Research Progress of Hemicyanine Dye for Molecular Imaging
WU Fang-zheng,ZHANG Le-le,HAI Zi-juan
(Key Laboratory of Structure and Functional Regulation of Hybrid Materials,Ministry of Education,Institutes of Physical Science and Information Technology,Anhui University,Hefei 230601,China)
Abstract:Molecular imaging has been widely applied to the research of fundamental biological processes,diagnosis,treatment design and therapeutic outcome assessment on many diseases.Hemicyanine(HCy)dye is a suitable bioimaging agent due to its good photophysical properties,biocompatibility and low toxicity to living systems.A huge amount of research work involving probes based on the HCy dye for molecular imaging has emerged in recent years.This review focuses on the development of probes based on HCy dye for fluorescence and multimodal imaging in a systematic manner.Design strategies of the activatable HCy probes and their successful applications as bioimaging agents are described in detail.In addition,the future development of HCy probes for molecular imaging is discussed.
Key words:hemicyanine;molecular imaging;fluorescence imaging;multimodal imaging
Studies have revealed that altered molecular profiles of the lesion site can indicate the incidence,progression,and treatment susceptibility of the disease before significant anatomical and physiological changes occur.Therefore,it is necessary to visualize molecular processes within cells,tissues,and living organisms[1-3].Molecular imaging refers to the characterization and measurement of biological processes at the cellular and molecular level.It has been widely applied to study the fundamental biological processes,diagnosis,treatment design and therapeutic outcome assessment for many diseases[4-6].Imaging techniques are generally divided into two categories,one is morphological/anatomical imaging and the other is molecular/functional imaging.Morphological/anatomical imaging techniques,including ultrasound(US),computer tomography(CT)and magnetic resonance imaging(MRI),can provide high spatial resolution but suffer from low sensitivity.Molecular/functional imaging techniques,such as optical imaging(OI),single photon emission computed tomography(SPECT)and positron emission tomography(PET),offers high sensitivity to detect molecular and cellular changes but with poor spatial resolution[1,4,6-7].Each imaging modality has its own strengths and weaknesses while multimodal imaging provides comprehensive information forin vi voresearch which can combine complementary advantages from two or more modalities[8-9].Therefore,one can choose the appropriate imaging modality based on the type of biological information one wishes to collect.
Among different imaging techniques,fluorescent measurement is preferable approach for real-time imaging because of its simplicity,low cost,noninvasive and high sensitivity.Therefore,abundant fluorescent probes with various dyes as signal reporters are prosperously developed[10].The cyanine dyes including cyanine,hemicyanine(HCy),and squaraine are suitable candidates due to their good photophysical properties,biocompatibility and low toxicity to living systems.As a main member of the cyanine family,HCy structure features the donor-π-acceptor(D-π-A)system(Fig.1).In detail,the positively charged nitrogen heterocyclic moiety(electron acceptor)and the terminal hydroxyl or amino group(electron donor)are connected by aπconjugated bridge.Based on the intramolecular charge transfer(ICT)mechanism,it has tunable optical properties in the near-infrared region.The excellent optical characteristics of HCy dye(such as high absorption coefficient,large Stokes shift and high fluorescence quantum yield)makes it widely applied in bioimaging analysis[11-12].
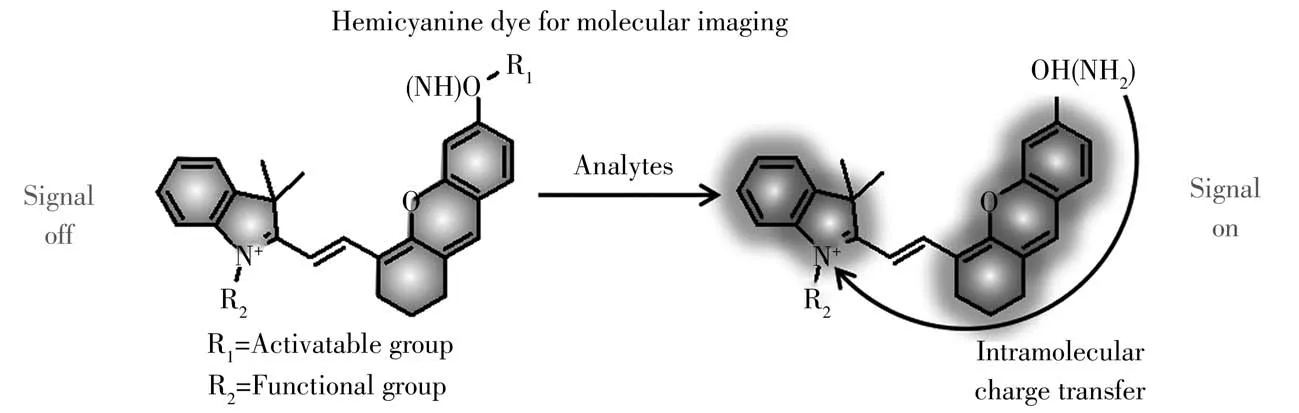
Fig.1 Chemical structure and the sensing mechanism of hemicyanine probes toward biological analytes for molecular imaging
In this review,we attempt to provide the recent development of activatable probes based on HCy dye for fluorescence and multimodal imaging in a systematic manner.Design strategies of the activatable HCy probes and their successful applications as bioimaging agents are described in detail.Then,the future development of activatable HCy probes for molecular imaging are given and discussed.
1 Hemicyanine dye for fluorescence imaging
Fluorescence imaging(FLI)has become one of the most powerful techniques for monitoring biological targets and processes in real time.It has high sensitivity but its spatial resolution decreases rapidly with the depth.Compared with shorter emission wavelengths,FL in the near-infrared(NIR)region has higher tissue penetration[6,10].Therefore,HCy dye with tunable NIR absorption and emission has been widely applied in FLI.In terms of its signal characteristics,FL probes based on HCy dye can be roughly divided into two types:“Turn-on”and ratiometric.
1.1 “Turn-on”fluorescence imaging
The FL signal of“Turn-on”probe is“off”and can be activated after interacting with the specific target.Therefore,“Turn-on”probe with high signal-to-noise ratio has significant advantages in the field of molecular imaging.In 2016,Ma et al.developed a water-soluble NIR FL probe 1 for monitoring the activity ofγglutamyl transpeptidase(GGT)[13].In the presence of GGT,theγ-glutamyl bond of the glutathione(GSH)moiety in probe 1 is rapidly cleaved,followed by spontaneous intramolecular cyclization and the release of the fluorophore HXPI.This probe shows high sensitivity to GGT with a detection limit of 0.50 U/L.Notably,the probe has been used to image GGT in zebrafish and evaluate the inhibition ability of three common inhibitors of GGT bothin vitr oandin vivo.Then,they designed the probe CYLP for imaging pantetheinase in cells and mice in 2020[14].By using pantothenic acid with a self-immolation linker as a recognition group,CYLP produces a sensitive FL off-on response at 710 nm with a detection limit of 0.02 ng/mL.Moreover,they observed obvious enhancement of pantetheinase in the tissues of mouse inflammatory models as well as in the intestines of mice with inflammatory bowel disease.In 2021,they further developed two probes(Rma-1 and Rma-2)for the selective analysis of monoamine oxidase(MAO)in living biosystems[15].Both probes showed selective and sensitive NIR FL responses at 708 nm toward MAO-A rather than MAO-B while Rma-1 with a self-immolation linker exhibited superior analytical performance with a detection limit of 4.5 ng/mL and 13-fold specificity for MAO-A over MAO-B.Furthermore,Rma-1 has been successfully utilized to image the MAO-A activity in cells,zebrafish and tumor-bearing mice.In the same year,they reported a new FL probe BHXP with tumor targeting ability[16].As shown in the Fig.2,BHXP is consist of biotin as the tumor-targeted group,HCy dye as the FL signal group and triarylphosphine as the HNO sensing group.It shows an NIR fluorescence off-on response toward HNO with high selectivity and sensitivity.BHXP is capable of imaging HNO in cells and HNO release from its donors in tumors of micein vivo.This probe may also be useful for assessing the potential therapeutic effects of HNO donors for cancer treatment.
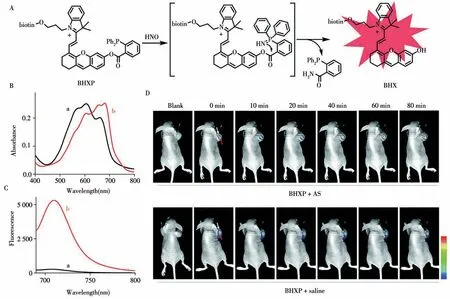
Fig.2 The sensing mechanism of BHXP toward HNO(A),absorption(B)and fluorescence spectra(C)of BHXP(10μmol/L)without(a)and with(b)AS(20μmol/L)in PBS,representative in vivo fluorescence images of tumor-bearing mice(n=3)(D)[16]
In 2017,Ye and colleagues reported a probe GANP for real-time imaging of endogenous GGT activity[17].GANP is very stable under physiological conditions and it can be effectively activated by GGT with 100-fold FL enhancement at 720 nm.The detection limit is 3.6 mU/L.Living cell imaging indicated that the membranebound GGT in living tumor cells can quickly recognize GANP and release the hydrophobic HCy with FL signal.Moreover,GANP can detect GGT activity in HCT116 tumor tissue with enhanced penetration depth(>100μm).Based on above work,they further reported the probe 1 with tumor targeting ability for non-invasive imaging of GGT in 2018[18].As shown in the Fig.3,probe 1 is divided into three parts:GGT cleavable substrateγglutamic acid(γ-Glu),NIR fluorophore HCy and tumor targeting group RGD.After cleavage by GGT,it has a significant 230-fold increase of NIR FL at 712 nm.Cell studies showed that probe 1 could induce bright NIR FL distributed mainly in the lysosome of U87MG tumor cells,which was not observed in the GGT-positive butαvβ3-deficient normal cells.Furthermore,probe 1 has been successfully applied to detect GGT activity in the xenograft U87MG tumors following intravenous(i.v.)administration.
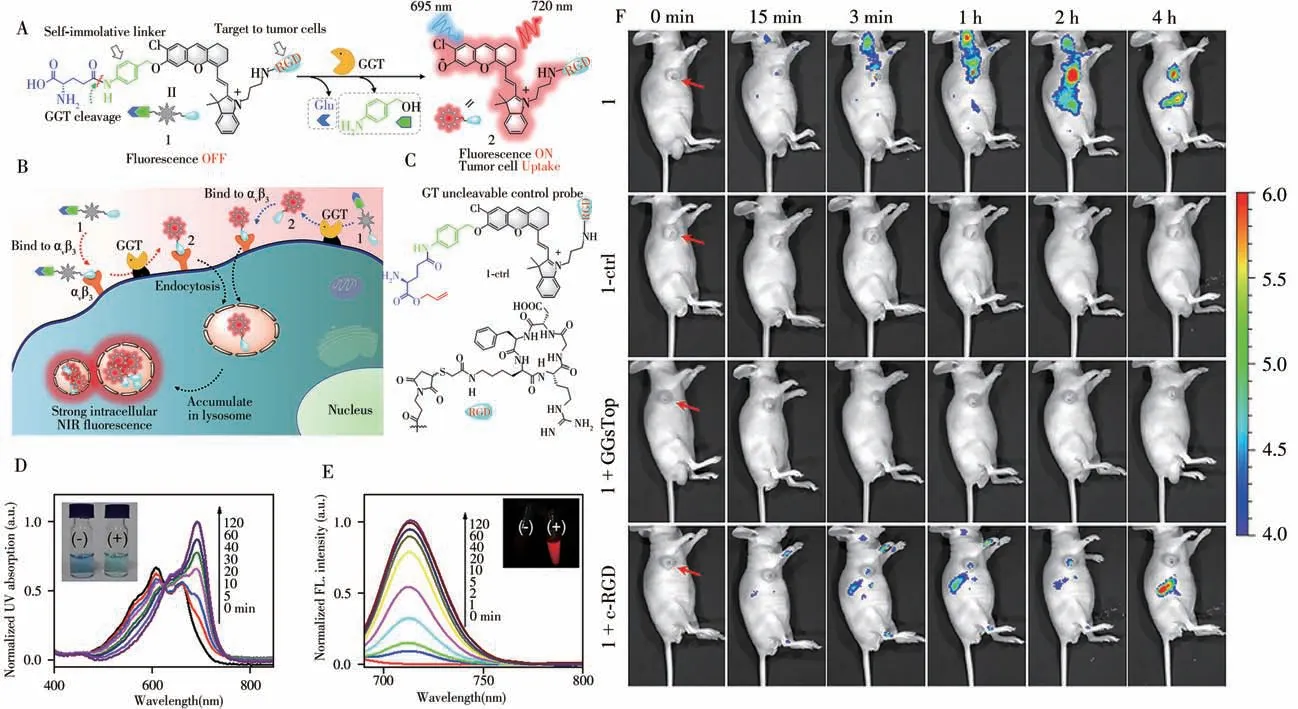
Fig.3 Chemical structure and proposed GGT-triggered conversion of probe 1 to fluorescent product 2(A),schematic illustration of GGT activation andαvβ3 integrin-targeted delivery of probe 1 for tumor cell NIR-fluorescence imaging(B),the chemical stucture of the control probe 1-ctrl(C),UV-Vis absorption(D)and fluorescence spectra(E)of probe 1(5μmol/L)following an incubation with GGT(100 U/L)at 37℃for different amounts of time,real-time fluorescence images(F)of U87MG-tumor-bearing mice receiving iv injections of probe 1(25μmol/L,150μL),probe 1-ctrl(25μmol/L,150μL),probe 1 with i.t.injections of GGsTop(5 mmol/L,100 μL),or probe 1 with i.t.injections of free c-RGD(2 mmol/L,100μL)[18]red arrows indicate the locations of the tumors
In 2018,Pu and colleagues reported two activatable FL probes(CyTF and CyBA)to distinguish keloid cells with abnormally high reactive oxygen and nitrogen species(RONS)level from normal skin cells[19].CyTF is highly sensitive to peroxynitrite(ONOO-),while CyBA is responsive to both ONOO-and hydrogen peroxide(H2O2).Both CyTF and CyBA show a 15-fold FL increment at 717 nm upon reaction with RONS.The FL increment of CyTF is higher than that of CyBA in stimulated dermal fibroblasts and keloid fibroblasts.Furthermore,CyTF permits specific detection of implanted keloid fibroblasts in a xenograft live mouse model.Encouraged by above study,in the same year,they further designed a FL probe FNP1 to specifically detect keloid-derived fibroblasts which have high expression levels of fibroblast activation protein-alpha(FAPa)[20].The hydroxyl group of FNP1 is caged with a FAPa-cleavable peptide substrate linked by a self-immolative group.In the presence of FAPα,FNP1 quickly and specifically turn on a 45-fold FL enhancement at 710 nm.Moreover,by integrating with a simple microneedle-assisted topical application,FNP1 enables sensitive detection of keloid cells in metabolically active human skin tissue with a theoretical limit of detection down to 20 000 cells.In 2020,they designed renal clearable macromolecular probes(CyGbPF and CyGbPP)to specifically detect an immune activation related biomarker granzyme B(GranB)[21].The hydrophilic poly(ethylene glycol)(PEG)passivation chain is linked to probes,which can passively target the tumor of living mice after systemic administration.In detail,CyGbPF and CyGbPP specifically turn on their FL signal at 707 nm by more than 20-fold in the presence of GranB and could effectively distinguish the activated immune cells(CTLs)from other cellsin vitro.Importantly,the high renal clearance efficiency of both CyGbPF and CyGbPP(60%injected doses at 24 h post-injection)enabled optical urinalysis of immune activation,which was noninvasive and more convenient than immunofluorescence staining.Thus,this study paves the way to advance the application of molecular optical imaging probes in facilitating the development of immunotherapeutic agents.After that,in the same year,they further reported a macromolecular probe CyP1 with high renal clearance efficiency to image bladder cancer(BC)in situ in a mouse model[22].As shown in Fig.4,CyP1 includes three functional components:the substrate of aminopeptidase N(APN)as cancer biomarker response part,the HCy dye as NIR FL signal part and 2-hydroxypropyl-β-cyclodextrin(HP-β-CD)as the renal clearance part.CyP1 has a high renal clearance efficiency(94%of the injection dosage at 24 h postinjection),after systemic injection into live mice,it can be specifically excreted through the kidneys,temporarily accumulate in the bladder and react with APN to turn on its NIR FL signal to report the presence of BC.
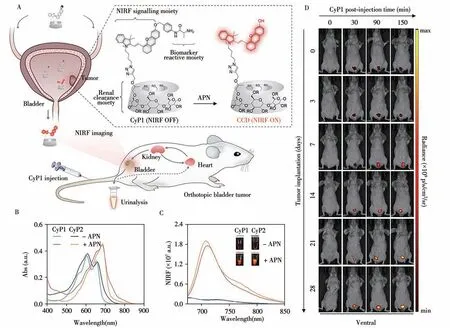
Fig.4 Design and mechanisms of the renal-clearable macromolecular reporter(CyP1)for NIRF imaging and urinalysis of BC in living mice(A),UV-Vis absorption(B)and fluorescence spectra(C)of CyP1 and CyP2(30μmol/L)in the absence or presence of APN(0.5μg/mL)in PBS(10 mmol/L,pH 7.4)at 37℃,representative NIRF images of living mice at t=0,30,90,or 150 min post-injection of CyP1 at different time points post tumor implantation(D)[22]
In 2019,Li et al.designed a FL probe Cy-Azo to image the activity of azoreductase(AzoR)[23].Itself is non-fluorescent due to the inhibited ICT effect.After the reduction by AzoR,the free amino group of Cy-Azo is released with 17-fold FL enhancement at 735 nm because of the recovery of ICT process.Due to its high selectivity to AzoR over other biological substrates,Cy-Azo is successfully applied in visualizing the activity of AzoR in two cell lines and three types of bacteria.In addition,it can monitor AzoR activity in acute and chronic ulcerative colitis(UC)mice models,which can provide much important information for the diagnosis and treatment of UC.Based on this work,they further developed a hypoxia-activated FL probe Cy-AP in 2020[24].As shown in Fig.5,the azo group of Cy-AP is reduced to the amino group in a hypoxic environment and immediately releases strong FL.Cy-AP with high sensitivity and selectivity to hypoxia can be used to visualize the existence of the“gut-liver axis”and to study its mechanism by monitoring hypoxia.In 2021,they exploited a FL probe QX-B for the detection of H2O2[25].The borate ester of QX-B is cleaved after reacting with H2O2and expose the hydroxyl group with 10-fold FL enhancement at 772 nm.It has been successfully applied in imaging exogenous and endogenous H2O2in different living cells and zebrafish.Furthermore,QX-B can be used in monitoring H2O2in diabetic mice for the first time,which provides very important information for the diagnosis and treatment of diabetes and its complications.After that,in the same year,they further designed a water-soluble NIR FL probe QX-P with simple synthesis[26].The hydroxyl group of QX-P is blocked by a phosphate group and the hydrolysis by alkaline phosphatase(ALP)release a strong fluorescence signal at 770 nm.Due to its high sensitivity and selectivity to ALP,QX-P has been successfully applied to real-time visualize of ALP activity in different cells and diabetic mouse models.
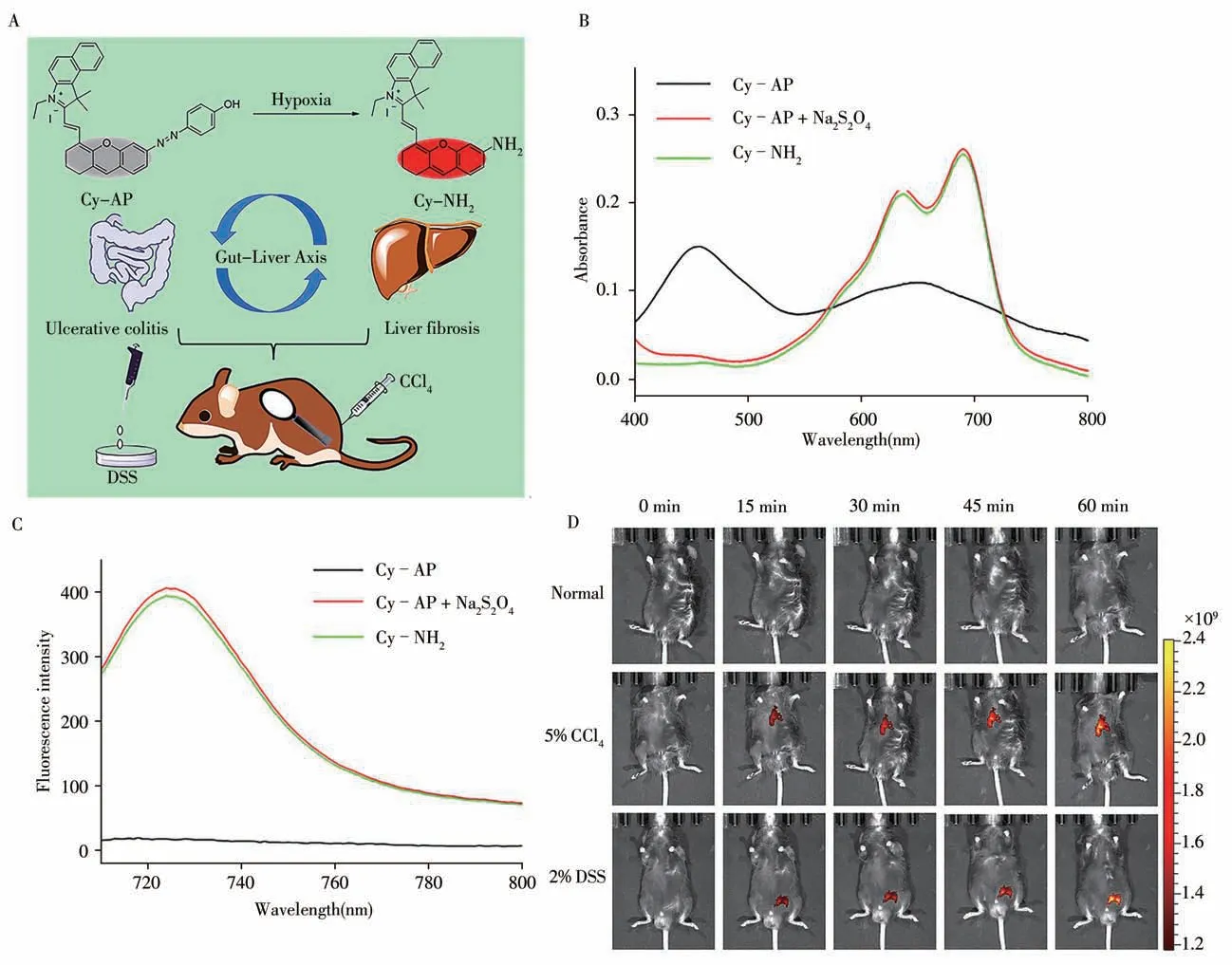
Fig.5 Design of probe Cy-AP to image the“Gut-Liver Axis”by detecting hypoxia(A),absorption(B)and fluorescence spectra(C)for Cy-AP(10μmol/L)before and after addition of Na2S2O4(5.0 mmol/L)and Cy-NH2(10μmol/L)in PBS buffer solution(pH 7.4,10 mmol/L,30%CH3OH),time-dependent in vivo fluorescence imaging(D)of the normal group,5%CCl4 group,and 2%DSS group mice after tail vein injection of Cy-AP(250μmol/L)[24]λex=640 nm,λem=695-770 nm
In 2019,Zhang et al.employed two FL probes NIR-LAP and NIR-ONOO-for sensing the markers of hepatotoxicity leucine aminopeptidase(LAP)and ONOO-[27].NIR-LAP and NIR-ONOO-provide lower detection limits for LAP(80 mU/L)or ONOO-(90 nmol/L),which can detect small fluctuations of LAP or ONOO-in living cells.More importantly,these two FL probes can evaluate the hepatoprotective effects of hepatoprotective drugs in living animals.This NIR dye platform with an optically tunable group could provide a convenient and efficient tool for the development of future probes applied in the pathological environment.In the same year,they engineered a“double-lock”molecular probe(NML)for accurate bioimaging[28].As shown in the Fig.6,NML consists of three parts:leucine aminopeptidase(LAP)cleavage substrate,MAO cleavage substrate and HCy fluorophore.Different from probes based on single-lock strategy,NML with double-lock strategy needs to be activated when both LAP and MAO are stored,but keep fluorescence quenching when either enzyme is missing or inhibited.The FL intensity of NML at 720 nm is correlated with the pathologically high levels of LAP and MAO expression in liver disease.Equipped with the“dual lock”mode,the imaging accuracy of drug-induced liver injury of NML is higher than that of the“single lock”probe.Through the changes in the intensity of LAP and MAO in the serum,NML can not only distinguish mice with liver cirrhosis and drug-induced liver injury at different stages,but also distinguish normal,drug-induced liver injury and liver cirrhosis mice.
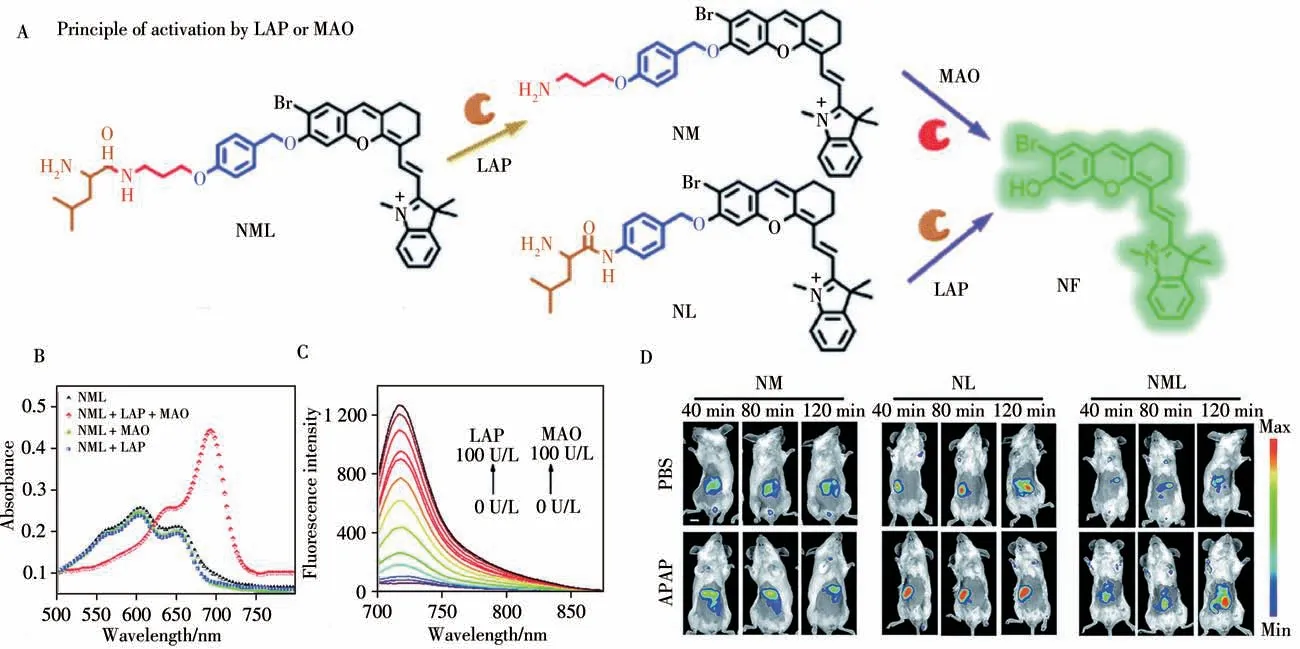
Fig.6 Design strategy of the enzyme-activated molecular probe and its activation by target enzymes(A),normalized absorption spectra of NML(10μmol/L)before and after the reaction with LAP(100 U·L-1)or MAO(100 U·L-1)(B),emission spectra of NML(10μmol/L)at different concentrations of LAP and MAO(C),representative images of BALB/c mice which received PBS and APAP(200 mg/kg,100μL,i.p.)for 1 h,followed by NM,NL and NML(50μL,100μmol/L,i.v.)for different durations,respectively(D)[28]λex=640 nm andλem=695-770 nm,scale bar:1 cm
1.2 Ratiometric fluorescence imaging
These“turn-on”probes relying on the FL change in single channel can be prone to suffer the fluctuations from instrumental parameters,the concentration of the probe,excitation intensity and biological environment.Fortunately,ratiometric probes by adjusting the emission signals of two channels at the same time can effectively overcome the interference of external factors and provide built-in correction for quantitative analysis[29].In 2014,Ma and colleagues reported a ratiometric probe Lyso-pH with insertion morpholine into a stable HCy skeleton,which is a new lysosome-targeting probe[30].Lyso-pH was successfully used to study the change of lysosomal pH with temperature.With the help of FL imaging,they revealed for the first time that the increase of lysosomal pH during heat shock and the irreversibility of this process.Lyso-pH may have more applications in detecting pHi n vivoand exploring the function of acid lysosomes in cells.After that,Yang and colleagues studied a series of resolution-tunable ratiometric NIR FL probes to monitor pH changesin vi voin 2015[31].The probes with different substituents show different red shifts from 31 to 76 nm between the two NIR channels.Among them,NIR-Ratio-BTZ modified with benzothiazole electron-withdrawing substituent shows two well-resolved emission peaks(the maximum emission shift is about 76 nm),which can be considered as one of the high-resolution pH imaging of living cells and organisms.In addition,it has an ideal pKa value(pKa≈7.2),which can be used to monitor small fluctuations in physiological pH close to neutral.In 2018,Zhang et al.designed the probe NIR-HMA,which has dual recognition sites for simultaneous visualization of different degrees of hypoxic microenvironment[32]. In a hypoxic microenvironment, over-expressed nitroreductase(NTR)catalyzes the conversion of NIR-HMA to NIR-MAO,resulting in significant FL enhancement at 710 nm.In the process of hypoxia-induced mitochondrial phagocytosis,the hydroxyl group of NIR-MAO protonated to NIR-MAOH with FL at 675 nm.Therefore,NIR-HMA can be used to observe hypoxia induces mitochondrial phagocytosis of cancer cells and normal cells.Recently,Ye and colleagues reported a GGT-specific ratiometric FL probe 1 which combining a BODIPY fluorophore with HCy dye in 2021[33].As shown in the Fig.7,probe 1 consists of four parts:a GGT cleavable substrateγ-Glu,an autolyzed linker 4-aminoase alcohol(PBAB),a quenched NIR fluorophore mCy-Cl and a“always on”BODIPY fluorophore.BODIPY fluorophore with high fluorescence quantum yield and high light stability to biological environment serves as fluorescent internal standard.Probe 1 initially showed a strong FL of BODIPY at 517 nm while the NIR FL of mCy-Cl is quenched.After interacting with GGT,theγ-Glu is cleaved and then triggers the spontaneous elimination of PBAB to form the FL product 2 containing uncaged mCy-Cl.Therefore,the NIR FL at 735 nm is restored and the FL of BODIPY at 517 nm is maintained,providing an activatable ratio signal(I735/517)for the detection of GGT activity.Through the ratiometric imaging of GGT activity,probe 1 can reveal the different expression levels of GGT in different tumor cells and biological tissues.
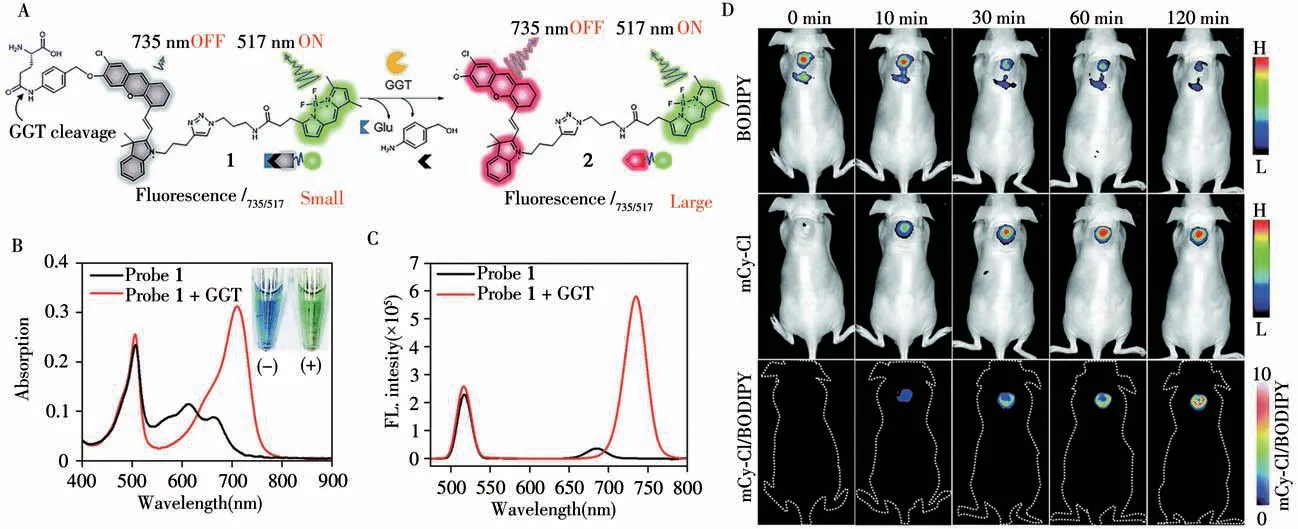
Fig.7 The chemical structure of probe 1 and the proposed chemical conversion into 2 in response to GGT(A),UV-Vis absorption of probe 1(5μmol/L)before(black)and after(red)incubation with GGT(500 U·L-1)at 37℃for 90 min(B),fluorescence spectra and probe 1(2μmol/L)before(black)and after(red)incubation with GGT(500 U·L-1)at 37℃for 90 min(C),fluorescence imaging of GGT activity in subcutaneous HeLa-tumor-bearing mice with probe 1(D)[33]BODIPY:λex/λem=480/520±20 nm;mCy-Cl:λex/λem=660/710±20 nm
2 Hemicyanine dyes for multimodal imaging
No single modality imaging is perfect and sufficient to obtain all the necessary information.Multimodal molecular imaging is the synergistic combination of two or more detection techniques,which enhance the visualization of biological materials and the reliability of collected data.The advantages of one technique can be combined with the advantages of another,while at the same time reducing the disadvantages of both.Therefore,multimodal molecular imaging may offer the prospect of improved diagnostic and therapeutic monitoring abilities[8-9].
2.1 Fluorescent/photoacoustic(FL/PA)dual-modal imaging
In the past few years,fluorescent/photoacoustic(FL/PA)dual-modal imaging has emerged as a promising method.Fluorescence imaging has superb sensitivity,but it is insufficient to visualize deep tissues.By contrast,PAI is an emerging imaging modality that combines laser excitation with acoustic detection,which offers large penetration depth(7-10 cm)and high spatial resolution(100μm).Therefore,various organic and inorganic materials with NIR absorption have been used for FL/PA dual-modal bioimaging analysis.Among them,HCy dye is a kind of good candidate,which has been widely exploited for FL/PA imaging[34-36].In 2018,Wu and colleagues developed two probes(C1X-OR1and C2X-OR2)for detecting and imaging liver injury and tumor metastasis[37].Under the catalysis of ALP,C1X-OR1can be used to diagnose and monitor drug-induced liver injury and subsequent recovery by imaging liver ALP activity.After the cleavage byβgalactosidase(β-Gal),C2X-OR2can be applied to image metastasis from abdominal ovarian cancer and lymphatic metastasis.The structural transformation mediated by these biomarkers leads to the generation of FL/PA signal thereby realizing dual-mode sensing/imaging of biomarkers.After that,they reported the FL/PA probe DLP which can diagnose drug-induced liver damage by imaging the increased levels of LAP in the liver of mice in 2019[34].The presence of LAP mediates the cleavage of the amide bond of DLP and initiates the release of HCy with FL/PA signal.DLP can track endogenous LAP in living cells and detect the increase in LAP levels to diagnose and monitor drug-induced in vivo liver damage.
In 2018,Pu et al.reported a probe CySO3CF3for FL/PA dual-modal imaging endogenous ONOO-in living systems[38].CySO3CF3does not have FL/PA characteristics because of its caged state and the electrondonating ability of oxygen atom is weak.In the presence of ONOO-,CySO3CF3is converted to CySO3OH with enhanced electron-donating ability of oxygen atom through a series of cascade oxidation elimination reactions.Thus,CySO3CF3not only specifically detects ONOO-in solution and cells with the limit of detection down to 53 nmol/L but also allows for FL/PA dual-modal imaging of ONOO-in the tumors of living mice.After that,in 2020,they developed a FL/PA polymeric renal reporter(FPRR)with a renal clearance enabler polymeric moiety(dextran)for real-time non-invasive imaging drug-induced acute kidney injury(AKI)[39].The hydroxyl group of CyOH is encapsulated by a self-immolative linker connected to the GGT-cleavableγ-Glu moiety to reduce the electron-donating ability of the oxygen atom.In the presence of GGT,the amide bond next toγ-Glu of FPRR can be specifically cleaved.FPRR not only showed high renal clearance efficiency(78%ID 24 h after injection)in live mice,but also specifically reacted with biomarkers in the kidney,turning on its FL/PA signals and reporting the occurrence of AKI.
In 2020,we designed a mitochondria-targeted probe TPP-HCy-BOH for FL/PA dual-modal imaging of excess H2O2in inflammation[40].After enters the cell,TPP-HCy-BOH can effectively accumulate on the mitochondria.The excess H2O2produced in inflammatory cells will quickly release free HCy(TPP-HCy)by removing the BOH part,which turns on FL/PA signal on the mitochondria with excellent sensitivity.Compared with the control group HCy-BOH without mitochondria targeting,TPP-HCy-BOH is more competent to image LPS-induced acute inflammationi n vivowith additional 1.6-fold higher sensitivity of FL in abdomen and 2.0-fold higher sensitivity of PA in liver.Encouraged by this work,we then developed two FL/PA probes(HCy-Cit-Val and HCy-Gly-Leu-Phe-Gly)to image the over-expressed cathepsin B(CTB)i n vivoin 2021[41].As shown in the Fig.8,probes are composed of two parts:HCy dye is used as the source of FL/PA signal and Val-Cit and Gly-Leu-Phe-Gly are the specific substrate of CTB.Due to the caged amino group of HCy,the FL/PA signal of HCy-Cit-Val or HCy-leucine-glycine is“off”.After entering tumor cells,HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly can be specifically cleaved by the over-expressed CTB in the lysosome and release free HCy-NH2that activate FL/PA signals.HCy-Cit-Val and HCy-Gly-Leu-Phe-Gly successfully monitor CTB activity with high sensitivity and spatial resolutioni n vi vo.Moreover,the property of HCy-Cit-Val is superior to HCy-Gly-Leu-Phe-Gly due to the higher catalytic efficiency of CTB toward HCy-Cit-Val than HCy-Gly-Leu-Phe-Gly.
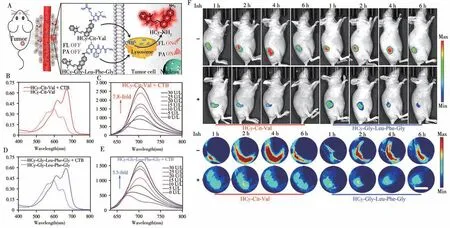
Fig.8 Schematic illustration of HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly for FL/PA imaging of CTB in tumor cell(A),visible absorption spectra(B)or(D)and FL spectra(C)or(E)of 25μmol/L HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly without or with 30 U/L CTB for 2 or 16 h,time-course FL images and MSOT images(F)of HeLa tumor-bearing nude mice in group HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly which were i.t.injected with HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly(250μmol/L,40μL)and group Inh+HCy-Cit-Val or Inh+HCy-Gly-Leu-PheGly which were i.t.injected with CA-074-Me(40 mmol/L,40μL)for 30 min and followed by HCy-Cit-Val or HCy-Gly-Leu-Phe-Gly(250μmol/L,40μL)[41]scale bar:3 mm
2.2 Fluorescent/magnetic resonance(FL/MR)dual-modal imaging
Magnetic resonance imaging(MRI)can produce anatomic images with unlimited tissue-penetration depth and high spatial resolution to promote preoperative detection of deep tissues while NIR FLI can generate images with high sensitivity for detecting low concentrations of biomarkers and image-guided therapy.Therefore,FL/MR dual-modal imaging possess high sensitivity and spatial resolution for molecular imaging and surgical guidance.In 2019,Ye and colleagues designed and synthesized a small molecule-based activatable FL/MR dual-modal probe P-CyFF-Gd for molecular imaging[42].As shown in Fig.9,P-CyFF-Gd consists of three parts:(1)quenched NIR fluorophore(HCy-Cl)with ALP recognition group(-H2PO3);(2)paramagnetic DOTA-Gd chelate;(3)hydrophobic dipeptide Phe-Phe(FF)linker that promotes selfassembly.In the presence of hydrophilic-H2PO3and DOTA-Gd groups,P-CyFF-Gd was a water-soluble small molecule probe exhibiting weak FL and lowr1relaxivity.After dephosphorylated by ALP,P-CyFF-Gd can self-assemble into nanoparticles with enhanced FL andr1relaxivity.P-CyFF-Gd can be used for noninvasive measurement and localization of ALP activity in live tumor cells and live mice.Moreover,it has been successfully used to locate the edge of orthotopic liver tumors in mice during surgery and allow for real-time image-guided liver tumor resection.
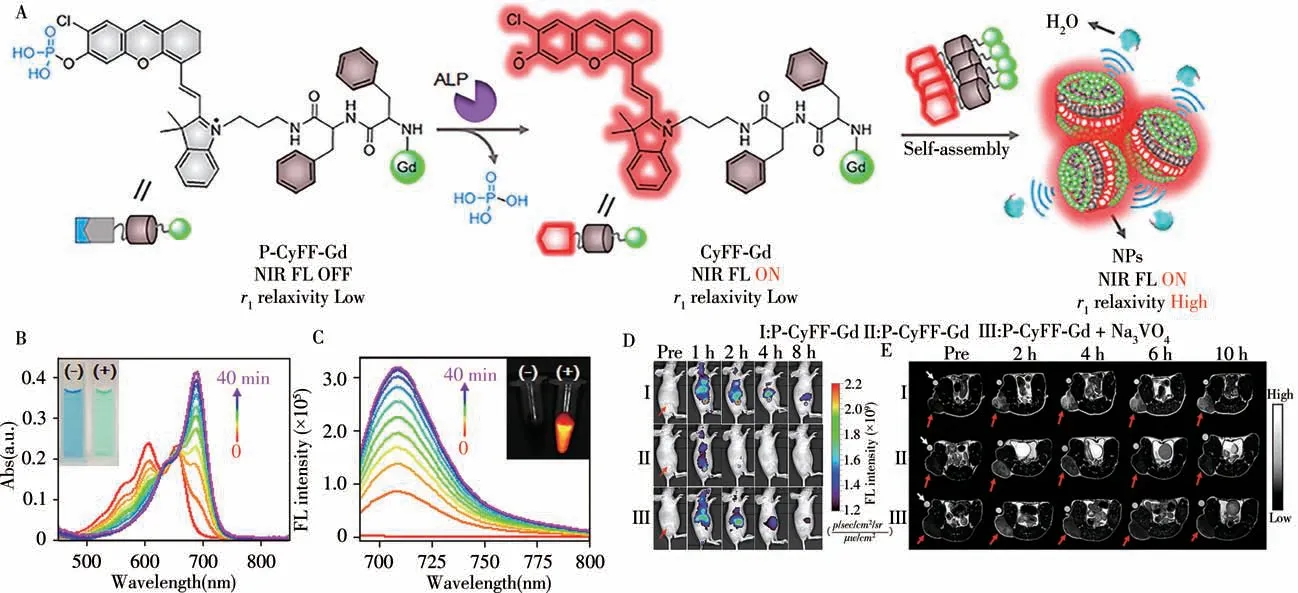
Fig.9 Chemical structure of P-CyFF-Gd and proposed ALP-mediated NIR fluorogenic reaction and in situ self-assembly of P-CyFF-Gd into NPs that show increased NIR FL and r1 relaxivity(A),UV-Vis absorption(B)and fluorescence spectra(C)of P-CyFF-Gd(5μmol/L)incubated with the ALP(0.1 U/mL,37℃)for 0-40 min,longitudinal FL imaging(D)of mice receiving i.v.injection of P-CyFF-Gd(I),P-Cy-Gd(II),or P-CyFF-Gd(50μmol/L,200μL)together with i.t.injection of 10 mmol/L Na3VO4(50μL)(III)at 0,1,2,4,and 8 h,T1-weighted MR images(E)of HeLa tumor-bearing mice receiving i.p.injection of P-CyFF-Gd(I),PCy-Gd(II),or P-CyFF-Gd(0.015 mmol·kg-1 Gd3+)together with i.t.injection of Na3VO4(10 mmol/L,50μL)(III)[42]
2.3 Fluorescent/chemiluminescence(FL/CL)dual-modal imaging
In contrast to FL,the signal-to-noise ratio of chemiluminescence(CL)is greatly improved because of the reduction or suppression of autofluorescence without light excitation.FL/CL dual-modal imaging probes has been rarely exploited for molecular imaging.In 2019,Pu and colleagues reported a unimolecular chemo-fluoroluminescent reporter(CFR)for crosstalk-free duplex imaging of drug-induced hepatotoxicity(DIH)[43].This is the first optical small molecule probe with two independent activation channels.As shown in the Fig.10,CFR simultaneously detects superoxide anion(O2·-)and caspase-3(casp3)through respective activation of its independent CL and FL channels.This crosstalk-free duplex imaging capability of CFR enables longitudinal measurement of two correlated biomolecular events(oxidative stress and cellular apoptosis)during the progression of DIH.Importantly,this study provides a general molecular design strategy for duplex imaging.In the same year,they further reported a renal-clearable duplex reporter(ADR)for real-time imaging of contrast-induced acute kidney injury(CIAKI)in a mouse model[44].The FL/CL signal are turned on in the presence of superoxide anion(O2·-)and N-acetyl-β-D-glucosaminidase(NAG)respectively.When applied to real-time imagingin vivo,ADR can detect sequential upregulation of O2,O2·-and NAG in the kidneys of living mice prior to a significant decrease in glomerular filtration rate(GFR)and tissue damage in the course of CIAKI.
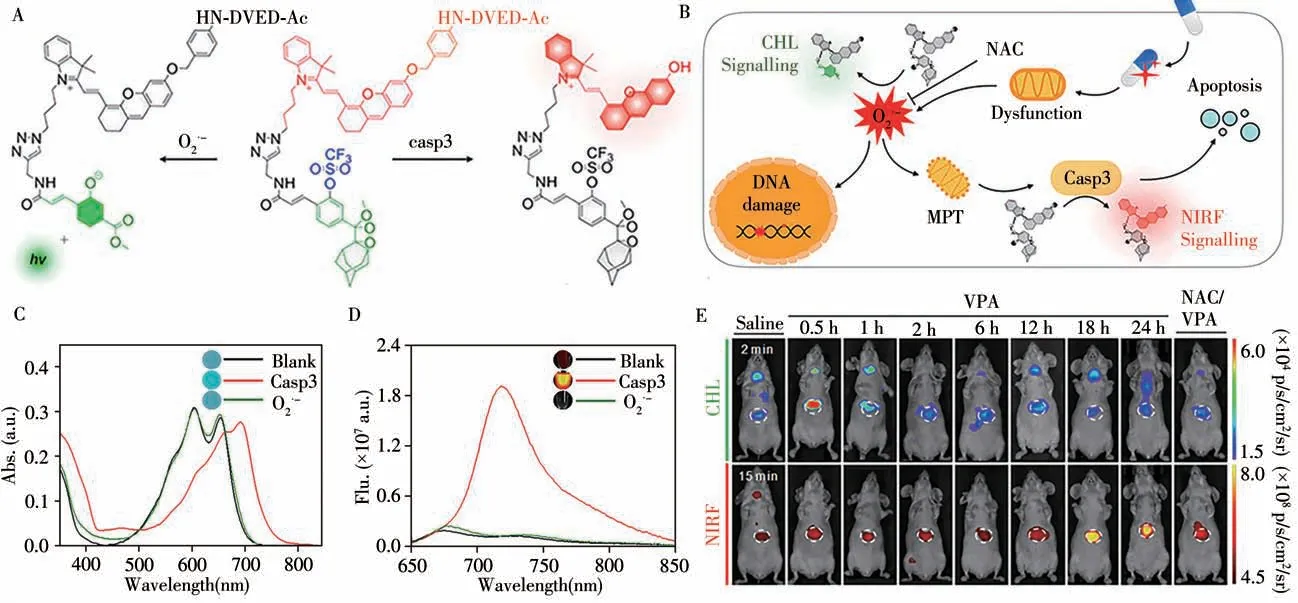
Fig.10 Duplex imaging mechanism of CFR(A),schematic illustration of how CFR sequentially detects the upregulation of O2·-and casp3 during the progression of DIH(B),UV-Vis absorption spectra(C)and NIRF of CFR(10μmol/L)(D)in the absence or presence of casp3(0.1 U/mL)or O2·-(50μmol/L)in PBS(10 mmol/L,pH 7.4)at 37℃,representative CHL images(E)of living mice at 2 min and NIRF images at 15 min after i.v.injection of CFR in different treatment groups[44]the white circles indicate the site of livers
3 Conclusion and outlook
This review summarized a series of activatable probes based on HCy dye for FL and multimodal imaging(Table 1).Particular attention was given to their design strategies,detection mechanisms,and their applications as bioimaging agents.A number of HCy probes have been reported through influencing the ICT process within these molecules,which always display large stokes shifts and ratiometric features toward a variety of biological analytes and processes.Despite great advances that have been made in this area,there is still much scope for improvement.For instance,only a few HCy probes were reported to realize bioimaging within mitochondria or lysosomes,and none in the endoplasmic reticulum,Golgi apparatus,and others.Therefore,activatable HCy probes of subcellular imaging expectantly been designed for biological applications.Moreover,the relatively short excitation and emission wavelengths of HCy embarrass the biological uses.Thus,future efforts aimed at designing new HCy probes will need to focus on how to red shift their absorption and fluorescence spectra.
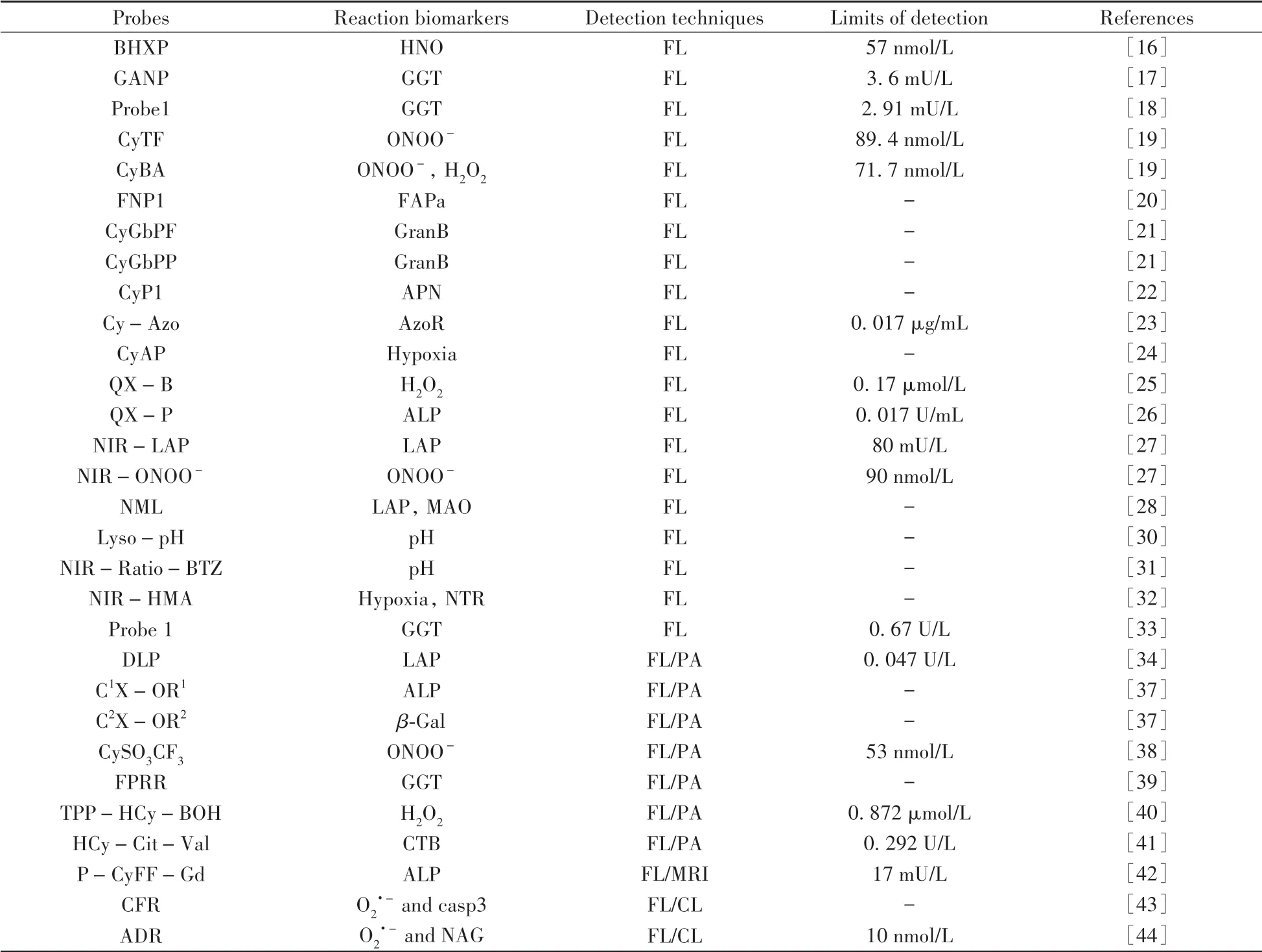
(Table 1 to be continued)
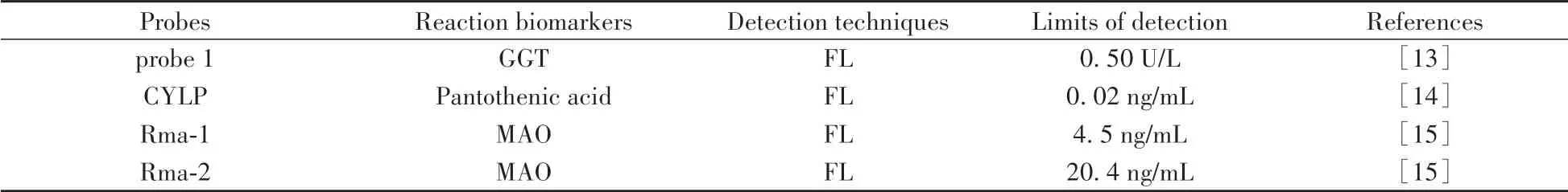
Table 1 The summary of reaction biomarkers,detection techniques and detection limits for probes mentioned in this review
- 分析测试学报的其它文章
- 碱性磷酸酶的体外检测和体内成像研究进展
- Recent Progress in Nanoscale MOFs for Biological Imaging of Tumors and Tumor Markers
- I-Motif-based Nanosystems for Biomedical Applications:p H Imaging,Drugs Controlled Release and Tumor Theranostics
- A Low-cost,Automated Nucleic Acid Extraction System Converted from the Open-Source Rep Rap 3D Printer
- Research Progress on Analytical Methods for Deciphering Adenosine-to-inosine RNA Editing
- CRISPR/Cas Based Biosensing Platforms for Molecular Diagnosis

