain as challenges for its commercial use.
Virus-like particle (VLP) is used to produce vaccines for the VNN disease. To date, VLPs of NNV expressed inE. colihave been tested in Malabar grouper (Epinephelus malabaricus), giant grouper (Epinephelus lanceolatus) (Liu et al., 2006) and orange-spotted grouper (Epinephelus coioides) (Lai et al., 2014). These vaccines showed high protection ef ficiency in protecting the larvae from NNV infection. A DNA vaccine experiment showed that the serum antibody level was significantly up-regulated after a DNA vaccine named pFNCPE42-DNA was injected into Asian seabass juvenile (Vimal et al., 2016).
Many researches have been conducted for developing NNV vaccines.Experimental tests show promising results of these vaccines against NVV in several fish species. However, current vaccines show some disadvantages including: i) all highly efficient vaccines have to be applied by injection. This is laborious and can result in wound infection and mortality due to the small size of fish larvae or juveniles. ii) some vaccines are only useful on adult fish to prevent VNN disease from vertical transmission, while most VNN disease usually occurs in horizontal transmission. iii) due to delayed adaptive immune activity in larval fish,vaccine application is unable to ensure long time protection (Brudeseth et al., 2013). In addition, the NNV is very diverse among different fish species (Munday et al., 2002; Nishizawa et al., 1997; Thiery et al.,2004). Vaccines against one type/species of NNV may not work against other type/species of NNV. Therefore, it is essential to study various types of NNV in natural and cultured populations of aquaculture species using sequencing and bioinformatics technologies. Currently, only a few NNV vaccines have been commercialized, including one inactivated RGNNV vaccine against NNV of sevenband grouper in Japan (Brudeseth et al., 2013). Nevertheless, researches using omics-approaches to understand immune mechanisms during NNV infection will improve the development of effective vaccines for NNV. It is also essential to conduct more studies on VNN disease to find simple and cost-effective approaches (e.g. immersion or oral vaccines) for vaccination in broodstocks or fry.
6.Host response to NNV infection
The genomic replication of betanodavirus and host response to NNV infection are illustrated in Fig. 1. After NNV infection, the betanodaviruses initially gain entry into cells by micropinocytosis and macropinocytosis pathways (Liu et al., 2005). Once viral particle enters cytoplasm of a cell, the virus uncoats the capsid proteins and release its viral genome for transcription and translation. Viral RNA1 translates NNV RdRp and guides it to mitochondrial membrane to synthesize viral RNA (Wu et al., 2010). During this period, Mx protein, one of the immune molecules in most fish species, interacts with RdRp and transfers RdRp protein for degradation through autophagy and lysosomes to attempt to inhibit viral replication, as shown in NNV-infected grouper,Asian seabass, European sea bass Gilthead sea-bream and turbot (Chen et al., 2008; Lin et al., 2006; Montes et al., 2010; Poisa-Beiro et al., 2008;Scapigliati et al., 2010; Wu et al., 2010; Wu et al., 2016). In addition,some hosts immune responses are more susceptible to certain virus strains than others. For example, the Mx protein is increased to a greater extent in RGNNV-infected than in SJNNV infected European sea bass(Carballo et al., 2016).
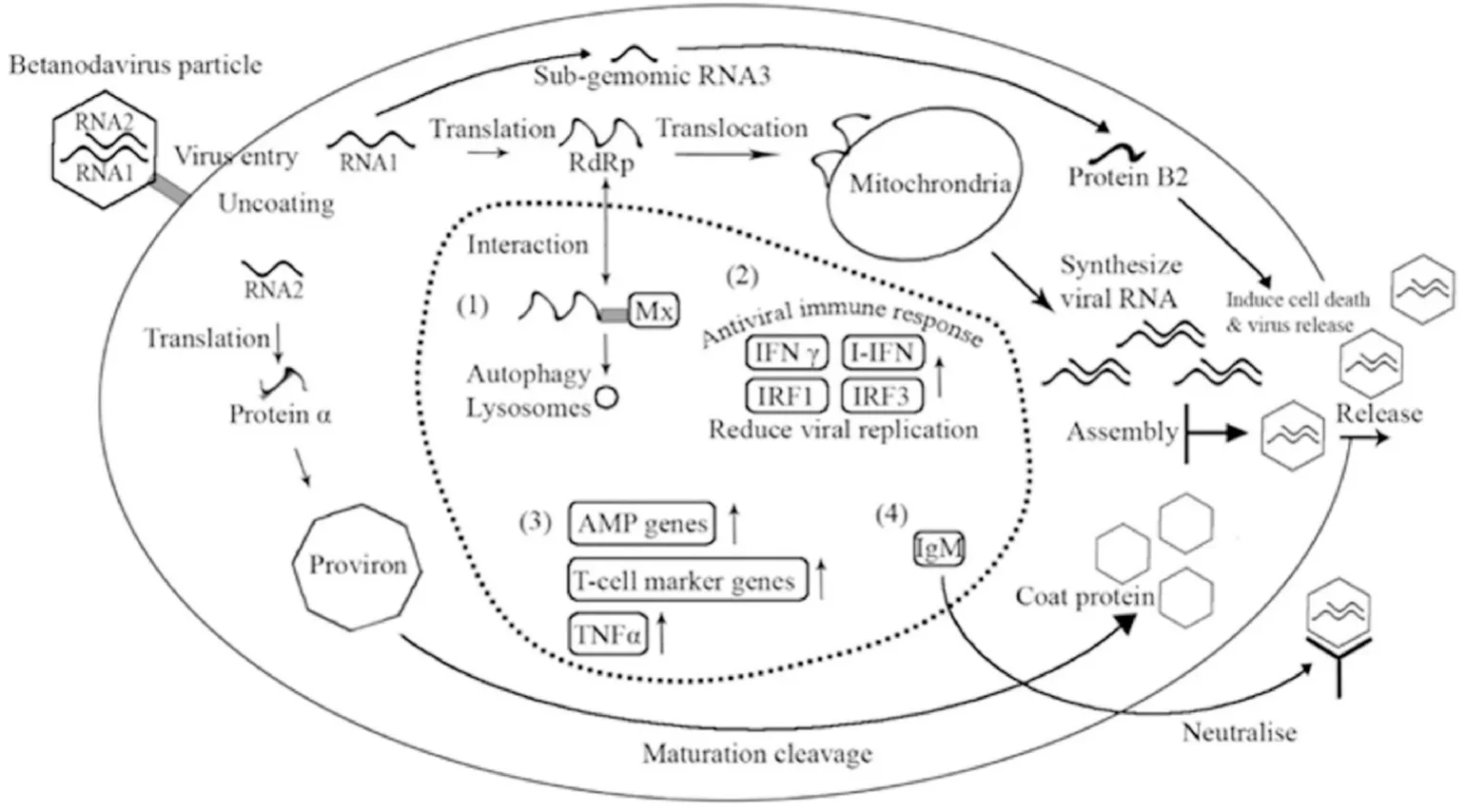
Fig. 1.Schematic representation of betanodavirus genomic replication. The RNA1 encodes RNA-dependent RNA polymerase(RdRp) and RNA2 encodes capsid protein.The sub-genomic RNA3 from RNA1 encodes protein B2. Inside of dotted line is the summary of host response to NNV infection. (1)Endogenous Mx protein can sequester viral RdRp for degradation through autophagy and lysosomes. (2) After NNV infection,Interferon (IFN) type I (I-IFN) and type II(IFN γ) can trigger innate and adaptive immune responses and the (interferon regulatory factor 3) IRF3 can reduce the virus replication in infected cell or fish. (3) Antimicrobial peptides (AMPs), T-cell marker genes and tumour necrosis factor (TNFα) are up-regulated in partial organs after NNV infection and function as activators of the immune response. (4) IgM and other antibodies produced by fish can neutralize the virus to against NNV for preventing from damage.
After viral genome replication in the host, the viral RNA begins to translate into viral proteins and capsid proteins start to assemble viral particles, leading to apoptosis and necrosis in host cells (Chen et al.,2007). Numerous viral particles assemble and capsid proteins triggering the death signal in the host induce innate immune responses, resulting in activating of cytokines, generation of cytotoxic T cells and antibody production (Koussounadis, 2006; Reyes-Cerpa et al., 2012). The host responds to NNV infection through various intercellular signalling molecules including the interferon (IFN), interleukins (IL-1) or tumour necrosis factor (TNF) (Costa & Thompson, 2016). Interferon type I (IFN α) and type II (IFN γ) are the main cytokines to induce antiviral innate immune responses and adaptive immune responses, respectively, in NNV–infected fish species. In diseased Atlantic halibut (Øvergård et al.,2012) and turbot (Montes et al., 2010), the expression of IFN γ and interferon regulatory factor 1 (IRF1) is significantly increased by NNV.Several researches in grouper and European sea bass indicated that NNV infection can induce IFN and IFN-related gene expression (Chen et al.,2014; Huang, Huang, Cai, et al., 2015).
Several kinds of cytokines are involved in anti-viral response. TNFα is induced in NNV-infected fish species and mediated through signal transducer and activator of transcription 3 (STAT3), which are involved in the formation of vacuolisation in the brain (Huang, Huang, Yang,et al., 2015; Poisa-Beiro et al., 2008). Besides this, antibody response is a common way for host response to NNV infection. IgM is employed to prevent host from dying, through neutralising the virus, as reported in VNN diseased Asian seabass, grouper and Atlantic halibut (Grove et al.,2006; Jaramillo et al., 2016; Yamashita et al., 2009).
Central nervous system is the main target of betanodavirus. The eggs and sperms are also affected by NNV due to vertical transmission.Antimicrobial peptides (AMPs) and IFN are highly expressed in the gonad of NNV-infected European sea bass and Gilthead sea bream(Valero, García-Alcázar, et al., 2015; Valero, Morcillo, et al., 2015).
7.Status of breeding for NNV resistance
Conventional selective breeding is also called artificial selection. It is the process the desired phenotypes of economically important traits (e.g.growth, appearance and disease resistance) are selected to improve the performances of selected traits of offspring (Gjedrem & Baranski, 2010).In conventional selective breeding, precise phenotypic records and quantitative genetics analysis play a critical role in selecting desired individuals based on the estimated breeding values (Gjedrem & Baranski, 2010). Conventional selective breeding is very effective in improving easily measurable traits such as growth (Gjedrem & Baranski,2010). Because diseases are the major challenges in aquaculture, genetic improvement of disease resistance is an important task in aquaculture breeding. Recent studies have shown that there are genetic variations in the resistance against some diseases in aquaculture species, including Asian seabass, European sea bass and Atlantic cod (Gadus morhuaL.)(Ødegård et al., 2010; Palaiokostas et al., 2018; Wang et al., 2017).Therefore, it is possible to select and improve fish for increased disease resistance (Gjedrem, 2015).
Genetic improvement for disease resistance through breeding provides permanent and additive increase of resistance for generations(Gjedrem, 2015). Thus, it offers more benefits compared to other methods, including vaccination and treatments with drugs, in reducing the risks of diseases in aquaculture. The use of resistant brooder stocks for producing resistant offspring for production will not only reduce disease outbreaks, but also increase the sustainability and profitability of aquaculture production (Ødegård et al., 2010; Yáñez et al., 2014).However, only the resistance to a few diseases in aquaculture, such as lymphocystis disease in Japanese flounder (Fuji et al., 2007) and infectious pancreatic necrosis in salmon (Houston et al., 2008, pp.199–204), are determined by major genes and have been relatively easily improved. The traits of resistance to most diseases are quantitative traits, which are determined by genetics factors, environmental factors and their interactions (Gjedrem, 2015). Conventional phenotypic selection for disease resistance is difficult as it is not easy to measure the phenotypes of disease resistance precisely and it takes a very long time to achieve disease resistance (Gjedrem, 2015). In this subsection, we introduce the approaches (Fig. 2) and status of the conventional and molecular breeding for NNV resistance in aquaculture and discuss some issues and solutions of breeding for NNV resistance.
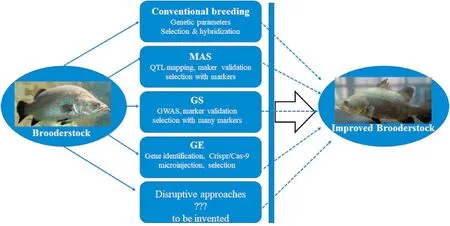
Fig. 2.General approaches to improve NNV resistance in aquaculture species. Each breeding approach can improve the resistance against NNV, whereas combination of conventional breeding, MAS (marker-assisted selection), GS (genome selction), GE (genome editing) and other disruptive breeding approaches will accelerate the improvement of NNV resistance in aquaculture species.
7.1.Heritability of NNV resistance in aquaculture species
Genetic variation within a population is the basis for genetic improvement (Gjedrem & Baranski, 2010) of traits, including qualitative traits and quantitative traits. A qualitative trait is a trait that can be classified into discrete categories (Falconer, 1996). Qualitative traits are simply inherited traits, which are controlled by one or a few major genes(Falconer, 1996). A quantitative trait is a measurable phenotype that depends on the cumulative actions of many genes, the environment and their interactions (Falconer, 1996). These traits can vary among individuals, over a range, to produce a continuous distribution of phenotypes, such as body weight and body length in aquaculture species. One important parameter to measure the additive genetic variation in total phenotypic variation is the heritability of the trait in question (Falconer,1996). It is relatively easier to improve quantitative traits with higher heritabilities than traits with lower heritabilities (Gjedrem & Baranski,2010).
In Atlantic cod, high heritabilities (0.43–0.91) for NNV resistance were reported in several studies (Ødegård et al., 2010b; Bangera et al.2011; Bangera et al. 2014). Bangera et al. (2011) reported a high heritability (0.68 ±0.14) for NNV resistance. Later, in another study, a very high heritability (0.91) for NNV resistance was reported (Bangera et al.,2014).
In European sea bass, experiments on offspring from different populations challenged by infection to W80 betanodavirus strain showed a high variation of NNV resistance between the four populations tested.The intrapopulation heritability of NNV resistance was 0.26 ±0.11(Palaiokostas et al., 2018). In addition, moderate negative genetic correlations (−0.28 ± 0.20, −0.35 ± 0.14, respectively) between NNV resistance and daily growth coefficient, and body weight have been established.
In Asian seabass, the mortality rates of juveniles from different families after NNV challenge were significantly different and the heritability of resistance to NNV was>0.25 (Wang et al., 2017), suggesting that there is sufficient genetic variation in NNV resistance in Asian seabass for genetic improvement of resistance to NNV.
All these data on the heritabilities and genetic variations of NNV resistance in aquaculture species, including cod, European sea bass and Asian seabass demonstrate that there are genetic variations in NNV resistance and thus selective breeding for improving NNV resistance is possible.
7.2.Status of conventional breeding for NNV resistance
Conventional breeding for disease resistance in aquatic species is mainly based on within-family selection and sometimes based on mass selection. Most aquaculture species are highly fecund and culture offingerlings are at low cost. The selection intensity can be extremely high(e.g. only 0.1%) in aquaculture species. With disease infection testing in each family and selection each family over generations, the disease resistance traits can be accumulated in offspring (Gjedrem & Baranski,2010). In this case, survivors from disease challenge tests could be considered as selection candidates in each family.
Conventional breeding for NNV resistance was conducted in Atlantic cod. Over 4700 fish from 50 families from two genetic groups (coastal cod and north east Arctic cod (NEAC)) were challenged with the NNV strain H-NV/RI97. At end of the challenge experiment, 56% of the fish from the coastal cod was still alive, while only 10% of NEAC survived.Their F1crosses were intermediate with 31% survival. (Ødegård et al.,2010). However, no detailed outcome of this breeding program has been reported. In European sea bass, there are also attempts to improve NNV resistance through breeding (Scapigliati et al., 2010), but there is no report on its outcome. It seems that these conventional breeding programs for NNV resistance have not reached the stage for commercialization, suggesting that more time is required for breeding disease resistance. It is also to note that although conventional breeding for NNV resistance could work, conventional breeding for NNV resistance using challenge testing confronts several disadvantages. First, the inbreeding depression is the most serious challenge in conventional breeding for NNV resistance. Although mass selection obtains the strain that can be resistant to NNV disease, inbreeding is quickly accumulated in a closed population. With high inbreeding, the species genetic diversity is compromised for the sake offighting a particular disease and the offspring may become susceptible to other pathogens. Therefore,without meticulous analysis and monitoring of genetic changes, the selective breeding might be risky in view of emerging infectious disease.Also, the breeding candidate from VNN disease for selection may become virus-carrier and vertically transmit virus to next generation in hatchery. Therefore, to overcome these challenges and to shorten the time required in the conventional breeding for NNV resistance, it is essential to integrate molecular breeding into conventional breeding programs (Houston et al., 2020; Yue, 2014).
7.3.Molecular breeding for NNV resistance
Molecular breeding refers to using molecule/DNA markers for the selection of preferred individuals for further genetic improvement of target traits. Molecular breeding could solve the problems in conventional breeding for disease resistance (Houston et al., 2020; Shen & Yue,2019; Yue & Wang, 2017; Yue, 2014). This is because, in the molecular breeding, once DNA markers can be reliably predict the resistance to diseases, selection is based on genotypes rather than phenotypes (Shen& Yue, 2019; Yue, 2014). Therefore, the tedious phenotyping of disease resistance with virus-challenging experiments is replaced with genotyping of DNA markers in fingerlings. Since genotypes is usually not affected by environment factors and genotyping can differentiate heterozygotes and homozygotes at targeted loci (Houston et al., 2020).Selection based on DNA markers for disease resistant individuals could be more precise than the conventional selective breeding if the DNA markers could precisely predict the breeding vales of each selection candidate. Therefore, in molecular breeding, identification of DNA markers, which can precisely predict the breeding values is an important task (Yue, 2014). Thirdly, selection based on DNA markers can be conducted at fingering stage and not necessary to wait for the appearance of phenotypes, thus reduces generation interval. Basically, there are two general approaches of molecule breeding: marker-assisted selection (MAS) and genomic selection (GS) (Yue, 2014) (Fig. 3). MAS refers to using a few markers with large effects on phenotypic variations,while GS uses many markers covering the whole genome for selection.Genome wise association study (GWAS) is much more powerful than QTL mapping for detecting DNA markers associated with traits, thus DNA markers identified in GWAS enable improvement of accuracy of GS. Here, we briefly elaborate on approaches (Figs. 2 and 3) and the status of MAS and GS in selection for NNV resistance in aquaculture species and discuss issues in molecular breeding for NNV resistance.
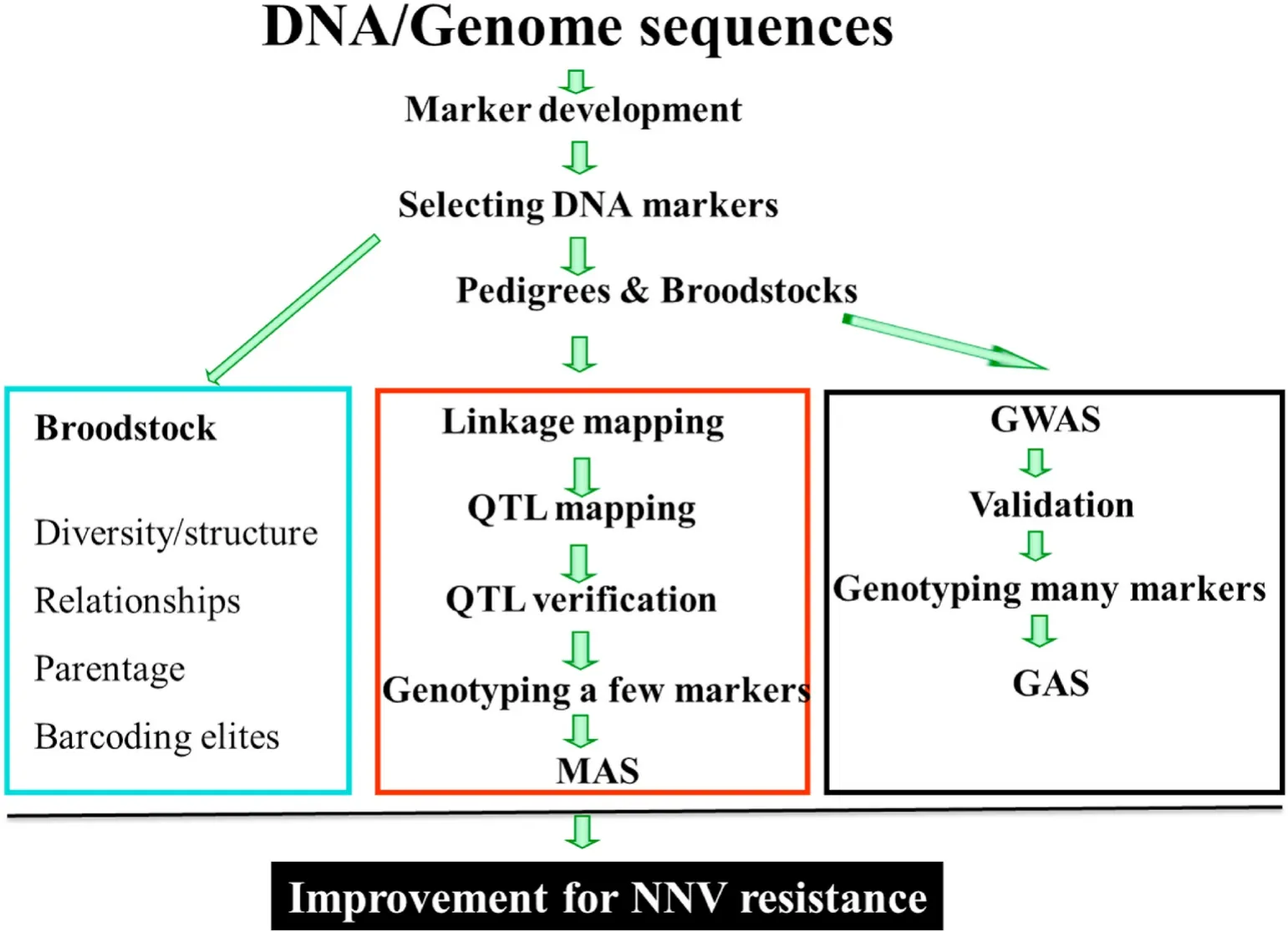
Fig. 3.Ways towards MAS and GS for genetic improvement of NNV resistance in aquaculture species. MAS: Marker assisted selection; GS: Genomic selection;GWAS: Genome wide association study; QTL: quantitative trait locus/loci; GAS: Genome assisted selection.
NNV disease resistance is a quantitative trait and is controlled by many genetic factors, including gene expression change, DNA methylation and histone modification, environmental factors and their interactions (Liu, Wang, Wong, & Yue, 2016; Liu, Wang, Wan, et al., 2016;Wang et al., 2017). The process of identification of DNA markers associated with QTL in pedigreed populations using DNA markers is called QTL mapping (Geldermann, 1975). QTL mapping is the prerequisite study to using QTL in marker-assisted-selection (MAS), which is employed to accelerate selection breeding for certain traits. Once DNA markers in significant QTL for NNV resistance are identified and verif i ed, they can be used for selection of NNV resistance in juveniles without NNV challenge (Yue, 2014). QTL mapping for NNV resistance has been conducted in some aquaculture species (Table 1). In Atlantic cod, using one hundred and ten polymorphic markers, five genome-wide significant QTL for resistance and survival time against VNN, with 68% phenotypic variance explained (PVE) have been identified (Baranski et al., 2010). QTL mapping and fine mapping were conducted using a high-resolution linkage map and trait values for NNV resistance in Asian seabass (Liu, Wang, Wong, & Yue, 2016; Liu, Wang, Wan, et al., 2016).In this study, four QTL were identified. One of these, located in linkage group 23, was for survival time, while the other three, located in linkage groups 4, 10 and 20, respectively, were for both resistance and survival time. The QTLqNNV-Su_20.1for survival time, was detected in LG20,had the highest PVE (phenotypic variance explained) of 10.9%. For resistance, the QTLqNNV-Re_20.1, located in LG20, had the highest PVE of 11.0% (Liu, Wang, Wong, & Yue, 2016; Liu, Wang, Wan, et al., 2016).DNA markers significantly associated with NNV resistance identified in QTL mapping provide an important tool for selection offingerlings for NNV resistance. To date, MAS for NNV resistance has being conducted in some aquaculture species including European sea bass and Asian seabass. Some preliminary data of promising experimental results of selection on NNV resistance using DNA markers identified in QTL mapping in Asian seabass have been reported on international meetings (Yue et al., 2017, pp. 85–85). However, to date, there is no report on commercial success of MAS for NNV resitance in aquaculture species.
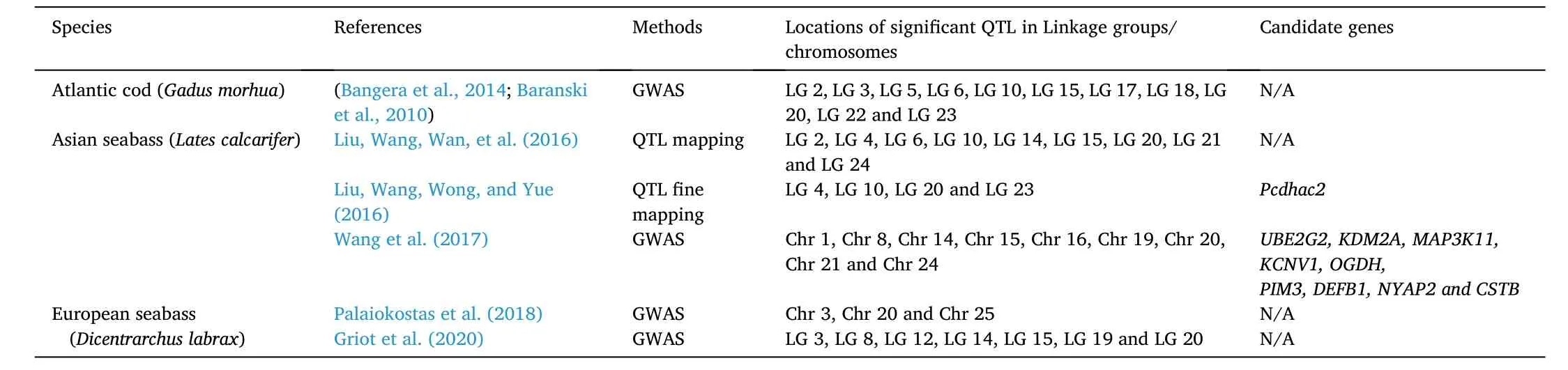
Table 1Summary of QTL mapping and GWAS for NNV resistance in aquaculture species.
In recent years, due to the rapid development of next generation sequencing and SNP genotyping, GWAS has become a powerful tool in genetics and commonly applied in various animals for interesting traits(Correa et al., 2017; Kong et al., 2019; Wan et al., 2019; Zhou et al.,2017). For NNV resistance in aquaculture species, GWAS has been conducted in Atlantic cod, Asian seabass and European sea bass(Table 1).
In Atlantic cod, using a linear mixed model approach, over 704 offspring from multiple families were challenged under controlled conditions with NNV. Two traits, including binary survival (BS) and number of days alive (ND) were recorded. All 704 fish were genotyped using 8358 SNPs covering the whole genome of Atlantic cod. Twentynine and 36 genome-wide significant SNPs (P <0.0022) were detected for BS and ND, respectively. The heritability was 0.49 for BS and 0.81 for ND. GWAS using alternative models was used and confirmed the associations. (Bangera et al., 2014). These significant SNPs have the potential to be applied in the MAS breeding for NNV resistance. However, just using only 29 or 36 significant SNP markers might not reach a high accuracy of selection. In Asian seabass, a GWAS was conducted to identify DNA markers associated with NNV resistance. In the study, over 2000 fingerlings from two batches of mass crosses between crossing 15 females and 15 males were challenged with NNV. A total of 986 individuals from 43 families were genotyped with 44,498 bi-allelic DNA markers using GBS. The GWAS identified three genome-wide significant loci on chromosomes 16, 19, and 20, respectively, and six suggestive loci on chromosomes 1, 8, 14, 15, 21, and 24, respectively. The nine significant QTL were identified and showed PVE for virus resistance ranging from 0.016 to 0.026 for quantitative trait and from 0.016 to 0.027 for binary trait (Wang et al., 2017; Yue & Wang, 2017). Using the 500 most significant markers in combination with a training population of 800 samples could reach a genomic prediction accuracy of 0.7 in Asian seabass. Using SNP genotyping, offspring from selected brooders has not experienced any outbreak of VNN in hatchery in Singapore since 2016 (Yue et al., 2017, pp. 85–85). A new line of VNN disease resistant Asian seabass has been released for commercial production (Tan, 2020).However, no commercial data of NNV resistance has been released. In another study in European seabass, a total of 1538 juveniles from a cross between 48 males and 17 females were challenged with NNV and mortalities and survivors were recorded. These juveniles were genotyped using RAD sequencing (Palaiokostas et al., 2018). GWAS identif i ed genome-wide significant QTL for NNV resistance on chromosomes 3, 20 and 25, respectively. Weighted GBLUP (genomic best linear unbiased predictor) also identified QTL on chromosome 3. The QTL on chromosome 3 explained 4% of the additive genetic variation. Several approaches of genomic prediction were investigated to examine their potential of using SNP data to estimate breeding values for resistance to VNN. Results showed that genomic prediction increased by 13% in successful classification of resistant and susceptible animals compared to pedigree-based methods. The Bayes A and Bayes B gave the highest predictive ability. Recently, a study in European sea bass (Dicentrarchus labrax) for NNV resistance has identified several QTL using GWAS with 57 K SNP chip (Griot et al., 2020). Among the total of eight QTL, one QTL located in chromosome 12, was identified in all tested population and explained 9.21% of the total genetic variance. Other SNPs showed that PVE from 0.39 to 1.1 for resistance to VNN disease. The loci identified from these two studies are essential for providing the genetic information for NNV resistance and set the foundation for selective breeding of genetic improvements against VNN disease in fish species. It seems that GS for NNV is promising for improving NNV resistance.However, GS for NNV resistance has been only tried in a few aquaculture species, and no report on commercial success of GS for NNV resitance in aquaculture species has been released yet.
Although MAS and GS are promising in breeding for NNV resistance in aquaculture species, there are a few challenges in MAS and GS. In MAS, only a few QTL with major effects are used in selection and some QTL are family-specific. Thus, the precision of MAS is not very high and may be effective in certain families but not necessarily effective in population level. GS using genome-wide markers is more powerful and precise in selection, but is much more expensive than MAS using a few markers. Thus, reducing the cost of SNP genotyping is a major task to make GS economically feasible in aquaculture species. Tagged SNP genotyping (e.g.GT-seq) (Campbell et al., 2015) may be an effective way to substantially reduce the cost of SNP genotyping for aquaculture species.
8.Genome editing
Using CRISPR/Cas9, genome-editing provides a new method and pathway for rapidly improving disease resistance in aquaculture species(Shalem et al., 2015). First step is to find out the candidate genes for disease resistance using QTL mapping, GWAS, RNA-seq or other screening experiments. In Asian seabass, fine mapping of QTL for NNV resistance has found that protocadherin alpha-C 2-like (Pcdhac2) contained a 6-bp microsatellite and is significantly associated with disease resistance (Liu, Wang, Wong, & Yue, 2016). Later, after a combination of results of GWAS and QTL mapping for VNN disease in Asian seabass, the growth factor receptor bound protein 2-associated -binding protein 3(GAB3) showed significant association with disease resistance and over-expressedGAB3in Asian seabass cell line could induce NNV replication (Yang et al., 2020). In addition, the candidate resistance gene, receptor transporting protein 3 (rtp3), was identified from RNA-seq in Asian seabass cell line (Liu et al., 2017). In addition, through virus overlay protein binding assay (VOPBA), grouper heat shock cognate protein 70 (GHSC70) was identified as NNV receptor or co-receptor protein in grouper cell line (Chang & Chi, 2015). With these candidate genes in hands, the next step is to design gRNA for CRISPR-Cas9 to edit target sequences in candidate genes in fertilized eggs to obtain mutagenesis. After one to two germline transmissions, the resistance ability of mutant fish can be verified in virus challenge experiments. If successful, the resistant population can have potential commercial application in the aquaculture industry. However, there are some challenges in genome editing in aquaculture fish species for NNV resistance. Firstly, only a few genes (e.g.Pcdhac2, GAB3,rtp3, GHSC70)involved in NNV resistance have been identified. Therefore, more genes involved in NNV resistance should be identified and their functions in NNV resistance must be analysed. Second, most of identified genes involved in NNV resistance show moderate or small effect on NNV resistance (Liu et al., 2017; Wang et al., 2017; Yang et al., 2020).Therefore, the genome editing in multiple genes in the same fish is essential but difficult (Shen & Yue, 2019). Third, it takes a very long time to obtain genetically stable broodstock due to long generation interval of most aquaculture fish. Therefore, to ensure that the selected genes are the right ones for genome editing for improving the targeted traits, it is better to try to edit the genes in model fish, including zebra fish. It is also to note that although gene editing using Crispr/Cas9 is more specific than other genome editing approaches, erroneous editing could happen (Shalem et al., 2015).
9.Future directions of genetic improvement for NNV resistance in aquaculture
VNN disease is spread worldwide and prevention of VNN disease using selective breeding in aquaculture is pressing. In disease resistance breeding, conventional selective breeding confronts challenges,including accumulation of inbreeding and contamination of vertical transmission and very slow genetic improvement. Therefore, molecular breeding using DNA makers identified in QTL mapping and GWAS for NNV resistance trait should be applied to breeding programs for rapid improvement of NNV resistance in aquaculture species and for identifying causative genes for NNV resistance. For MAS and GS, it is essential to validate whether DNA markers associated with NNV resistance detected in experiment families/populations are still associated with NNV resistance in other families/populations. Once the effects of DNA markers are validated, they can be used in MAS and GS in other populations (Fig. 3). In aquaculture species, the cost of genotyping DNA markers is a bottleneck for molecular breeding, especially in GS, where many DNA markers are used. Therefore, it is essential to substantially reduce the cost of SNP genotyping. Targeted SNP genotyping may be an effective way to reduce the cost. Fortunately, several methods, including GT-seq (Campbell et al., 2015), MTA-seq (Onda et al., 2018) for targeted SNP genotyping are available. In addition, the prediction models for GS must be calibrated and improved to reach a high level of precision (>0.70) for selection.
Causative genes for resistance can be used in genome editing for rapidly improving NNV resistance. According to the significant allele identified from QTL mapping and GWAS, the target-editing of favourable deletion or insertion in different traits may quickly achieve the purpose of breeding for disease resistance (Shen & Yue, 2019). These methods and approaches will become an opportunity for aquaculture breeding integration of new techniques to improve disease resistance in fish species. A recent study showed that MmHSP90ab1 was a functional part of the RGNNV receptor complex and involved in the internalization of RGNNV via the clathrin endocytosis pathway (Zhang, Jia, et al.,2020). This gene may be important gene for genome editing in NNV resistance. However, NNV resistance is a trait determined by multiple genes, it may be necessary to edit many genes to reach NNV resistance.Genome-wide CRISPR knockout (GeCKO) approach (Shalem et al.,2014) may be used for this purpose. This method utilizes a library of gRNA targeted to most genes in corresponding fish species and transfection into cell line, resulting in one gRNA per cell for genome editing.Then, the transfected cells can be challenged with virus to screen and select the surviving cells. After genotyping the surviving cells, the role of gRNA targeted genes in disease resistance can be further studied and may be used in selective breeding in the future. This approach has been conducted in murine cell line and identified Norovirus receptor gene(Orchard et al., 2016). However, various challenges still remain,including off-target editing, suitable fish cell line and the high cost of whole genome re-sequencing.
In summary, genome editing, combined with conventional breeding,MAS and GS, will accelerate the improvement of NNV resistance in aquaculture species (Fig. 2). Genetic improvement for NNV resistance will contribute to the sustainability of world aquaculture. Other disruptive approaches to improve NNV resistance need to be invented to accelerate genetic improvement of NNV resistance in aquaculture.
Declaration of competing interest
The authors have declared no conflicts of interest.
CRediT authorship contribution statement
Zituo Yang: Data curation, Writing – original draft. Gen Hua Yue:Software, Writing – review & editing, Supervision, Writing – review &editing, Conceptualization, Methodology. Sek-Man Wong: Software,Writing – review & editing, Supervision, Writing – review & editing,Conceptualization, Methodology.
Acknowledgements
This study was supported by the internal fund of the Temasek Life Sciences Laboratory, Singapore and a graduate research scholarship from Tropical Marine Science Institute, National University of Singapore.
 Aquaculture and Fisheries2022年2期
Aquaculture and Fisheries2022年2期
- Aquaculture and Fisheries的其它文章
- An overview of disruptive technologies for aquaculture
- CRISPR-Cas9 sgRNA design and outcome assessment: Bioinformatics tools and aquaculture applications
- The integrated analyses of metabolomics and transcriptomics in gill of GIFT tilapia in response to long term salinity challenge
- Phenotyping and phenomics in aquaculture breeding
- LAMP for the rapid diagnosis of iridovirus in aquaculture
- Insects as a feed ingredient for fish culture: Status and trends
