Insects as a feed ingredient for fish culture: Status and trends
Yuzer Al fiko, Dizhi Xie, Retno Tri Astuti, Joey Wong, Le Wang
aBiotechnology Research and Development Division, Seed Wilmar Indonesia Ltd, Cikarang, Bekasi, 17530, Indonesia
bCollege of Marine Sciences, South China Agricultural University, Guangzhou, 510642, China
cDepartment of Fishery Product Technology, Faculty of Fisheries and Marine Science, Brawijaya University, East Java, 65145, Indonesia
dSchool of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, 637551, Singapore
eTemasek Life Sciences Lab, 1 Research Link, National University of Singapore, 117604, Singapore
Keywords:
Fish
Feed
fly
Worm
Performance
A B S T R A C T
Fish culture plays an important role in supplying high-quality proteins and omega-3 (n-3) long-chain polyunsaturated fatty acids. Aquaculture has been the fastest growing sector in agriculture. Fishmeal is an important component of commercial fish feeds, but ensuring a sustainable supply of fishmeal is in question. A good source of proteins in aquafeeds is insects. For the past 20 years, investigations have been carried out on insect meals as alternative sources for fishmeal. Thus far, the results on replacing fishmeal in feeding aquaculture species have been promising. However, some challenging issues including costs and scaling up of insect production remains.In this review, we aim to summarize the status of applying meals of eight insect species in formulated aquafeeds.We also discussed issues in replacing fishmeal in fish feeds with insect meals and listed some future research directions to make insect meals an important source of proteins for green, profitable and sustainable (GPS)aquaculture. It is certain that in the near future, large-scale insect farming and processing to produce insect meals as an ingredient of fish feeds will have positive impact on the sustainability and profitability of aquaculture.
1.Introduction
Aquaculture of fish is an important source of high-quality proteins for humans (Stankus, 2021). Due to rapid growth of the human population and increasing standard of living, there is a rising demand for seafood. With the declining availability of wild fish and crustaceans, the only way to satisfy the increasing demands for animal protein is aquaculture (Daniel, 2018; Stankus, 2021). Aquaculture relies heavily on the consistent supply of fishmeal (FM), which is a major component in commercial fish feeds. Aquaculture has placed an ever-increasing pressure on wild fish to sustain farmed fish, which has led to the rapid decline of wild fish stocks (Stankus, 2021). The further development of aquaculture development is inhibited by the increasing costs of aquafeeds, including meat meal, FM and soybean meals, which account for 60–70% of total aquaculture production cost.
Due to the current scarcity of FM, alternative protein sources that provide similar nutritional benefits to FM have been explored extensively (Daniel, 2018). To ensure aquaculture production is green, profitable and sustainable (GPS) in a long run, searching for protein sources containing similar levels of nutritional components (essential amino acids, phospholipids and fatty acids) is important. Plant-based materials such as soybeans, oil seeds and cereal gluten are increasingly used in animal feeds (Daniel, 2018). However, it appears that substitution of a large amount of plant-based material and fish oil for FM is not feasible in the aquaculture industry. This is mainly because plant-based feeds contain anti-nutritional components and non-starch polysaccharides, as well as the fatty acid and amino acid profiles, which are less suitable for fish (Daniel, 2018). A more suitable alternative will be insect meals as the nutritional components, which are more similar to FM. Although,since 1969, insects have been evaluated and used as a potential food component in formulated feeds for animals (Calvert et al., 1969), their incorporation into aquafeeds has not received much attention until the 2000s (Ogunji et al., 2008). For the past 20 years, studies have been carried out to find out the effects of replacing FM with insect meals on growth and health in aquaculture species (Daniel, 2018). There are also many other research topics, including insect species identification, culture methods, nutritional value, large scale farming and breeding of insects, as well as safety issues (de Souza-Vilela et al., 2019). Recently,an increasing number of feeding trials have been performed using insect meals to replace partial FM in aquaculture species. Generally, most experiments showed promising results of replacing a part of FM with insect meals, although depending on fish and insect species. It seems that a substitution of more than 30% FM with insect meals has reduced fish growth (Hua, 2021; Liland et al., 2021). To date, several companies have supplied small amount of insect meals to aquafeed manufacturers. More investors are investing in various start-up companies to produce insect meals (Rumbos et al., 2021). Therefore, using insect meals to replace FM can revolutionize the aquaculture industry (Yue & Shen, 2021).
Although many published papers/reports detailed the use of insects as protein sources in fish feeds and the experimental results are promising, the knowledge gathered from different reports are largely fragmented. This review aimed at surveying and compiling comprehensively the information regarding (1) major insect species, which have been used in producing insect meals to replace FM in aquaculture feeds; (2)the status of research on feeding trials on replacing FM with insect meals; and (3) future directions of research on the use of insects as a replacement of FM in aquafeeds.
2.Insect species used in aquafeeds and their nutritional compositions
Insects are the most diverse group of animals, and a natural food source for fish, especially for carnivorous and omnivorous fish, as these fish species need relatively high amount proteins in their diets (Nogales-Mérida et al., 2019; Tran et al., 2015; van Huis, 2020). Humans have been using insects as a source of food in various countries for more than 2000 years. Although the use of insects in aquafeeds only started for less than 40 years ago; incredible breakthroughs on species selection, culture, maximizing production, nutritional values and field tests have been reported (Daniel, 2018). Insect meal production is developing rapidly in China, Europe, North America, Australia and Southeast Asian countries.Until now, at least 16 insect species have already been evaluated for an alternative protein source in aquafeeds (Henry et al., 2015; Nogales-Mérida et al., 2019). Among the species tested and used for industrial aquafeed production, eight species (Fig. 1), including 1) silkworms(Bombyx mori) (Duan et al., 2010), 2) black soldier fly (Hermetia illucens)(Barry, 2004), 3) house fly (Musca domestica) (Malik et al., 2007), 4)yellow mealworm (Tenebrio molitor) (Li et al., 2013), 5) lesser mealworm(Alphitobius diaperinus) (Rumbos et al., 2019), 6) house cricket (Acheta domesticus) (Hessler Frelinckx, 2019), 7) banded cricket (Gryllodes sigillatus) (Vandeweyer et al., 2018), and 8) Jamaican field cricket(Gryllus assimilis) (Masson et al., 2020), are the most promising. These eight species are the most studied species to date in replacing protein sources (e.g., to replace FM) in aquafeeds (see Fig. 2). These species were approved for the production of feed in aquaculture under EU legislation(Daniel, 2018). Details of the biology and culture methods of these insect species are may be found in other published papers (Gasco et al., 2018;Tran et al., 2015). These eight insect species have been analysed for nutritional composition, including contents of crude protein, amino acids, fat content, fatty acid profiles and minerals (Sanchez-Muros et al.,2014). Their major nutritional composition is shown in Table 1. Details on each of the eight insect species’ nutritional components can be found in many published papers/reports (Allegretti et al., 2017; de Souza-Vilela et al., 2019; Henry et al., 2015; Sanchez-Muros et al.,2014). Here, we briefly summarize the major nutritional composition in the eight species and point out some potential issues on nutritional components.

Fig. 1.Eight important insect species used in producing meals to replace fishmeal in aquafeeds. 1. Silkworms (Bombyx mori), 2. Black soldier fly (Hermetia illucens), 3. House fly (Musca domestica), 4. Yellow mealworm (Tenebrio molitor) 5. Lesser mealworm (Alphitobius diaperinus),6. House cricket (Acheta domesticus), 7. Banded cricket (Gryllodes sigillatus), and 8) Jamaican field cricket (Gryllus assimilis). 9. Insect meal, 10.Aquafeeds with insect melas, and a hungry fish waiting for feeds. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
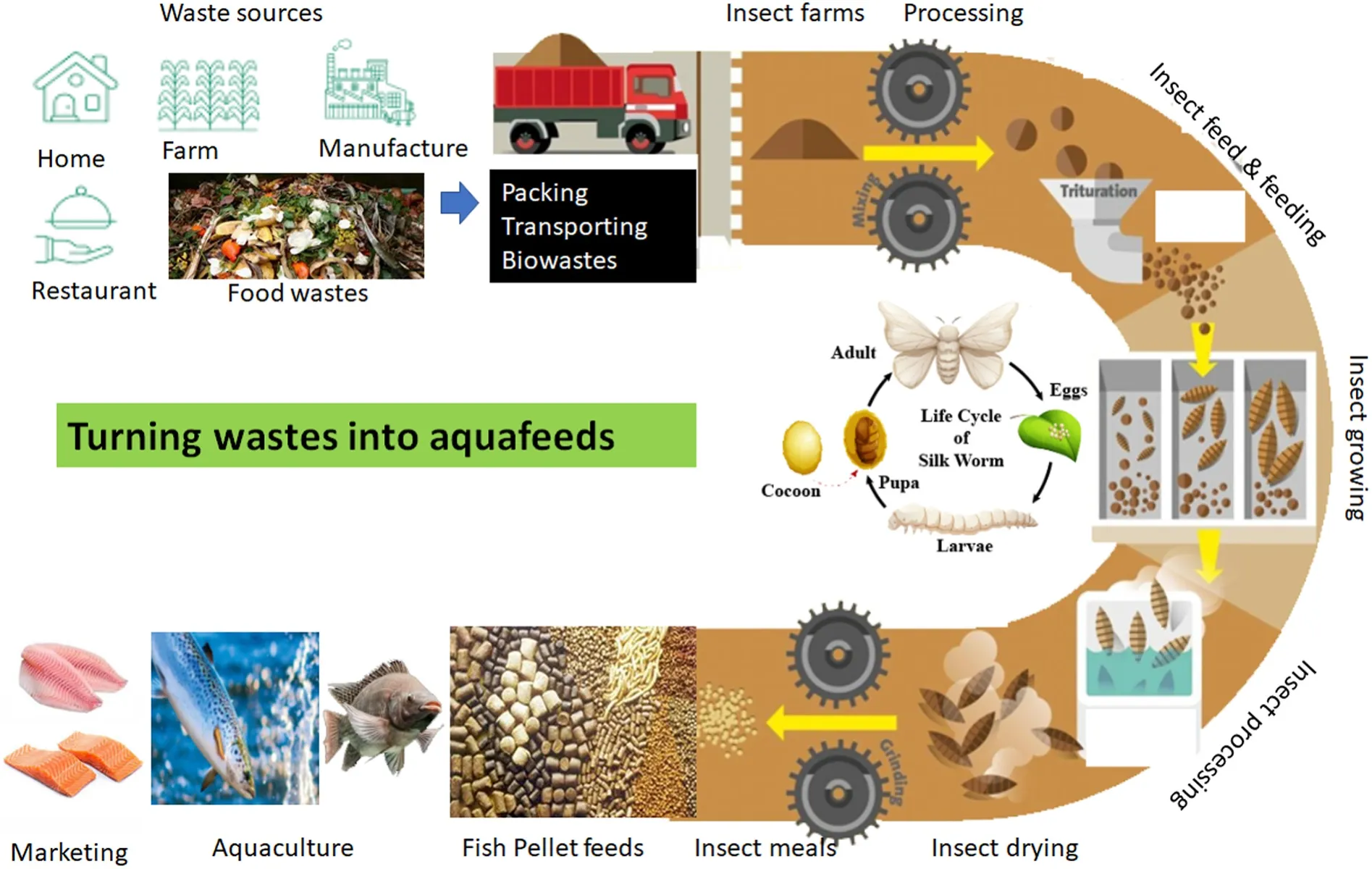
Fig. 2.Turning wastes into proteins using insects to produce insect meals to replace fishmeal in aquafeeds for fish culture.
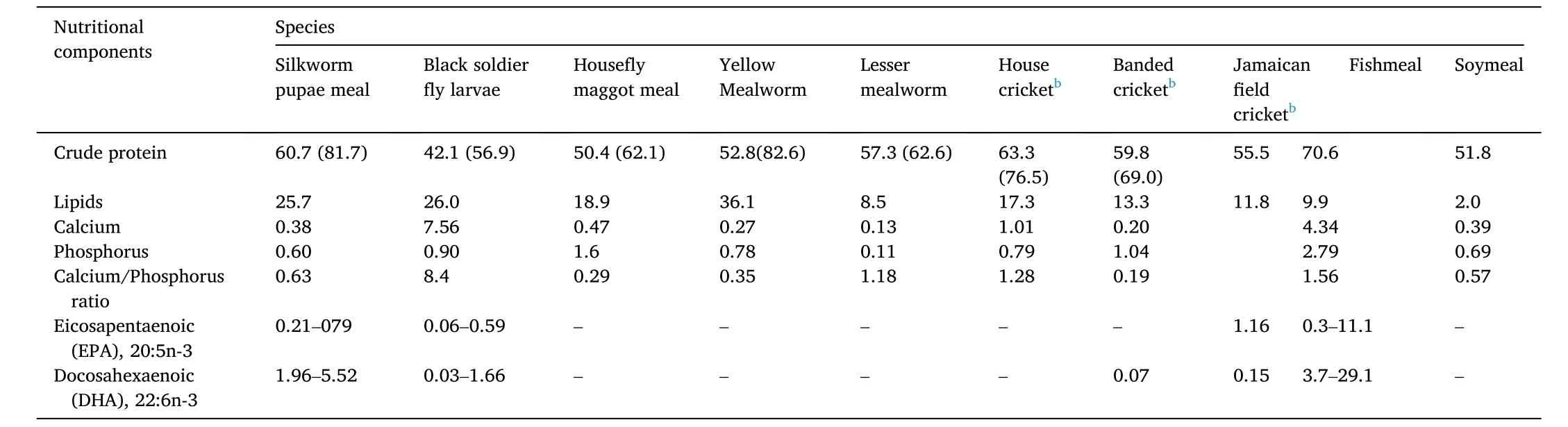
Table 1Main nutritional components (%) in meals of eight insect species, fishmeal and soymeal a.
These eight insect species possess a high crude protein (CP) level,which ranges from 42.1% to 63.3% (Table 1). This level of CP is less than that in FM, but is similar to soybean meal (SM) (Allegretti et al., 2017;Henry et al., 2015). Adult pupae of silkworm, locusts and rickets possess lesser CPs than the larvae of black soldier fly and house fly. The amino acid profiles of various insects varied in the eight species. Orthoptera (i.e., locusts and crickets) and mealworms’ CPs contain less lysine than FM, whereas Diptera (e.g., black soldier fly and house fly) and silkworms’ CPs are rather rich in lysine. Except for silkworms, the contents of sulphur amino acids in insects are lower than in FM. Threonine levels are similar in the seven insect species, while the silkworms have higher threonine (Henry et al., 2015; Sanchez-Muros et al., 2014). Other than the silkworms and house fly maggot meal, tryptophan levels in the other six insect species are generally lower. Supplementation with synthetic amino acids may be recommended for optimal growth, depending on the unique requirements of the fish species. Silkworms, black soldier fly and house fly have a superior amino acid profile than SM (Table 2). Therefore, these insects are better alternatives to SM for the replacement of FM in aquafeeds (Henry et al., 2015; Sanchez-Muros et al., 2014).
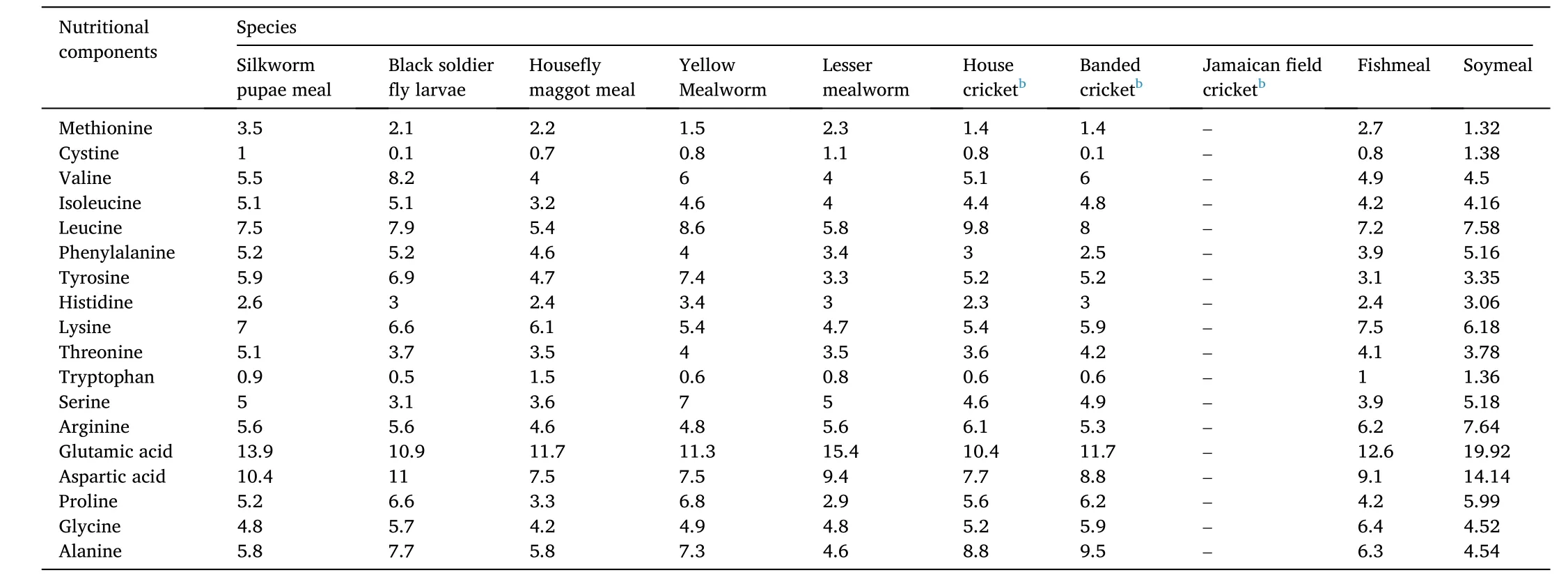
Table 2Amino acid composition (g/16 g nitrogen) of insect meals, soybean meal and fishmeal a.
All the eight insect species have lower fat content as compared to FM.Insects accumulate fat in their body, especially in their embryonic stages(Sanchez-Muros et al., 2014). The fat content in the eight insect species varied across different species, ranging from ~8% for mature locust to~36% for mealworm larvae (van Huis, 2020). It is important to note that even within a species, there is a lot of variation in lipid concentration.The fat content is affected by several factors, including the stage of development and the feed, which insects eat (Barros-Cordeiro et al.,2014; Barroso et al., 2019). In fish oil, the amounts of omega-3 fatty acids are much higher than in insect meals (Makkar et al., 2014), while in insect meals, there are large quantities of saturated fatty acids. Unsaturated fatty acid concentrations are higher in three insect species,including house cricket, mealworm and house fly maggot meals (about 60–70%). While in black soldier fly larvae (BSFL), the unsaturated fatty acid content is low (only ~19–37%) (Gasco et al., 2020; Hawkey et al.,2021; van Huis, 2020). These insects contain higher levels of polyunsaturated fatty acids (PFA) (i.e. n-6 PFA), and lesser contents of EPA(eicosapentaenoic acid 20:5n-3) and DHA (docosahexaenoic acid 22:6n-3) than fish oil (Gasco et al., 2020; Hawkey et al., 2021; van Huis,2020). The paucity of EPA and DHA in the eight insect species limits their utility in aquafeeds as an oil source. Although salmonids may synthesise EPA and DHA from ALA (Alpha-Linolenic Acid, 18:3n-3),dietary sources of EPA and DHA are more efficient in depositing them in fish meat and oil (Tocher et al., 2019). It is now well-known that the lipid contents and fatty acid profiles in insect meals rely heavily on their food, and it can be changed by altering the substrate composition(Makkar et al., 2014). Some reported data on fatty acid composition in the eight species are summarized in Table 3. It is to note that the composition of fatty acids is affected by many factors, including feeds for insects, culturing conditions and the stage of insect harvest.
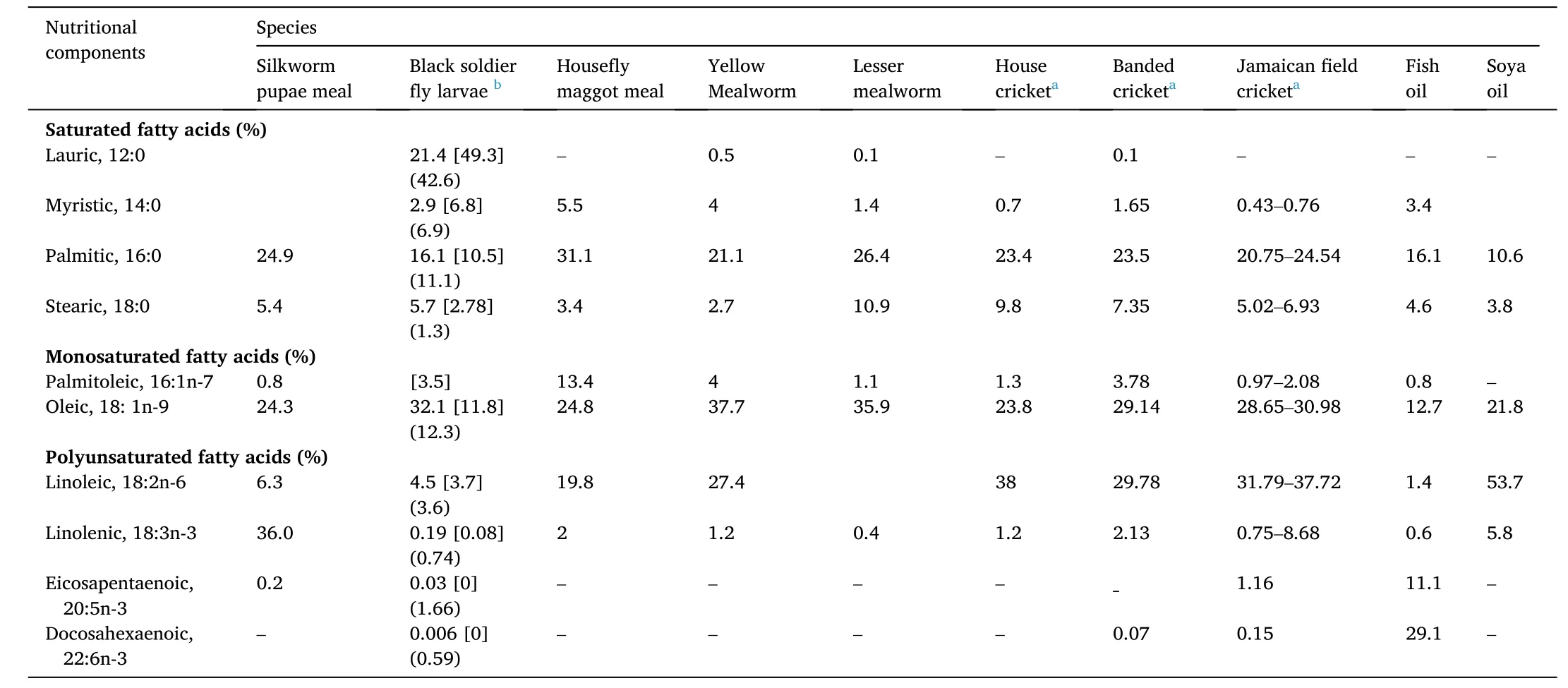
Table 3Fatty acid composition of insect oil (total lipids), soya oil and fish oil.
The ash content of the eight insect species is minimal, except black soldier fly larvae (BSFL), which has more than 15% ash. BSFL contains relative higher calcium (7.6% of dry matter), while other insect species have very low calcium levels (Table 1). Therefore, when replacing FM with insect meals, it is necessary to add calcium in aquafeeds. The calcium content of insect larvae meals can be increased by fortifying the rearing substrate with calcium. In the eight insect species, except for BSFL, which has a calcium/phosphorus ratio of 8.4, the calcium/phosphorus ratios of other species range from 0.2 to 1.2. These ratios (i.e. 0.2 to 1.2) are lower than the suggested nutritional levels (1.1–1.4) required for fish (Allegretti et al., 2017; de Souza-Vilela et al., 2019; Henry et al.,2015; Sanchez-Muros et al., 2014). It is also known that the phosphorus levels are very high in house fly maggot meal (1.6%) and Mormon cricket(1.04%).
In the eight mentioned insect species, the levels of carbohydrates are low, in most cases, the levels are lower than 20% (Barroso et al., 2014).Chitin is a polymer of glucosamine in the exoskeleton of insects (Lindsay et al., 1984). Therefore, insect meals usually contain some amount of chitin. The percentage of chitin in insect meals varies across different species and stages of development. There have been reports of both extremely high (>10% of dry matter) and extremely low values (100 mg/kg of dry matter) in different insect species (Gasco et al., 2019b;Lindsay et al., 1984; Mousavi et al., 2020; Nikoletta, 2019; Zlotko et al.,2021). Most fish species are not able to absorb chitin. However, there are some fish species, such asSebastolobus alascanus,Sebastes diploproa, andAnoplopoma fimbriathat have chitinase activity (Gutowska et al., 2004),therefore are able to use feeds with some chitin. The benefits of adding a small amount of chitin in aquafeeds have been recorded (Ringø et al.,2012; Tran et al., 2015). However, it is generally accepted that chitin is a limiting factor, which restricts the application of insect meals in aquafeeds (Ng et al., 2001; Sanchez-Muros et al., 2014).
It is worth noting that these insects have several properties that are not compatible with FM (de Souza-Vilela et al., 2019; Sanchez-Muros et al., 2014). Therefore, when using insect meals to replace FM, different nutritional components must be supplemented into aquafeeds depending on the insect species.
3.Status of use of insects in fish feeding
Recently, an increasing number of studies focused on feeding trials have been carried out to determine the effects of adding insect meals in aquafeeds on many aquaculture species. Here, we briefly summarize the current knowledge on the use of the eight insects in aquafeeds.
3.1.Silkworms
Silkworm pupa meal (SPM) has been applied in nutrition studies in many aquaculture fish species for over 40 years in China (Hu & Yang,1984) and other Asian countries (Akiyama et al., 1984). Unlike other insect meals, SPM, whether defatted or not, is able to produce decent outcomes in fish feeding trials and the fat of silkworm pupa is considered beneficial (Makkar et al., 2014; Sun et al., 2014). Silkworm pupa oil(SPO) is able to attract common carps (Cyprinus carpio) and stimulated their growth (Nandeesha et al., 1990). When sardine oil was substituted with SPO in the feed of common carp, similar growth and organoleptic parameters were observed. This implies that SPO is suitable for usage in cyprinids and that SPO might be used to replace both FM and fish oil(Nandeesha et al., 1999). Increased dietary non-defatted SPM or SPO enhances lipid digestibility without increasing fat deposition of fish(Nandeesha et al., 1999; Nandeesha et al., 1990). Furthermore, feeding defatted or non-defatted SPM also resulted in high digestibility in tilapia(Feng et al., 2021; Salem et al., 2008) and cat fish (Karthick Raja et al.,2019; Nuswantoro & Rahardjo, 2018). The digestibility of both defatted and non-defatted SPM in cyprinids is better than FM (Karthick Raja et al., 2019). However, in an earlier investigation on common carp,defatted SPM was less digestible than FM (Kim, 1974). Several studies have demonstrated the benefits of including SPM in the diets of fish larvae or juveniles, in cyprinids and many other aquaculture species.Even when not defatted, the dietary inclusion of SPM at a level of<50% in aquafeeds did not negatively affect growth performance, FCR (Feed conversion rate) and organoleptic quality (Karthick Raja et al., 2019). In the carnivorous chum salmon (Oncorhynchus keta), feeding trials with inclusion at low levels (from 5 to 12%) were successful (Akiyama et al.,1984). In olive flounder (Paralichthys olivaceus), when the two amino acids Lys and Met were supplemented to the feeds containing SPM, the growth performance was not affected (Lee et al., 2012). Positive results were reported for inclusion levels of 30–50% of defatted or non-defatted SPO in feeds of various feeding experiments on Rohu (Labeo rohita),common carp, Mahseer putitora (Tor putitora) and rainbow trout (Astuti et al., 2020; Karthick Raja et al., 2019). In common carp and Japanese sea bass (Lateolabrax japonicus), 100% FM replacement was possible without losing growth performance (Jeyachandran & Paulraj, 1976). In tilapia, the inclusion of 5% of SPM drastically reduced fish development in comparison with fish fed with normal fish feed (Boscolo et al., 2001).Similarly, very low inclusion levels (6%–9%) of SPM in feeds for Jian carp (Cyprinus carpio var Jian) dramatically reduced growth, activity of superoxide dismutase, intestinal protease activity, and elevated heat shock protein (Ji et al., 2015). In young abalones (Haliotis discus hannai Ino), a combination of SM and SPM substituted FM, resulted in a slightly higher survival rate and better growth performance (Cho, 2010). In sea cucumber (Apostichopus japonicus), using four diets, including Diet 1 served as the control diet containing 5% FM; Diet 2 with 3.75% FM and 1.25% SPO; Diet 3 containing 2.5% FM and 2.5% SPM and Diet 4 with 5% SPM. The feeding experiments revealed that sea cucumbers fed with the four diets had no differences in body composition. When FM was partially or entirely substituted with SPM, phagocytosis and serum alkaline phosphatase activity were not impacted. The study suggests that SPM might be a viable alternative to FM in sea cucumber aquaculture (Sun et al., 2014). In mirror carp (Cyprinus carpio), an eleven-week feeding trial found that SPM was an appealing sustainable functional feed component in carp diets, with benefits in terms of improving growth performance and particular physiological parameters(Wan et al., 2017). In rainbow shark (Epalzeorhynchos frenatum), feeding experiments revealed that SPM was able to substitute up to 30% of FM in the diet (Raja et al., 2020).
Overall, SPM seems to be a good protein source to replace part of FM in aquafeeds (Karthick Raja et al., 2019). However, silk worm pupa is also a good protein source for human consumption (Wu et al., 2021).The use of silk worm pupa for human consumption is much more cost-competitive. Currently, in the global market, the price of dried silkworm pupa is much higher than FM (USD 3500/t for dry silkworm pupa vs USD 1505/t for FM) in Jun 2021. In addition, in a green and circular economy perspective, bioactive peptides from silkworm pupae have industrial uses as a source of high-value proteins and bioactive peptides (Altomare et al., 2020), which is much more profitable than the use of SPM in aquafeeds. Therefore, from an economic point of view,replacement of FM with SPM is currently not economically viable.
3.2.Black soldier fly larvae (Hermetia illucens)
Black soldier fly larvae (BSFL) feed on many types of organic matters from food and animal wastes (Sheppard et al., 2002). Their larvae take around three weeks to mature (Tomberlin et al., 2002). At the pre-pupa stage, the larvae of the fly instinctively leave the substrate and migrate to a high and clean location, which eliminates the labour-intensive step to harvest them during farming (Sheppard et al., 2002). All of these advantages make BSFL a valuable insect species as a potential source of sustainable animal feed and a useful tool for waste valorization (Cickova et al., 2015; Romano et al.; Wang & Shelomi, 2017; Wardhana, 2016).Feeding tests have been conducted in many fish species to understand how the supplement of FM with black soldier fly larvae meal (BSFLM)affects growth performance, feed consumption and plasma parameters.An eight-week feeding trial in juvenile yellow cat fish (Pelteobagrus fulvidraco) revealed that 20% of the FM in the fish feed could be substituted with BSFLM without affecting growth performances, including weight gain, FCR, as well as whole body and muscle proximate composition.Other parameters, such as dry matter, CP, crude lipids, gross energy, and ADCs (amino acids apparent digestibility coefficients) were not altered.The feed with 30% FM substitution considerably enhanced cholesterol and nitric oxide concentration in plasma, along with the prevention of superoxide radical anion production. In yellow cat fish, up to 20% of the FM in traditional yellow cat fish diets was replaceable with BSFLM,without significantly reducing growth performance (Hu et al., 2017). In juveniles of the Jian carp (Cyprinus carpiovar. Jian), a 59-day feeding research revealed that dietary defatted BSFLM had no effect on Jian carp development, while increasing their antioxidant status led to an increase in catalase activity. When replacement amount approached 75%, signs of dietary stress and intestinal histological damage were detected. The research showed that defatted BSFLM could replace up to 50% of dietary FM (Li et al., 2017). In rainbow trout (Oncorhynchus mykiss), two studies were conducted to evaluate a partially defatted BSFLM as a prospective feed ingredient diet. The study revealed that a defatted BSFLM can be applied in trout diets to a maximum of 40% without affecting growth,the survival rate, condition factor, somatic indexes, fillet quality parameters and intestinal morphology (Renna et al., 2017). Another seven-week on-farm feeding experiment looked at the influence of the BSFLM on growth, as well as its proximate composition, FCR, fatty acid content and organoleptic qualities. The experiment showed that replacing FM with BSFLM (up to 50%) could significantly improve growth, FCR, and meat quality. However, the authors noticed that a reduced protein utilization efficiency in BSFLM fed fish might reduce production efficiency when BSFLM was applied across the whole production cycle, not just the first seven weeks (Stadtlander et al., 2017).Another feeding study in rainbow trout (Cardinaletti et al., 2019), found that the addition of dietary full-fat BSFLM did not degrade the quality of the fish fillets but had some negative effects on lipid metabolism. In Atlantic salmon (Salmo salarL.), a control feed with 20% FM was substituted with BSFLM at 25, 50 and 100% FM replacement in feeding experiments. The study showed that replacement of FM with BSFLM(25–100% replacing FM) did not change the fillet development, parameters of sensory tests and did not result in histopathological alterations while FCR increased. The authors reported that the methods of BSFLM preparation could have an impact on the performance of fish(Lock et al., 2014). A feeding study in Atlantic salmon found that a complete replacement of FM with BSFLM did not affect the quality offillets. In particular, neutral n-3 PUFA (polyunsaturated fatty acids)increased significantly in the fillets of Atlantic salmon fed with diets with BSFLM. The diets with different amount of BSFLM had no effect on the volatile organic composition (Bruni et al., 2020). The effect of graded quantities of BSFLM and BSFL paste on physical pellet quality,digestibility and nutrient utilization in extruded diets, as well as growth performance ofSalmo salarwas explored in a study (Weththasinghe,Lagos, et al., 2021; Weththasinghe, Hansen, et al., 2021). This study found that substituting conventional protein sources with low to moderate amounts (6.25% and 12.5%) of BSFLM or BSFL paste (3.75% and 6.75%) reduced enterocyte steatosis in the pyloric caeca, while replacing up to 25% with BSFLM or 6.75% with BSFL paste improved distal intestine histology. Furthermore, in Atlantic salmon, nutritional inclusion of BSFLM and BSFL paste exhibited minimal impacts on the skin mucus proteome and immunological response (Weththasinghe, Lagos,et al., 2021; Weththasinghe, Hansen, et al., 2021). The growth, nutritional utilization, liver health, and fillet sensory parameters ofSalmo solarfed diets with progressive substitution of FM with BSFLM were all tested in a recent feeding trial. The ADC (apparent digestibility coefficients) of protein, lipid, amino acids, and fatty acids, as well as the digestive enzyme activities, were not affected when FM in feeds was replaced with BSFLM. The introduction of insect meal in the diets had no effect on feed intake, growth rate, or FCR. Dietary substitution of BSFLM with FM had little effect on whole body protein, lipid, or amino acid composition, but whole-body fatty acid composition generally matched the diets. Both histological and chemical investigations revealed that replacing FM with BSFLM had no effect on liver lipid profiles. The sensory assessment of the fillet indicated very minor differences in the sensory quality of the fillet. In general, this study found that replacing FM with BSFLM in the diets of sea-water Atlantic salmon could be done without compromising growth, feed utilization, nutrient digestibility,liver characteristics, or fillet sensory qualities (Belghit, Lock, et al.,2019; Belghit, Liland, et al., 2019). Feeding experiments in the European sea bass (Dicentrarchus labrax) revealed that partial replacement of FM with defatted BSFLM (up to 50%) did not significantly affect growth performance, but did reduce feeding costs by 15.6% when compared to the FM-control diet (Abdel-Tawwab et al., 2020). Another feeding study was conducted to determine the effect of replacing dietary FM with BSFLM on oxidative stress indicators and fillet quality traits inDicentrarchus labraxjuveniles over a 62-day period. Overall, replacing FM with BSFLM up to 19.5% (~22.5% of total dietary protein) had no effect onD. labraxfillet quality or shelf life (Moutinho et al., 2021). In juvenile turbots (Scophthalmus maximus), feeding trials revealed that this fish tolerated diets containing 33% defatted BSFLM as a replacement for FM.However, at all the inclusion rates, the specific growth rate was lower.The acceptance of the diet was reduced as the inclusion rate increased,leading to lower feed consumption and lower growth rate, which might be due to the presence of chitin (Kroeckel et al., 2012). In the African cat fish (Clarias gariepinus), the effect of BSFLM in diets was investigated.FM may be replaced with BSFLM up to 50% without impacting the growth, nutritional utilization, survival rate and welfare ofC. gariepinusfingerlings (Adeoye et al., 2020). In the yellow cat fish (Pelteobagrus fulvidraco), feeding trials showed that 25% replacement of FM with BSFLM did not affect growth index or immunity index (Xiao et al.,2018). A feeding experiment was carried out to examine the feasibility of partially replacing FM with defatted BSFLM. The study revealed that replacing 10% of dietary FM with BSFLM is viable for Chinese soft-shelled turtles (Pelodiscus sinensis) and has a good impact on growth,antioxidant capacity and nutritional value. InIctalurus punctatus, results of feeding trials utilizing chopped black soldier fly larvae (produced on hen manure) on channel cat fish showed that the body weight and total length of cat fish was comparable to the control diets. A comparison of FM with BSFLM revealed that the latter could be beneficial as an FM replacement up to a 7.5% inclusion rate (Newton et al., 2005). In the freshwater cray fish (Cherax cainii), the dietary supplementary effects of BSFLM on the bacterial communities in the distal gut, immune response and growth of freshwater cray fish were evaluated in feeding experiments. The researchers discovered that in culture, the poultry byproduct meal with BSFLM feed was superior to other feeds. This feed led to increased health, gut microbiota, and up-regulated expression of cytokine genes (Foysal et al., 2019). In the Siberian sturgeon (Acipenser baerii), a study was conducted to understand the effects of full-fat BSFLM andTenebrio molitor(TM) meal on the intestinal health of young Siberian sturgeon. The introduction of insect meals (replacing 15% of FM) did not alter the growth performance and survival rate of this fish. The study also found that a diet with 15% replacement of FM with BSFLM improved the gut microbiota composition and intestinal shape of young Siberian sturgeon without causing villus height alterations (Józe fiak et al., 2019). In Chinese soft-shelled turtles (Pelodiscus sinensis), a recent study demonstrated that replacement of 10% dietary FM with BSFLM is feasible (Li et al., 2021).
Many studies in several aquaculture species have proven that BSFLM has the potential to partially or completely replace FM in aquafeeds. To discover more effects and economic benefits of replacing FM with BSFLM in aquafeeds, additional feeding trials and economic analyses are required. It is important to note that diminished performance of aquaculture species fed with feeds with BSFLM has also been reported in some circumstances. It is highly likely that the rearing substrates and processing method for BSFLM may have an impact on economic traits of aquaculture species. Therefore, optimizing and standardizing rearing substrates and processing methods for BSFLM are essential in ensuring constant and resilient performance of fish.
3.3.House fly maggot meal and house fly pupae meal (Musca domestica)
The application ofMusca domesticaas a fish food supplement has primarily been explored in tilapia and cat fish species, as well as some other aquaculture species. In two cat fish species (Clarias gariepinusandHeterobranchus longifilis) and their hybrids, many feeding experiments have been carried out to examine the effects of the application of house fly maggots in their feeds on their growth performance. The results of feeding trials are generally encouraging in cat fish species, but maggot meal inclusion cannot exceed 30% because higher inclusion rates tend to lower growth performance (Aniebo et al., 2009; Fasakin et al., 2003;Okore et al., 2016; Saleh, 2020). For example, a 70-day feeding experiment showed that live maggot was not able to provideC. gariepinuswith sufficient nutrients for optimal growth. The authors concluded that live maggots could only be used as a supplementary diet (Emeka & Oscar,2016). In another study to assess the cost-effectiveness and utilization of maggot in the fish diets, as well as to investigate the rate of larval(maggot) production from various culture enclosures, feeding trials were performed. The results showed that the aluminium box was the best option, producing the most maggots and being the most cost-effective of all the culture enclosures tested. The study demonstrated thatMusca domesticamaggots could be used as a partial replacement for FM inClarias gariepinusfingerlings at 60%–70% inclusion level for good growth and nutritional utilization (Kolawole &Ugwumba, 2018). A study used house fly maggots cultured from poultry waste to replace FM in aquafeeds at varied inclusion levels of 0, 25, 50,75, and 100% of maggot inclusions. The study indicated that 75% of wet maggots should be included in commercial fish feed to ensure adequate utilization byC. gariepinusjuveniles (Ipinmoroti et al., 2019). Under laboratory conditions, feeding studies were conducted to examine whether the following factors, including feeding fresh house fly maggots with or without artificial diet, affected water quality, growth performance, survival percentage and feed utilization in African cat fish fry.The study found that fingerlings ofC. anguillarisfed with frozen maggot larvae had the highest specific growth rate and the best mean weight(Achionye-Nzeh & Ngwudo, 2021). InOreochromis niloticus, fish supplied with a feed of ratio 4 to 1, consisting blend of wheat bran and live maggots, outperformed fish fed with simply wheat bran in areas of growth, specific growth rate, feed conversion ratio, and survival (Ebenso& Udo, 2003). When maggot meal was substituted for FM in the diet at a rate of 15%–68%, the best growth performance and survival rate were seen at a rate of 34% substitution of FM, with no deleterious effects on homeostasis. It was noticed that to improve the fatty acid profile of fish,it is essential to add n-6 and n-3 fatty acids to the diet with maggot meal(Ogunji et al., 2008). The practicality of employing house fly maggot meal as a partial (replacing up to 270 g/kg FM) substitute of FM in aquafeeds was confirmed in a 10-week feeding trial on Nile tilapia(Oreochromis niloticus) (Wang et al., 2017). To assess the effects of dietary FM partially substituted with house fly maggot-based meal (HMM),feeding studies were carried out on growth, fillet composition and physiological responses of the Asian bass juveniles. The study showed that up to 300 g/kg of house fly maggot meal could be utilized to replace dietary FM without affecting growth performance (Lin & Mui, 2017). In swamp eel (Monopterus albus), an eight-week rearing experiment showed that house fly larvae dietary supplementation (larvae supplemented up to once every third day) has favourable benefits on swamp eel growth and immunity, and we recommend supplementing house fly larvae every third day (Xiang et al., 2020).
In conclusion, multiple feeding tests in several aquaculture species have shown that inclusion of house fly maggot in fish diets can boost growth and FCR while avoiding physiological stress. In addition,incorporating house fly maggot meal into fish diets reduced feed costs.Depending on nutritional value, availability, growth and feed efficiency,maggot meal is an alternative protein source that is applicable to replace FM in aquafeeds. This is particularly advantageous in developing countries where FM imports require great costs. Further studies should focus on determining the maximal amount of house fly maggot meal as substitute to FM and discuss the potential economic benefit of such a replacement.
3.4.Mealworm (Tenebrio molitor)
Feeding trials in several aquaculture species proved that fresh and dried mealworms are acceptable as an alternative protein source for aquaculture. In the study of African cat fish (Clarias gariepinus), the growth and feed utilization efficiency of cat fish fed with diets with up to 40% worm meal replacement were not statistically different from fish fed with a control diet without any worm meal (Ng et al., 2001).Mealworm appears to be a viable alternative protein source for African cat fish. Another 90-day feeding trial revealed that a diet containing mealworm to replace half of the FM in common cat fish (Ameiurus melasRaf.) could maintain growth in fingerlings while fish fed with a diet containing FM grew faster than those fed with only mealworm(Roncarati et al., 2015). In yellow cat fish juvenile (Petteobagrus fulvidraco),the impact of diets including mealworm meal as a partial substitute of FM on growth performance and immunological responses was studied in feeding trials. The results showed that replacing up to 75% of FM in the diet with mealworm meal did not affect the growth of juveniles. The study found that incorporating 18% of mealworm meal in the diet improved the immune response and bacterial resistance of yellow cat fish without affecting their growth (Su et al., 2017). In gilthead sea bream(Sparus aurata) juveniles, feeding experiments showed that 25% substitution of FM with mealworm meal did not affect their growth and final weight, while 50% substitution resulted in growth reduction, lower FCR and less protein efficiency ratio (Piccolo et al., 2014). Feeding trials with feeds of different amount of mealworm meal on gilthead sea bream revealed that the free amino acid profiles of the diets and fish muscle were affected by the inclusion of mealworm meal (Iaconisi et al., 2019).In rainbow trout (Oncorhynchus mykiss), a feeding experiment demonstrated that it was possible to incorporate up to 50% of mealworm meal(to replace for FM) in the feeds without compromising growth performance (Gasco et al., 2014). In feeding trials conducted with partial substitution of FM with increasing levels of full-fat mealworm diet, after 90 days of rearing, there were no statistical differences detected for morphometric and slaughter traits in gilthead sea bream and rainbow trout. When mealworm meal incorporation in feeds increased, the fatty acids, including C16:0, C18:1n9, and C18:2n6 were elevated whereas EPA and DHA gradually decreased in fillets. The PUFA/SFA and n3/n6 ratios gradually reduced as the amount of FM replacement in the diets increased. It was concluded that mealworm meal might partially (up to 50%) replace FM in feeds of rainbow trout (Iaconisi et al., 2018). A recent 8-week feeding trial in rainbow trout with diets containing graded levels of full fat mealworm (0, 7, 14, 21, and 28 percent to replace FM) revealed that growth traits increased significantly with increasing dietary mealworm ingredient in the diet up to 14% (Jeong et al., 2020). Feeding tests on the juvenile Baltic prawn (Palaemon adspersus) were conducted to determine the effects of mealworm meal on the growth of the species. The feeds with mealworm meal resulted in similar great growth performance and dramatically reduced their mortality (Mastoraki et al., 2020). In European sea bass (Dicentrarchus labrax), the use of up to 25% mealworm as a substitute of FM in diets had no negative impact on weight gain. Growth and feed consumption ratio were all lowered by the substitution of 50% FM with mealworm meal while protein efficiency ratio, feed consumption, and body composition were not affected. The fatty acid profile of fish was changed by mealworm inclusion (Gasco et al., 2014). Another feeding experiment revealed that replacing dietary FM with defatted mealworm meal up to 80% in diets had no effect on food intake or central homeostatic regulation, indicating that defatted mealworm meal can be used as an FM substitute in European sea bass diets (Basto et al., 2021).
Mealworms based meals have been introduced as substitute to FM.Many studies on the use of mealworm meals in aquafeeds have been conducted. It seems that the percentage of dietary inclusion depends on feeding habits and farming conditions. Many studies focused on the effects of the substitution of FM with mealworm meals on growth without knowing the mechanisms underlying the effects. It is essential to look into the true modes of action, digestion and absorption of mealworm meal in the digestive system of different aquaculture species by using molecular genetics and genomic approaches (Terova et al., 2021). So far,it seems that there are some efforts to clarify the detailed impacts of mealworm meal on the digestion, absorption, metabolic activities, and gene expressions in complete bodies of aquaculture species. It is also important to note that in 2020, the different market prices of meal worm were 8.4–9.4 USD/kg in China, 10.8 to 14 USD/kg in the United States,12.9 to 20 USD/kg in Europe, and 65 to 70 USD/kg in South Korea(Hong et al., 2020; Sha fique et al., 2021). High prices will hinder the use of mealworm meal as a replacement of FM in aquafeeds. Therefore, it is urgently needed to discover ways to reduce the production costs, and to improve the yield and nutritional composition of mealworms using novel technologies, including genome editing and internet of things(Yue & Shen, 2021).
3.5.Lesser mealworm or litter beetle (Alphitobius diaperinus)
Alphitobius diaperinushas recently gained a lot of attention because it is now allowed to be used in the manufacturing of insect meal as an aquafeed element.A. diaperinus, particularly its larvae, are farmed for various reasons, mostly for feed, due to their high nutritional value (Yi et al., 2013). Nonetheless, there is a scarcity of data from research examining the usage ofA. diaperinusas an aquafeed (Rumbos et al.,2019). Recent studies found that chitin in adultA. diaperinuswas high,while chitin was low in pupa (Kurečka et al., 2021; Rumbos et al., 2019).Some studies have shown that chitin has negative effects on growth performance of fish (Khayrova et al., 2021).
3.6.House cricket (Acheta domesticus)
The house cricket (Acheta domesticus) meal has been used as a protein source in feeds of livestock, while its use in fish feed is just in infancy(Nikoletta, 2019). Feeding trials have been conducted in a few fish species. In hybrid tilapia, the impact of house cricket (A. domesticus)meal on the growth of red hybrid tilapia (Oreochromissp.) has been studied. In a feeding trial, the feed containing 60% ofA. domesticusand 40% of rice bran yielded the best results in terms of survival and growth rate (Lee et al., 2017). In perch (Perca fluviatilis), a 12-week feeding trial was done, with 25% of the FM replaced with a mixture of house cricket(A. domesticus). Between the control and experimental fed groups, the study found no significant differences in survival rates, but decreased fish growth and increased FCR. In comparison to fish fillets from the control group, feeding with insect pellets resulted in a considerable rise in linoleic fatty acid and a total level ofn-6 fatty acids in fish fillets.However, the changes in fatty acid composition were minimal, and the nutritional value of the fish fed a diet with house cricket meal for human consumption was unaffected (Tilami et al., 2020).
It seems thatA. domesticusis a promising insect species to replace a part of FM in aquafeeds. Additional feeding studies on the effects of replacing FM withA. domesticusmeal on growth and health might be required to ensure a sustainable and profitable use ofA. domesticusmeal in aquafeeds.
3.7.Tropical house cricket or banded cricket (Gryllodes sigillatus)
In pets and livestock, the banded cricket (Gryllodes sigillatus) has been employed as a protein source. Although this species has been proven to be used in animal feed by EU, research on its application in aquafeeds is still in its early stages. In rainbow trout, a 71-day experiment was done to compare the inclusion of 20%G. sigillatusmeal to a control diet, that did not contain any insect-based materials and relied only on FM for protein. The experiment findings suggest that substitutingG. sigillatusfull-fat diet for FM had no positive effects on survival or growth performance indicators (Józe fiak et al., 2019). More feeding trials are required to examine the effects of replacing FM withG. sigillatusmeal in various aquaculture species to ensure the safe and profitable use ofG. sigillatusin aquafeeds.
3.8.Jamaican field cricket (Gryllus assimilis)
The studies on the use ofGryllus assimilismeal (GAM) in replacing FM in aquafeed just began recently. In Nile tilapia male fingerlings feeding trials with various insect meals, including GAM showed that all the insect meals, including GAM tested may be used to feed Nile tilapia fingerlings (Fontes et al., 2019).
So far, research on insect meal inclusion in aquafeeds has concentrated on FM replacement and growth performance, as well as fish health, including intestinal health, microbiome alterations and immunology. In general, most studies have indicated that replacing partial FM has no negative effect on fish growth, flesh quality, or health. However,replacing all the FM appears to be impossible in most aquaculture species. Furthermore, there are two drawbacks in using insects as a source of protein in aquafeeds. Chitin is thought to be an anti-nutritional component that prevents fish from absorbing nutrients. Carbohydrates make up less than 20% of an insect’s biomass, while chitin makes up the bulk. As a result, chitin is a limiting factor for employing insects in aquafeeds. Low calcium and phosphorus levels may also be a limiting factor for using insect meals to substitute FM. Insects, on average, have lesser calcium than FM, though there are outliers, such as black soldier fly larvae. The calcium content of insects can be modified by the calcium content of the substrate on which they are raised. When compared to FM, phosphorus levels in insects are higher. The calcium-to-phosphorus ratio is a crucial aspect in fish feed. Therefore, when replacing FM with insect meals it is essential to adjust the calcium-to-phosphorus ratio to 1.1 to 1.4, as seen in FM. More research into the effect of insect meals on fish health and meat quality, including fatty acid profile, is required.Interdisciplinary approaches, including genomics and metabolomics methods, may be used (Yue & Shen, 2021). In any case, the approval of the use of insect meals in aquafeeds in EU, China and other countries will de finitely lead to thriving in both demand and supply for insect meals.
4.Insect farming and insect processing
Because many insects are only available in nature during specific seasons or months, insect farming in a controlled or indoor environment is an important way to make them available all year (Cadinu et al., 2020;DiGiacomo & Leury, 2019; Hawkey et al., 2021; van der Fels-Klerx et al.,2018; van Huis, 2020). One of the essential stages of insect farming is to ensure efficient production of eggs in quantity and quality to recover large volumes of organic matter, ensuring consistent larval production and maintaining progenitors (Pastor et al., 2015). For optimum insect growth and development, indoor rearing requires ambient environmental management (temperature, relative humidity, photoperiod),high-quality feed, and parasitoid and disease prevention (Hawkey et al.,2021; van Huis, 2020). In such instances, technical supervision can be used to conduct long-term intensive insect farming.
Insect farms abound in China and Thailand, including small and medium-sized businesses as well as large-scale operations. Thailand is a major producer of insects, with an estimated 20,000 food insect farms,primarily raising crickets and palm weevils (Hanboonsong et al., 2013,pp. 1–57). Cricket farming in Thailand, Cambodia, Lao People’s Democratic Republic (Lao PDR), the Democratic Republic of the Congo(DRC), and Kenya was studied. In most these countries, their infancy status was reported (Halloran et al., 2018). The European Commission(EC) is also pushing for insect inclusion in aquafeeds (Charlton et al.,2015). In fact, insect farming is practiced on practically every continent(Hawkey et al., 2021).
Significant investments in insect rearing start-up enterprises have recently been made all around the world. At the beginning of 2019, at least 42 European enterprises were active in the manufacture of insect meal (Mancuso et al., 2019), producing roughly 6000 tonnes of insect meal per year, according to the International Platform of Insects for Food and Feed (IPIFF). Ynsect, a French start-up company that specializes in breeding insects for aquaculture with the objective of expanding into pig, chicken, and pet feeds in the future, raised AUD50 million from private investors in 2017 (Jasper, 2017). To date, no data on large-scale commercial insect rearing is available, while the IPIFF estimates that insect meal production was ~10000 tonnes in 2020 and will rise to 0.5 million tonnes in 2030 (https://ipiff.org/). These volumes are still much lower than the present production levels of protein feeds and co-products (~5 million tons of FM used/year). This young industry will require significant investment, research, and development to mature into a viable competitive commodity. To minimize the existing high production costs, more research into automation processes is required. As long as production levels remain low, the price will continue to be a barrier to wider adoption of insect proteins. European prices for BSF larvae range from €2 to 9/kg (approximately. AUD3 to 14)(Mancuso et al., 2019). It is worth considering the novel industry’s environmental and socioeconomic benefits, which could outperform current forecasts based merely on input versus output costs. As seen by conflicting results from life cycle assessments, quantifying the environmental advantages of insect farming systems is more difficult (van Zanten et al., 2018).
Insect farming is still mostly labour intensive or non-automated around the world. In China, the United States, and Europe, large-scale cricket, mealworm, and waxworm (Galleria mellonellaL.) production has existed (Hawkey et al., 2021; Nikoletta, 2019; van Huis, 2020). The developing insect-based food and feed sector is based on many industry technologies. The development and implementation of new tactics are now ramping up, with an eye towards insect product industrialization(Hawkey et al., 2021; Nikoletta, 2019; van Huis, 2020). Currently, a few industrial insect farming firms are in various stages of growth (Tegtmeier et al., 2021). Critical factors such as insect biology studies,appropriate rearing conditions, and feeding formulations are necessary for large-scale manufacturing (Beesigamukama et al., 2021; Hawkey et al., 2021).
To produce insect meals, there are several essential steps (van Huis,2020) (Fig. 2). The first step is to ensure the availability of biomass,which should be continuously available since the nutrient composition of growing substrates has a great influence on critical production factors like total larvae yield, individual larva body weight, and nutrient composition of yielded insect larvae (Tschirner & Simon, 2015). The second step is decontamination, which is carried out using thermal or radiation processes. Drying the insect pupa or whole body with convection, and contact/radiation is the third step. The fourth step is breaking the insects or pupa into small pieces using grinding. In some insect species (e.g., yellow mealworm), it is essential to have the fourth step to extract fat from insects, which is the defatting process. Defatting is usually done with pressing mechanically and by aqueous and solvent based methods (Soxhlet and supercritical carbon dioxide) (Rumpold et al., 2017). Many approaches are available to extract proteins (Pojić et al., 2018), such as enzyme-, microwave-, ultrasound-, pulsed electric energy- and high pressure assisted extraction-approaches. For some species containing chitin in their body, it is essential to remove chitin from their meals. There are several methods to extract chitin chemically or biologically, one example will be fermentation with microorganisms and enzymes. (Rumpold et al., 2017). Details of the different steps required for processing can be found in the published paper (Rumpold et al., 2017). One important issue in producing insect meals is the cost of energy. It is essential to optimize every step of the processing with novel technologies, including automation with IOT (Internet of things) (Yue &Shen, 2021).
In order for insects to be economically viable in the feed industry,improved raising, harvesting, and processing tools and other processes must be created (Hawkey et al., 2021). Feed producers have expressed a strong desire for such processes as the volumes continue to increase,reaching 40,000 metric tons or more (Fletcher, 2021). When compared to other protein sources typically utilized in aquaculture feeds, an increase in insect meal production will ultimately lead to a decrease in insect meal selling price, which is currently not competitive. Finally,preliminary consumer acceptance experiments of insect-fed fish revealed a favourable response (Bazoche & Poret, 2021; Gasco et al.,2019a). There are currently no best practices developed anywhere on the planet. Insect mass raising and breeding will not require a complicated infrastructure, but environmental conditions must be controlled to optimize insect growth. Animal proteins of high quality will be produced as a result of the development and use of innovative technologies throughout insect farming industries.
5.Conclusion
The eight insect species discussed here have been used in replacing FM in aquafeeds. These insects possess high protein, fats and calories,which make them excellent components in aquafeeds. Many feeding trials with various aquaculture species have shown that these insects could successfully replace partial FM in aquafeeds. Replacement rates of less than 30% are recommended in most studies. Higher rates of substitution for FM, and 100% substitution, have been found to be technically possible in some aquaculture species. However, using insects to replace FM to feed farmed fish has some issues. One is the nutritional values of insects, which are different among species and stages of development within a species. Therefore, when designing feeds on an industrial scale production of insects, it is essential to have constant substrates to feed insects. Another issue is that none of the eight species discussed here are perfect substitutes for FM. Regarding the composition of amino acids and the digestibility of proteins, BSFLM is the most similar to FM. All the eight insect species discussed in this article, except for SPM, have lower sulphur amino acid contents than FM. The fat of the eight insect species lacks n-3 LC-PUFA (EPA and DHA), which makes them unsuitable for inclusion in marine fish diets. However, then-3/n-6 fatty acid profiles of house crickets, lesser mealworms and black soldier flies were significantly modified by dietaryn-3 fatty acids (Oonincx et al., 2020), suggesting that the finishing diets (enrichedn-3 LC-PUFA and sulphur amino acid) is a sensible approach to improve the accumulation ofn-3 LC-PUFA and sulphur amino acid in terrestrial insects.Encouragingly, comparing to terrestrial insects with trace amounts ofn-3 LC-PUFA, the aquatic insects have much higher levels ofn-3 LC-PUFA (Twining et al., 2018). To date, the all studied insect meals in aquafeeds are selected from terrestrial insects. It is worthy to investigate into effect of aquatic insect meals on the growth, health and flesh quality of farmed fish.
Besides experimental or pilot studies on feeding trials, the viability of enlarging the production of insects into an economically viable scale,which is capable of providing insect meals in industrial quantities (i.e.,total>1 million tons/year) must be examined. This includes developing low-cost insect meals and designing particular infrastructures, such as automation of insect culturing systems, to reduce the cost of manpower and energy. To compete with traditional protein sources, insect meals must have advantages in nutritional value and price, as well as yearround availability and consistent quality. More research is needed to optimize the nutritional values of insect meals for fish feeding. Several factors, including these affecting chemical composition, nutrient and energy bioavailability of insect meals have to be considered. The amino acids, fatty acids and mineral profiles as well as the processes of producing insect meals must be optimized to fit the nutritional requirements, palatability and feeding preferences of aquaculture species.Since the eight insect species discussed here are able to convert biowastes into valuable protein sources, sanitation measures for the safe use of substrate must be developed to ensure that insect meals are free of diseases and undesirable elements. The use of insect meals as a replacement of FM in aquafeeds requires the development of a legal framework and legislation, as well as the improvement of risk assessment procedures. It is also necessary to conduct research on the impact of feeding aquaculture species with insect meals on the safety, quality,and societal acceptance of seafoods.
The volume of insect meals is currently still minimal, but is increasing. The production of insect meals in the next a few years will still be small in compared to other ingredients in aquafeeds. The insect farming industry is likely to flourish in the next 10 years. It is certain that in the near future, insect farming for insect meals as a fish feed ingredient will affect aquaculture substantially and make aquaculture green,pro fitable and sustainable.
CRediT authorship contribution statement
Yuzer Al fiko: Reference collection and selection, Writing – original draft, preparation, Writing – review & editing. Dizhi Xie: Reference collection and selection, Writing – original draft, preparation, Writing –review & editing. Retno Tri Astuti: Reference collection and selection,Writing – original draft, preparation, Writing – review & editing. Joey Wong: Conceptualization, Reference collection and selection, Writing –original draft, preparation, Writing – review & editing. Le Wang:Conceptualization, Reference collection and selection, Writing – original draft, preparation, Writing – review & editing.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
We thank experts on insect feeds for supplying references.
 Aquaculture and Fisheries2022年2期
Aquaculture and Fisheries2022年2期
- Aquaculture and Fisheries的其它文章
- An overview of disruptive technologies for aquaculture
- CRISPR-Cas9 sgRNA design and outcome assessment: Bioinformatics tools and aquaculture applications
- The integrated analyses of metabolomics and transcriptomics in gill of GIFT tilapia in response to long term salinity challenge
- Phenotyping and phenomics in aquaculture breeding
- VNN disease and status of breeding for resistance to NNV in aquaculture
- LAMP for the rapid diagnosis of iridovirus in aquaculture
