CRISPR-Cas9 sgRNA design and outcome assessment: Bioinformatics tools and aquaculture applications
Mingkun Luo, Jun Wng, Zijie Dong, Chenghui Wng, Guoqing Lu
aKey Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Freshwater Fisheries Research Centre of Chinese Academy of Fishery Sciences, Ministry of Agriculture and Rural Affairs, Wuxi, 214081, China
bKey Laboratory of Freshwater Aquatic Genetic Resources, Ministry of Agriculture and Rural Affairs / National Demonstration Center for Experimental Fisheries Science Education / Shanghai Engineering Research Center of Aquaculture, Shanghai Ocean University, Shanghai, 201306, China
cDepartment of Biology, University of Nebraska at Omaha, Omaha, NE, 68182, USA
Keywords:
CRISPR-Cas9
Genome editing
sgRNA design
Outcome assessment
Bioinformatics tools
Aquaculture applications
A B S T R A C T
The CRISPR-Cas9 genome editing has many advantages over its counterparts and could ultimately assist in disease control and molecular breeding in aquaculture. Single-guide RNA (sgRNA) design is presumably the most crucial task in a typical CRISPR-Cas9 experiment; an understanding of algorithms behind sgRNA design programs is thus essential for improved efficacy and specificity. We focus this review on the bioinformatics aspects in genome editing experiments and describe commonly used computational approaches and tools of sgRNA design and outcome assessment. We show an example of sgRNA design with optimal parameter settings and appropriate interpretation of results and present a brief overview of CRISPR-Cas9 applications, such as genetic improvement and sustainability in aquaculture. We discuss challenging issues, particularly in the context of computational biology, in the use of computational tools in CRISPR-based genome editing. This review provides a synthesis of bioinformatics tools used for CRISPR-Cas9 sgRNA design and outcome assessment and offers a general view of CRISPR-based applications in farmed fish, which are expected to facilitate genome editing programs and hence improve aquaculture breeding, production, and sustainability.
1.Introduction
Genome editing can modify DNA in a targeted manner and holds grand promise for improved aquaculture breeding and production(Canário, 2019). The commonly used genome editing technologies include, but not limited to, ZFNs (Zink- finger nucleases), TALENs(transcription activator-like effector-based nucleases), and CRISPR-Cas(clustered regularly interspaced short palindromic repeats, CRISPR and its associated endonuclease, Cas) (Kim & Kim, 2014; LaFountaine et al., 2015). The CRISPR-Cas9, recognizing DNA by a programmable guide RNA and cleaving by a structurally bonded enzyme, has many practical advantages over its counterpart genome editing tools (Canário,2019; Cui et al., 2018). However, there are challenges encountered by practical scientists in the CRISPR experiments, including how to design optimal guide RNAs with high on-target activity and low off-target effects. Fish, known for whole-genome duplication and diverse types of eggs (transparent vs. opaque, sticky vs. non-sticky), add more challenges in the sgRNA design. A review of sgRNA design tools is thus essential,and a subsequent guideline of how to choose from many existing tools and use the most suitable ones can assist genome editing applications in aquaculture.
The CRISPR-based genome editing can rapidly introduce genomic changes and thus has many applications in aquaculture, including genetic improvement and disease resistance (Blix et al., 2021; Gratacap et al., 2019; Wargelius, 2019). The editing of genes underlying aquaculture traits such as growth and reproduction has the potential to improve aquaculture breeding and production (Blix et al., 2021; Wargelius, 2019). CRISPR has enabled the production of a breed of red sea bream with an increase of skeletal muscle mass and reduced body length(Kishimoto et al., 2018; Ohama et al., 2020), muscle mass enhancement in the olive flounder (Kim et al., 2019) and yellow cat fish (Zhang et al.,2020), and the effective disruption of reproductive competence in Nile tilapia (Jin, 2018). A recent meta-analysis of CRISPR-based genome editing studies found more than half of the studies attempted to deal with aquaculture challenges while the rest aimed to address fundamental questions in genetics, physiology, or technology (Blix et al.,2021). Multiple reviews on the application of CRISPR-based genome editing in aquaculture are available (Gratacap et al., 2019; Wargelius,2019; Yang et al., 2021); however, less effort has been made on the computation approaches and associated tools involved in the experiments.
Two important bioinformatics predictions in CRISPR-Cas9 genome editing experiments are sgRNA design and outcome assessment (Hanna& Doench, 2020). The CRISPR-Cas9, adopted from the prokaryotic immune system and used in the laboratory, uses a sgRNA and the Cas9 protein guided by sgRNA to recognize and cleave the target DNA sequence (Jinek et al., 2012). The sgRNA madein vitroorin vivoconsists of a custom-designed CRISPR RNA (crRNA) sequence fused to atrans--activating crRNA (tracrRNA) (Mojica & Rodriguez-Valera, 2016). The tracrRNA is a constant part of sgRNA, forms several stem-loops and is required for Cas9 binding, crRNA processing, and Cas9-mediated target cleavage (Jiang & Doudna, 2017). The crRNA sequence, a 5′- end 20 nucleotides variable part known as gRNA spacer, is complementary to the target DNA sequence with a protospacer adjacent motif (PAM) - an essential targeting component. Although the design of sgRNAs appears to be an intuitive task, a number of challenges exist and may affect the efficacy and specificity of genome editing. For example, the Cas9 endonuclease was found to exhibit variable efficiency at different target sites and accept few base mismatches and DNA/RNA bulges (Lee et al.,2016). In addition, once cellular DNA is cleaved, the cellular repair machinery can add or delete pieces of genetic material or make changes to the DNA by replacing an existing segment with a customized DNA sequence, resulting in a variety of genome editing outcomes (Choudhary et al., 2020; Cui et al., 2018). Assessing genomic editing outcomes is often critical for follow-up experiments.
Extensive research has been conducted to identify nucleotide features associated with sgRNA efficiency and develop corresponding models for on-target prediction (Doench et al., 2014, 2016; Hanna &Doench, 2020). Many computational tools related to CRISPR-Cas9 sgRNA design have been developed, and a number of reviews on commonly used tools and resources are made available (Alkhnbashi et al., 2020; Chuai et al., 2017; Cui et al., 2018; Graham & Root, 2015;Liu et al., 2020). In this review, we focus on bioinformatics aspects of CRISPR-Cas9 genome editing with relevance to aquaculture applications. We introduce a work flow in genome editing experiments, describe major bioinformatics resources related to sgRNA design and outcome prediction in the CRISPR-Cas9 system, demonstrate a case study of sgRNA design, and briefly summarize applications of CRISPR-Cas9 in aquaculture. We discuss areas that need to be considered or improved for the efficient use of genome editing in aquaculture. This review is expected to provide a glimpse of computational approaches and tools used in the CRISPR genome editing experiments, through which we expect to promote further genome editing research and applications in aquaculture.
2.CRISPR-Cas9 experimental design
A minimum of three phases are involved in a CRISPR-Cas9 genomic editing experiment, including sgRNA design, CRISPR-Cas9 laboratory experiment, and selection and application (Fig. 1) (Yang et al., 2021).The design and synthesis of sgRNA consists of target gene selection,sgRNA design, sgRNA synthesis. The CRISPR laboratory experiment contains processes of artificial fertilization, sgRNA and Cas9 delivery whereas the selection and application stage includes mutagenesis analysis, selection of phenotypes with CRISPR-induced mutations, and the establishment of new varieties with improved quality or quantity values in aquaculture. Several protocols have been published for guiding CRISPR genome editing experiments (Li et al., 2021; Vejnar et al., 2016).
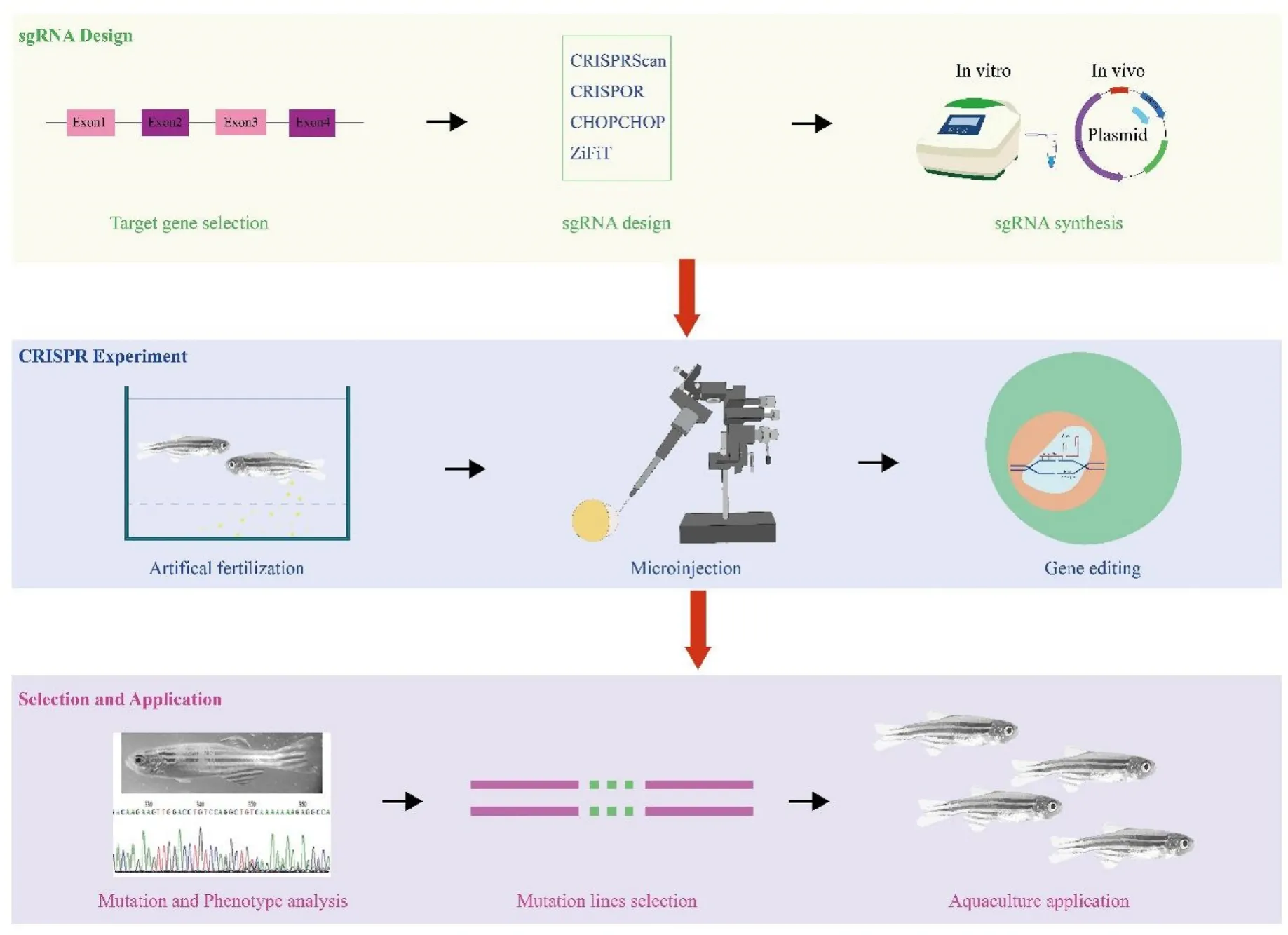
Fig. 1.A typical work flow of genome editing using CRISPR-Cas9 and microinjection in fish. The three phases are sgRNA design, CRISPR experiment, and selection and application.
Using an optimized CRISPR-Cas9 genome editing system in zebraf i sh, Vejnar et al. (2016) describes how to generate and genotype mutants. It explains how to construct extremely efficient sgRNAs through the web application CRISPRscan, how to manufacture sgRNAsin vitro,and how to identify heterozygous fish with the desired mutations. Li et al. (2021) detailed a procedure for CRISPR-Cas9 mediated gene editing in tilapia. Selection of target sites,in vitroRNA transcription,artificial insemination, and microinjection of one cell stage embryos are all part of this process. This protocol facilitates application of CRISPR-Cas9 in studies of other aquaculture fishes. Graham and Root(2015) reviewed major considerations in the design of genome editing experiments, and surveyed tools and resources available to assist users of CRISPR technology (Graham & Root, 2015). Computational methods and resources for CRISPR-Cas investigations, including sgRNA design,repair outcome prediction, editing outcome evaluation, and sgRNA associated repositories and databases, were also discussed (Sledzinski et al., 2020).
3.CRISPR-Cas9 sgRNA design
sgRNA design is presumably the most critical step in CRISPR-Cas9 experiments. In principle, it is relatively easy to predict sgRNA candidates simply by finding the PAM site (i.e., 5′-NGG-3′) and identifying the 20 nucleotides upstream (Doudna & Charpentier, 2014; Jinek et al.,2012). However, in practice, only a certain number of sgRNA can ef ficiently target DNA on the desired site in genome editing, and nucleotide mismatch in sgRNA and the PAM motif may result in off-target editing(Doench et al., 2016; Hsu et al., 2013). It is thus essential to take into consideration the cleavage efficiency of sgRNA and the potential for off-target activity in CRISPR-Cas9 genome editing (Doench, 2018;Hanna & Doench, 2020).
3.1.On-target prediction: efficacy
CRISPR-Cas9 systems exhibit a variable degree of variability in their cleavage activity. The likelihood of Cas9 enzyme precisely cutting target DNA is determined by a number of variables, including nucleotide composition, chromatin accessibility, and thermodynamic stability(Table 1) (Doench et al., 2014; Lee et al., 2016; Wilson et al., 2018).Doench et al. (2014) analyzed sgRNA sequences of several mouse and human genes in cellular assays, identified sequence features, and developed a prediction model called Rule Set1 for sgRNA on-target eff i cacy. Several new features, such as the number of position-independent nucleotides, target location in the corresponding gene, and melting temperature, were incorporated later into Rule Set 2(Table 1) (Doench et al., 2016). A number of additional nucleotide features affecting efficiency were observed (Gagnon et al., 2014;Heigwer et al., 2014; Moreno-Mateos et al., 2015; Wang et al., 2014; Xu et al., 2015) (Table 1). These features have been implemented in tools such as E-CRISP (Heigwer et al., 2014), CHOPCHOP (Labun et al., 2016,2019; Montague et al., 2014), and CRISPOR (Haeussler et al., 2016) for predicting sgRNA on-target efficiency (Table 1).
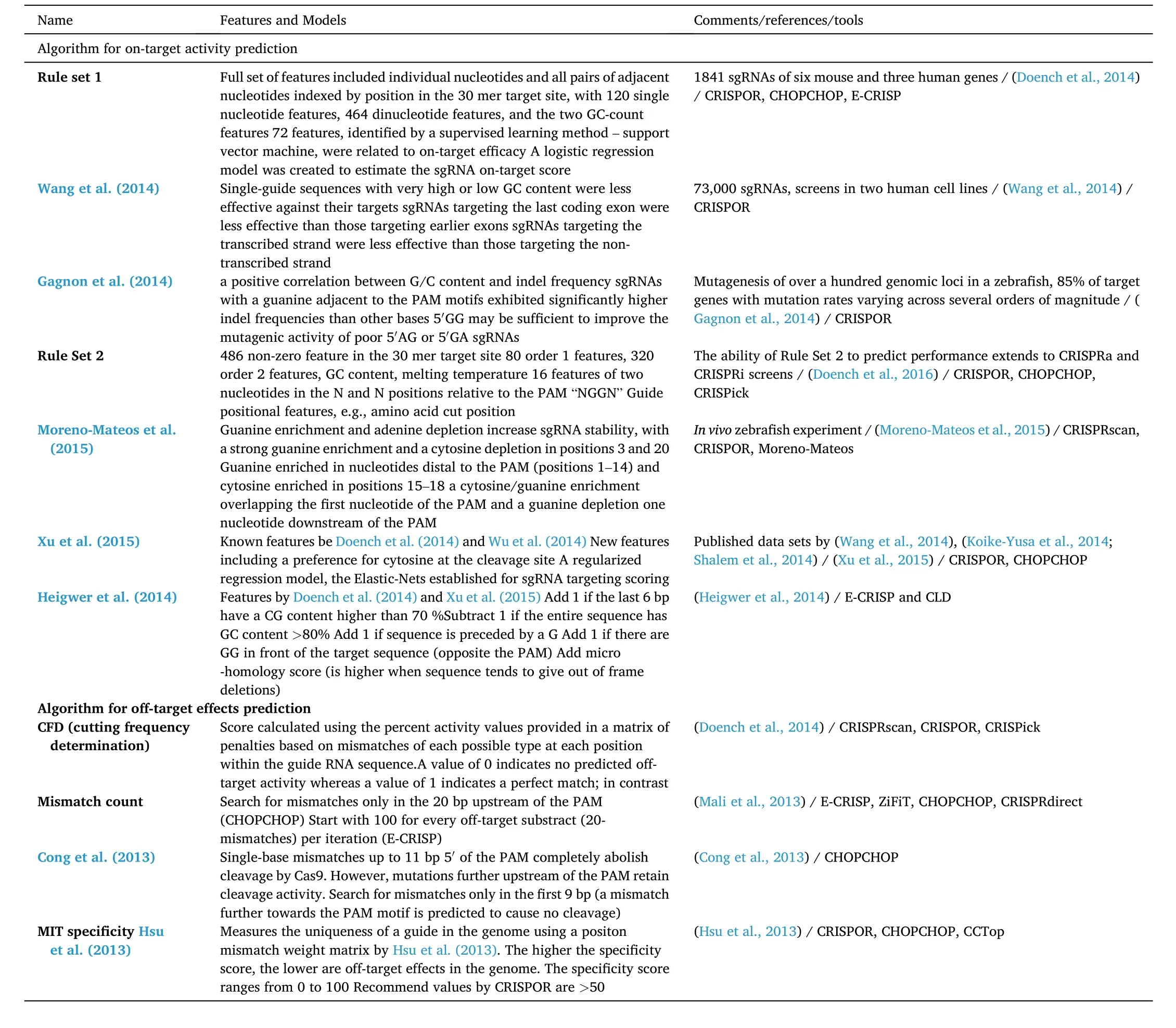
Table 1Major features and models used for on-target and off-target prediction.
Based on a large-scale analysis of sgRNA mutagenesis activity in zebra fish, Moreno-Mateos et al. (2015) found guanine enrichment and adenine depletion contribute to the stability, loading and activity of sgRNA (Table 1). The logistic regression analysis was conducted to find features, mononucleotide and dinucleotide, associated with sgRNA activity and a regression linear model was implemented in CRISPRscan for sgRNA efficacy prediction (Moreno-Mateos et al., 2015). CHOPCHOP and CRISPOR also use this model to predict sgRNA activity (Table 1).The effect of sequence context on sgRNA efficiency was found to differ significantly between CRISPR knock-out and CRISPR activation/inhibition (Xu et al., 2015). The corresponding model was implemented in SSC (Xu et al., 2015), CRISPR-FOCUS (Cao et al., 2017), and CRISPR-DO(Ma et al., 2016).
3.2.Off-target effects: specificity
Specificity is another critical factor affectingin vitroandin vivogenome editing. CRISPR-Cas9 can tolerate nucleotide mismatches,which means sgRNA may guide Cas9 to other possible loci in addition to its targets, leading to unexpected gene knockout or knock-in activity(Hsu et al., 2013; Park et al., 2017; Wu et al., 2014). There are two widely used methods in specificity prediction of CRISPR sgRNAs,alignment-based and scoring-based (Liu et al., 2020). The offen used approaches to estimate sgRNA specificity is to compare off-target sequences, identify the number, position and distribution of mismatches,and come up with a penalty matrix (Hsu et al., 2013). This penalty matrix corresponds to each position and can be used to estimate a score for each sgRNA according to its potential off-target sites, an indicator to choose appropriate sgRNAs. It has been used to calculate sgRNA specif i city by tools such as CRISPRscan (Moreno-Mateos et al., 2015),CHOPCHOP (Montague et al., 2014), CCTop (Stemmer et al., 2015), and CRISPOR (Labun et al., 2016; Montague et al., 2014).
Cutting frequency determination (CFD) is another popular score for off-target evaluation. Sung et al. (2014) profiled the off-target activity of thousands of sgRNAs and developed a metric to predict off-target sites.Doench et al. (2016) added PAM and other features in the scoring matrices for CFD estimation. Validation with GUIDE-seq showed the CFD score performed better than those proposed by Doench et al. (2016)and Hsu et al. (2013). The CFD was included in CRISPRscan (Moreno-Mateos et al., 2015) and CRISPOR (Labun et al., 2016; Montague et al., 2014). It has been found that a single off-target model is not enough to design reliable sgRNA (Haeussler et al., 2016). Most tools offer advanced options for the off-target detection model or show several scores accepted by the community. For example, CRISPOR (Haeussler et al., 2016) offers scoring models by Hsu et al. (2013) and Doench et al.(2016).
3.3.sgRNA design tools
Many sgRNA design tools have been developed to support the CRISPR-Cas9 experiments and a number of resource reviews are available (Chuai et al., 2017; Cui et al., 2018; Graham & Root, 2015; Hanna &Doench, 2020; Liu et al., 2020; Sledzinski et al., 2020). Cui et al. (2018)reviewed various sgRNA tools, with a focus on-target efficiency prediction and off-target algorithm. Liu et al. (2020) compared computational methodologies for effective CRISPR sgRNA design and evaluation,finding that the majority scores are empirical or trained on experimental datasets, and that scores are implemented using a variety of computational methods. Yan et al. (2018) benchmarked CRISPR on-target sgRNA design and concluded that the reported sgRNA design guidelines are not reproducible across different sgRNA libraries, cell types and organisms.CRIPRScan was the only tool with predicted on-target efficiency scores correlating within vitroobserved cleavage activities, according to a zebra fish evaluation of CRISPR genome editing tools. There is no surprise with this observation because the bulk of on-target prediction systems were developed based on rules inferred from datasets of human or mouse cells whereas CRISPRScan based onin vivozebra fish knockout experiments. Table 2 lists commonly used web tools for sgRNA design in the CRISPR-Cas9 experiments with detailed information of input,parameter settings, output and comments; an extended list is available in Table S1 (Supplementary File 1).
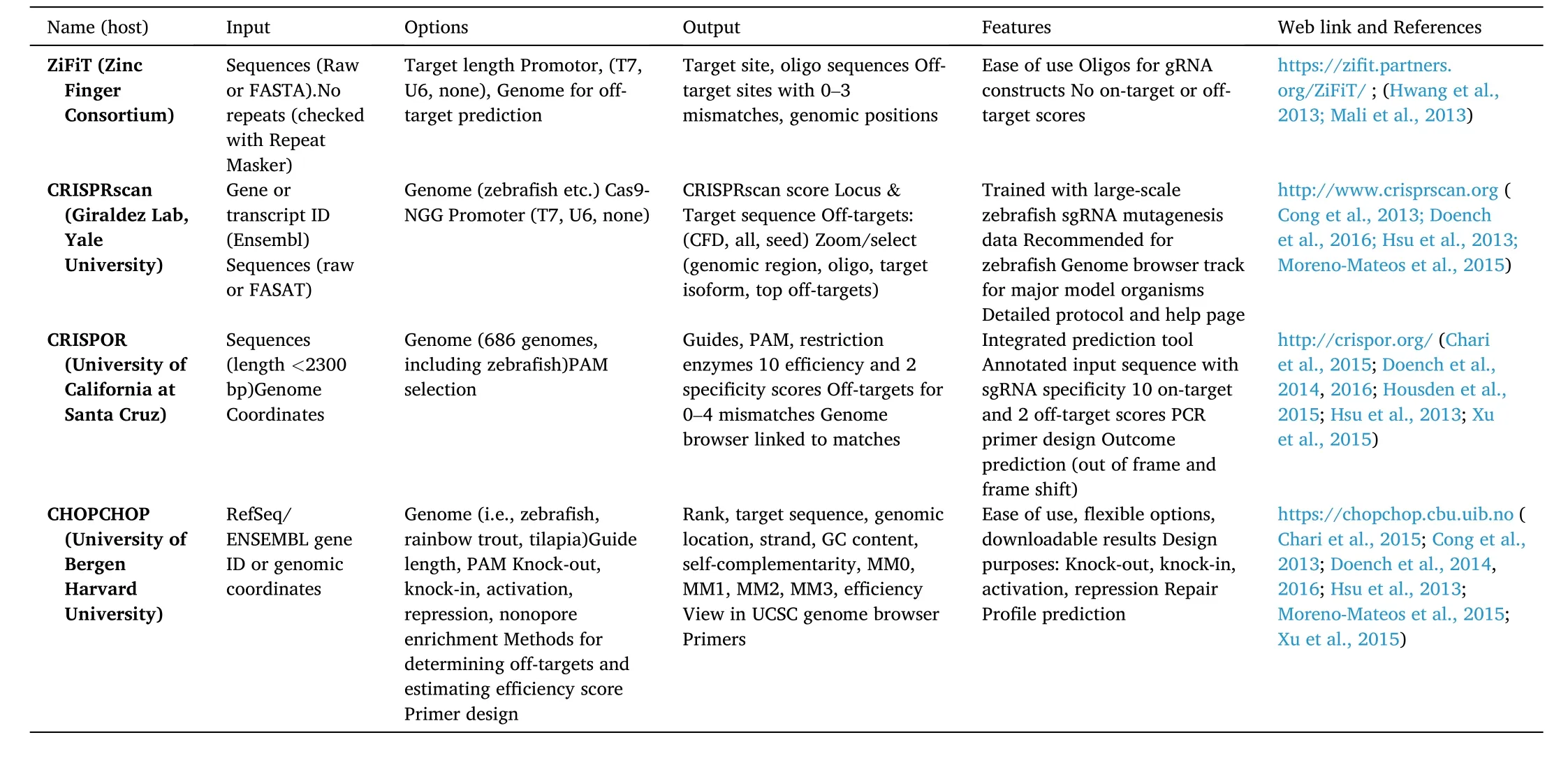
Table 2Representative online tools for sgRNA design in the CRISPR-Cas9 experiment.
4.Genome editing outcome assessment: Sanger or nextgeneration sequencing
CRISPR-Cas9 genome editing often causes uniform biallelic and heterozygous mutations in diploid organisms (Liu et al., 2015). The assessment of editing outcomes is based on the alignment or mapping of resulting sequences to the template and conducted by computing the proportion of indels and repair types (NHEJ or HDR). Direct sequencing of PCR products containing such mutations results in superimposed sequencing chromatograms, which can be analyzed by TIDE or ICE(Fig. 2). TIDE estimates the frequency of targeted small nucleotide changes introduced by CRISPR and reports the identity of the detected indels and their frequencies (Brinkman et al., 2018). The next-generation sequencing (NGS) data can be used for assessing genome editing results with tools such as CRISPResso2 and CRISPR RGEN tools. CRISPResso2 is a web tool designed to enable rapid and intuitive interpretation of genome editing results produced by amplicon sequencing (Clement et al., 2019). CRISPR Cas-Analyzer is another online tool that uses NGS data to assess genome editing results. It is a JavaScript-implementation that runs on a client-side web browser and eliminates the need to upload huge NGS datasets to a server, which is a time-consuming step in genome editing analysis with other applications such as CRISPR-GA or CRISPResso (Park et al., 2017).
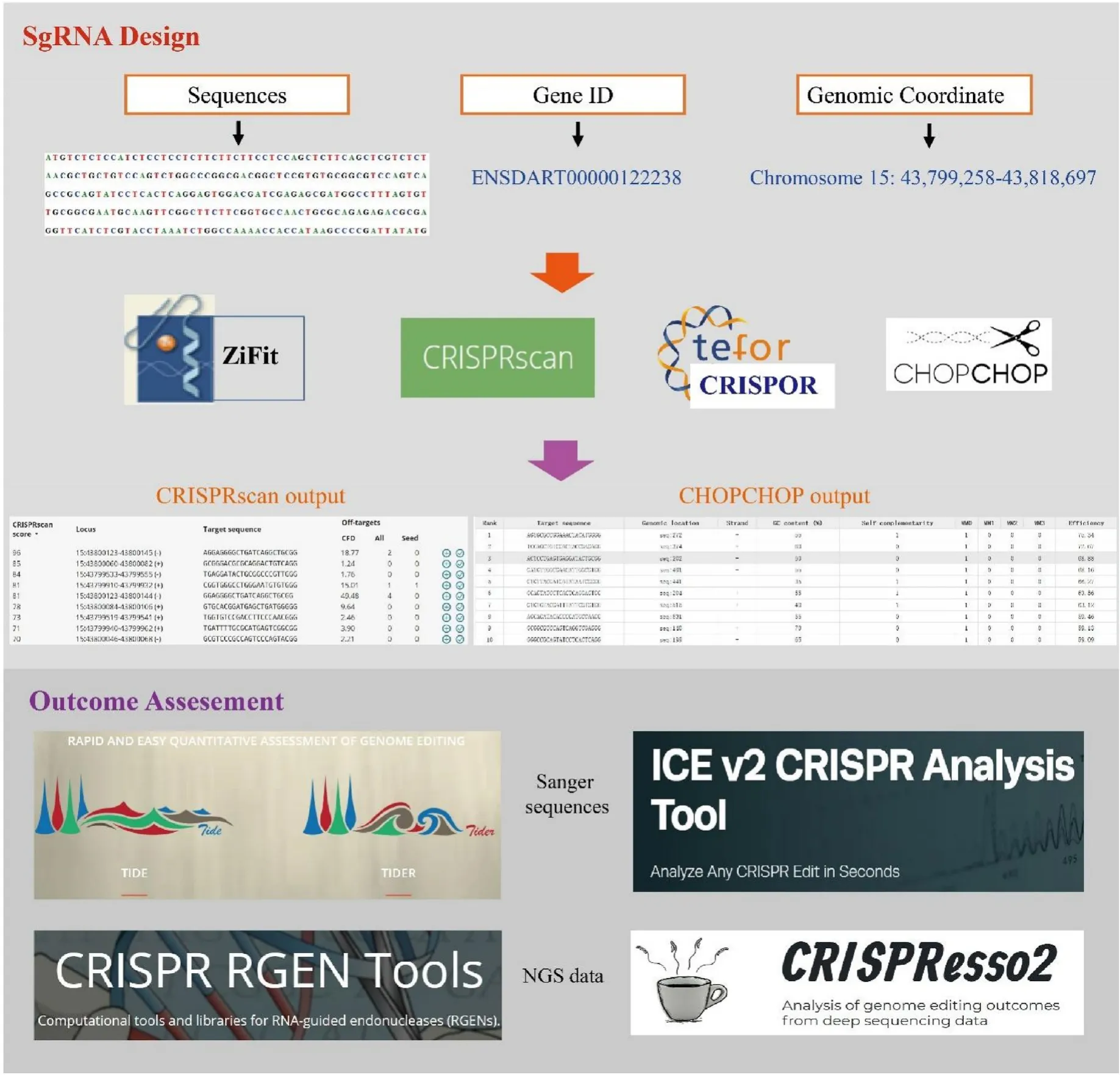
Fig. 2.An example pipeline of sgRNA design and outcome assessment of genome editing in aquaculture, with prediction programs denoted.
5.Use of bioinformatics tools for sgRNA design of the Tyr gene:an example
To illustrate how to use sgRNA design tools effectively, we further show a case study of tyrosinase gene (tyr) and compare the results and comment on their different features. Thetyrgene of zebra fish has five exons and the first protein-coding exon was used. We used four sgRNA design tools, ZiFit, CRISPRscan, CRISPOR, and CHOPCHOP. For the input, all tools accept raw sequences, ZiFit also accepts in FASTA format,and others accept gene names, gene IDs, or genomic coordinates. For parameter setting, only CHOPCHOP allows users to upload their own reference genome if the genome of interest is not on the list. Currently,there are only nine and 18 reference genomes, respectively, in the ZiFit and CRISPRscan genome databases. More reference genomes are available in CRISPOR and CHOPCHOP. Users can have the option to select Cas9 nucleases (e.g., PAM-NGG) and promoter information (e.g., T7 promoter) according to the experimental needs. The program ZiFitappears to have a strict 5′requirement of sgRNA, “GG” present at 5′-end of sgRNA. The output of sgRNA design varies depending on the software used (Supplementary File 2). The output by ZiFit contains the sequences of sgRNA and oligos used for PCR validation and provides concise information related to sgRNA, which contains the fewest number of sgRNA compared to other tools (Supplementary File 2: Table S2). The program CRISPRScan outputs detailed information about the designed sgRNAs,including CRISPRScan score, target sequences, off-target CFD score, offtarget numbers, and so on (Supplementary File 2: Table S3). The output by CRISPOR contains the most comprehensive information including sgRNAs along with MIT specificity Score, CFD score, predicted ef ficiency, predicted outcome, and off-target information; it also provides enzyme and cloning information for sgRNA synthesis and PCR primers for the evaluation of sgRNA efficiency (Supplementary File 2: Table S4).The output by CHOPCHOP has information of sgRNA, mismatch (offtarget), efficiency, and PCR primers for evaluation. In short, the output by CHOPCHOP consists of most of the sgRNA information needed and is more concise than those by CRISPRScan and CRISPOR (Table 3). We suggest multiple sgRNA design tools be used to improve on-target ef ficiency and off-target specificity.
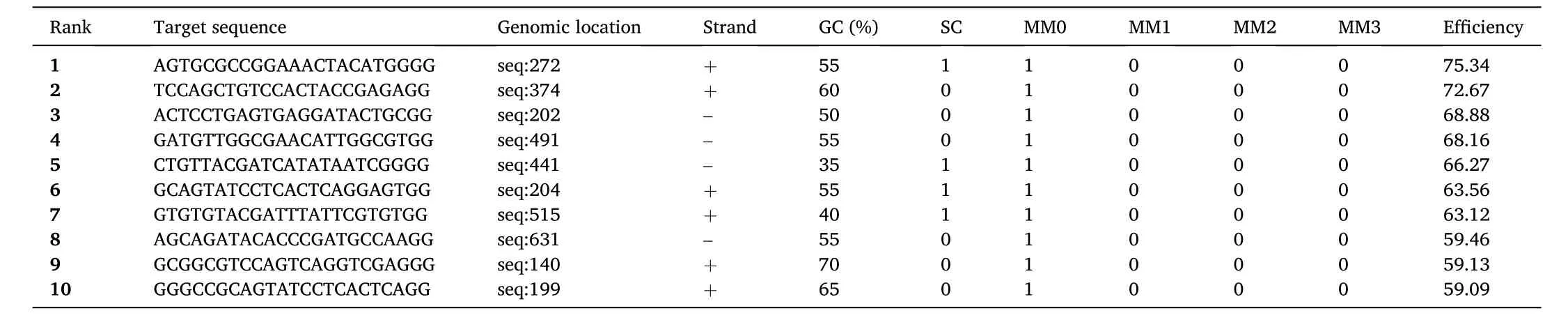
Table 3Top 10 predicted sgRNAs by CHOPCHOP too. Full lists of the predicted sgRNAs can be found in Supplementary File 2.
6.Applications of CRISPR-Cas9 in aquaculture
Multiple excellent reviews on genome editing and its broad application in aquaculture have been published (Gratacap et al., 2019; Luo et al., 2021; Yang et al., 2021). Zhu and Ge (2018) described advancements and applications of genome editing technologies, with a focus on understanding reproductive gene functions. Blix et al. (2021) conducted a literature mining on the current status of genome editing in farmed fin fish species and concluded that there remained a focus on reproductive traits, but this had been expanded to include genes related to other traits such as disease resistance. They also found more than half of the studies focused on practical aquaculture problems and the rest belonged to basic genetic, physiological or technological research (Blix et al.,2021). The potential of genome editing to improve aquaculture breeding and production discussed by Gratacap et al. (2019) highlighted that the high fecundity and external fertilization of most aquaculture species were suitable for the application of CRISPR-Cas9 technologies,functional disease resistance alleles could be identified throughin vivoandin vitroscreenings for functional testing and application, and using sterilized fish in aquaculture could prevent interbreeding of genome edited individuals with wild stocks (Gratacap et al., 2019). Yang et al.(2021) reviewed genome editing and its application in genetic improvement in aquaculture and were convinced that the application of CRISPR-Cas9 technologies would transform aquaculture breeding that will produce more, high-quality seafood. While research progress and important findings about the applications of genome editing in aquaculture were detailed in the above reviews, we provide below a glimpse of CRISPR-based applications in aquaculture.
The CRISPR-based genome editing technologies have been applied to more than 20 economically important, aquaculture fish species,including Atlantic salmon, tilapia, common carp and grass carp (Table 4)(Lu et al., 2021; Yang et al., 2021). The aquaculture traits studied in these genome editing experiments include pigmentation, disease resistance, reproduction, feed utilization, growth, muscle development, etc.Most studies used ZiFit, CRISPRScan, and CRISPOR for sgRNA design.We observed the mutation efficiency of CRISPR-Cas9 varies largely depending on the species and genes. For example, thetyrgene has an efficiency of 22% in Atlantic salmon but 60% in white crucian carp(Edvardsen et al., 2014; Liu et al., 2019).Mstn(myostatin) a and b,muscle growth-related genes that prevent the formation of skeletal muscle, appear to have a relatively high mutation efficiency, 94% and 88%, respectively (Kishimoto et al., 2018). Among many applications,the most exciting case is the complete knockout of muscle growth inhibitor (Pm-mstn) with CRISPR-Cas9 has allowed creating a new breed of red seabream (Pagrus major). The pure genetic mutant breed were established within two years, which is much faster than the time needed with traditional breeding methods (Kishimoto et al., 2018).

Table 4Application of CRISPR/cas9 genome editing in major aquaculture fish species.
Sexual dimorphism observed in at least 20 farmed fish is another important trait in aquaculture, since males and females grow at different rates with dissimilar body sizes (Mei & Gui, 2015). Molecular mechanisms underlying sex determination and differentiation were studied with genome editing of sex-related genes in yellow cat fish, tilapia, and Atlantic salmon (Dan et al., 2018; Li et al., 2014, 2020; Wargelius et al.,2016). In addition, fish exhibit a wide variety of pigment cells and the CRISPR editing of genes involved in the functional regulation such as pigment cell formation, migration and pigmentation is a research area with much attention (Luo et al., 2021). For example, Du et al. (2021)knocked out theScarb1andScarb1-like genes and observed the gene disruption induced a break of the red coloration and a decrease of astaxanthin and lutein, suggesting the twoScarb1genes might act as a“switch” in the red carotenoid ornamentation in Oujiang color common carp. Although much progress has been made in genome editing of farmed fish, there are challenges in its practical applications. For example, the success rate of microinjection in embryos of many fish species remains very low. In addition, the genome editing efficiency needs to be improved while decreasing the off-target effects.
7.Discussion
The CRISPR-Cas9 genome editing has become a dominant technology for genetic perturbations, including gene knock-out and knock-in,regulatory activation and inference (Graham & Root, 2015). This review focused on two fundamental aspects related to the computational analysis in the CRISPR-Cas9 experiments, i.e., sgRNA design and outcome assessment. We reviewed the nucleotide rules and models of sgRNA, which will help understand the underlying algorithms of different sgRNA prediction tools (Doench et al., 2014, 2016; Gagnon et al., 2014; Heigwer et al., 2014; Lee et al., 2016; Moreno-Mateos et al.,2015; Wang et al., 2014; Wilson et al., 2018; Xu et al., 2015). The prediction models created based mainly on two types of datasets,in vitrohuman and mouse cell lines versusin vivozebra fish, were implemented in popular sgRNA design programs CHOPCHOP and CRISPRScan,respectively (Labun et al., 2016, 2019; Montague et al., 2014). Gene editing outcome assessment is based mainly on the alignment or mapping of resulting sequences to the target (Liu et al., 2015). The underlying algorithms of alignment or mapping can be different, and the results may vary among various tools. A rule of thumb with sgRNA design in aquaculture-related CRISPR experiments is to use CRISPRScan for sgRNA design in companion with other similar tools such as ZiFit,CRISPOR, or CHOPCHOP.
Future sgRNA designs should consider cell-type heterogeneity, the epigenetic environment of different cell types, and personalized sgRNA design rules for specific cell types to achieve better sgRNA design performance (Chuai et al., 2017). Benchmark analysis of CRISPR sgRNA design demonstrated sgRNA prediction results varied largely among different tools using the same set of sequences, highlighting the importance of understanding features adopted by each tool (Uribe-Salazar et al., 2020, pp. 2020–10). The design rules derived from specific cell types or organisms may not be applied well to others (Yan et al.,2018); the quality of the assays where the training data came from is important to build models (Haeussler et al., 2016). Heuristic methods currently used for sgRNA prediction shall be improved with data-driven models through comprehensive experimental evaluation of on-target modification efficacy and target-site specificity across many contexts(Graham & Root, 2015). The integration of multiple assays and data sources likely lead to more comprehensive and accurate sgRNA prediction (Yan et al., 2018).
Regarding the genome editing outcome assessment, TIDE appears to be the most straightforward and practical tool; however, it may not be well suited for the analysis of sequences containing tandem repeats and does not seem to be applicable in the analysis of larger rearrangements(Sledzinski et al., 2020). The latest NGS technology has made it easy to assess genome editing outcomes, which has been broadly used in model organisms but has not been well adopted for genome editing in aquaculture (Park et al., 2017). The limitation with NGS is its cost,time-consuming process and often the need for external service (Sledzinski et al., 2020).
There are many aspects of genome editing beyond the focus of this review, including nuclease systems other than CRISPR-Cas9, genome wide CRISPR screen, and base editing. Studies have considered the design of a double nickases system, the modification of existing Cas9 protein (Chuai et al., 2017). The desire to expand the list of recognized PAM sequences spurred both the development of Cas variants engineered to recognize more flexible PAM sequences as well as the characterization of additional Cas enzymes (Graham & Root, 2015). A major recent focus has been the development of comprehensive tools for use on data from large-scale CRISPR-based genetic screens (Hanna & Doench,2020). Another direction of genome editing, which consist of deactivated Cas9 (dCas9) or Cas9 nickase (nCas9) linked with a cytidine or a guanine deaminase, is precise base editing and the corresponding tools were discussed in (Hwang et al., 2018).
Current research of genome editing in aquaculture focuses on the knockout experiments with the disruption of gene functions. However,CRISPR technology can be designed for gene knock-in, CRISPR interference (CRISPRi), CRISPR activation (CRISPRa), or base-editing, which shall benefit more to aquaculture selection programs as compared to the gene knock-out, because this could take full advantage of the phenotype and genotype association results from previous genetic mapping and GWAS studies (Houston & Macqueen, 2019). Almost all the current research in aquaculture focuses on single gene knockouts; however,most aquaculture-related traits such as growth and reproduction are determined by many genes. Due to the availability of genomic resources(Lu & Luo, 2020), whole-genome CRISPR screening technology is of great promise in aquaculture in that it produces many mutants for future selection and breeding (Cuellar et al., 2018, p. 209; Uribe-Salazar et al.,2020, pp. 2020–10; Zhao et al., 2020). It is critical to reduce possibilities of off-target effects and minimize the issue of mosaicism in the founder generation (Yang et al., 2021). As this technology improves, we hope that researchers will be able to continuously update and improve the CRISPR/Cas9 system, which will benefit research and practice in aquaculture.
8.Conclusions
The CRISPR-Cas9 has revolutionized genome editing technologies and brought great promise into aquaculture selection and breeding.Besides technical challenges, sgRNA design and knockout outcome assessment are two significant computational problems. We reviewed nucleotide features and the learned rules or models of sgRNA and described commonly used tools for sgRNA design and genome editing assessment. We presented a case study of sgRNA design with several web tools, compared their results and commented on their pros and cons from a practical point of view. We overviewed the applications of CRISPR-Cas9 in aquaculture, which is still at its early stage compared to its use in biomedical research. Taking advantages of ongoing genetic mapping and GWAS results, the CRISPR technology can be improved to promote aquaculture selection and breeding programs.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. G Yue for his invitation to submit this review. This publication was made possible through funding support from the National Science Foundation (DBI-1919574) and the University of Nebraska at Omaha.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aaf.2021.10.002.
 Aquaculture and Fisheries2022年2期
Aquaculture and Fisheries2022年2期
- Aquaculture and Fisheries的其它文章
- An overview of disruptive technologies for aquaculture
- The integrated analyses of metabolomics and transcriptomics in gill of GIFT tilapia in response to long term salinity challenge
- Phenotyping and phenomics in aquaculture breeding
- VNN disease and status of breeding for resistance to NNV in aquaculture
- LAMP for the rapid diagnosis of iridovirus in aquaculture
- Insects as a feed ingredient for fish culture: Status and trends
