Detection of Broad Spectrum Bacteria Using a FITC-Lysozyme and Positively Charged AuNPs Constructed FRET Platform
WANG Ling,ZHANG Ya-qing,TAO Xiao-qi,SONG Er-qun*
(1.College of Food Science,Southwest University,Chongqing 400715,China;2.College of Pharmaceutical Sciences,Southwest University,Chongqing 400715,China)
Abstract:This manuscript demonstrated a universal platform for bacteria assay by using a fluorescein isothiocyanatelabeled lysozyme(FITC-Lys)and polyethylenimine-modified positively charged gold nanoparticles(PEI-AuNPs)constructed fluorescence resonance energy transfer(FRET)sensor based on its electrostatic binding with bacteria.In the presence of the bacteria,the energy donor of positively charged FITC-Lys bond with the bacteria through both the recognition to peptidoglycan of cell wall and electrostatic interaction while the energy acceptor of positively charged PEI-AuNPs bond with the bacteria based on the electrostatic interaction,which decreases the distance between energy donor and acceptor and then causes fluorescence quenching,showing FRET“on”signal readout mode with the low fluorescence intensity.PEI-AuNPs were synthesized by hydrothermal method and characterized by ultraviolet spectrum,transmission electron microscope,and laser particle size analyzer.The results shown that PEI-AuNPs with diameter about 9.6 nm had maximum ultraviolet absorption at 526.5 nm,and they were positively charged.The method developed in this study could detect 10 different kinds of bacteria such as St aph yl ococc us aur eus,S treptococcus
Key words:bacteria;lysozyme;gold nanoparticle;FRET
Due to the lack of on-site effective supervision of food safety in the processing environment and processing procedures[1],pathogens contamination causing byS.aureus,E.col i,L.monocyt ogenes,S al monel l a,V.parahaemolyticus,Bacillus cereus(B.cereus)in milk,meat,poultry and egg products[2-4]can cause gastrointestinal infections[5],purulent skin infections[6],pneumonia[7],meningitis[8],sepsis[9]and many other diseases seriously threaten food safety and public health[10-20].There are about 23 000 deaths caused by antibioticresistant bacterial infection in the United States every year,and the death rate caused by pathogens is increasing yearly[21].It is estimated that there will be about 10 million deaths every year in the world by 2050[22].Due to the great harm caused by bacteria to health and the complex of contaminated bacteria,it is particularly important to develop universal method for detecting bacteria.
Plate counting is classical method of identifying bacteria,but it is limited by long incubation time(24-48 h),the requirement of absolutely clean experimental platform,and tedious operation step[23].Therefore,some rapid detection methods have been developed,such as polymerase chain reaction(PCR),but is limited by professional instruments for DNA extraction and false positive[24-26].Immunological detection method is limited by the great difference between antibody batches and poor stability[27-28].Electrochemical detection and colorimetric detection are susceptible to external interference[29-30].Flow cytometry and gene chip method need large instruments and are limited by their specialty[31-33].What′s more,current methods mostly focus on the detection of a specific kind of bacteria,a universal method is needed due to the wide distribution of bacteria.
Fluorescence resonance energy transfer(FRET)is a non-radiative energy transfer process through longrange dipole-dipole interaction between donor-acceptor pair[34].FRET-based strategies have achieved sensitive and rapid detection of various targets such as organophosphate pesticides[35-37],veterinary drugs[36-37],mycotoxins[37-39],heavy metal ions[39-40],biomarker[41-42],virus[43]and pathogenic bacteria[44]with high accuracy.
Lysozyme(Lys)is a protein that can specifically hydrolyze theβ-1,4 glycosidic bond between N-acetyl muramic acid and N-acetyl glucosamine in peptidoglycan of bacterial cell wall[45].In previous studies,fluorescein isothiocyanate-labeled lysozyme(FITC-Lys)was used to detect Gram-positive bacteria(G+)bacteria based on the recognition of lysozyme to the peptidoglycan of bacteria by recording the fluorescence intensity of supernatant or precipitate after centrifugation[46-47].However,this method has the limitation of bad accuracy and only detecting some G+bacteria.At present,a lot of studies have confirmed that modification of lysozyme can broaden its ability to act on Gram-negative bacteria based on electrostatic binding between the modified lysozyme and Gram-negative bacteria[45,48-51].Therefore,adjusting pH to make lysozyme positively charged can broaden its antibacterial spectrum.
The emission wavelength of fluorescein isothiocyanate(FITC)overlaps with the absorption spectrum of gold nanoparticles(AuNPs),which is a common donor-acceptor pair[52].In this study,FITC-Lys and polyethyleniminemodified positively charged gold nanoparticle(PEI-AuNPs)constructed FRET universal platform for broad spectrum bacteria detection based on electrostatic interaction between each other.Specifically,after adjusting pH of the reaction buffer lower to the isoelectric point of FITC-Lys and making the FITC-Lys positively charged,FITC-Lys could bind to all bacteria through both the peptidoglycan recognition and electrostatic interaction with bacteria.Other hand,PEI-AuNPs could bind with bacteria through electrostatic interaction.In the presence of bacteria(no matter of G+or G-bacteria),FITC-Lys and PEI-AuNPs would close each other due to their corresponding binding with bacteria,resulting in fluorescence quenching and showing FRET“on”signal readout mode(Fig.1).The results showed that,based on the FITC-Lys and PEI-AuNPs FRET platform,a series of pathogenic bacteria(no matter of G+or G-bacteria)could be detected simply,showing universal assay platform for broad spectrum bacteria.
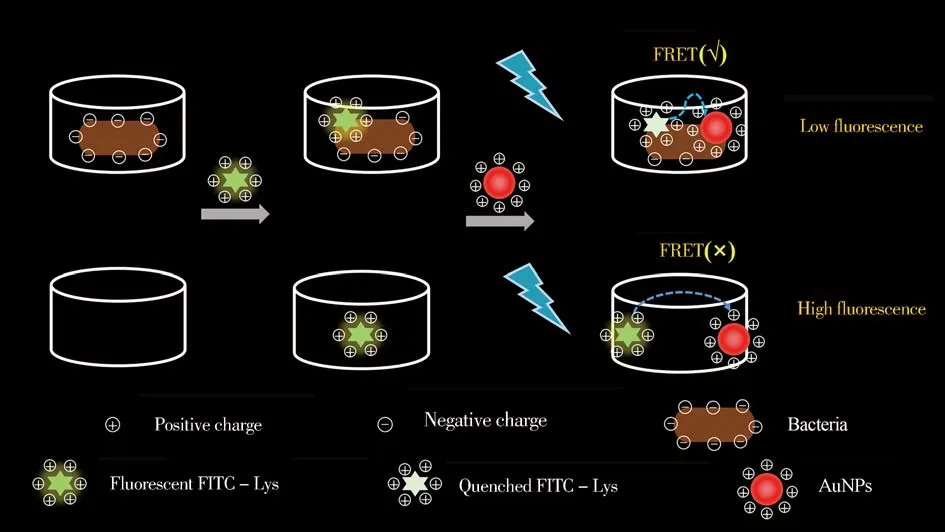
Fig.1 Illustration for the detection of bacteria based on FITC-Lys and PEI-AuNPs constructed FRET platform
1 Experimental
1.1 Apparatus and reagents
All experimental water was prepared by Milli-Q ultrapure water system(Millipore Company,USA)with a resistance of 18.2 MΩ·cm.Fluorescent spectroscopy was recorded on a fluorescence spectrophotometer(F-7000,Hitachi).UV-Vis absorption spectra were obtained using an ultraviolet spectrophotometer(UV-2450,Shimadzu company).The hydrated particle size andζ-potential were measured using a dynamic light scattering Malvern instrument(NanoZS ZEN3600,United Kingdom).Fluorescence intensity were obtained by multifunctional microplate reader(SpectraMax iD3,Meigu molecule). Morphology was characterized using a transmission electron microscope(TEM)(JEM1200EX,JEOL,Japan).
Chloroauric acid(HAuCl4·4H2O),polyethylenimine(PEI)and casein were provided by Shanghai Macklin Biochemical Co.,Ltd.Lysozyme labeled with fluorescein isothiocyanate(FITC-Lys)was obtained from Xi'an Ruixi Biology Co.,Ltd.Fluorescein isothiocyanate(FITC),bovine albumin(BSA)and bovine albumin labeled with fluorescein isothiocyanate(FITC-BSA)were purchased from Beijing Solarbio Science&Technology Co.,Ltd.β-Lactoglobulin was provided by Shanghai Yuanye Biology Science and Technology Co.,Ltd.Glucose and sucrose were purchased from biofroxx Biotechnology Co.,Ltd.Ascorbic acid,calcium chloride,dipotassium hydrogenphosphate,potassium chloride and sodium chloride were obtained from Chongqing Chuandong Chemical Industry Group Co.,Ltd.S.aureusATCC 29213,S.pneumoniaATCC 10015,E.faecalisATCC 19433,L.monocytogenesATCC19115,S.typhimuriumATCC 27853 andV.parahaemolyticusATCC17802 were obtained from China General Microbiological Culture Collection Center.E.col iO157∶H7 CICC 21530 andS.paraty phoi d ACICC 21501 were obtained from China Center of Industrial Culture Collection.S.e pid ermidisCCTCC AB 91100 andP.aer u gi nosaCCTCC 93078 were obtained from China Center for Type Culture Collection.
1.2 Synthesis of PEI-AuNPs
PEI-AuNPs were prepared according the previous published work[53].Specifically,PEI(69.4 mg)dispersed in 9.5 mL of primary water was mixed with 823μL of 1%HAuCl4,reacting at 65℃for 1 h with vigorous stirring.After removing supernatant by centrifugation at 18 000 r/min for 20 min,the precipitate was dispersed with ultrapure water and stored in a refrigerator at 4℃.
1.3 Binding of PEI-AuNPs with bacteria
Bacteria(109cfu/mL)were incubated with PEI-AuNPs(4 nmol/L)at 37℃for 1 h,and then the samples were washed by centrifugating(2 000 r/min,5 min)for 3 times.
1.4 Detecting bacteria with FITC-Lys and PEI-AuNPs
FITC-Lys(70μg/mL)was incubated withS.aureus,S.pneumoniae,E.f aecali s,S.e pider midis,L.monocytogenes,E.coli,S.typh imur ium,S.paratyph oid A,V.par ahaemolyticusandP.aeruginosa(108cfu/mL)for 30 min,and then the mixture was incubated with PEI-AuNPs/AuNPs(10 nmol/L)for 1 h,following measuring their fluorescence intensity.
2 Results and discussion
In our previous work,the dual-molecule recognition-based strategies were developed to detect bacteria[54-56].In these strategies,specific antibiotics and aptamer which both could only recognized some special kinds of bacteria were employed as recognition molecules,meaning that different other recognition molecules are required to employed if detecting other types of bacteria.Therefore,a universal detection platform for broad spectrum bacteria assay is needed to explored.In this study,energy donor of FITC-Lys and energy acceptor of PEI-AuNPs were employed to construct FRET universal platform for broad spectrum bacteria detection.
2.1 Potential distribution of different bacteria and protein at p H 4
The isoelectric point of lysozyme is 11.0,which has high positive charge[57].Bacterial cell walls contain various functional groups,which provide binding sites for solutes in the environment.Therefore,under most pH conditions,bacterial cell walls are often negatively charged and have strong affinity with positively charged substances[58].As shown in Fig.2,bacteria ofS.aureus,S.pneumoniae,E.faecalis,S.epi dermi d i s,L.monocytogenes,E.coli,S.typhimurium,S.paratyphoid A,V.parahaemolyticus,P.aeru ginos a,and FITCBSA were all negatively charged at pH 4,while FITCLys was positively charged at this condition.
2.2 Characterization of PEI-AuNPs
PEI-AuNPs was synthesized by using PEI as protective agent and reducing agent according to the literature with slightly modification[53].The PEI-AuNPs has the maximum ultraviolet absorption at 526.5 nm(Fig.3A).The hydrodynamic size(about 11.22 nm)of PEI-AuNPs measured by dynamic light scattering(Fig.3B)is consistent with the size about 9.6 nm shown in the TEM image(Fig.3C).The PEI-AuNPs is positively charged with zeta potential about+33.7 mV.To verify the binding of bacteria with PEI-AuNPs,the mixture of bacteria with PEI-AuNPs was centrifuged to observe the precipitate after incubation.As shown in Fig.3D,compared with the yellow or white precipitate from the pure bacteria samples,the mixed samples of bacteria and PEI-AuNPs showed red precipitate,which was attribute to the binding of PEI-AuNPs with bacteria.
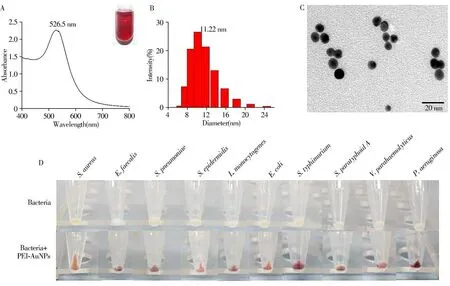
Fig.3 UV-Vis absorption spectrum(A),hydrodynamic size distribution(B)and TEM image(C)of PEI-AuNPs;digital photos of each sample treated by centrifugation(D)the inset in Fig.3A is a digital photo of PEI-AuNPs
2.3 Selection of the procedures to detect bacteria
By takingS.aur eusas a model,we tried to detect the bacteria according the procedure(FITC-Lys and PEI-AuNPs mixed firstly and then co-incubated withS.aur eus)published in our previous work.Unfortunately,there wasn′t any change of the fluorescence intensity.Then another two different detection procedures(PEI-AuNPs andS.aureusmixed firstly and then co-incubated with FITC-Lys,FITC-Lys andS.aureusmixed firstly and then co-incubated with PEI-AuNPs)were studied.The results showed that,if PEI-AuNPs incubated withS.aur eusfirstly then incubated with FITC-Lys,there also wasn′t any change of the fluorescence intensity.Fortunately,when FITC-Lys incubated withS.aur eusfirstly then incubated with PEI-AuNPs,an obvious change of the fluorescence intensity was observed,suggesting that using this procedure might be feasible to detect bacteria.This phenomenon might be attribute to that the electrostatic binding capacity between PEI-AuNPs andS.aureusmight be stronger than that between FITC-Lys andS.aureusaccording the results shown in Fig.2.If PEI-AuNPs bind to bacteria firstly,the steric hindrance of PEI-AuNPs also might hindered the recognition of Lys toS.aur eus.
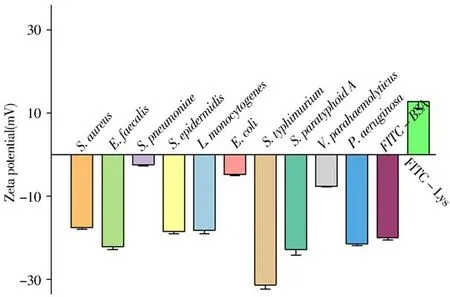
Fig.2 Zeta potential distribution of different bacteria,FITC-BSA and FITC-Lys
2.4 Feasibility of detecting S.aureus based on the FRET strategy
The feasibility of detectingS.aur eusbased on the proposed strategy was further verified by setting different control groups of blank(FITC-BSA protein and negative charged AuNPs were employes). Compared with the blank group(FITC-Lys+ PEI-AuNPs),the fluorescence of the system(S.aureus+FITC-Lys+PEI-AuNPs)was quenched(Fig.4),withΔFof 1 877(ΔF=F0-F,whereF0andFare the fluorescence intensities before and after addingS.aureus).However,other control groups shown no difference,suggesting that FRET strategy is feasible.
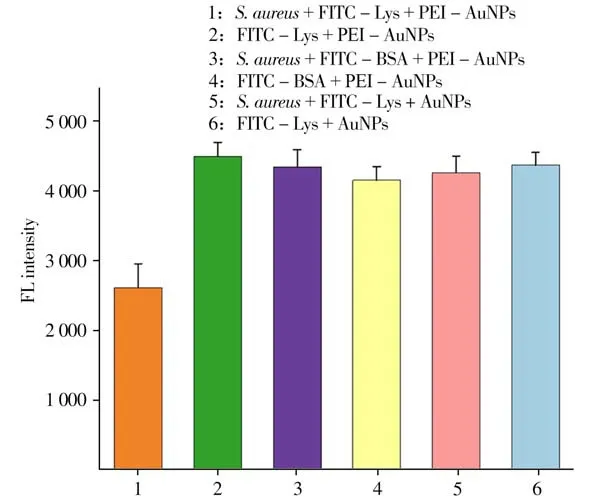
Fig.4 Fluorescence intensity of different probes after incubating with or without S.aure us
2.5 Detection of broad spectrum bacteria
The proposed strategy for detecting broad spectrum bacteria was studied by employed both G+(S.pneumoniae,E.f aecali s,S.e pid ermidisandL.monocytogenes) and G-bacteria(E.col i,S.ty ph imurium,S.par at yphoi d A,V.par ahaemolyt icusandP.aer uginosa)as models.As shown in Fig.5,ten different types of bacteria showed obvious fluorescence intensity after treated with FITC-Lys and PEI-AuNPs.More interestingly,the fluorescence intensity of the group containingE.f aecal i sshowed the greatest change.The difference value of fluorescence signal is related to the content of peptidoglycan in cell wall,the amount of charge,the surface area of bacteria and hydrophobic interaction etc[59].The cell wall of G+bacteria is mainly peptidoglycan,while the main component of Gbacteria is lipopolysaccharide,with only two thin layers of peptidoglycan in the inner layer,so the content of peptidoglycan of G+bacteria is higher than that of G-bacteria.FITC-Lys can specifically act on peptidoglycan of bacteria,so FITC-Lys is easier to label G+bacteria,especiallyE.f aecali sandS.aureus[60].BecauseE.faecalisis G+bacteria and has more negative charges,so there are more fluorescence donors and receptors bound toE.faecal i s,showing the strongest fluorescence signal.
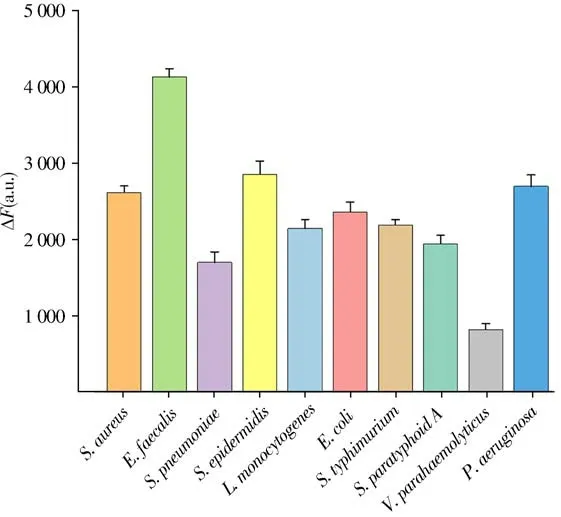
Fig.5 Fluorescence intensity change(ΔF)of different types of bacteria treated with FITC-Lys and PEI-AuNPs
2.6 The interference experiment
To verify the application of the proposed sensor of broad spectrum bacteria in real samples,the potential interference of protein,metal ions,sugar and vitamins in real samples(taking milk and juice as examples)on the assay was investigated. According to the published work[61-62],the contents of main protein and ion in milk and the contents of sugar and vitamins in juice were as following:casein(28 g/L),β-lactoglobulin(3.5 g/L),bovine albumin(0.35 g/L),Ca2+(1.04 g/L),PO43-(0.73 g/L),K+(1.09 g/L),Na+(0.37 g/L),glucose(4.6 g/L),sucrose(9.8 g/L),and ascorbic acid(0.15 g/L).When the above-mentioned substances were added into buffer and subjected to analysis with PEIAuNPs and FITC-Lys,we found some substances really could bring interference.Then all the substances were ten-times diluted and subjected to analysis again.Compared with the buffer sample and the group withS.aureus,all the tested substances showed no obvious interference,suggesting that the proposed method could be applied for detecting bacteria in real sample with appropriate dilution.
3 Conclusions
In summary,we have developed a universal platform for rapid and simple bacteria detection by using FRET sensing in which the donor of FITC-Lys and the receptor of positively charged AuNPs was employed.The proposed strategy showed feasibility of detection of broad spectrum bacteria.
- 分析测试学报的其它文章
- 阿尔兹海默症生物标志物和早期诊断新技术
- 40周年刊庆 引 言
- 微管纸喷雾质谱法快速筛查血液中5种强极性毒物
- 基于核酸分子光开关的闭管可视化环介导等温扩增检测方法
- Detection of Biomarker Protein PDGF-BB in Esophageal Squamous Cell Carcinoma Using a DNA Biosensor Based on Enzyme Cycle Amplification
- Quantification of Carcinoembryonic Antigen by Single Particle Inductively Coupled Plasma Mass Spectrometry

