Simple Synthesis of Ultra-small Size Focused Sulfur Quantum Dots for Fluorescence Sensing
ZHENG Qiong-hua,SHENG Yi-lun,ZHANG Shan-biao,
HUANG Zhong-nan,CHEN Wei*,PENG Hua-ping*
(Higher Educational Key Laboratory for Nano Biomedical Technology of Fujian Province,Department of Pharmaceutical Analysis,Fujian Medical University,Fuzhou 350004,China)
Abstract:A facile assembly-fission method combined with dialysis post-treatment strategy was adopted to prepare a kind of ultra-small size focused fluorescent sulfur quantum dots(SQDs).The as-prepared SQDs were characterized by transmission electron microscopy(TEM),energy-dispersive X-ray spectroscopy(EDX),UV-Vis absorption spectrometry(UV-Vis)and fluorescence spectrometry.The photoluminescence properties of the SQDs were investigated in detail,which indicated that the SQDs with the average diameter of 2.2 nm exhibited an excitation-independent emission and an optical stability in aqueous solution.Meanwhile,the fluorescence of the SQDs could be quenched by Fe3+significantly,and the mechanism for the fluorescence quenching was via inner filter effect(IFE).Based on the fluorescence quenching effect of the Fe3+/SQDs system,a facile fluorescence sensing platform was successfully constructed for the highly selective and sensitive detection of Fe3+.The proposed SQD-based sensor showed a wide response to Fe3+in the range of 5-600μmol/L,with a detection limit(LOD)of 1.19μmol/L.In addition,this method,with significant advantages of high sensitivity,high selectivity,simplicity and economy,was successfully applied to the detection of Fe3+in human serum,avoiding the deficiencies of complex sample preparation and expensive detection conditions.Thus,this method has a potential application prospect in clinical diagnosis,and opens up a new way for the design of effective fluorescent probes for other biologically related targets.
Key words:sulfur quantum dots;assembly-fission;dialysis post-treatment;ferric iron
As one of the essential trace elements in human body,iron is involved in many physiological processes in the body,such as oxygen transport,oxygen metabolism and electron transfer[1-3].The participation of ferric ion(Fe3+)ensures the successful expressions of functional proteins.The lack/excess of Fe3+in the body may lead to anemia fatigue,decreased immunity,hypersideremia and other diseases,which may eventually lead to diseases like hepatitis and cancer[4].In addition,Fe3+in water can multiply of iron bacteria which harms to human body[5].Consequently,monitoring of Fe3+has important environmental and biomedical significance.By now,several available methods have been proposed for the detection of Fe3+,such as voltammetry,colorimetry and mass spectrometry[6-8].Among these methods,fluorescence detection technique has attracted wide attention due to its high sensitivity,fast analysis and high spatial resolution[9-10].However,traditional nanomaterial fluorescent probes such as organic molecules,silicon nanocrystals,metal nanoclusters,organic chromophores etc,have seriously hindered their practical application because of their characteristics of toxicity,biological incompatibility and water insolubility[11-14].
Fluorescent single-element quantum dots(QDs)have become a promising fluorescent probes owing to their size-controlled luminescence and good optical stability,which show the potential to serve as nontoxic replacements for traditional heavy-metal-based QDs[15].Among these single-element QDs,sulfur QDs(SQDs),as a new type of metal-free QDs,have recently attracted intensive attention due to their unique optical property,low toxicity,easy availability and bactericidal nature[16-19].To date,limited passivator and stabilizer have been used to synthesize SQDs,and polyethylene glycol(PEG)is the most studied ligand[20].In recent years,exploring novel and effective synthetic methods of PEG-SQDs with high fluorescent property has been strongly desired.In 2014,Li′s group reported that SQDs could be prepared directly from elemental sulfur for the first time[21].Subsequently,Shen′s group proposed a strategy based on assembling of SQDs in fission of sublimed sulphur[22].Recently,Ding′s group proposed post-synthesis of SQDs by Cu2+-assisted etching of the assembled S dots[23].Interestingly,we found that a high performance SQDs could be obtained only by dialysis treatment of the assembled S dots.Thus,we proposed a facile approach for the post-synthesis of SQDs,where dialysis posttreatment of S dots could turn on their luminescent.In addition,the obtained SQDs with the average diameter of 2.2 nm show excitation-independent emission,low-toxicity and good stability,which make the as-prepared SQDs have great potential for biosensing applications.Based on the fluorescence quenching effect of Fe3+on SQDs,a novel SQD-probe-based sensing platform has been proposed.Therefore,this work not only provides a facile strategy for the synthesis of SQDs,but also shows their potential applications in biosensing,life analysis and other fields.
1 Experimental
1.1 Materials and reagents
Sublimed sulfur was purchased from Hengxing Chemical Reagent Co.Ltd(Tianjin,China),sodium hydroxide(NaOH)was purchased from Aladdin Reagent Co.Ltd(Shanghai,China),polyethylene glycol(PEG-400)was purchased from Sinopharm Chemical Reagent Co.Ltd(Shanghai,China).The serum samples were obtained from Fujian Provincial Hospital.All other chemical reagents were of analytical grade and directly used without additional purification.
1.2 Instrument
The UV-visible and fluorescence spectra of SQDs were determined by UV-2450 spectrophotometer(Shimadzu Japan)and Cary Eclipse fluorescence spectrometer(Agilent USA),respectively.The morphology and particle size of SQDs were characterized by JEM-2100F transmission electron microscope(TEM,JEOL Japan).Time-resolved PL decay curves were recorded using a FLS980 spectrometer(Edinburgh Instruments).
1.3 Preparation of SQDs
Briefly,1.4 g of sublimed sulphur powder,3 mL of PEG-400,4 g of NaOH,and 50 mL of ultra-pure water were mixed,and then the mixture was stirred at 70℃for 72 h(the product was recorded as"S dots").No obvious luminescence was observed for the S dots.After dialyzing of the S dots for 24 h,the SQDs were obtained.An intense blue emission of the SQDs could be observed under the UV light(365 nm).
1.4 Detection of Fe3+using SQDs
Different concentrations of Fe3+(30μL)was added into a mixture of SQDs(100μL)and acetic acid buffer solution(50 mmol/L,1.37 mL,pH 5.0).After 5 min at room temperature,the fluorescence emission spectra were recorded(365 nm of excitation wavelength).The selectivity measurements of Fe3+detection were conducted in a similar way.The standard curve of Fe3+was determined by measuring the change in relative fluorescence intensity(F0/F)at different Fe3+concentrations,in whichF0andFwere corresponded respectively to the fluorescence intensity before and after Fe3+was added into the SQDs solution.
1.5 Serum sample detection
In order to evaluate the feasibility of this method in real samples,we carried out the standard addition experiments in serum samples for Fe3+detection.First,the pretreated samples were obtained by ultrafiltration of the serum samples.The pretreated samples were then diluted 300 times with acetic acid buffer solution(50 mmol/L,pH 5.0).Finally,different concentrations of Fe3+were added into the mixture to carry out the standard addition experiments.Subsequent fluorescence spectra were recorded with the protocol for the above Fe3+detection.
2 Results and discussion
2.1 Characterization and optical properties of SQDs
A novel fluorescent SQDs has been synthesized by"assembly-fission"method and dialysis post-treatment strategy.The morphology of the product was characterized by TEM.The SQDs were well-dispersed and showed near-spherical morphology(Fig.1A).Average diameter of the SQDs was(2.213±0.061)nm(Fig.1B),indication ultra-small SQDs with size-focusing has been synthesized by this facile process.The energydispersive X-ray spectroscopy(EDX)were conducted for the elemental analysis of the product,which showed obvious C,O,and S peaks.The relative contents of C,O and S elements were 83.33%,14.62%and 2.05%,respectively(Fig.1C).These results demonstrated the successful formation of the SQDs.As shown in Fig.1D,the SQDs had the characteristic absorption peaks at 260 nm and 350 nm,which was corresponded to the reported result[24].In addition,compared with S dots,the SQDs exhibited excellent photoluminescence(PL)under the UV light(Fig.1E),and the maximum emission wavelength was 446 nm(excited at 365 nm,Fig.1F).This results indicated that the dialysis post-treatment could effectively promote the formation of the ultra-small SQDs and thus enhance their PL intensity.The three-dimensional excitation and emission matrix(3D-EEM)fluorescence spectra indicated that the dialysis post-treated product was excitation-independent emission(Fig.1G),which could mainly due the size-focusing effect of the SQDs.Moreover,the relative quantum yield of the SQDs was examined to be 4.84%using quinine sulfate as a reference whose quantum yield was 54%in 0.1 mol/L H2SO4solution.
The stability tests showed that after xenon arc light exposure for 1 h and after stored at room temperature for 9 months,no distinct change of fluorescence intensity was observed,indicating that the SQDs had good light stability and time stability(not shown).Besides,as depicted in Fig.1H,no distinct change of the PL intensity was observed with pH varied from 4 to 12,suggesting their wide ranging applications under different harsh environment.

Fig.1 TEM image(A),the size distribution(B)and EDX spectrum(C)of SQDs;UV-Vis absorption spectrum of SQDs solution(D);fluorescence spectra of S dots and SQDs(E);fluorescence excitation and emission spectra of SQDs(F);3D-EEM fluorescence spectra of SQDs(G);pH stability of SQDs(H)
2.2 Fluorescence sensing of SQDs for Fe3+
2.2.1 Fluorescence quenching effect and mechanism study of SQDs/Fe 3+systemBased on the good PL properties of the as-prepared SQDs,we further used the SQDs for sensing application.As shown in Fig.2A,when addition of Fe3+into SQDs solution,the PL of SQDs quenched significantly.Thus,a SQDbased fluorescence sensor could be developed to achieve the high efficiency and simple detection of Fe3+.We further explored the quenching mechanism of this SQDs/Fe3+system.
To further gained insight into the quenching mechanism of SQDs/Fe3+system,corresponding experiments were performed.First,the UV-Vis absorption spectrum of Fe3+was studied,which overlapped widely with the excitation spectrum of SQDs(Fig.2B).Thus,we speculated that the quenching mechanism of the SQDs due to Fe3+was considered to internal filtration effect(IFE)[25].IFE,also known as apparent quenching,does not belong to static or dynamic quenching process.In addition,no new substance will be formed and the fluorescence lifetime of SQDs will not be changed[26].To verify the speculation,we monitored the UV-Vis spectroscopy of the mixture of SQDs and Fe3+.No new absorption peak was observed in the mixture of SQDs and Fe3+(Fig.2C),which indicated that no interaction was existed between SQDs and Fe3+,and no new product was formed as well.Furthermore,the average fluorescence lifetime of SQDs was almost unchanged after addition of Fe3+,which clearly proved that there was no energy transfer process between them(Fig.2D).Therefore,the PL quenching mechanism of Fe3+to SQDs was attributed to IFE(Fig.2E).

Fig.2 Fluorescence spectra of SQDs in the absence of Fe3+(green curve)and in the presence of 300μmol/L Fe3+(blue curve)(A);UV-Vis absorption spectra of Fe3+and fluorescence spectra of SQDs(B);UV-Vis absorption spectra of Fe3+,SQDs and SQDs/Fe3+system(C);the fluorescence lifetime curve of SQDs before and after adding Fe3+(D);an illustration of fluorescence quenching mechanism of Fe3+on SQDs(E)
2.2.2 Optimization of detection conditionsConsidering the effect of hydrolysis,masking,oxidation or other side reactions,the pH value and the ions contained in the solution could influence the quenching effect of Fe3+on SQDs.Thus the pH value and the type of buffer solution(50 mmol/L)were investigated.In phosphate buffer(PB)solution,citric acid-HCl-NaOH buffer solution,britton robinson(BTR)buffer solution and phosphate buffered saline(PBS)buffer solution,the values ofF0/Fwere close to 1 with different pH buffer solutions(Fig.3A).It might be due to the masking effect of citric acid and phosphoric acid in buffer on Fe3+.Interestingly,while in HCl-NaAc buffer solutions,the fluorescence intensity of SQDs was obviously effected by the pH value,and pH 5.0 is the optimal pH condition(Fig.3A).In addition to the buffer solution and pH value,the reaction time was another factor for the sensing system.As demonstrated in Fig.3B,the value ofF0/Fincreased with the increasing of reaction time,and reached a plateau at 5 min.Finally,pH 5.0 HCl-NaAc buffer solution,and 5 min of reaction time were used in the subsequent Fe3+assay experiment.

Fig.3 Effects of different buffer solutions with different pH values(A)and reaction time(B)on F0/F valueⅠ:BTR;Ⅱ:PBS;Ⅲ:PB;Ⅳ:citric acid-HCl-NaOH;Ⅴ:HCl-NaAc
2.2.3 SQDs-based fluorescence sensing for Fe3+According to the above results,the linear plot and detection limit of the proposed fluorescence sensing platform were further investigated under the optimal conditions.As demonstrated in Fig.4A,the quenching efficiency was proportional to the Fe3+concentration ranging from 5μmol/L to 600μmol/L(r=0.997).The limit of detection(LOD,S/N=3)was 1.19μmol/L,which is comparable to,or lower than the reported Fe3+sensing methods(Table 1).To evaluate the potential applicability of the proposed SQDs-based PL sensing platform for determination of Fe3+,the selectivity was confirmed by addition of the common ions.The concentration of interfering ions was 5 times than Fe3+.As shown in Fig.4B,no apparent PL quenching effects were observed in pH 5.0 HCl-NaAc buffer solution with common ions.Notably,although Hg2+could also quench the PL of SQDs,the interference from Hg2+could be eliminated by KCl(Fig.4C).Therefore,the proposed PL sensor could be applied to detect Fe3+with high selectivity in complex samples.
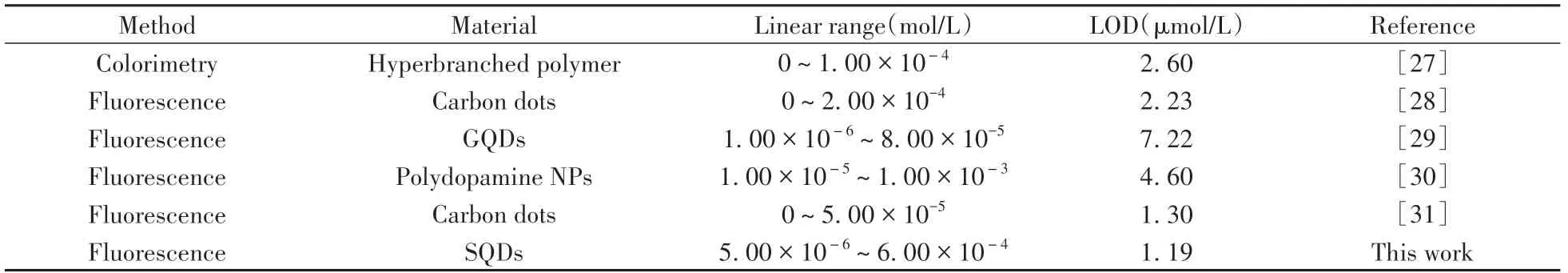
Table 1 Comparison of the detection performances for Fe3+detection
2.3 Serum sample detection
Encouraged by the above results,the practicality and accuracy of this method for the determination of Fe3+was evaluated by standard addition methods in blank serum samples.Table 2 showed an acceptable recovery values of Fe3+in serum samples were between 95.8%and 104%,with relative standard deviations(RSD,n=3)in the range of 0.20%-1.8%.The results suggested that the present PL sensor might provide a feasible assay method for Fe3+detection in real serum samples.
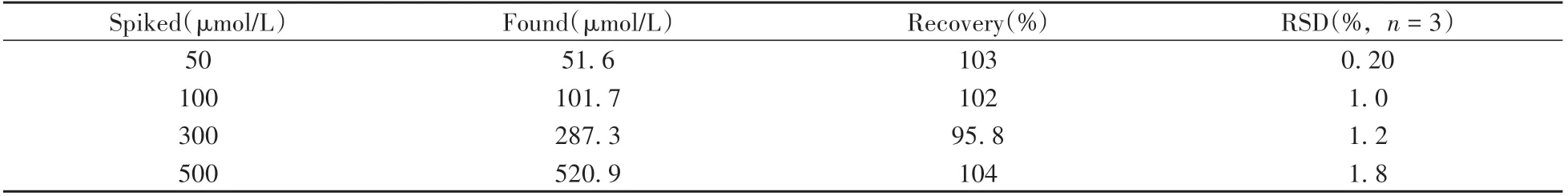
Table 2 Recoveries and relative standard deviations of Fe3+in serum samples
3 Conclusions
A novel and simple assembly-fission synthesis method combined with dialysis post-treatment strategy was introduced to obtain ultra-small size-focusing SQDs fluorescence probe.The SQDs showed a single emission wavelength,enhanced PL intensity and high stability.Based on the excellent fluorescence performance of the SQDs,the SQDs were used for the determination of Fe3+based on IFE between Fe3+and SQDs,and could apply for Fe3+detection in real serum samples.This work not only provides a facile pathway for the preparation of SQDs and their composites,but also shows promise for applications in life analysis and other fields.
- 分析测试学报的其它文章
- Research Progress of Hemicyanine Dye for Molecular Imaging
- 碱性磷酸酶的体外检测和体内成像研究进展
- Recent Progress in Nanoscale MOFs for Biological Imaging of Tumors and Tumor Markers
- I-Motif-based Nanosystems for Biomedical Applications:p H Imaging,Drugs Controlled Release and Tumor Theranostics
- A Low-cost,Automated Nucleic Acid Extraction System Converted from the Open-Source Rep Rap 3D Printer
- Research Progress on Analytical Methods for Deciphering Adenosine-to-inosine RNA Editing

