I-Motif-based Nanosystems for Biomedical Applications:p H Imaging,Drugs Controlled Release and Tumor Theranostics
MA Wen-jie,HUANG Jin,CHENG Hong,JIA Rui-chen,CHEN Biao,SUN Huan-huan,WU Yu-chen,HE Xiao-xiao,,WANG Ke-min
(1.College of Biology,Hunan University,Changsha 410082,China;2.State Key Laboratory of Chemo/Biosensing and Chemometrics,College of Chemistry and Chemical Engineering,Hunan University,Key Laboratory for Bio-nanotechnology and Molecule Engineering of Hunan Province,Changsha 410082,China)
Abstract:I-motif,as a novel pH-sensitive element,has attracted extensive attention and research upsurge in the field of biomedicine due to its ability of fast conformation alteration at different pH conditions,as well as the advantages of automatic synthesis,excellent biocompatibility and flexible functional integration.In this review,the basic performances of i-motif are firstly described.Then,the applications of i-motif-based nanosystems in biomedical field are reviewed from three aspects of cellular pH imaging,pH-controlled drugs release and pH-responsive tumor theranostics applications.Finally,some of the current challenges of the i-motif-based nanosystems for the biomedical applications are pointed out,and an insight into the future prospects of i-motif-based nanosystems for precision medicine is provided.
Key words:i-motif;pH imaging;pH-controlled drugs release;pH-responsive tumor theranostics
pH is the most basic physiological parameter in organisms,which regulates the activity of most intracellular biomolecules and is crucial for measuring normal life activities[1-3].Sensitive and efficient response to the changes of cellular pH is of great significance for understanding the pathogenesis and physiological processes of diseases.At present,numerous of pH-sensitive intelligent elements have been developed for pH detection and biomedical applications[4-6],such as pH-sensitive chemical bonds[7],organic small molecules[8],polymers[9],nanomaterials[10]and DNA elements(e.g.,A-motif[11],triplex DNA[12],and i-motif[13-14],etc.).Among them,the DNA i-motif,bearing cytosine(C)-rich sequences,which can be folded into a tetramer structure by forming hemiprotonated C-neutral C(CH+·C)base pairs under acidic environment in an antiparallel orientation,has sparked a great deal of interest of researchers[15]. Taking advantage of easy synthesis,facile functionalization and excellent biocompatibility,as well as the advantage of fast conformation alteration at different pH conditions,the i-motif could be used as one of the promising stimuli-responsive molecule tools for the construction of pH-sensitive activatable probes in response to acidic microenvironment in the field of biomedicine.
Up to now,there are some reports about the review of i-motif.However,most of these reviews focus on the summarization of fundamental aspects of i-motif[16-17],conformational transition study of i-motif structures[18],or the i-motif-based DNA nanomachines[13,19-20],i-motif-based biosensors[21-22],and so on.In this report,we review and summarize the most significant progress made by our group and others in the biomedical applications of i-motif-based nanosystems for the first time.Firstly,we briefly summarize the superior properties and structural features of i-motif tetramer.Subsequently,the most recent achievements of i-motif-based nanosystems for cellular pH imaging,pH-controlled drugs release,and pH-driven tumor theranostics applications are described by typical examples.Finally,this review discusses some of the current challenges and future prospects of i-motif-based nanosystems for the biomedical applications.We hope that this review will benefit for the application of smart response elements,allowing the development of new strategies for establishing activatable nanosystems and achieving more precise and efficient treatment of tumors.
1 The i-motif structure
In 1993,Gehring and co-workers found that the C-rich DNA sequences of 5′-d(TCCCCC)could form into the quadruplex structure via a stack of intercalating CH+·C in an antiparallel orientation under slightly acidic pH conditions or even neutral pH,it was named as i-motif[15].Under alkaline pH conditions,the cytosine will be deprotonated and the tetramer structure is re-opened in a random coil state.Since then,a great deal of imotif structures has been determined and the results showed that the C-rich single-stranded DNA molecules could form into a universal quadruplex i-motif structure under acidic conditions[23].Moreover,the C-rich DNA fragments are widely presented in telomeres and the promoter region of various tumor-related genes[24].In the last two decades,i-motif,as a non-canonical secondary structure of DNA,has attracted extensive attention from researchers[25-26].Generally,the i-motif structures can be made up of one,two,or four DNA strands(Fig.1).Therefore,the i-motif sequence is flexible to design and easy to functionalize,and usually is stabilized in the pH range of 3-7 depending on sequences[13].

Fig.1 Schematic diagram of i-motif conformations[13]
The protonation of CH+·C base pairs is a prerequisite for the formation of i-motif structures.Hence,the pH value of the medium plays an important role in i-motif formation.Recently,researchers have proved that the formation and stability of i-motif structures do not solely rely on pH but also on various microenvironments such as DNA topology,ionic environments,temperature,crowding,and others[23,27].Moreover,some other studies have shown that the number of C bases on C-rich DNA fragments(C-tracts),the sequence length and composition of loop-region play significant parts in pH response range and conformations stability of imotif[28-29].Using poly T as the loop-region sequence,it was found that the thermal stability and conformation conversion pH value(pHT,the pH value at the midpoint of conformational transition)of i-motif gradually decreases with the extension of the loop sequence,implying that the short loop-region sequence base composition is more favorable for the formation of stable i-motif structure[30].While the loop-region remains unchanged,the pH responsiveness of i-motif is more sensitive with the number of CH+·C base pairs increase in the folded structure,and the pH response interval will shift to a neutral direction[31].Furthermore,the report has indicated that the folding of intra-and intermolecular also mainly depends on the length of the C-tracts[32].
Until now,different techniques have been used to characterize the formation of i-motif structures.The nuclear magnetic resonance(NMR)was the first approach used to demonstrate the crystal structure of the i-motif formation under acidic pH,the amino protons involved in the CH+·C base pair show a characteristic NMR signal at 15 ppm[15,25,33].X-ray diffraction was also used to analyze the i-motif structure[16,34].In addition,the imotif structures possess specific UV absorptions spectra[28-29]and circular dichroism(CD)spectra[35-36].With the decrease of pH value,the UV absorption at 295 nm increased,while the CD spectra of the negative band near 245 nm and the positive band near 275 nm red-shifted to near 260 and 290 nm,indicating the formation of i-motif tetramer structure.Fluorescence methods have also been used to monitor the folding/unfolding of i-motif structure by modifying pH-insensitive dyes at the 5′and/or 3′ends of i-motif sequences,which provides a more sensitive and simple approach[37-38].Moreover,other methods such as mass spectrometry(MS)[39],polyacrylamide gel electrophoresis(PAGE)[29,40],Raman spectroscopy[41]and so on,have been extensively used to elucidate the formation of the i-motif structures.
2 I-motif-based nanosystems for biomedical application
On the basis of the aforementioned advantages and characteristics,the i-motif has been utilized as an important pH-responsive element for the construction of intelligent nanosystems for the biomedical research in the fields of cellular pH imaging,pH-controlled drugs release,and pH-driven tumor theranostics applications.
2.1 I-motif-based nanosystems for cellular p H imaging
2.1.1 Intracellular p H imagingThe changes of pH in cells are closely related to the occurrence and development of tumor[42-43].In recent years,many important efforts have been put into obtaining intelligent nanosystems for intracellular pH imaging of tumor cells based on DNA i-motif[4-6].In 2014,our group chose i-motif as a pH-sensitive element,and then designed an i-motif-based nano-flare to image intracellular pH changes[44].At alkaline pH,the nanoprobe was stabilized as a double-stranded structure,resulting in Rhodamine Green in close proximity of the gold nanoparticles(AuNPs)surface,thus generating quenched fluorescence.At acidic pH,the i-motif sequences trended to form into an intact intramolecular quadruplex structure and fell down from the nano-flares,leading to fluorescence recovery,thus achieving pH imaging in living cells.However,such a nanoprobe labeled with only one dye,which was susceptible to interference in a complex environment and might not be conducive to accurate imaging of tumor cells.Therefore,we subsequently developed a two-dye-labeled nano-flare for ratiometric intracellular pH imaging[45].It consisted of an AuNP that was functionalized with single-stranded DNA sequence X,the X was designed to complementary with a pH-responsive i-motif sequence X*,which was modified with pH-insensitive Rhodamine Green and Rhodamine Red as fluorescence resonance energy transfer(FRET)pairs at its 5′-and 3′-end,respectively.At alkaline pH,the X was hybridized with X*,leaving Rhodamine Red detached from Rhodamine Green and generated low FRET efficiency.While under acidic pH,the X*folded into an intramolecular i-motif tetramer structure,causing the separation of X and X*,thus resulting in high FRET efficiency for effective intracellular pH imaging(Fig.2A).Li and coworkers developed a controllable DNA nanomachine for time-resolved pH imaging in live cells and animals[46].The nanomachine was elaborately designed based on the integration of a UV-light-activatable DNA i-motif probe and upconversion nanoparticles(UCNPs).The activatable DNA probe(I-PD)was made up of an i-motif strand(I-strand)and a PC-group-contained complementary DNA(PC-strand).At physiological pH,the I-PD was stabilized as a double-stranded structure,leading to a quenched fluorescence signal.It could be delivered to a specific site in a living cell or in vivo without actuating functions to pH during the delivery process.Nevertheless,once encountering target sites,the I-PD could be specifically activated for cell pH imaging with high precision in a temporally programmable manner(Fig.2B).These efforts demonstrated that the i-motif-based nanosystems could be successfully used for pH imaging in living cells and showed high temporal-spatial resolution.
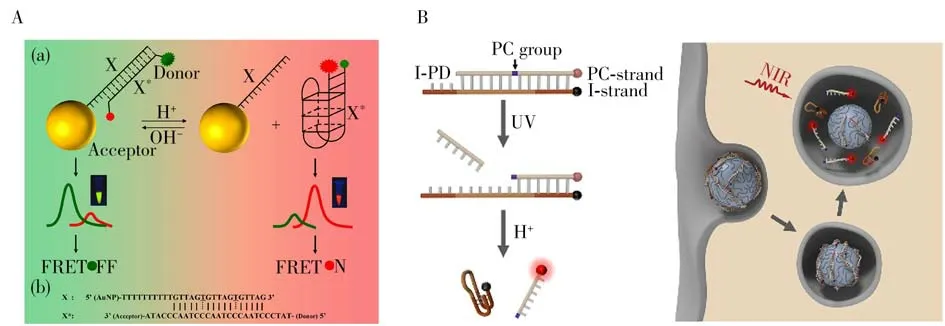
Fig.2 I-motif-based nanosystems for intracellular pH imaging
In addition,the i-motif-based acid response strategies were also widely used for accurate imaging of intracellular biomolecules or dynamic manipulation of biological behavior in subcellular organelles[47-48].For example,Li et al.engineered an i-motif-guided model to mimic the pH-responsive dimeric behavior of natural spidroins by using a framework nucleic acid(FNA)nanoplatform,which could be dynamically manipulated with lysosomal pH and ATP within living cells[48].In 2020,they further constructed a FNA nanocarrier using the truncated square pyramid(TSP)cage encoded an i-motif and ATP aptamer as logic-computing elements for the response of endogenous pH and ATP in different subcellular compartments,while the release of the molecular payloads allowed for precise fluorescent imaging of mRNA[49].These kinds of logic-controlled FNA nanoplatforms based on i-motif hold the ability of fast pH responsiveness and effective endocytosis,as well as the advantages of inherent biocompatibility and stability.
2.1.2 Extracellular p H imagingExtracellular pH also plays a crucial role in cell physiological and pathological processes,including adhesion,migration and drug resistance[43,50].The precise and dynamic monitoring of extracellular pH is of great significance for the diagnosis and therapy of tumors.In 2016,benefiting from the flexible acid-induced allosteric properties of i-motif,our group designed a cell-surface-anchored FRETbased i-motif sensor for extracellular pH imaging based on the interactions between streptavidin and biotin[51].Systematic experimental results revealed that the i-motif probe possessed high sensitivity and excellent reversibility for the imaging of extracellular pH.Moreover,the FRET strategy could avoid the influence of environmental effects,thus providing a precise approach for extracellular pH imaging.Similarly,Nie and co-workers constructed a cell-surface-anchored ratiometric DNA tweezer for real-time monitoring of extracellular pH[52].The i-motif sequence in the middle was used to dynamically manipulate the on/off states of the tweezer and the cholesterol was used as an anchoring element to attach the tweezers on the cell surface.Living cells studies suggested that the tweezers could respond to extracellular pH in the pH range of 5.0-7.5 quickly and reversibly,performing real-time imaging of cell-surface-pH changes with excellent temporal-spatial resolution(Fig.3A).However,the poor specificity and complicated anchoring procedures impeded their further applications in distinguishing different tumor cells.
Hence,in order to improve the specificity of nanoprobes in the process of cellular pH imaging,our group introduced aptamer as a specific targeting recognition element and integrated it with i-motif sequence to develop a new pH-activatable aptamer probe(pH-AAP)for cell-anchored specific pH imaging of tumors[53].By rationally design of the i-motif sequences,the pH-AAP strategy achieved extracellular pH-activated,membrane-anchored high-contrast imaging of different tumor cells in vitro and in vivo with excellent specificity(Fig.3B).Further,inspired by the stimuli-responsive behavior of mimosa,we developed a cell-surfaceanchored ratiometric DNA nanomimosa(DNM)for the precise extracellular pH imaging of tumors by using the flexible and controllable design of i-motif sequences and DNA nanostructures[54].Four different DNA strands was self-assembled into a DNM with an open state at neutral pH.Once encountering target cells,the DNM would be in a closed state on the surface of cells owing to the specific anchoring of the aptamer and pH-stimuli conformation conversion of the i-motif,thus resulting in the recovery of the FRET signal and accurate imaging of tumor extracellular pH with high specificity and low background signal.More recently,Li et al.engineered an extracellular pH and membrane receptor bispecific-driven DNA logic nanorobot(pH-RE)with improved accuracy for in vivo tumor imaging[55].Due to the unique microenvironment of tumors,the logic design of pHRE would not only be unaffected by the spatial barrier between different biomarkers,but also could avoid the on-target off-tumor effects,significantly improving the accuracy of tumor imaging.
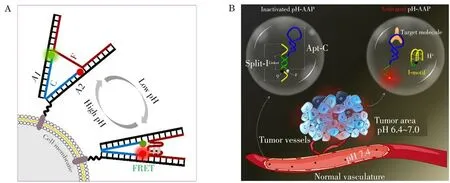
Fig.3 I-motif-based nanosystems for extracellular pH imaging
Although several efforts of extracellular pH-responsive i-motif strategies have been made recently,most of them still presented a relatively wide pH response range,or their response range were within the nonphysiological pH,making it difficult to respond to small pH changes outside the tumor cells sensitively[53].Recently,some works have focused on improving sensitivity and narrowing the pH response interval of i-motif sequence by optimizing its base composition or structural design of related functional domain.Particularly,Nesterova and co-workers reported a rational principle for designing highly responsive pH probes based on the DNA i-motif.They found that the hairpin-contained i-motif was conducive to the folding of i-motif tetramer structure due to the embedding of hairpin structure in the sequence,which could significantly improve its sensitivity to small pH changes and reduce the pH response range[37,56],providing new opportunities for the design of acid-sensitive activatable probes.Encouragingly,our group engineered a fluorescent ratiometric probe based on hairpin-contained i-motif for high-resolution imaging of small pH changes on the tumor cells[57].An almost 70-fold change in the signal-to-background ratio was observed in a significantly narrow pH response range(0.90 units).We believe that this design might lay a foundation for the construction of acid-activated theranostics probes for imaging and killing of tumor cells.
Collectively,these proof-of-concept studies of i-motif-based nanosystems offer sustained impetus to design the pH-responsive smart nanodevices for accurate diagnosis of tumor in the future.
2.2 I-motif-based nanosystems for p H-controlled drugs release
I-motif was often used as a gate-like molecule for the construction of pH-controlled drug release systems due to its ability of flexible and rapid conformational transition under different pH conditions[58-60].For example,Qu et al.designed a simple,pH-fueled molecular gate-like delivery system using i-motif as caps for the controlled release of Rodamine B(RhB)from mesoporous silica nanoparticles(MSN)[61].Under acidic pH,the C residues in the C-rich sequences were partially protonated and folded into a closed i-motif structure,then the RhB loaded in the MSN pores were strongly capped by the i-motif tetramer structures.With the pH increases to alkaline,the C+residues were deprotonated and the i-motif structures were opened to a random coil state,resulting in rapid release of RhB from the mesopores.Accordingly,Song et al.developed an intelligent pH-responsive nanocarrier and release system by using AuNPs modified C-rich sequences and its complementary strands as gated molecules to protect the RhB released from the pores(Fig.4A).Under acidic pH,the C-rich sequences reconfigured to the i-motif quadruplex structure,leading to the separation of the AuNPs caps and the release of RhB from the MSN pores[62].In these reports,using i-motif as switch primitives,the on/off cycle of the nanosystems could be realized by adjusting pH,accompanied by efficient encapsulation and rapid release of drug molecules.
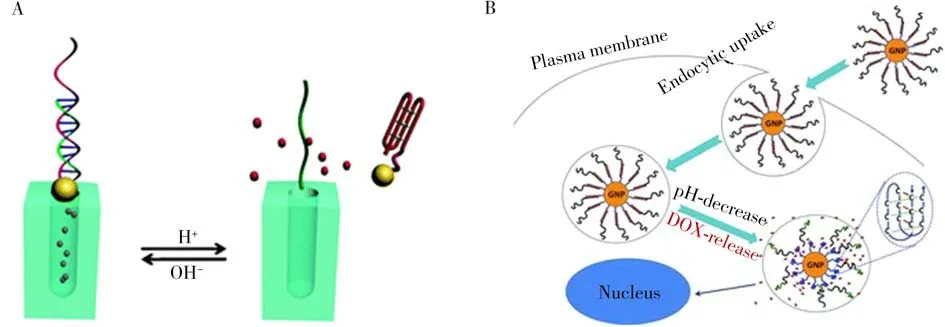
Fig.4 I-motif-based nanosystems for pH-controlled drugs release
Moreover,the i-motif-based nanosystems were also widely used for the targeted controlled released of chemotherapeutic drugs in cancer cells due to the distinctive physiological microenvironments of tumors[58,63].Zhou and co-workers designed a pH-triggered drug delivery system for storage and controlled release of doxorubicin(Dox)by using an i-motif-conjugated gold nanoparticle[58].At alkaline pH,the Dox could be intercalated into DNA double-stranded GC base pairs,serving as a carrier for drug delivery.When it internalized into cells,the Dox would be released from the nanocarriers due to the formation of i-motif in the acidic environment of late endosomes/lysosomes,achieving precise drug release(Fig.4B).Recently,Duan et al.developed a multifunctional DNA nanohydrogel for targeted drug delivery of tumors[64].At alkaline pH conditions,the pH-responsive i-motif sequences,unmethylated cytosine-phosphate-guanine oligonucleotides(CpG ODNs)and MUC1 aptamer were integrated into DNA nanostructure by one-step self-assembly for the construction of intelligent DNA nanohydrogels that could provide sites for Dox intercalation.At acidic endosomes/lysosomes,the DNA nanohydrogels would be disassembled due to the formation of i-motif quadruplex structures,causing the release of Dox and CpG ODNs.Such a method demonstrated high loading capacity,targeted delivery,and controlled release of drugs,which enabled precise treatment of cancers with high efficacy and low adverse effects.
A different approach was applied to photonically trigger the folding/unfolding of i-motif to realize the controlled release of drug molecules.Our group designed a photon-fueled gate-like MSN-based delivery system that the malachite green carbinol base(MGCB)molecules were loaded in MSN as a photoinduced hydroxide ion emitter and the i-motif sequence was modified on the surface of MSN as a gated molecule[65].Under dark conditions,the low pH could induce the formation of i-motif structures,resulting in drug molecules being trapped in the mesopores.However,under ultraviolet light irradiation,MGCB could release OH-,causing the i-motif structure unfolding into single-stranded DNA due to the increase of the pH.Consequently,the pores were opened,leading to the rapid release of the entrapped cargo from the pores.This design provides a novel approach to develop i-motif-based controlled-release systems for efficient drug delivery and holds great promising application in accurate therapy of tumor.
2.3 I-motif-based nanosystems for tumor theranostics
In 1998,“theranostics”as a new concept,which combined with diagnosis and therapy,was firstly termed by John Funkhouser,the Chief Executive Officer of PharmaNetics[66].Since then,theranostics has become one of the core keywords in tumor research.The development of theranostics strategies that can simultaneously realize sensitive imaging and precise therapy against tumor is expected to significantly facilitate advances in personalized medicine[67].In recent years,with the rapid development of bio-nanotechnology and molecular engineering technology in the biomedical field,a great deal of novel and efficient theranostics nanosystems based on i-motif have been developed[68].In this part,we will classify and describe in detail about i-motif-based nanosystems in tumor theranostics applications,including imaging-guided chemotherapy,gene silencing,and synergetic therapy.
2.3.1 Imaging-guided chemotherapyChemotherapy,as one of the most common tumor therapy models,has been widely used in clinical treatment of various tumors,which is used to kill tumor cells by delivering chemotherapeutic drugs into tumor tissues such as Dox[69-70].However,the nonspecific adsorption and adverse effects of free Dox remain challenges for specific therapy.Therefore,several studies have focused on engineering smart nanosystems to improve the accuracy of drug delivery and treatment of tumor cells.In particular,the development of i-motif-based nanosystems triggered by tumor acidic microenvironment provides a new opportunity for the construction of activated tumor theranostics strategies.
For example,our group developed an i-motif-based in situ multivalent theranostics system for contrastenhanced imaging and killing of tumor cells[71].We firstly designed an aptamer and split i-motif functionalized DNA monomer(AptDM),allowing for pH-responsiveness and Dox loading capacity.In the extracellular acidic microenvironment of the tumor,the AptDM tended to crosslink with each other on the cell surface to form multivalent DNA assemblies(MDAs)due to the formation of intermolecular i-motif structure.The results successfully demonstrated that the MDAs possessed enhanced imaging,effective drug delivery and killing of target tumor cells compared to AptDMs,providing new insights for the construction of pH-manipulated theranostics probes.Jiang et al.constructed an AS1411 aptamer functionalized DNA-conjugated BSA nanocarrier with a pH-responsive i-motif sequence for targeted Dox delivery and efficient treatment of tumor cells[72].The in vivo studies showed that the nanocarrier system could efficiently inhibit tumor growth.More recently,Li and co-workers engineered an i-motif based,GD2 aptamer-mediated Dox delivery system(named as IGD-Targeted)for imaging-guided neuroblastoma(NB)antitumor therapy[73].At physiological pH,the Dox was retained in IGD-Targeted and the hybridization of i-motif sequence prevented the GD2 recognition domain from forming the correct three-dimensional structure for GD2 recognition.At acidic environment of tumor,the two split i-motif fragments tended to form into a complete intermolecular i-motif tetramer structure,enabling restoration of the targeting ability of IGD-Targeted,accompanied by Dox release around NB tumor cells for the specific therapy.In a subsequent work,in order to improve theranostics accuracy,we skillfully engineered a facile pH-responsive aptamer“moleculedoctor”(termed as pH-Apt-MD)with hairpin-contained i-motif for bispecific tumor cells imaging and killing[74].As displayed in Fig.5,the pH-Apt-MD was made up of two DNA strands,one was cell-specific aptamer sequence,another was pH-sensitive i-motif strand complementary to the aptamer sequence partially.When the pH-Apt-MD encountered to the target tumor cells,it could achieve bispecific cells imaging and in situ drug release,owing to the formation of the hairpin-contained i-motif and the membrane receptor recognition-initiated disassembly.
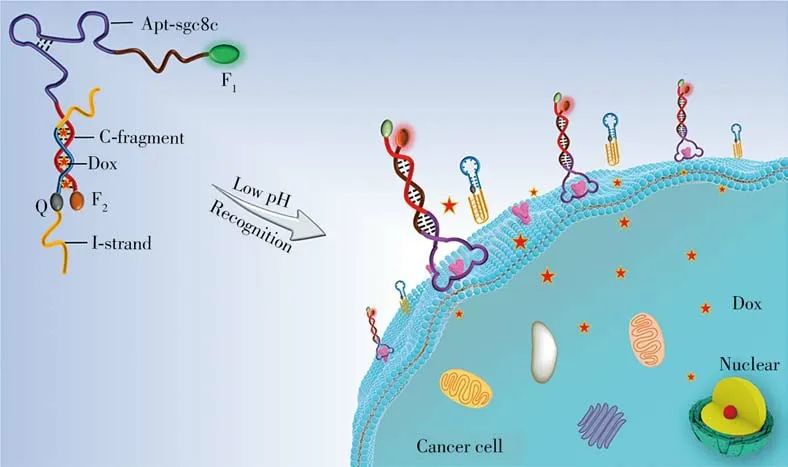
Fig.5 I-motif-based aptamer“molecule-doctor”for accurate imaging and chemo-killing of tumor cells[74]
These targeted theranostics strategies could effectively reduce the adverse effects of chemotherapy drugs on normal tissues and increase the concentration of drugs in tumor sites,thus improving the bioavailability of antitumor drugs.We believe that these strategies based on aptamer and i-motif might open a novel approach for the design of intelligent molecular probes,which would possess great potential in clinical accurate diagnosis and treatment of tumors in the future.
2.3.2 Imaging-guided gene silencingGene silencing,a molecular process involved in the down regulation of target genes,has attracted extensive attention in the field of tumor therapy because of its advantages of lasting and potential one-time cure,and is considered as the mainstream treatment mode of tumors in the future[75-77].By combining i-motif with different oligonucleotide drugs(e.g.,siRNAs,DNAzymes or antisense oligonucleotides(ASOs)),a series of pH-sensitive smart therapeutic agents would be established for gene silencing of tumor cells.For instance,in view of the homogeneity of i-motif and oligonucleotide drugs,we developed an aptamer-targeted,i-motif-driven in situ bipedal hybridization chain reaction(pH-Apt-BiHCR)strategy for specific activatable imaging and improved gene silencing of tumor cells[78].First,three DNA single strands were used to self-assemble into a“Y-shaped”DNA scaffold(pH-Apt-Y6)with pH-responsiveness and aptamer target recognition capability.When encountering target cells,the aptamer domain on the pH-Apt-Y6 probe could bind to the receptors on the cell membrane.At the same time,the i-motif blocking sequence formed into an intact intramolecular hairpin-contained i-motif structure and fell down from the pH-Apt-Y6 due to the acidic microenvironment around cell surface,exposing the HCR triggers and initiating the HCR on both sides of the“Y-shaped”DNA scaffold to form the pH-Apt-BiHCR probe.The pH-Apt-BiHCR not only contained repeated FRET units for high-contrast imaging of target cells,but also carried a large number of ASOs as interfering molecules for enhanced cell growth inhibition(Fig.6A).The results showed that the i-motif-based dual-HCR system could significantly increase the imaging/gene silencing effect and hold great progress in early diagnosis and therapy of tumors.
Very recently,taking advantage of the specific recognition and cleaving ability of DNAzymes to tumorigenic RNA(mRNA or miRNA)for the downregulation of intracellular gene expression[80],our group engineered a tumor acidic microenvironment activated DNAzyme-mediated nanodevices(sDz-ND)based on imotif,enabling specific imaging and gene silencing of tumor cells[79].As illustrated in Fig.6B,the sDz-ND was made of two split DNAzyme-functioned modules,where the recognition region of DNAzymes were blocked by i-motif sequences.At acidic pH,the two different modules would crosslink of each other to form integrated sDz-ND through released sticky ends hybridization owing to the formation of i-motif quadruplex structure,resulting in the recovery of fluorescence signal and activation of theranostic function.Such a strategy provides new insights for i-motif-mediated activity modulating of DNAzymes,and holds great promise for accurate imaging and gene therapy applications.
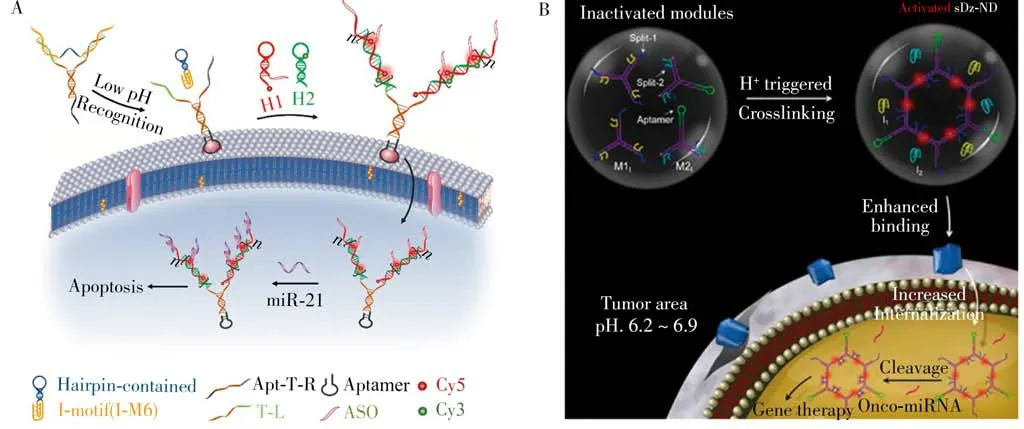
Fig.6 I-motif-based nanosystems for imaging-guided gene silencing
In addition,several studies have focused on developing i-motif-conjugated DNA nanostructures for si RNA gene delivery and tumor theranostics.For example,Qi and co-worker constructed a series of DNA tetrahedron nanocages(Td)that gifted with different numbers of i-motif DNA structure for helping therapeutic siRNA get rid of being entrapped in endosomes,thus achieving efficient siRNA delivery and therapy in vivo[81].The results suggested that the i-motif-conjugated Td could escape from endosome in tumor cells quickly.Further,the carried siRNA could effectively down-regulate the expression of tumor-associated mRNA and achieved effective gene therapy for tumors.Owing to the excellent biocompatibility,good stability,and effective cellular uptake,the i-motif-based DNA nanostructures could be a promising therapeutics carrier for siRNA delivery and show great application potential in tumor medicine field.
2.3.3 Imaging-guided synergetic therapyDue to the genetic heterogeneity and complexity of tumor cells,the practical effect of single-mode treatment of tumors is very limited,which caused a series of problems such as multi-drug resistance and insufficient therapeutic efficacy[82-83].Moreover,the sensitivity of tumor cells in the same tissue to drugs is also different.Therefore,it is difficult to achieve the desired therapeutic effect with a single treatment mode.As an alternative,synergetic therapy,combining several tumor therapeutic models into a single nanoplatform,has been developed as a promising candidate for tumor therapy[84-85].In recent years,i-motif as one of popular functional nucleic acid molecules,has been widely used in developing multifunctional nanosystems to improve the therapeutic efficacy.
For example,Su et al.fabricated an i-motif and aptamer functionalized gold nanostar(GNSs)as pH and near-infrared dual-responsive drug delivery systems(A-GNS/DNA/Dox)for combined chemo-photothermal tumor therapy[86].The Dox was intercalated into the GC base pair of the double-stranded DNA,which was conjugated with GNS nanocomposites.After uptake by targeted endocytosis,the i-motif sequence of A-GNS/DNA/Dox tended to form into intact i-motif structures in acidic endosomes/lysosomes,thus triggering Dox release.Furthermore,the GNSs converted the external NIR irradiation to heat,promoting the disassembly of double-stranded DNA,further leading to Dox release,accompanied by the recovery of the fluorescence.It was demonstrated that the therapeutic performance of the A-GNS/DNA/DOX was significantly improved by stimuliresponsive and combined chemo-photothermal therapy.Similarly,Guo et al.further developed a targeted imotif DNA-conjugated AuNP as a stimuli-responsive drug nanocarrier(Dox@AuNP-MUC1)for synergistic chemo-photothermal therapy[87].The results exhibited that the enhanced Dox release and synergistic chemophotothermal therapy were realized by the formation of i-motif structures and excellent photothermal conversion efficiency of AuNPs.
In 2014,Kim et al.constructed an i-motif-based Au nanomachine in programmed siRNA delivery for gene silencing and photothermal killing of tumor cells[88].The i-motif-conjugated siRNA was immobilized on the AuNPs.While in acidic endosome,the i-motif fragments formed into an intermolecular tetraplex structure,resulting in the release of siRNA,accompanied by gene silencing.At the same time,the formation of i-motif structure would promote the aggregation of AuNPs,which accelerated photothermal killing of cells when irradiated with laser.Recently,we have reported an i-motif-driven and aptamer-functionalized DNA nanostructure for synergistic chemo-gene killing of tumor cells[89].We firstly designed a pH-sensitive and aptamer-integrated tile motif(pH-Apt-TM),which could load Dox in GC base pair of double-stranded region.When it was in proximity to the target cells,the pH-Apt-TM could recognize cells through the interaction between aptamer and ligands on the cell surface.Meanwhile,the extracellular acidic pH crosslinked pH-Apt-TM into a multivalent hand-in-hand DNA tile assembly(HDTA)owing to the split i-motif fragments formed into an intact intermolecular i-motif.The HDTA strategy not only introduced multivalent aptamer on the probe through pH-driven DNA tile assembly,improving the recognition affinity and imaging contrast,but also the combination of chemo-gene killing was realized by Dox loading and the introduction of antisense sequences,which enhanced the killing efficiency.
In addition,Kim and co-workers designed a DNA-decorated Au nanomachine modified with pHresponsive i-motif and photosensitizer-loaded G-quadruplex for the combinatorial chemo,photodynamic,and photothermal therapy[90].The i-motif structure was used as a pH-responsive drug delivery carrier,Gquadruplex structure was used to interact with photosensitizer(ZnPc)and the Dox was loaded in i-motif/cDNA duplex.When the nanocomposites internalized into tumor cells,the i-motif DNA would form into tetraplex structure in the acidic endosomal pH,leading to the aggregation of AuNPs,which enabled heat generation under NIR irradiation,thus resulting in photothermal killing of tumor cells.Moreover,the Dox released from i-motif/cDNA duplex would realize chemotherapy and the ZnPc loaded in the G-quadruplex would produce photodynamic therapy effects under laser irradiation.Therefore,the use of this strategy could achieve multimode collaborative treatment.
Overall,significant progress has been made in i-motif-based smart nanosystems for synergistic tumor therapy.By combining the activated imaging performance and excellent synergistic effects,we believe that it could become as one of the most promising strategies for precise theranostics applications in tumor and personalized medicine.
3 Conclusions and perspective
In this review,we have briefly summarized the structural characteristics and basic properties of i-motif.Subsequently,the recent advances of i-motif-based nanosystems in the field of cellular pH imaging,pHcontrolled drug release and pH-driven tumor theranostics applications were systematically discussed.As an ideal molecular tool,i-motif not only possesses favorable physical properties,but also has excellent biocompatibility.In addition,the facile synthesis and modification of i-motif makes it easy to integrate with other nanomaterials or functional nucleic acid structures,so as to construct multifunctional intelligent nanosystems for precise diagnosis and treatment of tumors.We believe that the current and imminent i-motifbased nanosystems will have a great impact on clinical translation and precision medicine.
Although several achievements have been made in the biomedical field,the further development of i-motif still faces severe challenges:(1)Cell homeostasis is strictly regulated by small pH changes.How to obtain more sensitive i-motif sequences that respond to small pH variations in tumors is crucial and challenging.By reasonably designing the base composition of each functional domain(C-tracts and i-loop)in the i-motif sequences,it is expected to obtain satisfactory i-motif sequences to meet the requirements of rapid and accurate pH response.(2)The intracellular microenvironment is complex and changeable.The DNA i-motif,as a nucleic acid molecule,is easy to be degraded by ribozymes,resulting in false positive signals,which is not conducive to accurate signal acquisition.Therefore,prolong the circulation time in vivo and increase the stability of DNA imotif are also urgently needed.By functional modification such as locked nucleic acid(LNA)or combined with other biocompatible and stable nanomaterials,it is expected to improve the stability of i-motif and promote its clinical transformation.Moreover,the i-motif could be combined with other endogenous molecules(such as ATP,GSH or enzymes,etc.)responsive elements or integrated with other spatiotemporal controllable stimuliresponsive(such as temperature response,light response,etc.)molecular tools to build multi-factor cascadedriven and multi-functional intelligent system to meet the needs of fast and accurate tumor theranostics.In addition,the i-motif structures are widely presented in telomeres and the promoter region of various tumor-related genes,which involved in the occurrence and development of tumors by regulating the expression of downstream genes,thus could be used as an important target for anticancer drug design in the field of precision medicine.In conclusion,with the comprehensive development of theoretical basis,DNA nanotechnology and other technology in biomedicine,the functional nucleic acid molecule of i-motif will provide a new opportunity for biomedical research,and open up a new approach for intracellular sensing,imaging and therapy.
- 分析测试学报的其它文章
- Research Progress of Hemicyanine Dye for Molecular Imaging
- 碱性磷酸酶的体外检测和体内成像研究进展
- Recent Progress in Nanoscale MOFs for Biological Imaging of Tumors and Tumor Markers
- A Low-cost,Automated Nucleic Acid Extraction System Converted from the Open-Source Rep Rap 3D Printer
- Research Progress on Analytical Methods for Deciphering Adenosine-to-inosine RNA Editing
- CRISPR/Cas Based Biosensing Platforms for Molecular Diagnosis

