Recent Progress in Nanoscale MOFs for Biological Imaging of Tumors and Tumor Markers
LIAO Xue-wei,LIU Yang,WANG Chen
(1.Analytical&Testing Center,Nanjing Normal University,Nanjing 210023,China;2.School of Chemistry and Materials Science,Nanjing Normal University,Nanjing 210023,China;3.School of Environment,Nanjing Normal University,Nanjing 210023,China;4.State Key Laboratory of Analytical Chemistry for Life Science,Nanjing University,Nanjing 210023,China)
Abstract:Metal-organic frameworks(MOFs)have played important roles in a wide range of fields,such as gas storage and separation,fuel cells,energy conversion,and chemical/biological sensors.When one of the dimensions is scaled down to nanometer,MOFs can be named as nanoscale MOFs(nMOFs).Compared with the traditional MOFs,nMOFs retain the advantages of highly ordered porosity,structural tunability,more effective surface modification sites and improved biological distributions.nMOFs are biocompatible molecular nanomaterials with great potentials in various biomedical applications,especially for detection of tumors and tumor markers.In this review,recent progress in nMOFs for biological imaging of tumors and tumor markers is summarized.Different bioimaging techniques including fluorescence imaging,magnetic resonance imaging(MRI),photoacoustic imaging(PAI),computed tomography imaging(CTI),positron emission tomography imaging(PETI)are introduced.After that,multimodal imaging superimposed by two or more imaging modalities is highlighted.Finally,a brief conclusion about this special field is provided.Priorities of research in nMOF-mediated therapies and the prospects of clinical applications of nMOFs as delivery carriers and cancer therapeutics are also outlined.This review is expected to advance our understanding of this special field,and promote the applications of nMOFs in early diagnosis of disease and tumor therapy.
Key words:nanoscale metal-organic frameworks(nMOFs);biological imaging;tumor markers;biomedicine
Metal-organic frameworks(MOFs),which are an emerging class of crystalline porous compounds composed of metal-containing nodes(known as secondary building units,SBUs)and organic linkers,have the advantages of both organic and inorganic materials[1-3].In 1995,the first highly porous and stable MOF-1 was synthesized[4].Afterwards,a large number of MOFs of varied topology structures and sizes have been designed via diverse synthetic strategies[5-9].Thanks to the extraordinarily high surface areas,tunable pore sizes,and adjustable surface properties[10],MOFs have received extensive development over the past few years and played important roles in various fields[11-18].In the wake of development in nanotechnology,nanoscale MOFs(nMOFs)with one of the particle dimensions in the range of tens to hundreds of nanometers emerged[17].Compared with the traditional MOFs,nMOFs have highly ordered porosity,enhanced chemical/colloidal stability,more effective surface modification sites and improved biological distribution[9,18],which are the reasons that found wide applications in energy conversion[14],catalysis[15],chemical sensors[16],and biomedicine[18].
Early and precise diagnosis of cancer based on detection of tumors and tumor markers contribute to prompt treatment and therapy.There are a variety of bio-molecules which are used as tumor markers including antigens,DNA,mRNA,and enzymes.Detection of tumors and tumor markers is a powerful medical tool for early diagnosis and treatment of diseases.For example,visualized bioimaging technologies play important roles in the field of biomedicine because they can facilitate the diagnosis of diseases using visual imaging of tumors and biomarkers[19].Quick treatment of critical areas after diagnosis is also one of the advantages of imaging technologies[20].Moreover,bioimaging can be used for in vivo tracking and monitoring the dynamic process of molecules in real time.The accuracy of targeted therapy can thus be efficiently improved[21].So far,fluorescence imaging[22],magnetic resonance imaging(MRI)[23],X-ray computed tomography imaging(CTI)[24],photoacoustic imaging(PAI)[25],positron emission tomography imaging(PETI)[26]and other imaging modalities have been applied in diagnosis and treatment of diseases.As an outstanding platform,nMOFs with high porosity can not only help encapsulate large amounts of contrast agents in the pores,but also prevent the agents from agglomeration[27].Compared to other nanoparticles,nMOFs can be properly tailored to possess multifunctions for synergistic applications.The inherent imaging capabilities of metal centers or organic ligands have also played critical roles in the promotion and applications of nMOFs for bioimaging.In addition,customizable functional groups on the surface of nMOFs can also be connected to specific molecules for targeted imaging.
In this review,we summarized the recent developments in the nMOFs based nanoplatform for biological imaging of tumors and tumor markers(Fig.1).Various imaging technologies including fluorescence,MRI,CTI,PAI,PETI and the multi-modal imaging are introduced.The important roles of various bioimaging modalities including mono-and multi-modal imaging modalities in tumor diagnoses and therapies are also well explained.Finally,a brief conclusion and the future developments of nMOFs for biological imaging are presented.It is hoped that this review can shed some light on the superiority of nMOFs applied in the fields of biological imaging.

Fig.1 Nanoscale MOFs(nMOFs)and their applications in biological imaging of tumors and tumor markers
1 Fluorescence imaging
Fluorescence bioimaging is one of the most popular imaging modalities with relatively simple instrument.Organic fluorophores encapsulated in nMOFs can provide effective guidance for imaging.As extracellular pH is lower for solid tumors than for normal body tissues,pH sensing can provide useful information for tumor detection and analysis.For example,Rhodamine B isothiocyanates(RBITC)were integrated in pocket-channel frameworks(PCN)-224 for pH sensing in a wide range(pH 1.7-11.3)[28].RBITC and tetrakis(4-carboxyphenyl)porphyrin(TCPP)in PCN-224 were capable of imaging in acid and basic solutions,respectively.In addition,pH-responsive nMOFs have been fabricated and used for intracellular fluorescence imaging and drug delivery[29]. Cellular uptake studies showed that the fabricated nMOFs had good biocompatibility.
In addition to pH sensing,imaging of intracellular DNA and microRNA is also possible with nMOFs[30-31].For example,Li′s group encapsulated light-switchable sensing frame within dissociable nMOFs to construct autonomous three-dimensional DNA walkers(Fig.2A).Through endocytosis,a self-driven pattern in the acidic environment of the cell lysosome was achieved.Biological imaging of a model biomarker(microRNA-21)was made available(Fig.2B).The proposed sensing platform enables highly sensitive and efficient detection of tumor markers,offering a powerful assay strategy for intracellular imaging.In another example,ZIF-8 nMOFs were employed as a delivery vehicle to construct a microRNA biosensor[30].Obviously,nMOFs could serve as a powerful imaging tool for sensitive and accurate determination of microRNA in living cells(Fig.2C)[31].
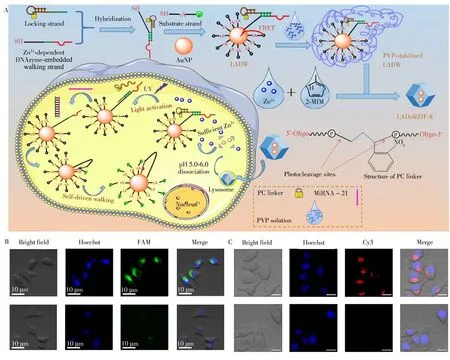
Fig.2 Schematically illustration of the intracellular sensing mechanism of light-activated and self-driven autonomous 3D DNA walkers(A).Confocal fluorescence images of living MCF-7 cells in the presence(top images)and absence(bottom images)of UV light irradiation by incubating with nMOFs for 4 h[30](B).Confocal images of nMOFs incubated A549 cells in the presence(top images)and absence(bottom images)of UV light irradiation using a photo-responsive DNA tetrahedral amplifier and nMOFs encapsulation[31](C)
For efficient imaging,some special strategies have been combined with fluorescence imaging technique,such as aggregation-induced emission(AIE)and aggregation-caused quenching(ACQ)[32-35].They selected MOF-199 for loading the photosensitizers 2-(4-(diphenylamino)phenyl)anthracene-9,10-dione(TPAAQ)with AIE and Ce6 with ACQ.After cellular uptake,endogenous glutathione(GSH)induced the decomposition of MOFs to release photosensitizers.Through the bright infrared(IR)/near-infrared(NIR)emission of Ce6 and TPAAQ in cancer cells,a cancer cell-specific image-guided photodynamic therapy was demonstrated,while the phototoxicity of normal cells was reduced.In addition,up-conversion luminescent nanoparticles(UCNPs),which can convert NIR radiation into visible light,have become a new class of bio-probes in biomedical applications.The unique optical characteristics decrease the auto-fluorescence background of optical imaging in vivo and reduce the photo damage to cells and living animals[33].Kuang′s group fabricated a hybrid nanoassembly consisting of UCNPs core and zeolitic imidazolate frameworks(ZIF)-8 shell encapsulated with chiral NiSx nanoparticles(NPs)(denoted as UCNP@ZIF-NiSx)(Fig.3A)[34].The up-conversion luminescence(UCL)signal of UCNPs at 540 nm was quenched by Ni Sx NPs,while the UCL signal at 660 nm hardly changed.Using fluorescent signals of UCNPs,the assembly was used for quantitative monitoring of reactive oxygen species(ROS)i n vivo.To track and evaluate non-small-cell lung cancer,Tang′s group used UCNPs as NIR antennas attached onto the surface of nMOFs containing a large number of oxygen indicators(Fig.3B)[35].In the process of fluorescence resonance energy transfer(FRET),the UCNPs upconverted NIR light excited the O2indicator,resultingi n vi votracking of non-small-cell lung cancer lesions through hypoxia imaging.
An IR/NIR imaging regent can also be incorporated into living cells for fluorescence imaging.For example,an unique MOF(Fig.3C)was prepared based on Yb3+NIR emitting lanthanide cations for NIR imaging[36].NIR microscopy allows for highly efficient discrimination between signals of nMOFs emission and the fluorescence background arising from cellular biological materials[36].These studies indicated the promising applications of NIR lanthanide emission in biological imaging of living cells.
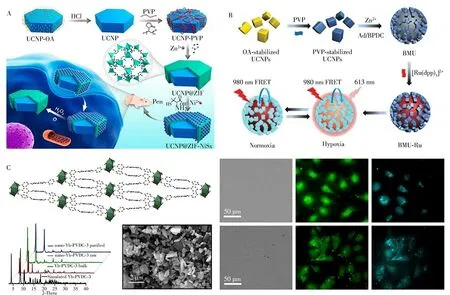
Fig.3 ROS detection using UCNP@MOF-Ni Sx nanoassemblies[34](A).Strategy for fabrication of sensors based on UCNPs,bio-MOF-100,and[Ru(dpp)3]2+Cl2 for cycling hypoxia response under NIR excitation[35](B).Left:Crystal structure of Yb-phenylenevinylene dicarboxylate(PVDC)-3 viewed along the a-crystallographic axis,the powder X-ray diffraction patterns for different samples,and SEM image of nano-Yb-PVDC-3.Right:Visible and NIR microscopy images of nano Yb-PVDC-3 in HeLa cells(Upper)and NIH 3T3 cells(Lower)(λex=340 nm)[36](C)
2 Magnetic resonance imaging of(MRI)
Noninvasive MRI with sub-millimeter spatial resolution is a powerful diagnostic tool in the medical field.MRI has been proved to be highly effective for evaluating anatomical changes and monitoring organ functions[37].However,in many cases,contrast agents are needed to shorten the longitudinal(T1)and transverse(T2)relaxation rates of water protons at the target tissues,thus improving the contrast between diseased tissues and normal ones.Paramagnetic or super-magnetic substances including Gd,Fe and Mn are good MRI contrast agents.Lin′s group designed a Gd3+based nMOF for MRI contrast agents with large relaxivities for the first time(Fig.4A)[38].This work suggested that the platform of nMOFs could be potentially used for multimodal imaging,especially doping with Eu3+or Tb3+centers as highly luminescent contrast agents.However,toxic Gd3+,when used as an MRI contrast agent,was likely to be leached from nMOFs and reduce biological safety.Ultra-small superparamagnetic iron oxide(USPIO),γ-Fe2O3(maghemite)coupled with drug carrier MIL(materials from Institute Lavoisier)-100(Fe),was a stable and safe nanovector for image-guided therapy[39].Such MIL-USPIOnanovector exhibited excellent relaxometric properties due to the high value of saturation magnetization.Similarly,Fe3O4@UiO-66[40],MnCo-MOF[41],Cu(Ⅱ)-TCPP[42]and PCN-222(Mn)[43]could also been used as MRI contrast agents for combining cancer imaging and treatments.Due to the good dispersibility of Mn3+in the framework as well as the high water affinity of the pores,PCN-222(Mn)showed high longitudinal relaxivity and good catalytic activity,providing good contrast of the tumor site for imaging(Fig.4B,C)[43].
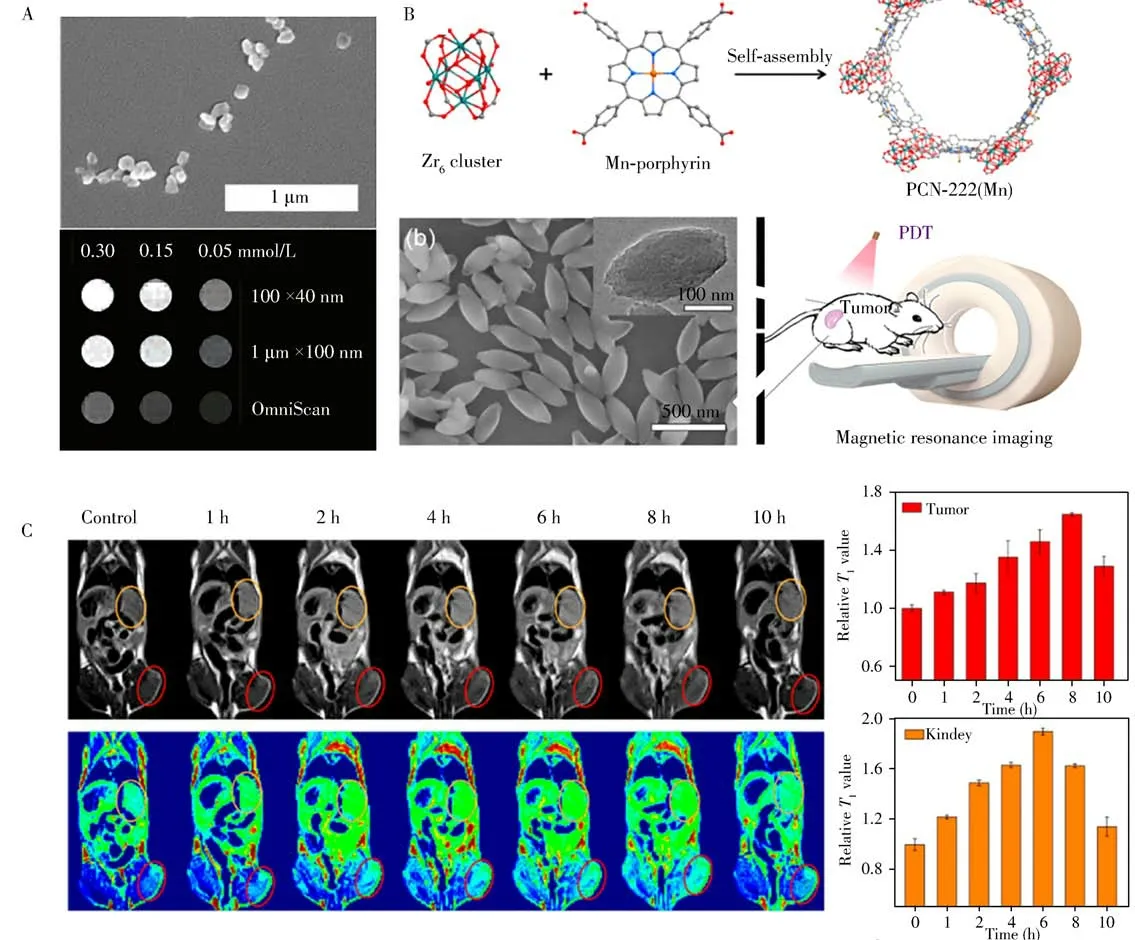
Fig.4 SEM images of the crystalline Gd3+based nMOF,and the corresponding MRI[38](A).Illustration showing the construction of PCN-222 and its SEM image,which was then used for MRI[43](B).MRI of tumor-bearing mice and the tumor(red circles),kidney(orange circles)sites[43](C)
3 Other means of imaging
As another noninvasive clinical diagnostic technique,CTI can provide high-resolution 3D structural details based on differential X-ray absorption of biological tissues[44].To provide high contrast between target tissues and adjacent tissues,contrast agents with high X-ray attenuation are ideal for CTI.But using smallmolecule contrast agents alone(such as barium sulfate and iodinated aromatic molecules)will cause adverse reactions in some patients due to non-specific distribution and extravasation from blood and lymphatic vessels[45].Incorporating imaging agents into nMOFs can serve as promising CT contrast agents.Based on the metal ion chelating ability of the porphyrin structure,2D Zn-TCPP was labeled with 99mTc(a gamma emission radioisotope)for single photon emission computer tomography(SPECT)imaging,achieving quantitative tracking in vivo after intravenous injection[46].Quercetin with excellent X-ray attenuation ability can be encapsulated into Zr-MOFs for in vivo CTI[47].This Zr-MOF-Quercetin provides an imaging platform for quantitatively monitoring Zr-MOF biodistribution and real-time monitoring of tumor treatment.Besides,gold nanoparticles can also be used for CTI owing to the large X-ray absorption coefficient.Han′s group developed gold nanorods(AuNRs)/ZIF-8 janus NPs with high contrast for CTI-guided liver cancer theranostics,which exhibited excellent performance as potential CTI agents[48].
Based on the photoacoustic effect of light absorbers,PAI is a new non-invasive and non-ionizing biomedical imaging technology with remarkably increased imaging depth and spatial resolution.One type of porphyrin-MOF containing porphyrin-like single atom Fe(Ⅲ)centers was expected to be active for PAI(Fig.5A)[49].Switches in the spin state of Fe(Ⅲ)in P-MOF under NIR could facilitate the generation of1O2in vivo and thereby enabled photoacoustic imaging of cancer cells.After injection,the average intensity of PAI in the tumor area increased by 12 folds.Moreover,a bacteriochlorin-based versatile MOF nanosheet(DBBC-UiO)was developed as O2-·generator under NIR laser irradiation for PAI-guided therapy for hypoxic tumor(Fig.5B)[50].The DBBC-UiO exhibited remarkable photoacoustic responses and the concentration-dependent PAI signal intensity was positively correlated with DBBC-UiO concentration(0-1 mg/mL).

Fig.5 Schematic illustration of NIR-stimulation of single atom iron centers in P-MOF,resulting in efficient cancer phototherapy via photodynamic therapy and photothermal therapy,as well as PAI cancer imaging[49](A).Schematic illustration of the synthetic procedure and photoinduced therapy mechanism of DBBC-Ui O[50](B).Schematic synthesis route of Zr-UiO-nMOFs conjugates and the corresponding crystal structure[51](C)
In addition,highly specific PETI has superior detection sensitivity and deeper signal penetration.By injecting metabolites marked with radionuclides into the human body,the status of metabolic activities can be directly reflected.Hong′s group reported an nMOF incorporated by the positron-emitting isotope zirconium-89(89Zr)for PETI of MDA-MB-231(triple-negative breast cancer cells,nucleolin)(Fig.5C)[51].The porous structure of nMOFs increased the loading rate of hydrophilic and hydrophobic drugs and the surface-modified polyethylene glycol(PEG)improved the stability and dispersion in biological media.PETI of the present nanoplatform with long half-life(t1/2=78.4 h)not only guided efficient drug delivery,but also monitored metabolic processesin vivo.
4 Multi-modal imaging
Imaging methods involving fluorescence,MRI and CT are the most commonly used technologies,but they have advantages and disadvantages,respectively.Fluorescence has a high sensitivity but a relatively low penetration depth.MRI can provide 3D soft tissue details but its planar resolution is limited.CT imaging is suitable for imaging bones and calcified parts but less sensitive to soft tissues.Thus,monomodal imaging alone fails to provide all the information needed for comprehensive imaging and diagnosis.To address these issues,multimodal imaging superimposed by two or more imaging modalities can provide more pathological information,and has been rapidly developing into a promising technology for clinical diagnosis and disease detection in recent years[52].
The combination of fluorescence and MRI will be popular in precise diagnosis.Prussian blue(PB)nanoparticles can serve as an imaging contrast agent due to the unique Fe(Ⅱ)-C≡N-Fe(Ⅲ)structure in which Fe(Ⅱ)was low spin(S=0)and Fe(Ⅲ)was high spin(S=5/2)[53].Moreover,carbon dots,PB analogues were capable of green fluorescence[54].It has been reported that PB@ZIF-8 core-shell could be used for MRI and fluorescence optical imaging[55].As another example,the GdMOF/Au nanocomposites were chosen as a multimodal contrast agent for MRI/CT dual-modal imaging(Fig.6A)[56].Gd(Ⅲ)is an ideal contrast agent candidate for CT imaging,exhibiting excellent MRI capacity.Owing to the higher Gd3+loading rate of GdnMOF,the retention time and relaxivities were improved.Lin′s group fabricated a novel yolk-shell Au@MOF nanocarrier with high anticancer drug delivery capacity and superior NIR-Ⅱ(1 000-1 350 nm)responsive photothermal conversion ability for imaging-guided monitoring and combined chemo-photothermal therapy(Fig.6B)[57].After efficient accumulation of Au@MOF in tumors tissues,the temperature elevation was quickly triggered under laser irradiation.Meanwhile,the tumor sites displayed strong PAI signals due to the intense NIR absorption capacity of Au@MOF.
Recently,CTI/PAI combined with thermal imaging was successfully applied by introducing the photoactive tetratopic chlorin(TCPC)and Hf atom into UiO-66(Fig.6C)[58].Due to the high photothermal conversion efficiency and strong X-ray attenuating ability of Hf element,the as-synthesized TCPC-UiO was capable of photodynamic therapy and photothermal therapy simultaneously.Thus,this excellent multi-modal imaging platform also manifested valuable therapeutic advantages under NIR irradiation.To reveal sufficient characteristics for diagnosis of different types of cancer,Au@MIL-88(Fe)core-shell nanoparticles were synthesized for the assembly of multifunctional imaging agents(Fig.6D)[59].AuNRs with photothermolysis and NIR(650-900 nm)absorptive properties can serve as contrast agents for X-ray CT and PAI contrast.The porous MOF shells prevent aggregation while exhibiting MRI property.Duringin vivostudies of gliomas,both CT and MRI showed clear structure with high depth of penetration and PAI exhibited clear detection,high spatial resolution,and high contrast(Fig.6D)[59].Fe2+adsorbed ZIF-8 was prepared fori n vivomultimodal imaging of cancerous cells for early diagnosis of target cancers[60].Unlike that in normal cells,the Fe2+species on ZIF-8 could be oxidized by ROS to Fe3+and then formed superparamagnetic Fe3O4in cancer cells.The Fe3O4nanoparticles increased the T2relaxation rate of H2O in tumor tissues and exhibited CT contrast.Simultaneously,the high concentration of GSH in cancer cells degraded ZIF-8,thereby allowing Zn2+to escape from the skeleton and eventually form fluorescent ZnO nanoclusters.Moreover,Dong′s group reported a novel multifunctional Zr-ferriporphyrin MOF nanoshuttle with tri-mode tumor-specific imaging capability[61].The nanoshuttle revealed stronger PA signals after tail vein injection and good CT capability,which might have resulted from the high-Zr component.Additionally,the good photothermal effect endowed its good in vivo photothermal imaging ability.The fascinating tri-mode imaging capability provided a powerful tool for accurate tumor diagnosis.It is important to form a clear imaging contrast between the target and surrounding tissues.Therefore,the development of effective contrast agents with good sensitivity and selectivity is critical to the efficiency of diagnosis and treatment.Due to their porosity and customization,nMOFs have great potentials as new imaging agents and promoting diagnostic medicine.Although multimodal imaging has been used for monitoring the degradation process of nMOFs,the comprehensive theoretical basis needs to be further explored by means of long-term monitoring of absorption,distribution,metabolism and excretion.
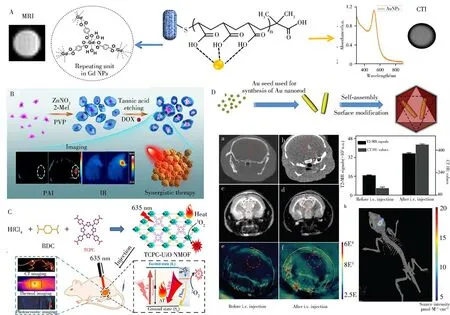
Fig.6 Schematic representation of the structure of hybrid GdMOF-poly(acrylic acid)-Au nanostructures and the application to multimodality imaging[56](A).Schematic illustration for the fabrication process,IR/PAI imaging effects,and synergistic anticancer therapy of Au@MOF-doxorubicin hydrochloride(DOX)[57](B).Synthesis and mechanism for cancer therapy of TCPC-Ui O by light activation[58](C).Schematic illustration of the synthesis of Au@MIL-88(Fe)nMOFs and the application to multimodality imaging[59](D)
5 Conclusions
In summary,we have outlined the latest biomedical developments of nMOFs for biological imaging of tumors and tumor biomarkers.Different biological imaging methods were introduced in this review.With a large specific area and high porosity,nMOFs have the special ability to act as imaging agents with high loading rates.Although tremendous progress has been made in this field,there are still some new challenges and issues that need to be addressed.
Firstly,the ultimate goal behind the use of tumors and tumor markers for diagnosis is to develop powerful and reliable detection techniques for early diagnosis.By using biomarker detection,physicians can monitor disease progression in time,and accordingly choose a precise and accurate therapeutic strategy for their patients.To achieve this goal,the detection platforms must be affordable,portable and adaptable to a point-ofcare testing,which is also one of the trends of future clinical diagnostics.
Secondly,for better performance,the size control and biodistribution of nMOFs need to be precisely designed and fabricated.The particle size of nanomaterials affects not only cells’uptake efficiency,but also the biological distributionin vivo.Kidneys are known to rapidly eliminate particles smaller than 6 nm via filtration,while particles larger than 200 nm can accumulate in the liver and spleen via rapid mononuclear phagocytic system clearance[61].Although MOFs have been confined to the nanoscale level,it′s essential to obtain nMOFs with more suitable hydrodynamic sizes for improved biodistributionin vivo.In addition,the biocompatibility,toxicity,and thein vivodegrading are worthy of contemplation.To date,the mechanisms and pathways by which nMOFs are biodegradedin vivoremain little known.
Finally,the surface modification is an important determinant of imaging quality.Total reliance on the inherent biocompatibility of nMOFs for biological applications is undesirable.To prevent the mononuclear phagoytic system clearance,modifying nMOFs with PEG or its derivatives is the most common method,which can not only improve biocompatibility,but also increase the stability of nMOFs.Other substances such as silica[62],cyclodextrin[63],heparin[64],hyaluronic acid[65]and cancer cell membranes[66]have also been used to modify nMOFs.In addition,the surface of nMOFs can also be modified with targeting molecules including folic acid[67],integrin targeting peptides[62]and aptamers[68]to increase tumor cell uptakes.Although significant progress has been made in surface modification,the accurate characterization of coating or targeting efficiency has been a formidable challenge in recent years.
- 分析测试学报的其它文章
- 阿尔兹海默症生物标志物和早期诊断新技术
- 40周年刊庆 引 言
- 微管纸喷雾质谱法快速筛查血液中5种强极性毒物
- 基于核酸分子光开关的闭管可视化环介导等温扩增检测方法
- Detection of Broad Spectrum Bacteria Using a FITC-Lysozyme and Positively Charged AuNPs Constructed FRET Platform
- Detection of Biomarker Protein PDGF-BB in Esophageal Squamous Cell Carcinoma Using a DNA Biosensor Based on Enzyme Cycle Amplification

