Recent Development in Defects Engineered Photocatalysts:An Overview of the Experimental and Theoretical Strategies
Zaiba Zafar,Shasha Yi*,Jinpeng Li,Chuanqi Li,Yongfa Zhu,Amir Zada,Weijing Yao*,Zhongyi Liu,and Xinzheng Yue*
Recently,defect architectured photocatalysis is proved to be the most versatile choice for high solar to chemical energy conversion processes.Defect engineering strategies are of great demand to effectively tune the electronic microstructure and surface morphologies of semiconductors to boost charge carrier concentration and extend light harvesting capability.This review provides a comprehensive insight to various kinds of defects along with their synthesis procedures and controlling mechanism to uplift photocatalytic activity.In addition,the contribution made by defects and material optimization techniques toward electronic band structure of the photocatalyst,the optimal concentration of defects,the key adsorption processes,charge distribution,and transfer dynamics have been explained in detail.Further,to clarify the relationship between photocatalytic activity and defect states in real,a comprehensive outlook to the versatile photocatalytic applications has been presented to highlight current challenges and future applications.Defect engineering therefore stands as the next step toward advancement in the design and configuration of modern photocatalysts for high efficiency photocatalysis.
Keywords
defect engineering,electronic structure,photocatalysis,vacancies
1.Introduction
Photocatalysis is considered as one of the most appealing advanced technology in solution to the environmental problems and non-renewable energy resources depletion with a variety of applications such as synthesis,industry,water,and environmental remediation.[1-5]Although enormous research has been carried out with impressive advancement in photoactive materials,the process of photocatalysis still suffers from low efficiency and poor stability that is far below the requisites for practical applications.The three main key steps that governs photocatalysis includes light absorption,generation of electron-hole pairs,followed by their migration from the bulk to the surface and initiation of interfacial redox reactions upon their arrival at the active sites.[6,7]In order to boost up the photocatalytic efficiency,various approaches have been adopted such as doping,crystal facet exposure,synthesis parameters,reaction environments,hybridization,dimensions,and morphology tuning of photocatalysts.[8-11]However,the efficiency of these photocatalysts is still limited by their cost,wide band gap,lower stability and poor charge transfer kinetics.[12]In order to address the aforementioned issues,extensive research has been conducted to increase the surface area,such as nanowires,nanosheets,nanotubes,and other hierarchical nanostructures are developed with abundant active sites for redox reactions.[13-17]Similarly,to enhance charge separation,hetrojunctions were explored utilizing different semiconductors with appreciable band alignment.[18-20]Alternatively,effective light absorption with undoubtedly decreased recombination and improved photocatalytic performance through defect engineering have been reported.[21,22]Defects in semiconductors greatly alter carrier concentration and interface reaction affecting the overall process efficiency of a photocatalyst.Thus,defects are the most frequently investigated scenario for tuning the properties of photocatalyst.[23,24]However,defects are detrimental to photocatalysis in regard of the recombination centers and lack detailed explanation in terms of carrier concentration,transfer dynamics,band structure,and interface profiles.[25]The emerging trend of defect engineering still brought the opportunity of deliberately manipulating the photocatalyst properties.Although the influence of defects on photocatalytic performance has been previously elaborated in some reviews,their optimization and intrinsic role in photocatalysis is still elusive.[12,25-28]Therefore,some novel strategies based on advanced modeling theoretical and experimental knowledge are of great need to elucidate the role of defects in photocatalysis.Subsequently,it has been suggested that different synthesis strategies offer different mechanisms thus altering the band structure,carrier mobility,and so reactivity(Figure 1).It therefore remains a challenging task to account the relationship between defect chemistry and performance of corresponding photocatalyst.Therefore,it is of great need to address the importance of defect chemistry with successive ratio and formation mechanism for a step forward photocatalysis.

Figure 1.Defects induced levels in semiconductor-based photocatalysts for water splitting.
This review focuses on state of the art defective photocatalysts,their classification relative to the atomic structure and location,synthesis strategies,and band structure modulation for photocatalytic applications(Figure 2).Moreover,recent advancement in defect optimization has been systematically summarized with combined theoretical and experimental investigations.Various characterization techniques for defect identification have been highlighted with an overview to the reaction profiles at semiconductor interfaces.Further,the crucial role of defects in photocatalytic processes such as water oxidation,CO2reduction,pollutant degradation,organic synthesis,and nitrogen fixation has been discussed.In the last,current challenges with future development have been prospected.

Figure 2.A schematic illustration of defect chemistry of photocatalysts from design to visualization and photocatalytic applications.
2.Defect Chemistry and Photocatalysis
2.1.Defect Types and Classification
Defects are generally classified into various categories based on their atomic structure or location in photocatalysts materials.The structural irregularities split into four main divisions based on dimensions and vacancy.Such as point defects(doping and vacancy)or line defects(edge or screw dislocation),[29,30]planer defects(grain boundaries),[31]volume defects(voids and lattice disorders),and manifold defects[32](Figure 3).These defects can be further subdivided into more types depending upon the verity in atomic structure of the defective-photocatalyst.Simultaneously,defects can also be classified with respect to their location in semiconductor materials.According to their location in photocatalyst,defects can be divided into surface,bulk,or interface defects,respectively.In a mono-component photocatalytic system,as illustrated in(Figure 4),all the three steps including charge generation,migration,and consumption would take place in same semiconductor material.These systems have defects located either on the surface or in bulk,thus called surface and bulk defects,respectively.[33-36]Generally,the aforementioned classification of defects is obviously relevant to the respective surface or bulk defects.For instance,both kinds of point defects in photocatalysts can act as bulk and surface defects.[33,34]
Similarly,dislocations and boundaries can be regarded as surface defects owing to their exposed terminals on the crystalline surface.[37]Volume defects resemble much to point defects and can exist on both surface or in bulk of semiconductor materials.[38]It must be mentioned that,defects with different locations(bulk and surface)and atomic structures can co-exist in same photocatalyst.Like in case of multicomponent photocatalytic system where two or more elements combines to create a mutual interface.The photocatalytic charge generation and consumption would therefore proceed in different components via the interface.Thus,in multicomponent systems,defects can be created in other components than semiconductor such as co-catalyst[39-41]and photosensitization.[42-44]On the other hand,interfacial defects are also involved in hybrid photocatalytic systems(Figure 4b).[45-47]The interfacial defects are mainly originated from the surface defects of the components in contact.Similar to surface defects,interfacial defects can also exist as point defects,line and planer defects.

Figure 3.Schematic illustration of various defects with local atomic structures in the photocatalysts.Reproduced with permission from Ref.[12]Copyright 2020,Wiley-VCH.

Figure 4.Schematic illustrating the defects at the different locations in a)mono-component and b)multicomponent photocatalytic materials.Reproduced with permission from Ref.[25]Copyright 2018,Elsevier.c)Recombination pathways of photogenerated electrons and holes on TiO2with surface and bulk defects.Ref.[56]Copyright 2013,Royal Society of Chemistry.
2.1.1.Influence of Surface/bulk Defects on Photocatalytic Activity
The role of surface and bulk defects in photocatalysis is extensively researched,though the exact role is still not very explicit.[48-51]Typically,both surface and bulk defects are considered as recombination centers reducing the overall photocatalytic performance.[1]However,it has been found that anion vacancies highly contribute to enhanced photocatalytic efficiency.Kong et al.reported that an increased ratio of surface defects could lead to a higher photocatalytic activity and efficient charge migration.[52]Similarly,Liu et al.demonstrated the higher stability and enhanced photocatalytic activity of defective TiO2via theoretical investigation.[53]It is also revealed that sub-surface VOare less vulnerable to reactive oxygen species than surface VOwith a certainly lower formation energy than the latter.Defects can greatly affect the band structure of photocatalyst with deliberately regulated positions.Zhang et al.reported that both surface and bulk defects alter the conduction band(CB)minimum in TiO2.[54]As explained earlier,bulk defects act as trap states for photogenerated charge carriers causing electronic delocalization,decreased reactivity,and low photocatalytic efficiency.For instance,Zhu et al.synthesized BiPO4with bulk defects inhibited charge separation and decreased photocatalytic performance.[55]Meanwhile,several studies have implied the synergistic role of surface and bulk defects in photocatalysis,promoting the absorption of light and donor density.[54]Therefore,more detailed studies are needed to comprehend the contrary opinions in the literature.
As a matter of interest,the typical role of surface and bulk defects in photocatalytic process have been reviewed.For example,in case of defect-free TiO2,the absorption of photon energy hν≥ Egresults in generation of photogenerated electron-hole pairs,some of which rapidly undergo recombination while a few reaches the surface of TiO2performing redox reaction.However,in case of TiO2with bulk and surface defects the situation is slightly altered by a number of events taking place in parallel to the photo-redox reaction.
The photogenerated charge carriers can get trapped in the bulk defects,while the trapped holes may act as new recombination centers as the latter is no more available for photocatalytic reaction,as illustrated in Figure 4c.Alternatively,the photogenerated holes can be captured by the surface defects thus facilitating electron-hole separation.Further,these captured photogenerated holes are readily available to redox reactions promoting the photocatalytic activity.Thus,the existence of surface defects in TiO2can highly promote the photocatalytic activity.[56]In addition,not only the sub-surface defects but even bulk VOin CeO2is found to significantly improve water oxidation performance.[57]
2.2.Anion Vacancies
Anion vacancies are one of the most widely investigated defects among metal oxides.In general O,N,and S vacancies are the most common anion vacancies in photocatalysts.[26]Among these anion defects,VOs are the most frequently studied for photocatalytic applications.Both experimental and theoretical calculations have shown that VOserves as backbone for heterogeneous catalysis by providing adsorption sites which strongly influences the photocatalytic activity.[58-61]The electronic structure and charge transport in TiO2are closely related to the vacant oxygen sites,[58,62-64]that leads to the formation of unpaired Ti3+centers and introduce donor levels to the electronic structure of TiO2.[65,66]Besides these,VOalso affects the rate of chemical reactions that depends on the recombination of electron-hole pairs.[67]The excess electrons due to VOgreatly affect the adsorption of key adsorbates such as TiO2,O2,and water on the surface.[60,68]VOis created via fast heating in atomically thin In2O3through phase transformation from In(OH)3.The existence of Vo could be estimated through X-ray photoelectron spectroscopy(XPS)and electron paramagnetic resonance(EPR)with their characteristic peaks at 531.4 eV and 2.004,respectively.[69]In addition to thickness,the electronic structure and Vo in ultrathin In2O3could also be controlled very well.[68]
Similarly,single crystal nanosheets(NSs)of tungsten oxide(WO3)with abundant surface VOhave been synthesized through a two-step post-treatment alcohothermal approach,followed by vacuum H2annealing(Figure 5a).The resultant-induced surface VOcould lead to the improved light harvesting due to creation of localized surface plasmon resonance(LSPR)in the corresponding robust 2D-semiconductor.[70]The introduction of VOcauses the resultant yellowish WO3-xto appear as drab olives with a dimeter in the microscale level to an average thickness of about 15-20 nm represented as WO3-x-VT and WO3-x-HT in Figure 5b.Furthermore,the high-resolution transmission electron microscopy(HRTEM)investigation has revealed that the corresponding vacuum and hydrogen treatments could lead to the formation of surface disorder layer,as shown in Figure 5b.[71,72]These anionic vacancies shift the optical absorption beyond the band edge position of 480-700 nm,due to the induced discrete energy levels below the CB.[73,74]Thus,theoretically there are three different channels(Figure 5c)available for light harvesting;i)excitation of electrons from VB maximum to CB minima,or ii)electronic excitation to the VOs,and iii)LSPR excitation.The light harvesting efficiency of tungsten oxides in the absence of a co-catalyst could be proved further via photocatalytic OER of water under UV or visible irradiation(Figure 5).No significant oxygen evolution could be observed under near-infrared irradiation,which could be due to the insufficient LSPR to carry oxidation of water(Figure 5e).However,under simulated light of 1.5 AM,WO3-x-HT shows highest specific activity of 1593 μmol h-1gcat.-1which is much higher than the reported WO3NSs under similar chemical environments.Also,it further increases with the corresponding H2and vacuum treatment.[75,76]Moreover,the effect of additional infrared light photocatalytic OER of WO3-x-HT has also been investigated.The results in Figure 5d clearly demonstrate that the additional infrared radiations could promote OER to a certain extent which remain unaffected in solely infrared light.This can be attributed to the increased charge carrier separation.WO3NSs-based catalyst systems should,therefore,be coupled with a suitable co-catalysts for potential photocatalysis.[70]However,despite these factors,high temperature treatment could also lead to unwanted phase transition.In order to address the issue,various strategies have been put forwarded.Han et al.synthesized Fe@TiO2nanoparticles(NPs)with surface VOs through simple pH treatment(Figure 5f).[77]The comprehensive structural and chemical elucidation have revealed that the optimization of pH could lead to the promotion of grain boundaries on anatase Fe@TiO2surface which predominantly increase the photocatalytic activity with significant stability in basic environment.The photocatalytic decomposition experiments(Figure 5g,h)under dark/UV irradiation have shown significant 4-CP degradation,owing to the increased density of VOs over the larger surface area with significant electron-hole pairs generation to produce appreciable amount of OH radicals to conduct degradation process.[78,79]The corresponding photocatalytic degradation system with conceptual band gap illustration has been shown in Figure 5i.

Figure 5.a)Schematic illustration describing the formation of tungsten oxide single crystal NSs.b)SEM and HRTEM images of WO3,WO3-x-VT,and WO3-x-HT NSs.c)Band level arrangements of tungsten.d)Promotion of photocatalytic oxygen evolution over WO3-x-HT under monochromatic light at λ=350,405,420,475,and 550 nm by the introduction of near-infrared light(780-2000 nm).e)Photocatalytic oxygen evolution over WO3,WO3-x-VT,and WO3-x-HT NSs under different illumination conditions.Reproduced with permission from Ref.[70]Copyright 2015,Wiley-VCH.f)Steps used to fabricate pH-modified Fe-doped TiO2NPs.g)Photocatalytic degradation of 4-CP and h)radical-induced formation of p-hydroxybenzoic acid(p-HBA)from BA reacted with three different Fe@TiO2NPs.[Fe@TiO2]=0.4 g L-1,[BA]0=10 mM.The photocatalytic degradation property of bare TiO2NP is included for comparison.i)Conceptual illustration of band gap structure and photocatalytic decomposition mechanism of Fe@TiO2NP treated under basic condition.Reproduced with permission from Ref.[77]Copyright 2019,Elsevier.
Besides VO,sulfur vacancies(VS)are the extensively studied defects in water splitting.For instance,Du et al.synthesized VSin monolayer ZnIn2S4with enhanced photocatalytic H2evolution owing to the unique structure of the photocatalyst with induced VS.[69]Similarly,VSrich hybrid molybdenum(Mo)structures with increased photocatalytic activity have been widely researched.[80]As a suitable alternative to nobel metals,VSengineered 2D-transition metal dichalcogenides(TMDs)have also been investigated for efficient H2evolution reaction.[81]Various strategies have been adopted to optimize charge transfer resistance and the intrinsic performances of these TMDs.For example,Voiry et al.elaborated the role of VSin MoS2NSs for efficient HER performance.[82]The HER activity is inferred from the existence of two different domains of vacancies,that is,point defects at low concentration of VS,while the higher concentration of VSresults in uncoordinated Mo regions due to stripping of the S atoms,as shown in Figure 6a-d.The intrinsic performance evaluated from the turnover frequency(TOF)demonstrated the significantly increased catalytic activity of the as-synthesized MoS2NSs(Figure 6e).Therefore,the combination of local point defects and the stripped Mo regions could serve as an efficient strategy for optimizing active sites in MoS2.Similarly,the catalytic performance of Ru/MoS2can be attributed to the two types of defects,that is,1T-phase MoS2and the corresponding VSwhich devotes to the increased active sites and good conductivity.[80]The higher concentration of defects leads to outstanding HER performance with lower onset potential,suggesting the vital role of VSdefects in catalytic performance of 2D-TMDsbased catalysts.
Nitrogen vacancies(VN)are another important class of anion defects in semiconductor materials.These VNcan highly overcome the recombination of photogenerated charge carriers thus promoting the overall quantum efficiency.For instance,g-C3N4enriched with VNhave been extensively studied with expanded optical response.Various methods are adopted for engineering N defects in g-C3N4,such as hydrothermal synthesis,[83]temperature-controlled polymerization,[84]and hydrogen reduction.[85]VNare also considered as one of the fundamental defects in design of metal-free catalysts,for example,poly(N-ethyl-benzene-1,2,4,5-tetracarboxylic diimide) and porous carbon with significant catalytic performance.[86,87]Metal-free catalysts are now being highly persuaded due to their unique physical and chemical characteristics.Among metal-free photocatalysts,polymeric carbon nitride(PCN)has attracted tremendous attention due to its promising visible light photocatalytic activity.[88]Furthermore,in contrast to conventional semiconductor photocatalysts,PCN exhibits low-defect formation energy.For instance,the loss of C or N atoms from PCN occur at considerable ease owing to their small atomic radii and weak lattice distortion thus making the creation of bulk defects comparatively easy than traditional semiconductors with more distorted lattice and greater vacancy formation energy.Generally,the intrinsic defects in PCN could be classified as VN,carbon vacancy and also including the derivative functional groups,that is,amino,cyanamide,and cyano groups,respectively.The introduction of these defects creates mid-gap states below the CB minimum affecting the catalytic activity.For example,Tu et al.reported PCN NSs with controllable VN.[89]The VB-XPS spectra were utilized to determine the position of these mid-gap states.As shown in Figure 7d,a gradual decrease in the position of these mid-gap states can be clearly observed relative to the increased concentration of VN.Nevertheless,further increase in concentration of these VNwould result in much deeper mid-gap states which latter contributes to the recombination of charge carriers.Besides VNdefects,the functional groups implanted over the edge of PCN NSs such as the amino groups(-NH/NH2)and cyano groups(-C≡N)are also considered as defects which actively participates in molecular adsorption.[90]For example,Liu et al.induced cyano groups on deficient carbon nitride(DCN)constructing a p-n junction(Figure 7h).[91]The corresponding cyano and amino groups could effectively impart nor p-type conductivity to PCN.Furthermore,the electron acceptor cyano groups highly promotes charge transfer and separation by traping the photogenerated charge carriers.Xue et al.synthesized porous PCN with VNand cyano groups with extended carrier lifetime and significantly decreased recombination.[92]Moreover,the introduction of mid-gap states inside the band gap further broadens the absorption spectrum while the catalyst can accept electrons in CB,thus preventing the recombination of photogenerated charge carriers.[93]For instance,the photocurrent density and electrochemical impedance spectroscopy(EIS)measurements of VN-deficient g-C3N4have revealed increased photocatalytic activity then the pristine g-C3N4(Figure 7i,j).Thus,on one hand,the induced mid-gap states accepts the electrons in CB while also reducing the migration path in porous NSs structure and therefore showed increased current density with a lower EIS radian.[93]
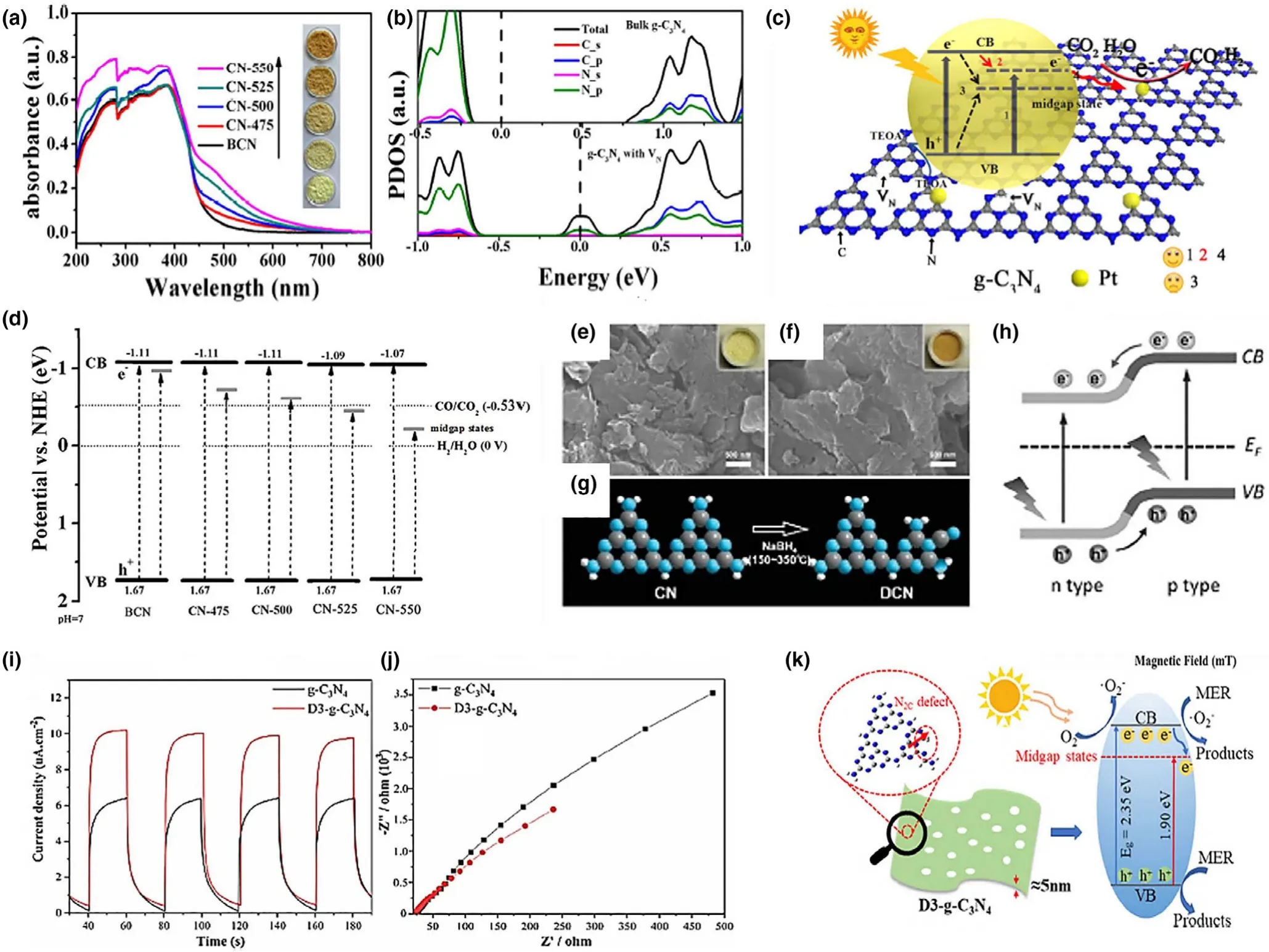
Figure 7.a)UV-visible absorption spectra of BCN and CN-x,and the photographs of the samples(the inset).b)Calculated PDOS of BCN and g-C3N4(CN)with VN.c)Schematic illustration of g-C3N4with VNfor photoreduction of CO2to CO and H2evolution.Paths 1,2,and 4 refer to the electron excitation from VB to mid-gap states,the electron trapping from CB to mid-gap states,and the electron transfer to reaction sites,respectively.Path 3 refers to deeper mid-gap states that act as recombination sites for trapping photogenerated electrons and holes.d)Schematic illustration of the electronic structure of BCN and CN-x.The gray dark lines refer to the band edge of mid-gap states,and a series of mid-gap states exist below the CB.Reproduced with permission from Ref.[89]Copyright 2017,American Chemical Society.e,f)SEM images of PCN and DCN.g)Fabrication process of DCN.h)Diagram of charge migration in pn homojunction of DCN.Reproduced with permission from Ref.[91]Copyright 2016,Wiley-VCH.i)Photocurrent density and j)EIS spectra of two catalysts.k)Photocatalytic degradation of MER over D3-g-C3N4.Reproduced with permission from Ref.[93]Copyright 2020,Elsevier.
2.3.Metal Vacancies
Besides anion vacancies,metal vacancies also have considerable effects on the physicochemical and electronic properties of metal compounds because of their specific orbital distribution with characteristic electronic configuration.[94]In contrast to anion vacancies,metal vacancies are more challenging to engineer owing to their high formation energy,hence it is more difficult to ascertain the function of positively charged voids.[95]Researchers have developed a variety of photocatalytic materials consisting of positively charged metals to investigate the role of cation vacancies in photocatalysis,for instance Ti vacancies in TiO2,[96]Zn vacancies in ZnO,[97]Bi6S2O15with Bi-vacancies.[98]Nevertheless,atomic level insights to the role of positively charged vacancies during the photocatalytic process,yet it is quiet a challenging field due to the presence of complex nanostructure like capping agents and grain boundaries.Among the low-dimensional materials,2D ultrathin atomic layers with confined positively charged vacancies serve as an ideal model for elucidation of the relationship between structure and activity.Furthermore,the atomic escape energy is relatively small in 2D ultrathin materials which permits the manipulation of appropriate cation vacancies more conveniently.Song et al.reported that Ti vacancies in single-layer H1.07Ti1.73O4·H2O NSs can contribute to the formation of ample active O species in the close proximity of the vacancy sites that binds H2O molecules by the development of surface coordination through hydrogen bonds.[99]Consequently,10.5-fold activity enhancement was observed for H2evolution as compared to its layered counterpart.The photocatalytic performance of materials like TiO2significantly depends on the electrical and optical properties which are determined typically by defects and crystal structure.[100]
Inherently,TiO2shows n-type conductivity because of the presence of VO.When the concentration of acceptor reaches to a certain level via doping,the Fermi level(EF)is shifted toward VB edge and hence alters the conductivity from n-type to p-type like that in TiO2.[101-103]Similarly,both Cr-and Fe-doped TiO2exhibits p-type conductivity and shows photocathodic current.[102]However,foreign acceptor has shown a determinant influence on the charge separation and transfer,which greatly influences the carrier mobility.[104]In addition to anionic defects in undoped TiO2,Ti has been projected as an active site for solar water splitting.[105,106]Jiao and co-workers fabricated one-unit thick cell ZnIn2S4layers with both rare and rich Zn vacancies(VZn-rich)through facile hydrothermal method under variant temperature.[107]Aberration-corrected high-angle annular dark- field scanning transmission electron microscopy(HAADF-STEM)has been applied to investigate detailed surface-atom arrangements in one-unit-cell of ZnIn2S4.The presence of VZnand the corresponding line-intensity profile have been illustrated in Figure 8.Further,to investigate additional features of both rare and rich Zn vacancies,zeta potential,photoacoustic spectroscopy(PAS),and EPR together concluded that one-unit thick cell ZnIn2S4layers with distinctive Zn vacancies have been successfully prepared.

Figure 8.Characterizations for VZn-rich one-unit-cell ZnIn2S4(ZIS)layers obtained at 200°C.a,b)HAADF-STEM images and c)intensity profile corresponding to the dark cyan arrow in b),demonstrating abundant zinc vacancies in the atomic layer,and d)the corresponding crystal structures,e)SAED pattern,f)atomic force microscopy(AFM)image and g)the corresponding height profiles,h)EPR spectra of VZn-rich and VZn-poor one-unit-cell ZIS layers.Reproduced with permission from Ref.[107]Copyright 2017,American Chemical Society.
Similarly,Zn-rich one-unit cell ZnIn2S4layers exhibited significantly improved separation efficiency of electron-hole pairs as measured by surface photovoltage spectroscopy(SPS),ultrafast transient absorption spectroscopy(UTAS),and photoluminescence(PL)spectroscopy.Moreover,the VZnincreases the light absorption capacity from 440 nm to near-infrared region and enhancing CO2adsorption capacity due to the negatively charged VZn-rich ZnIn2S4layers.[98]In addition,the spatial distribution of charge density at CB edge using DFT calculation revealed that VZncan gain charge density from nearby sulfur atoms,proposing the easy excitation of the electrons to CB.By virtue of this performance,the VZn-rich single-unit cell ZnIn2S4layers showed high CO2photoreduction activity with CO formation rate of 33.2 μmol g-1h-1,which was 3.6 times higher than that of VZn-poor counterpart.[107]In other cases,for example,vanadium(V)vacancies in mono-unit cell BiVO4layers can introduce a new defect-level in forbidden band and upsurge the concentration of hole nearby EF.[108]As a result,the electronic conductivity and charge separation efficiency of defective BiVO4layers are highly improved to contribute to the higher photocatalytic methanol formation.
2.4.Pores and Vacancy Clusters
When nearby surface atoms are escaped from crystal lattice,pits and vacancy clusters are created,which have a marked influence on both the photocatalytic performance and electronic structure of the defective material.[109-112]Generally,there are numerous benefits of the confined pits in photocatalysis:Firstly,the confined pits form defective sites close to EFthat rise the carrier concentration;second,atoms with small coordination number adjacent to pits incline to join target molecules to favor active sites for catalytic performance;thirdly,confined pits truncate the carriers migration paths and enhance separation efficiency of surface charges for enhanced photocatalytic activity.[113]Ultrathin WO3NSs with exposed(001)crystal facets and confined pits were developed by heating ultrathin WO3·2H2O at 400°C for a period of 0.5 h,and nonporous and bulk WO3at 500°C for 3 h and 10 min(Figure 9),respectively.[114]This topological chemical conversion greatly benefits the overall oxygen evolution reaction(OER)for photoanode.Under light irradiation,photogenerated holes move along the W-O-W chains in(001)facets(the x-direction),and the confined pits can therefore significantly reduce their migration path to encourage the charge separation in comparison to both bulk and nonporous WO3NSs.The pore-rich WO3thus possesses superior photocatalytic water oxidation performance.Moreover,the DFT computation depicted that the occurred pits lift the DOS to the VB top edge,which increases the number of hole carrier density and correspondingly speed up the O2evolution reaction.

Figure 9.a)Schematic illustration on the rational design thought and synthesis process of pore-rich WO3ultrathin NSs for realizing the simultaneously optimizing on the multi-limitation factors during overall oxygen-evolving photoanode reaction process.b)HRTEM of pore-rich WO3ultrathin NSs.c)The photocurrent versus applied potential curves of various WO3samples.Reproduced with permission from Ref.[114]Copyright 2016,Wiley-VCH.
2.5.Vacancy Associates
Besides mono-atomic lattice vacancies,vacancy associates can also significantly alter the characteristic properties of semiconductor materials due to multi-atomic vacancy coupling.[115]The main advantage of these defect-rich materials is the abundance of uncoordinated surface atoms in addition to more exposed surface-active facets.[115,116]For instance,morphology-controlled synthesis of defect-rich ultrathin Bi2WO6results in the formation of peony like aggregations with exposure of the(100)and(113)facets as have been confirmed through XPS,PAS as well as theoretical computations.[116]Similarly,nearly transparent vacancy rich monolayer BiO2-xhas been made through liquid exfoliation from the bulk BiO2--x(Figure 10a).[115]The ultrathin and single crystalline features of these NSs were evaluated by TEM,HRTEM,and associated fast Fourier transform(FFT)analyses and AFM(Figure 10b,c)which collectively supported the exposure of(111)and(011)facets of BiO2-xnanoplates(Figure 10d,e).Furthermore,the VOin both BiO2-xNSs and nanoplates were investigated through EPR and positron annihilation lifetime(PAL)measurements.The longer lifetime component(τ2,~601 ps)in these materials was attributed to the O defects and large vacancy voids,while the shorter lifetime(τ1,241 ps)was ascribed to the trapped positron annihilation.Theoretical modeling further confirmed that the longer positron lifetime(τ2,160 ps)was due to the trapped vacancy associates(Figure 10f-i),while the intensity corresponds to the abundance of these defects.[116,117]It is worth mentioning that decreasing the thickness of BiO2-xto monolayer results in partial exposure of Bi atoms that can escape from the lattice very easily carrying along O atoms to produce vacancy associates in BiO2-x.[115]Moreover,a noticeable shift in CB of BiO2-xmonolayer can be observed from 0.04 to-0.05 eV in comparison to BiO2-xnanoplates(Figure 10j),which greatly promotes the electron-hole pairs’separation and enhances the photocatalytic degradation of pollutant under both visible and near-infrared irradiation(Figure 10k).Similarly,by decreasing the thickness of BiOCl NSs up to the atomic level creates VBi‴V··o-VBi‴triplet vacancy in BiOCl.[117]The square like ultrathin NSs having size of 50-100 nm(Figure 11a,b)possess very obvious lattice fringes along the(001)plane that well match with the tetragonal BiOCl atomic(110)plane.In addition,the PAL measurements of BiOCl nanoplates yielded lifetime components,the longer lifetime components(τ3and τ4,around 590 ps and 2.5 ns,respectively)and four shorter lifetime components(τ1,250 ps),the later can be ascribed to the trapped positron(Figure 11c),while the other component(τ2,325 ps)is known to be associated with the respective vacancy associates VBi‴V··oVBi‴(Figure 11d).These triplet vacancies in ultrathin BiOCl have four negative charges that results in higher exposure of the(001)facet for ultrathin BiOCl NSs in comparison to the platelets,increasing the dye adsorption capability and hence improves degradation(Figure 11e).Furthermore,the XPS spectra reveals a shift in VB from 1.05 to 1.17 eV for ultrathin BiOCl NSs that greatly benefits the efficient oxidation of the holes(Figure 11g,h).[117]Therefore,the constructed VBi‴V··oVBi‴triplet vacancy alters the physiochemical as well as electronic configuration of materials.Thus,decreasing the material thickness to atomic scale level could cause a great disruption in the interatomic bonds,leading to the exposure of associates to produce more dangling bonds in ultrathin BiOCl NSs than nanoplates.The dangling bonds can behave as the active sites and improve photocatalytic activity.

Figure 10.a)Schematic diagram for solvent exfoliation of BiO2-xnanoplates into monolayer BiO2-x.b)Bright- field TEM,c)AFM,d)HRTEM and inset the corresponding FFT images of monolayer BiO2-x.e)Structure of monolayer BiO2-xgrows within(01-1)facet.f,h)Positron density distribution(yellow)in BiO2-xwith VO′′′and VBi-O′′′defects,respectively.g,i)Positron density in BiO2-xalong(01-1)plane for the trapped positrons of VO′′′defects and VBi-O′′′vacancies,respectively.j)Schematic diagram of the band structure of the two samples,the upshifting of VB maximum and CB minimum effectively separates the photogenerated electron-hole pairs in monolayer BiO2-x.k)Schematic illustration of the photocatalytic oxidation of pollutants by monolayer BiO2-x under UV,visible,and NIR irradiation.Reproduced with permission from Ref.[115]Copyright 2018,Wiley-VCH.
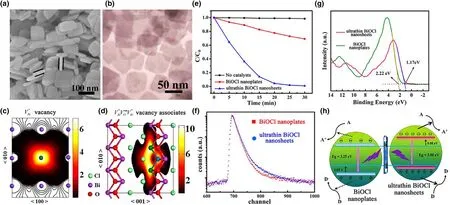
Figure 11.a)SEM image of BiOCl nanoplates.b)HRTEM image of ultrathin BiOCl NSs.c,d)Schematic representations of trapped positrons of VBi‴defect and VBi‴VO··VBi‴ vacancy associates,respectively.e)Comparison of photodecomposition of Rhodamine B with ultrathin BiOCl NSs and BiOCl nanoplates under simulated solar irradiation.f)Positron lifetime spectra and g)VB-XPS spectra of ultrathin BiOCl NSs and BiOCl nanoplates,respectively.h)Schematic illustration of the band structure of ultrathin BiOCl NSs and BiOCl nanoplates,the upshifting of VB maximum,and CB minimum effectively separate the photoinduced electron-hole pairs in ultrathin BiOCl NSs.Reproduced with permission from Ref.[117]Copyright 2013,American Chemical Society.
2.6.Lattice Disorders and Dislocations
Besides surface escape phenomenon of the atoms,many other types of lattice defects like disorder and distortion can significantly affect surface-active atoms for bonding.Therefore,the resultant crystals impart instability to the system with large specific energy as the system tends to attain thermodynamic stability by getting minimal value.Hence,lattice distortion causes many changes in local atomic arrangements,such as bond angle,coordination number and bond length.[118,119]These lattice distortions will obviously alter the electronic configuration to affect the photocatalytic performance.Recently,Ti3+accompanying lattice distortions with overall improvement in photocatalytic performance have been reported.[120]Although these distortions contribute very minimum toward the enhanced photocatalytic activity since the band gap cannot be further narrowed due to the absence of Ti3+defects,yet the distortions are thought to suppress the recombination of electrons and holes.One other example of disorder-engineered TiO2is the hydrogenated black TiO2nanocrystals with substantially increased light absorption in addition to charge carrier trapping.[121]As shown in Figure 12a,b,the lattice features of pure TiO2after hydrogenation and the increased surface disorder could be clearly observed in the latter case.As a result,black TiO2demonstrates much more outstanding photocatalysis than the white counterpart(Figure 12c).
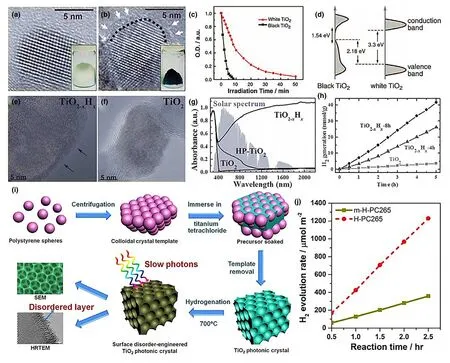
Figure 12.a,b)HRTEM images of TiO2 nanocrystals before and after hydrogenation,respectively.Inset:the photos comparing unmodified white and disorder-engineered black TiO2 nanocrystals.c)Photocatalytic activity of the black and white TiO2 nanocrystals under the same experimental conditions.d)Schematic illustration of the DOS of disorder-engineered black TiO2 nanocrystals,as compared to that of unmodified TiO2 nanocrystals.Reproduced with permission from Ref.[121]Copyright 2011,American Association.HRTEM images of e)black TiO2-xHxand f)pristine TiO2.g)DRS of 8h-H-plasma-reduced black titania(TiO2-xHx),the high-pressure hydrogenated black titania(HP-TiO2),and pristine TiO2.The background is the total solar spectrum.h)Solar lightdriven photocatalytic water splitting for H2generation over pristine TiO2,TiO2-xHx-4h,and TiO2-xHx-8h(TiO2-xHx).Reproduced with permission from Ref.[122]Copyright 2013,Wiley-VCH.i)Schematic procedure for the preparation of the surface disorder-engineered TiO2PC photocatalyst.j)Time-dependent H2 evolution of H-PC265 and m-H-PC265.Reproduced with permission from Ref.[123]Copyright 2017,Elsevier.
These induced disorders could yield mid-gap states and form a continuum called band tail that extends to the CB edge,which are not only the prominent centers for excitation and relaxation but also behave as trap states to prevent recombination processes for improved photocatalytic reactions.This can be attributed to the increased concentration of H2on the disordered surface as attracted by the dangling bonds(Figure 12d).It is thought that H2treatment may have stabilized these disorders through passivation of the dangling bonds.[121]Similarly,TiO2@TiO2-xcore-shell structure was synthesized through hydrogen plasma treatment showing the disordered surface layer of 2 nm surrounded the crystalline core(Figure 12e,f).[122]More interestingly,TiO2@TiO2-xcore-shell specimen possesses the highest absorption efficiency of 83% among all the reported black TiO2(Figure 12g).This can be attributed to the band gap narrowing through band tails and intermediate states induced by the Ti-H bonds through hydrogenation.As expected,TiO2-xHxexhibits far higher photocatalytic H2generation rate than the pristine TiO2(Figure 12h).
Besides increased light absorption and charge separation,hydrogenation also triggers the slow photon effect in photonic crystals(PCs),such as TiO2PCs synthesized through monodispersed polystyrene(PS)templates(Figure 12i).[123]These TiO2PCs(H-PC265)could serve as dual-functional photocatalysts and show significantly increased photocatalytic H2evolution rate than the sample with no photon effect(Figure 12j).In a similar case,the distinct surface distortion due to the change in local atomic arrangement could also be observed in a single layer of ZnSe when compared to its bulk phase.[124]Moreover,surface distortions in a single layer of ZnSe increase the DOS at the CB edge relative to the bulk counterpart that assures high rate of electron migration with robust photocatalytic water oxidation.Similar surface distortions have been reported in SnS,SnS2,and NiTi-LDH(layered double hydroxide)systems.[125,126]
2.7.Manifold Defects and Photocatalysis
In addition to the forecited defects,band gap tailoring through occupied atoms at anion vacancies has shown to be another viable strategy for defect engineering.For example,Yang et al.reported hydrogen-mediated oxygen-deficient TiO2(OVH-TiO2)with increased light harvesting ability,by placing a H atom at a defect site in TiO2(OV-TiO2)led to a new sub valence band and increased photocatalytic efficiency.[127]The color of TiO2turned blue or red upon formation of the hydrogen free or filled VO,respectively,as shown in Figure 13.The intrinsic band gap for OV-TiO2remains the same with a slight absorption tail in the visible region,whereas a significant decrease in band gap is observed for OVH-TiO2.Thus,a remarkable photocurrent response has been observed for the OVH-TiO2demonstrating the vital role of dopants in anion defects than the anatase TiO2and OV-TiO2,respectively,due to their negligible visible light absorption.

Figure 13.a)Color change from white TiO2to blue and red by introduction of hydrogen free oxygen and hydrogen filled-VOs,respectively,in TiO2lattice along with their associated atomic structure images.Ti,O,and H are indicated by blue,red and green balls in order.b,c)Band gap comparison between OV-TiO2and OVH-TiO2in reference to pristine TiO2.d,e)Photocurrent density measurements of OV-TiO2and OVH-TiO2under visible light irradiation,where the cell comprises TiO2,Pt,and Ag/AgCl as photoanode,a counter electrode and reference electrodes,respectively.Reproduced with permission from Ref.[127]Copyright 2018,Wiley-VCH.f)Band structure alignments of CN,Bt-CN,OA-CN,and Bt-OA-CN.g)Proposed mechanism for the photocatalytic behavior of Bt-OA-CN under visible light irradiation.Reproduced with permission from Ref.[128]Copyright 2019,Scientific Reports.
Likewise,a potential increase in light harvesting ability,more efficient charge separation and modulated energy band structure could be achieved through dual defect modification.Katsumata et al.synthesized dual defect modified g-C3N4(Bt-OA-CN)with corresponding O-dopants and VN.[128]In this dual modification,the cyano groups increase the charge separation and efficient surface arrival due to its strong electron withdrawing effect,resulting in 11.4 times higher degradation activity of bisphenol.This superior photocatalytic performance can be attributed to the synergetic relationship of the dual structural modification.Moreover,both PL and photoelectrochemical measurements have revealed the efficient charge carrier separation,while the quenching studies further confirmed that both the O2·-and holes are the predominant oxidizing species of the corresponding catalytic system(Figure 13).
3.Defect Synthesis and Controlling Strategies
3.1.Chemical Reduction and Surface Chemical Etching
Chemical reduction is one of the most effective approaches to engineer surface defects in semiconductors via various reducing solvents like ethylene glycol,glycerol or reducing agents like NaBH4,CaH2,N2H4,etc.[129-131]For example,Bi et al.reported defective K4Nb6O17ultrathin NSs through chemical reduction route using NaBH4as the reductant,leaving behind VOon the surface.Once this VOis introduced on the surface,the material’s light harvesting ability is enhanced narrowing its band gap by lowering the CB edge.[132]Moreover,the vacancies also serve to trap electrons to endorse charge carriers’separation that greatly improves H2evolution activity.The preparation of metal oxide by employing reducible solvents always involves O elimination to create vacancies.The reduction of BiOCl used ethylene glycol results in VOthrough reaction at the oxygen-terminated(001)surface under heating at 160°C.[133]The created VOnot only bestows the effective capture of photogenerated electrons and O2to produce superoxide anion radicals,but also extends light absorption edge up to 600 nm.Besides these reduction approaches,recently,aluminum reduction has been emerged as the most suitable route for creating VOlike that in TiO2-x.[134]The reduction mechanism could be ascribed to the maintenance of low partial pressure in order to release O from TiO2,giving a core-sell structure which contributes to the predominant enhancement from visible to NIR region.[134]Similarly,oxygen-deficient black BaTiO3can also be synthesized through the former reduction method.[135]Although these induced VOdoes not bring a noticeable change in morphology and crystal structure,yet the amorphous core-shell structure results in band gap narrowing with the corresponding increased light absorption.
3.2.Temperature-controlled Vacuum Synthesis
Vacuum synthesis is another universal strategy that is widely practiced to engineer defects in metal oxides.Xing et al.reported a cost-effective and low temperature-mediated TiO2with Ti3+and VOvia vacuum activation without altering the crystalline structure.[136]During vacuum activation,due to the low outside pressure,O atoms in TiO2escape from the lattice to restrain the outside pressure when heated at suitable temperature.The vacuum activation creates defect states that increase light harvesting and charge carrier trapping to promote both the photocatalytic degradation and H2evolution activities.However,as the vacuum activation is a mild surface treatment,these defects are gradually disappeared during prolong photocatalysis.[136]Similar activation treatments can be carried out for other metal oxides,such as MoO3,WO3,and ZnO.[8]Furthermore,the concentration of VOcan be tailored via controlling the activation temperature.What needs to be emphasized is that vacancies like sulfur,boron or even metal is difficult to engineer through this process.For example,VOinduced in Fe2O3through sintering under reducing environment of H2could lead to the formation of photoactive Fe3O4.[137]However,photoactive and highly conductive Fe2O3could be synthesized by thermally decomposing β-FeOOH in limited O2supply with a substantial increase in photoactivity in comparison to the pristine Fe2O3synthesized under ambient atmosphere.[138]It,therefore,emphasis the role of thermal activation for engineering VOin metal oxide,such as Fe2O3for water splitting.
3.3.High Temperature Hydrogen and Ammonia Treatment
High temperature H2treatment has been reported as one of the significant techniques to create defects particularly VOin metal oxides.[139-141]The introduction of both surface and sub-surface VOleads to efficient hydrogen evolution and reduced recombination,which involves high temperature reduction via H2to create structure disorders and produce VO.[113,142]Moreover,in case of TiO2,extended absorption band edges are observed along with color transformation,with improved photocatalytic activity.Such as,hydrogenation of TiO2NPs results in stoichiometric crystalline core and disordered surface with a remarkable decrease in band gap(1.85 eV),due to tailing.[113,140]TiO2NPs with characteristic defective shell and DOS are shown in Figure 14a-d.It is interesting to find that,in case of black TiO2,donor density increases by three orders of magnitude because of the introduced VO.Moreover,the H2annealed WO3photoanodes revealed distinguished stability for about 7 h without any significant loss in activity.[143]This high stability is attributed to the WO3-xwhich is highly resistant to dissolution and oxidation.[144]As the annealing temperature is increased,the color of the WO3film first changes to deep green(400°C)and then dark blue when heated above 450°C due to the partial reduction of WO3(Figure 14e),that improves the formed VO/Ti3+defects,the more Cu there is,the darker the sample is.The WO3-xcan be distinguished from the well-known HxWO3by applying positive bias as the latter is quickly bleached due to its electrochromic property thus proving the resistivity of the former toward oxidation(Figure 14f).Hydrogen-treated TiO2nanowires(H:TiO2)with significantly enhanced photocurrent density have also been reported owing to the negative shift in the saturation potential of H2and the higher donor density as a result of the induced VO(Figure 14g,h).[145]The increased activity in UV region is due to the induced VO,although the photoexcited electrons residing in these vacancies are not involved in the splitting reactions as their level are below the reduction potential for water splitting(Figure 14i).In addition,due to the low coupling between the localized VOand delocalized CB states,the corresponding electronic transitions could not be expected to occur which is the main reason of the weak activity of H:TiO2nanowires in the visible region with least contribution to the photocurrent.Similarly,Liu et al.demonstrated defective TiO2with various loading amounts of Cu(0,1 and 5% )via an in-situ thermal treatment under H2and He atmosphere.[146]The amount of Cu and gas environment have a marked influence on color change of the final material,due to reduction of Cu(Cu2+to Cu+or Cu0)and the localized states below the CB at 0.7-1.0 eV.[143]This result makes the transitions among both the VOand tailed states to CB and VOfrom tailed VB,respectively,thus extending the light absorption of black TiO2toward the NIR region.[140]Similarly,H2treatment can activate and simultaneously stabilize the photoactivity of WO3at different temperatures.[144]In addition,H2pretreatment could also lead to high concentration of H interstitials.[147]Besides VO,another type of vacancies like N in g-C3N4can also be created via the same thermal treatment.[148]Further,aside from reducing environment alone,calcination-assisted H2/N2mixed-atmosphere treatment have also been reported to efficiently engineer VOs in SrTiO3nanofibers,with significant improvement in H2evolution activity by decreasing band gap(1.85 eV)due to tailing.[149]

Figure 14.a,b)Schematic of nanoparticle’s structure and c,d)DOS for reference(P25)TiO2and black TiO2.Reproduced with permission from Ref.[140]Copyright 2012,American Chemical Society.e)Representative LSV curves measured under simulated solar light(100 mW cm-2,AM 1.5G).f)Comparative study of the electrochemical and PEC stability of the HxWO3sample and the hydrogen-treated WO3sample.Reproduced with permission from Ref.[144]Copyright 2012,Royal Society of Chemistry.g)SEM image of vertically aligned TiO2 nanowire arrays prepared on FTO substrate.h)LSV collected from pristine TiO2 nanowires(white)and H:TiO2 nanowires annealed at different temperatures.i)A simplified energy diagram of H:TiO2 nanowires.Reproduced with permission from Ref.[145]Copyright 2011,American Chemical Society.
3.4.Molten Salt Synthesis
Molten salt(MS)method is a unique and simple route to synthesize material with faster mass transfer reaction in liquid environments.Until now,various metal powders and ceramic crystals have been synthesized through different molten salt systems.[150,151]In contrast to solid-state synthesis,molten salt system offers low temperature synthesis with controlled size and desired morphology.Meng et al.reported molten salt strategy for self-doped TiO2using trifluoroacetic acid(TFA)as a reducing agent which leads to the formation of VOboth on the surface and bulk in TiO2.[152]A substantial enhancement in optical absorption in visible region can be observed clearly(Figure 15a),confirming the presence of oxygen or Ti3+defects,induced by the incorporation of continuous donor states below the CB.[140]The formation of these VOvia molten slat synthesis can be ascribed to the incomplete combustion of TFA,which causes consumption of lattice O hence creating vacancies both on the surface and in bulk.Interestingly,truncated bipyramidal TiO2could be seen clearly in TEM characterization(Figure 15b),revealing the molten salt synthesis.The resultant narrowed band gap is due to induced defects,which altered the electronic states(Figure 15c).Zhang et al.have successfully synthesized defective g-C3N4with two types of VNvia alkali-assisted thermal polymerization of urea,melamine or thiourea.The introduction of N defects results in red shift of the absorption edge in g-C3N4(Figure 15e).Also,the size of shift observed can be effectively tuned via KOH ratio.In addition,similar defects can be created through other alkali compounds,that is,NaOH and Ba(OH)2confirming the versatile synthesis strategy.The synthesized g-C3N4possesses enhanced light absorption and improved charge separation with superior HER performance than the pristine g-C3N4[153](Figure 15i).

Figure 15.a)UV-vis DRS.b)TEM image of P25 annealed in MS with TFA.c)Schematic illustrations of the energy bands.Reproduced with permission from Ref.[152]Copyright 2018,Royal Society of Chemistry.d)UV-vis DRS and e)plots of transformed Kubelka-Munk function versus photon energy for g-C3N4 and g-C3Nxprepared using urea as precursor and different amounts of KOH(ranging from 0 to 1.0 g).Inset in d)shows a digital photograph of samples prepared with different amounts of KOH(ranging from 0 to 1.0 g,from left to right).f)UV-vis DRS spectra of g-C3N4and g-C3Nxprepared with melamine as precursor and 1.0 g of KOH.Inset in bottom-left corner shows a digital photograph of samples g-C3N4(left)and g-C3Nx(right).g)UV-vis DRS spectra of g-C3N4and g-C3Nxprepared with urea as precursor and 1.0 g of NaOH or Ba(OH)2.Inset in left bottom in d)shows a digital photograph of samples g-C3N4(left),g-C3Nxprepared with NaOH(middle),and g-C3Nxprepared with Ba(OH)2(right).Insets in upper-right corner in f)and g)show plots of transformed Kubelka-Munk function versus photon energy for corresponding samples.h)Transient photocurrent response of g-C3N4and g-C3Nx-0.01 under visible light illumination(λ > 420 nm).i)Time course of H2evolution over 10 h for 1 wt% Pt/g-C3N4and 1 wt% Pt/g-C3Nx-0.01 in 25 vol% aqueous lactic acid solution under visible light irradiation(λ > 420 nm).Reproduced with permission from Ref.[153]Copyright 2017,Wiley-VCH.
3.5.Vapor Diffusion Method
Vapor diffusion is a novel synthesis strategy for defect creation in semiconductors with desired location and density.Generally,the synthesis process involves homogenous mixing of the precursors with comparatively identical liquification and sublimation temperatures thus avoiding inhomogeneous defects.The method has a potential of defect engineering in a facile manner.For example,Zhang et al.synthesized dual defects modified PCN through one step in-situ vapor diffusion approach with tunable VNand atomically dispersed Cu atoms[154](Figure 16).The existence of surface VN/band tailing devoted to enhanced full spectrum photocatalytic activity than p-PCN could be clearly observed.However,the PCN with two Cu(DPCN-Cu-2)exhibited decreased activity under UV irradiations than the p-PCN.This can be attributed to the coupling effects of VNwith Cu atoms affecting the overall HER performance illustrating the mismatch of enhanced light absorption and limited photocatalytic performance under full light spectrum.

Figure 16.a)Synthetic procedure for DPCN-Cu-x.b)UV-vis DRS spectra(inset:from left to right:p-PCN,DPCN-Cu-1,DPCN-Cu-2,and DPCN-1).c)Stability test under irradiation full light for DPCN-Cu-1.d)Full light and UV-cut 420 in the absence of Pt.e)Band structure alignments for different PCN samples.Reproduced with permission from Ref.[154]Copyright 2020,Elsevier.
3.6.UV-assisted Vacancy Sites
Another feasible way to build VOin metal oxides is the ultraviolet irradiation.For instance,Ye et al.and Zhang et al.individually created VOin BiOCl through UV irradiation.[155-157]Due to long bond length and lower bond energy of Bi-O bond,it can be easily broken via exposure to high powered UV light,leaving behind surface O sites.However,in some metal oxides,the energy of UV radiation is not enough to break the metal oxide bond.It is therefore established that UV radiations can induce surface defects in metal oxides that provides active sites for selective dissociation of H2O.[158-161]However,Mezhenny et al.demonstrated through STM analysis that UV irradiations are not enough to create surface VO,statistical calculation of defects for pre-cleaned TiO2(110)after UV treatment did not count for surface defects.[162]Furthermore,the formation energy of O defect has been reported to be~7 eV,which is larger than the energy of UV radiation.[163]Also,the theoretical investigation precluded the O defects via UV irradiation,which is in close agreement with the previous reports.[164]
3.7.Lithium Transformed Defects
Lithium-induced conversion is known to be the most versatile and novel strategy for defect generation in metal oxides,such as Co,Fe,Ni and their mixtures.[165,166]These transition metal oxide NPs(~20 nm)are transformed during this process into ultra-small NPs with diameter of 2~5 nm accompanied with grain boundaries,dislocations,and defects,serving as active sites for photocatalytic reaction.This method also provides remarkable interconnection among the NPs with large surface area.Furthermore,this approach is considered as the most magnificent way for inducing defect states in metal oxides,which can be extended in defect-rich crystals for improved photocatalysis.[166]
3.8.Fast Heat Transformation
Recently,pit engineering or VOgeneration via fast heating strategy have got immense practice because ultrathin NSs of Co3O4,CeO2,WO3,and In2O3can be easily synthesized via heat transformation strategy from their NSs precursors,such as CoO,CeCO3OH,WO3·2H2O,and In(OH)3,respectively.[69,110,114,167]Since the process involves direct sample heating,the drastic change in temperature leads to the formation of pits vacancy at numerous sites.For example,after fast heating In(OH)3at 400°C for only about 3 min,the phase transformation from In(OH)3to porous In2O3microstructure could be produced.[69]Besides,air decomposition produces VOs which rarely exists if calcined in the presence of O2.Therefore,it is more favorable to engineer pit defects into ultrathin nanomaterials via a phase transformation process,including other types of vacancies like N and S under NH3and H2S atmospheres,respectively.[8]
3.9.Ball Milling
Another useful way to create defects with considerable structural deformation is ball milling.During this process,the material suffers extreme reduction in volume resulting in defect exposure which is beneficial for optimizing the corresponding photocatalytic activity.[168]Zhu et al.demonstrated Bi defects and VOvia ball milling of BiPO4.However,since the defects generated is of bulk type,which considerably inhibits the separation of photogenerated charge carriers.[169]Aside from the bulk defects,in general surface defects at the atomic level in ultrathin NSs could result in exposure of a large number of surface atoms,boosting the photocatalytic efficiency.Moreover,the defect density can be handled carefully via controlled milling power and time;however,the type is hard to be tailored accordingly.
4.Plasma Treatment
Plasma etching stands to be the most adaptable method to introduce intrinsic defects in photocatalytic materials.Li et al.reported S vacancies in monolayer MoS2under mild treatment of Ar plasma.[170]The method provides a sound control over the density of VSs by varying the exposure period to Ar plasma.The resultant vacancies created can be confirmed by aberration-corrected STEM analysis that have a pronounced effect on the electronic structure of MoS2and are in close agreement with DFT calculations and STM characterization.[170]In addition to vacancy formation,the method can also be employed to engrave pits as well.For instance,plasma treated Co3O4NSs can be effectively build with pores via plasma treatment.[171]Furthermore,surfactant assisted template synthesis of mesoporous TiO2with predominant disorders through sol-gel method have been reported.[172]The methodology is preceded through N2/Ar plasma treatment which enables the successful doping of substitutional N2in the disordered-TiO2triggering the visible light water oxidation.Moreover,the defect types and concentrations can be satisfactorily adjusted by varying the type,intensity and treated time of the gas.[172]Similarly,some typical synthesis routes for photocatalysts with associated defects and their corresponding photocatalytic performances are summarized in Table 1.
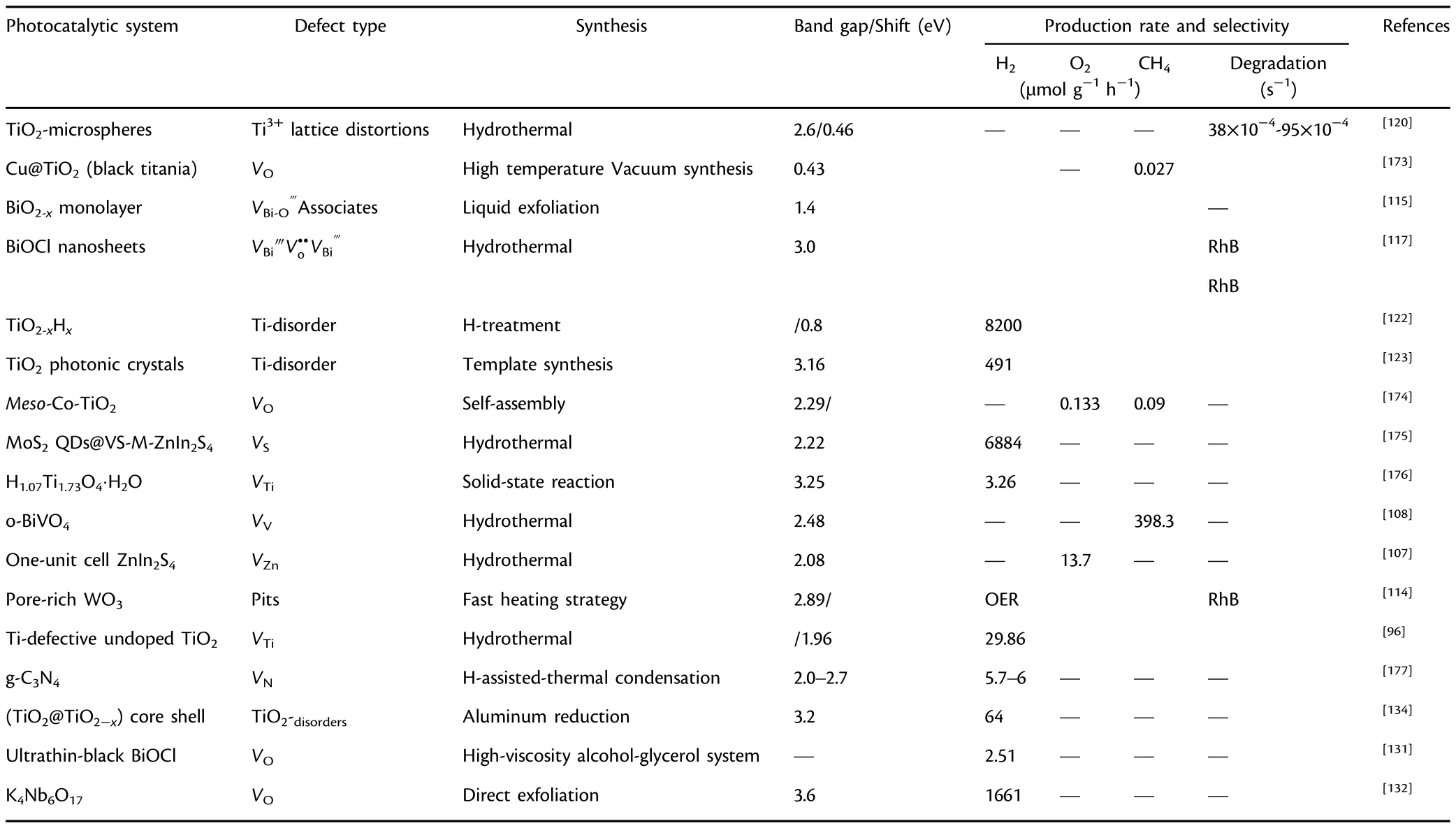
Table 1.Highlighted studies on defect synthesis and photocatalysis.
5.Properties of Defective Photocatalysts
5.1.Defect States and Electronic Band Structure
The light absorption and respective electronic processes in semiconductors including charge generation and migration are mainly governed by their electronic band structures.[178-180]In case of direct band gap semiconductors,the nature of the optical transitions is determined by the electronic band structure,which does not involve changes in the crystal vibrational properties and can absorb all of the radiations within micrometers.Therefore,the charge carriers relatively travel a shorter distance to reach the reaction sites compared to the indirect band semiconductors that involve changes in the vibrational modes of the crystal during optical transition,providing a longer path for charge carriers to travel through.[180]As a result,the probability of recombination in deep minority charges increase before they reach the surface of photocatalysts.For direct and indirect band gap semiconductors,the absorption coefficient(α(E))above the band gap is represented byand α(E)= α0((E-Eg)/Eg)2,respectively.[180]The energy of a photon is given by E=hν=hc/λ,where “λ” is the wavelength and “c”is the velocity of light.The electronic band structures have a significant influence on the charge carrier mobility that in turn depends on the widths of the VB and CB edges.Similarly,the mobility of charge carriers is shown to be inversely proportional to the effective masses of electrons and holes that are decided by the curvature of the individual bands.[181]So that the highly curved broad bands result in large carrier mobility and low effective masses(Figure 17).Besides carrier mobility,the electronic band structure also invites photocorrosion during photocatalysis.[182]The electronic band structure forms the principle basis for the spectral range of light absorption and the so-called theoretical solar to hydrogen efficiency(STH).[183-185]Therefore,the most effective strategy to increase the conversion efficiency is a band gap modulation to extend the light absorption with maximum harvesting in visible region.[183,186]However,the band gap should be large enough to conform the thermodynamic requirements for photocatalysis,[187]which is the minimum energy required to run over the thermodynamic losses(0.3-0.5 eV)and standard Gibbs free energy for water splitting(1.23 eV).The band edge positions of various semiconductors relative to the normal hydrogen electrode(NHE)at pH 0 and vacuum revealed an over potential of 0.4-0.6 eV for fast reaction kinetics.[188,189]Therefore,the most suitable band gap for solar water splitting is 1.9-2.3 eV.[182]TiO2is the most frequently researched semiconductor.However,its large band gap limits its practical applications as a photoelectrode material,making dopant-induced electronic changes necessary to benefit the photocatalytic activity.[179]

Figure 17.a)Schematic diagram of Shockley-Read-Hall(SRH)recombination mediated by mid-gap states.b,c)Recombination diagram of photogenerated electrons and holes.The larger sizes of electrons or holes represent the greater effective masses;the longer wave arrows represent the faster transfer rates.Reproduced with permission from Ref.[191]Copyright 2019,Royal society of chemistry.
Generally,defect states encompasses all those states which appear in the band gap of catalyst materials as a result of doping or actual vacancies in the material.Doping does not alter the electronic band gap but incorporates shallow or deep energy levels called mid-gap states,as shown in Figure 17a that are able to extend the absorption range from shorter to longer wavelength.[190]In comparison to the pristine semiconductor,dopant-induced counterpart constitutes an add on shoulder edge of the absorption spectra with a very small optical cross section of the introduced defect.[191]The induced shallow levels can greatly enhance the charge carrier mobility by increasing the diffusion of minority carriers.[192,193]Generally,defects are the main culprits behind the mid-gap states changing the overall electronic band structure of photocatalysts to facilitate charge separation with elongated lifetimes.[194]However,these defect states can also serve as the main repositories of trap sites,driving the carrier recombination processes and reducing their lifetimes.Thus,their positions and natures relative to the band gap provides significant details about the excitation and relaxation mechanism.[195-197]On the other hand,band tail states are the localized electronic states thatresides just below the CB or above the VB of a semiconductor(Figure 17b,c).Band tails usually arises as result of disorder caused by thermal,structural or impurity states.
In order to investigate the increased photocatalytic activity of doped aTiO2,a number of models have been reported in the literature to estimate the electronic structures.[198]For instance,Fe-doped TiO2with different concentration of the dopant have been reported with increased visible light absorption and can be attributed to the variable oxidation state of Fe(Fe(II),Fe(III),and Fe(IV))causing increased self-trap energy and distinct position of the defect-induced mid-gap states.[199]Further,to investigate about the localized tail states and band gap of the doped and undoped aTiO2,inverse participation analysis(IPR)is carried out.[200]The IPR analysis of the undoped aTiO2have revealed strong localization of the electronic states.[201]However,in case of the doped aTiO2,the localized VB and CB tails increase consequently due to the distribution of charges in respective occupancies.It could be observed clearly that for undoped aTiO2the valance tails are more localized than the CB(Figure 18bI),and become further localized in Fe(II)-doped aTiO2(Figure 18bII).However,in case of Fe(III)-doped a TiO2and Fe(IV)-doped aTiO2,the EFs are shifted toward the VB indicating increased mobility of charges in the respective systems(Figure 18bIII-IV).Further,the electronic density of the sates(DOS)and partial DOS(PDOS)for the d electrons of Ti and Fe,and for p electrons of O have been presented in Figure 13b both for the doped and undoped aTiO2,respectively.In case of pristine aTiO2,the highest VB comes from O 2p state while the lowest CB is mainly accompanied by the Ti 3d states(Figure 18aI).It has doped Fe(II),Fe(III)aVO(Figure 18aII-IV).Fe(IV)-doping introduces a mid-gap state of 0.4 eV below the CB edge,whereas Fe(II)-doping results in an impure state of 0.3 eV just above the VB maxima,while no significant effect on the CB minima is observed for both the later and the former.However,Fe(III)-doping not only produces mid-gap states at 0.8 eV below the CB but is also accompanied by the additional states at both CB and VB minima.[199]

Figure 18.Total density of states(TDOS)(black line,right scale)and the corresponding values of the inverse participation ratio(IPR)(blue dots,left scale)on the left side and the spin density(ρ↑ - ρ↓)on the right side for(I)undoped aTiO2,(II)Fe(IV)-aTiO2,(III)Fe(II)-aTiO2,and(IV)Fe(III)-aTiO2models.The zero-energy value is set at the Fermi energy represented by the vertical dashed line.The iso value used for spin density plots is 0.01 e˚A-3.Total density of states(TDOS)and the projected density of states on the p and d orbitals of(I)undoped aTiO2,(II)Fe(IV)-aTiO2,(III)Fe(II)-aTiO2,and(IV)Fe(III)-aTiO2 models.The zero-energy value is set at the Fermi energy represented by the vertical dashed line.Reproduced with permission from Ref.[199]Copyright 2016,American Chemical Society.
5.2.Defect States and Enhanced Light Absorption
Hoch et al.investigated the role of defects in the electronic structure of nanostructured In2O3-x(OH).[194]In order to address the role of these mid-gap electronic defects,both DOS and PDOS have been calculated through hybrid DFT approach.The results showed that due to the change in the initial potential energy of the charge carrier caused by defects,the distribution in the populated states could be tuned easily.Consequently,these shallow acceptor and donor states could be easily excited by near band gap excitation.[197]In addition,several transient absorption(TA)studies have indicated that VOinduces donor states right below the CB edge,while the surface OH group creates acceptor sites just above the VB.[195,197,202,203]This demonstration has been found to be true for defective In2O3(OH)yas well.[194]
For simplification,the DOS and PDOS have been calculated for different kinds of samples,including pristine or defect-free bulk In2O3surface,others is comprised of samples with only surface OH and VOor with different concentration of co-existed OH and VO.Comparing the DOS of pristine In2O3with that of the bulk,it has found to reduce the Egup to 1.1 eV through shifts in both VB and CB levels,leading to a Egof approximate 2.0 eV.[204]In case of only VO-surface In2O3(OH)y,more deep states are introduced to the surface probably above the VB minimum at 1.5 eV.Moreover,the position of the EFindicates that these states are completely filled as VObehaves as donors and their increasing concentration on the surface would lead to the accumulation of electrons in the material.However,the band does not shift significantly with an OH-vacancy.The only key difference is the charge accumulation(O p electrons)present together,which clearly shows that the presence of OH group highly moderate the effect of VOaccompanied by the occupied states near the CB edge.[194]
Similarly,surface VOin combination with interstitial defects(Ti3+),such as H2treated TiO2nanofibers(H:TiO2),have also been found to possess enhanced light absorption caused by the corresponding change in electronic band structure.[205]This can be attributed to both defect to VB and CB to defect transitions(Figure 19a,b)with much faster photocatalytic degradation activity,in contrast to pristine TiO2.[205]Similarly,Cussing et al.investigated the effect of H2treatment on the electronic band structure by creating the corresponding Ti3+vacancies.[206]An overall band shift of 0.1 eV could be observed by the respective VO;however,the increased optical absorption does not guarantee higher photocatalytic activity(Figure 14c,d).Further,the DFT calculation revealed that the photocatalytic activity of pristine TiO2up to 400 nm is associated with VB-CB transitions,while in case of H:TiO2the absorption edge is extended up to 440 nm due to the incorporated shallow traps.This allows the transition between the filled localized defects and CB(Figure 14e)with shorter lifetimes and negligible effect on photocatalysis.[206]Moreover,the Ti3+vacancies were found to be flat,with larger effective masses,thus limiting the mobility of the charge carriers.[207]Similar investigations concerning band gap manipulation revealed that the foreign Ti dopant greatly improve the conductivity in case of Fe2O3.Nevertheless,the deep levels can still serve as the recombination centers for electrons and holes.[191,208,209]

Figure 19.a)The schematic diagram of the possible band alignment between the surface defect(VO and Ti3+interstitial defect)and anatase TiO2.b)Illustration of the mechanism of photocatalytic degradation of organic dye over H:TiO2NFs.Reproduced with permission from Ref.[205]Copyright 2017,Nature Scientific Reports.c,d)Reduction of TiO2leads to a shift of the band edge from 390 to 440 nm as well as an increase in light absorption with wavelengths above 440 nm,despite the extended light absorption range,the photocatalysis was extended up to only 440 nm with a decrease in UV photoactivity.e)The GGA+U predicted band structure shows that the VOs allowed a decrease in the inter band transition energy as well as allowing new defect state-CB transitions.Reproduced with permission from Ref.[206]Copyright 2017,American Chemical Society.
5.3.Transition Levels and Band Gap Approximation
The accurate description of both donor and acceptor defects requires a detailed investigation of the band structure and transition levels.The defect-induced band levels are commonly interpreted by considering single particle Kohn-Sham eigenvalues.However,this approach is still not justified for the involved electronic transitions,revealing the approximation of different charge states with respective electronic transitions,[210,211]such as the two and three extrinsic defects in ZnO,[212]the interstitial Zni,VOand hydrogen Hiwith substitutional H and N,as shown in Figure 20.The shallow donor character of ZnO with H impurities is highly supported by experimental evidences from temperature dependent electron energy-loss spectroscopy (EELS) measurements.[213]The ionization energies of these donor states are found to be 25 and 35 meV[214,215]via EPR investigation,[216]and 51 meV by electrical conductivity experiments.[217]Besides transition level states approximations,DFT calculations based on periodic pseudo potential plane-wave have revealed H impurities in ZnO as shallow donors.[218]If one H atom is introduced at the interstitial Hiand substitutional O sites,then the charge density is delocalized over the sites with higher concentration at Zn and O components.[216]This electronic state induced by the H impurity is termed as the perturbed host state when compared to the band structure of bulk stoichiometric ZnO.[209]In the doped Hisystem,the occupation of CB bottom results in a slight decrease in energy with a small deformation of the state.Consequently,the greater the size of the super cell,the lesser will be the perturbation,which therefore supports the shallow donor nature of the infinitely diluted limit state with negligible perturbation.Similarly,the interstitial Zn defects in ZnO has been extensively studied as intrinsic donor sites via both theoretical and experimental calculations[219]revealing that the donor states lie to 30 meV below the CB level.[220]Conventionally,the sub-lattices of O and Zn ions provide two different sites for interstitial atoms,the octahedral one,and the tetrahedral voids.The first three nearest O ions do not belong to the sub lattice,as in case of Zn,showing the octahedral interstice to be 0.9 eV more stable than the tetrahedral cavity.[219]This is attributed to the shallow nature of Zniwith 3d energy level which is considerably lower relative to O 2p valance state and results in a slight mixing of the occupied 4s and CB levels.[218]As stated earlier,that among native defects,VOis probably the most extensively investigated one in semiconductors.[201,221]It was considered to be responsible for the n-type conductivity associated with semiconductors and classified as shallow donor specie,which are always characterized by EPR analyses having g value of~1.96.[222,223]However,this assumption was found to be ambiguous,as experimental analysis showed that the typical signal at g~1.96 is that of CB-electron that is coulombically attracted by the ionized donor center.[213,224]The same is true for Al-,H-,In,and Ga-doped ZnO systems.[216,225]Smith and Vehse reported the paramagnetic VO+through EPR studies using high energy electron irradiated single crystal.[226]Quantum mechanical calculations have also revealed that VOintroduces localized energy levels in the band gap that act as deep donors with a negative character,depicting the thermodynamic instability of VO+specie that can only be observed under photoexcitation.[209,218,227-229]Therefore,the VOis no more responsible for the n-type conductivity associated with that of ZnO.The induced VOcauses a moderate lattice relaxation along with the displacement of four Zn ions toward the Zn-O vacancy followed by the corresponding bond elongation.The singlet configuration creates a fully occupied localized electronic state above the VB level that is 2.9 eV more stable than the triplet configuration.Similarly,EPR and DFT analyses of N vacancy have witnessed the deep acceptor properties where the substituted lattice is much stable provided with well approximated experimental evidence.[230]In addition,DFT calculations using 72-atom super cell have established the charge trapping behavior of N-dopants with thermal ionization energy of 1.3 eV.[231]The same electronic transitions are observed for 108-and 192-super cells with 1.39 and 1.47 eV,respectively.This negligible influence of super cell size on the respective thermodynamic transition highly supports the localized character of deep acceptors.Therefore,N-doping is no more considered as a viable route for p-type conduction of ZnO.[231]

Figure 20.Structural models of a)stoichiometric bulk ZnO,b)interstitial H,Hi,c)substitutional H to O,HO,d)interstitial Zn in an octahedral void,Zni,e)VO,and f)substitutional N to O,NO;Zn,O,N,and H are represented by gray,red,yellow,and green spheres,respectively.g)The band structure of the pure bulk ZnO is reported as reference.h)Band structure of VOdefect in ZnO in various charge states(108-atom super cell).In the bottom scheme the Kohn-Sham eigenvalues at Г point are reported.Reproduced with permission from Ref.[212]Copyright 2010,American Institute of Physics.
5.4.Defect States and Charge Transfer Dynamics
5.4.1.Defect Density and Molecular Adsorption
Increased density of defects could lead to the recombination of charge carriers owing to the thermodynamically favored backward reactions.[232,233]Higher defect concentration,therefore,accelerates recombination events through multiple pathways thus decreasing the photocatalytic performance.[234]These desirable and undesirable electronic transport with controlled level of defect density have been illustrated in Figure 21a.However,system(a)represents a photocatalyst modified with a single co-catalyst,while(b)shows photocatalytic system with two co-catalysts at an optimum defect concentration.It can be observed from(i)that charge trapping is much predominant within the forbidden states in system(a)indicating excessive trap states.However,in system(b)step-(v)the modification via co-catalysts greatly lower the barrier for interfacial charge transport.Furthermore,step-(ii)in(a)would more probably lower the thermodynamic driving forces for the corresponding OER and HER,step-(iii)may further restrict HER while promoting water oxidation.Moreover,step-(iv)in(b)would rather occur with less probability due to low-defect density,setting the way to(v).Therefore,it can be clearly observed that the unstrained charge density could be overcome successfully via controlled defect concentration thus avoiding step-(i)to promote photocatalysis.[232]In addition,defects or surface VOhighly suffer from instability and corresponding deactivation during photocatalysis.[233]Chen et al.developed Bi-NPs and VOon pristine Bi2O2CO3(Bi@OV-BOC)with simultaneously increased stability and photocatalytic activity owing to the pivotal role of Bi.It has been demonstrated that H2O and O2(Figure 21c)easily get in to the VOsites to deactivate the OV-BOC.On the other hand,in case of Bi@OV-BOC the Bi metal serves as the center of adsorption for both H2O and O2therefore preventing the adverse occupation of VOby the respective molecules.[235]Further analyses on the combined effect of nanoparticles and VOimplied effective separation of charge carriers with effective electron transport pathways,where Bi-NPs mainly donates electrons to the surface VOwith altered charge difference,as shown in Figure 21b.These results suggest that VOmainly acts as the intermediate entity for electron collection to facilitate electron-hole pair separation as represented in Similarly,defects play a crucial role in controlling the adsorption and desorption processes of both the reactants and products.[236]Upon interaction of the adsorbed molecules with catalyst surface,the electron-rich vacancy sites induce a shift in LUMO to facilitate the rapid electron transfer reaction.For instance,cation defects result in electron delocalization and band broadening by up shifting the VB maxima with improved charge carrier migration.[96,97,117,237,238]
In addition,cation vacancies as shallow acceptors lead to higher electrical conductivities and increased donor densities to provide abundant active sites for initial activation of water molecules and a high degree of delocalization in contrast to anion vacancies.[239]It has been found that(101)-anatase is the most favorable coordination surface with a jagged twofold coordination of O2C atoms along the(010)plane(Figure 21d,e).[240]Both experimental and theoretical studies revealed that the water adsorption on TiO2surface could be either in the form of dissociative adsorption,molecular adsorption or an intermediate state of the two.However,the time lapse STM analyses have revealed the migration of water among the sub-surface defects(Figure 21f,g),ruling out the existence of intermediate adsorption.[236]Furthermore,the molecular adsorption of water falls out at the fivefold Ti5Csites while the H atom binds to the O2C(Figure 21h).[241]In addition,molecular adsorption mostly occurs in the form of surface radical groups such as-OH adsorption,where the water acts as a medium rather than a reactant.[236]However,most of the dissociative adsorption occurs on VOor Ti-related defects.[242-245]The water dissociates leaving behind a pair of-OH that helps in the redistribution of the trapped electrons toward the neighboring Ti4+sites.[237,239]Therefore,the pathways revealed that the adsorption energy of water on defect-free stoichiometric TiO2is much higher(Figure 21i)than the one bearing defects,supporting the evidence to alter the mechanism via introducing defects.[236]Similarly,further investigation of adsorption energies and reactant activation on photocatalytic activity,in-situ DRIFT analyses have revealed that N2O4is the main intermediate during active pollutant degradation by BOC and Bi@OV-BOC(Figure 21j).Although there is negligible energy barrier for NO2to NO3conversion on BOC and OV-BOC surface,however the energy barrier that exists for NO to NO2conversion have drastic effect on the actual photocatalytic activity limiting the formation of the main intermediate specie.In case of Bi@OV-BOC,the combined effects of Bi and VOresult in low activation barrier(Figure 21j),which highly favors the respective degradation reaction to the final products(NO-3).These analyses suggest that NO removal via BOC and OV-BOC could need to face some activation barrier in contrast to Bi@OV-BOC to trigger the photocatalytic activity,supporting the significance of co-existed metal NPs and VOfor enhanced pollutant degradation activity.[235]

Figure 21.a)Schematic representation of dual-functional photocatalysis.(a)with high defect concentration subjected(i)charge entrapment,(ii)inadequate energy offsets for HER and OER,and(iii)undesired thermodynamically supported reverse reactions in H2production perspective(eg:ORR)(b)with optimum defect concentration,(iv)lower defect density aiding charge separation,(v)low interfacial energy barriers by doping that support charge transfers and lower probability for undesired back reactions owing to fewer defects.CB,conduction band;VB,valence band;ηHER,the driving force for hydrogen evolution reaction;ηOER,driving force for oxygen evolution reaction.Reproduced with permission from Ref.[233]Copyright 2020,American Chemical Society.b)Charge difference distribution between the surface of Bi2O2CO3and Bi metal NPs,with charge accumulation in blue and depletion in yellow.c)Proposed carriers transfer mode on Bi@OV-BOC,Reproduced with permission from Ref.[235]Copyright 2020,Elsevier.d-i)View of the anatase(101)surface along the[010]direction(front view)and(d)top view,showing the twofold coordinated oxygen(O2c)and the fivefold coordinated(Ti5c)titanium atoms.Time lapse STM images showing a water molecule(red circle)migrating on the surface.The final adsorption site marked with a yellow arrow in(e)and the initial adsorption site(yellow arrow in(f)have the same appearance,indicative of a sub-surface defect.unevenness in the line profile is due to changes in the local electronic structure caused by sub-surface defects,and(h)after water dose at T~150 K and imaged at 182 K.(i)The water adsorption state on O vacancy,Ti interstitial and stoichiometric surface.Reproduced with permission from Ref.[236]Copyright 2009,American Chemical Society.j)DFT calculated adsorption energy of several major intermediate adsorption products of the BOC,OV-BOC and Bi@OV-BOC samples.Reproduced with permission from Ref.[235]Copyright 2020,Elsevier.
5.4.2.Carrier Trapping and Distribution of Charges
The irradiation of light with approximate wavelength results in the excitation of electron as HOMO-LUMO promotion.These photogenerated charge carriers could be trapped either in the bulk or migrate to the surface to proceed the upcoming redox reactions.[246]During trapping events,the main charge carrier sates are the trapped holes or more free electrons.The surface or bulk distribution of these temporal charge carriers could be evaluated further through TAS measurements which is highly dependent on crystal phase.[247]The PL emission from anatase TiO2has been found in the visible region of the spectra due to the recombination of the respective donor(VO)and acceptor(-OH)species,while rutile shows strong luminescence in the NIR region caused by the subsequent recombination of electrons and holes.In addition,the sub-surface trapping in anatase could elongate lifetimes to more than 1 ms that greatly benefits reduction reaction.[248]Simultaneously,the deeply trapped electrons in rutile highly promote water oxidation but are unable to enhance the overall activity.These particular characteristics of electrons and holes are associated with defects irrespective of the physical properties.[195]Figure 22a-f represent the total charge(Δq)distribution and activation energy over the BOC,OV-BOC,and Bi@OV-BOV surfaces for H2O activation and O2adsorption,respectively,which further certifies the electron transport from NPs to VOs.[235]Further,the PDOS states calculated for both H2O and O2clearly show(Figure 22g,h),no significant overlap over BOC surface;however,the peaks observed for OV-BOC and Bi@OV-BOC possess predominant overlapping suggesting chemical adsorption in the later while the former exhibits physical adsorption promoting the activation of molecules.Initially,amount of energy from photon could be consumed in lattice distortion,while the rest is utilized by the adsorbed charged species to overcome the surface barrier,the lattice distortions if co-exited with point defects could also improve crystallinity in mesoporous materials without Pt.deposition with enhanced photocatalytic activity.[246-252]For example,the trap states in P25-TiO2could serve as the charge accumulation centers,that later accomplish the photoreduction of mono and di- flour methyl acetophenone(AP)derivatives under UV irradiation.[253]As the trap states are situated just below the CB of P25-TiO2,the electrons could easily relax back to the trap states from CB to yield the accumulated electrons.This remarkable stability and increased lifetimes in deaerated ethanol[254,255]offers magnificent electron transfer to adsorbed AP to yield the reduced products.[256]Moreover,Yue et al.demonstrated three possible recombination pathways in semiconductors;i)recombination of excited electrons with holes situated in the empty VB following to trap states in the VB which is also limited reaction due to the concentration of electrons second order kinetics are limited by the availability of electrons and holes;ii)recombination due and holes elucidated through Shockley-Read-Hall Model;iii)in another situation,an excited electron can recombine with hole meanwhile releasing significant energy to the neighboring electron or hole.[256]As already mentioned,that VO”primarily acts as acceptor species,after accepting an electron,the formation of VOmakes it to behave more like a donor than acceptor site during photocatalytic reaction.[257]The extra electron in VOsituated in sub-surface could also recombine with a hole to further reduce Ti4+to Ti3+,hence preventing electrons to approach Ti at that site.The process continues to transfer the aligned Ti4+to regenerate the initial VOstate throughout the photocatalytic process.[257]However,this kind of migration increases the rate of recombination processes with longer travel pathways.Likewise,the electrons can be transferred to the nearby dissociative adsorbed species by the surface VOif the electrons are approaching along the surface Tiatoms.In this case,the resulting Ti3+could also act as active site for O2adsorption.Similarly,Ti vacancies are also known to highly assist in electron transport processes with involved surface-adsorbed species,these vacancies therefore elongate the lifetimes of photogenerated charge carriers and improve separation.[105,258]In addition,the photocatalytic processes mainly involve the sub-surface,surface VOor cation defects which are situated in bulk for photovoltaic processes.The resultant delay in the carrier transport in the former is due to trapping owing to the lattice distortion.[259]Furthermore,the presence of lattice defects could highly influence the distortion caused by the chemical tension.[253,260]However,at the same time these defect states are of vital importance for carrier accumulation and transport that functions as acceptor or donor sites in the interfaces.[249,261]

Figure 22.a)Charge difference distribution of optimized H2O adsorption between H2O and BOC,b)OVBOC and c)Bi@OV-BOC;d)charge difference distribution of optimized O2adsorption between O2and BOC,e)OV-BOC and f)Bi@OV-BOC;the PDOS of g)O2and h)H2O molecules adsorbing on BOC,OV-BOC,and Bi@OV-BOC surfaces.The calculated Bader charge(Δq)is the sum of Bader charge of several atoms of one O2or one H2O molecules and the negative values of Bader charge change mean the transfer of electrons from the photocatalyst surface to the adsorbed molecules.Reproduced with permission from Ref.[235]Copyright 2020,Elsevier.
5.4.3.Reaction Pro files and Processes at Semiconductor Interface
Surface adsorption and activation are the key steps in photocatalytic processes.[238,262]A detailed investigation of adsorption and surface activation of water molecules on Ru and Pt NPs-based cluster models have revealed the binding ability of water molecules to the catalyst surface which is highly influenced by both the metal d-band and the corresponding coordination environment that follows a linear relationship with energy of adsorption.[263]Lin et al.have systematically calculated the mechanism of water splitting via dispersion corrected techniques using g-C3N4catalyst system and proposed three main pathways referring to the activity,as illustrated in Figure 23a,b.[264]It can be noticed that due to sulfur doping,the step 2 of mechanism 1 becomes energetically least feasible with free energy(ΔG)equals to 0.53 eV.Similarly,in case of mechanism 2,step 3 remains uphill with a ΔG value of 2.27 eV;hence,the reaction is thermodynamically forbidden.However,in mechanism 3,the dissociation of water is a downhill process owing to the low energy for OH*adsorption,but is still limited by step 7,the O-O bond formation with ΔG of 0.77 eV,as the removal of the proton from water is more difficult after sulfur doping in all the three pathways.[264]

Figure 23.a)Three OER mechanisms on g-C3N4.Color scheme:O,red;H,white.b)Free energy(ΔG,eV)profiles of OER(pH=0,U=1.23 V)on S-C3N4.Color scheme:mechanism 1,green;mechanism 2,black;mechanism 3,red.Reproduced with permission from Ref.[264]Copyright 2015,Elsevier.c)Overall total-energy profile of the H2O=H2+1/2O2reaction on pure BiOCl(001)surface d)and BiOCl(001)surface with surface VO.Surface Bi,sub-surface Bi,surface O,H atom of water and O atom of water are in white,gray,red,blue,and green,respectively.Red ring represents surface VO.The remaining substrate is not shown for clarity.Reproduced with permission from Ref.[267]Copyright 2015,Wiley-VCH.e)Potential energy diagram for the proposed water dissociation path.Reproduced with permission from Ref.[242]Copyright 2003,AIP Publishing.
The existence of defects in photocatalysts exhibits a significant influence on the adsorption energies of certain species by changing the active sites and lowering the activation energy.[265,266]Furthermore,the first principle calculations for water dissociation on anatase TiO2have shown that less coordinated VOare more favorable for dissociation of water.[242]Likewise,the sub-surface VOin anatase-TiO2also facilitates the direct dissociation of water.[243]Another justification on the formation mechanism of O-O bond over VOin CeO2has also proposed some optimized routes for water oxidation with a significant decrease in activation energy.[238]Zhang et al.reported that VOpresent on BiOCl(001)mainly acts as the active sites for water oxidation.[267]However,majority of the calculations carried out were based only on the initial steps of water activation and dissociation involving a single or few water molecules.[238,244,268]A limited focus has given to water oxidation interaction between the catalyst surface and water molecules.[269]The interaction of water with both defective and defect-free BiOCl surfaces(Figure 23c,d)has shown that VOare the most favorable sites for water adsorption in ultrathin structures,where the adsorbed water could dissociate into OH and H for defective and perfect surfaces,with adsorption energies of 0.97 and 0.77 eV,respectively.[267]Thus,in case of perfect surface(Figure 23c),the resultant OH after dissociation will fall out at the bismuth site while the H will get bonded to the surface VO.Furthermore,the energy barrier for water dissociation(3.33 eV)is considerably higher,making the water splitting reaction to take place on a perfect surface with significant difficulty.However,in case of defective surface(Figure 23d),the dissociated OH falls out at VOwith a notable ease in water splitting activity.[267](Figure 23e)represents the corresponding reaction coordinates for the proton transfer up to transition state(TS)and the respective molecular(M)and dissociated(D)configuration states.As the potential energy of the dissociated metastable state(D2)falls close to the M2 molecular state,the free energy basin will comprise of the products with configuration closer to D2 or D3 to receive equal potential energy contributions from both the configurations.[242]Wang et al.demonstrated a versatile approach to investigate photocatalytic splitting using multipoint averaging molecular dynamics(MPAMD)technique and suggested two pathways for OER that could take place with considerable ease due to decreased energy barrier as shown in Figure 24a,b.[245]Although pathway i takes place along Ti-sites,pathway II occurs on bridged-oxygen.Pathway i takes start from OHtgeneration via protonation of H2O followed by hole trapping on the surface to form●OHt,the later deprotonates to yield Ot-radical that couples with another Ot-to produce O2after oxidation.[245]In case of pathway II,the●OHt-instead can couple directly with one of the bridged O on the surface without protonation.Subsequently,hole trapping and deprotonation produce O2molecule and generate a surface ionized VO,that can be readily filled up by adsorbing water molecules with successive deprotonation steps to regenerate the surface.[245]Therefore,all these three radicals,including terminal●OH,O-,and bridging O-are valued to be the essential species for surface reactions.Generally,the radicals weaken the Ti-O bonds to facilitate O2desorption and reduce the recombination of photogenerated charge carriers,thus promoting the photocatalytic activity.Likewise,the reaction coordinates and energy profiles of both the pathways(Figure 24c)obtained have revealed fast kinetics rate due to lower energy barrier in contrast to the general agreement of poor OER performance on TiO2,through micro-kinetics.[245]Accordingly,the analysis of devised mechanisms revealed that lower the efficiency of TiO2is mainly due to the lower concentration of the charge carriers and is negligibly affected by higher energy barrier.[242]Hence,the key step to increase the photocatalytic activity of TiO2(110)is to increase the concentration and diffusion of charge carriers.[245]
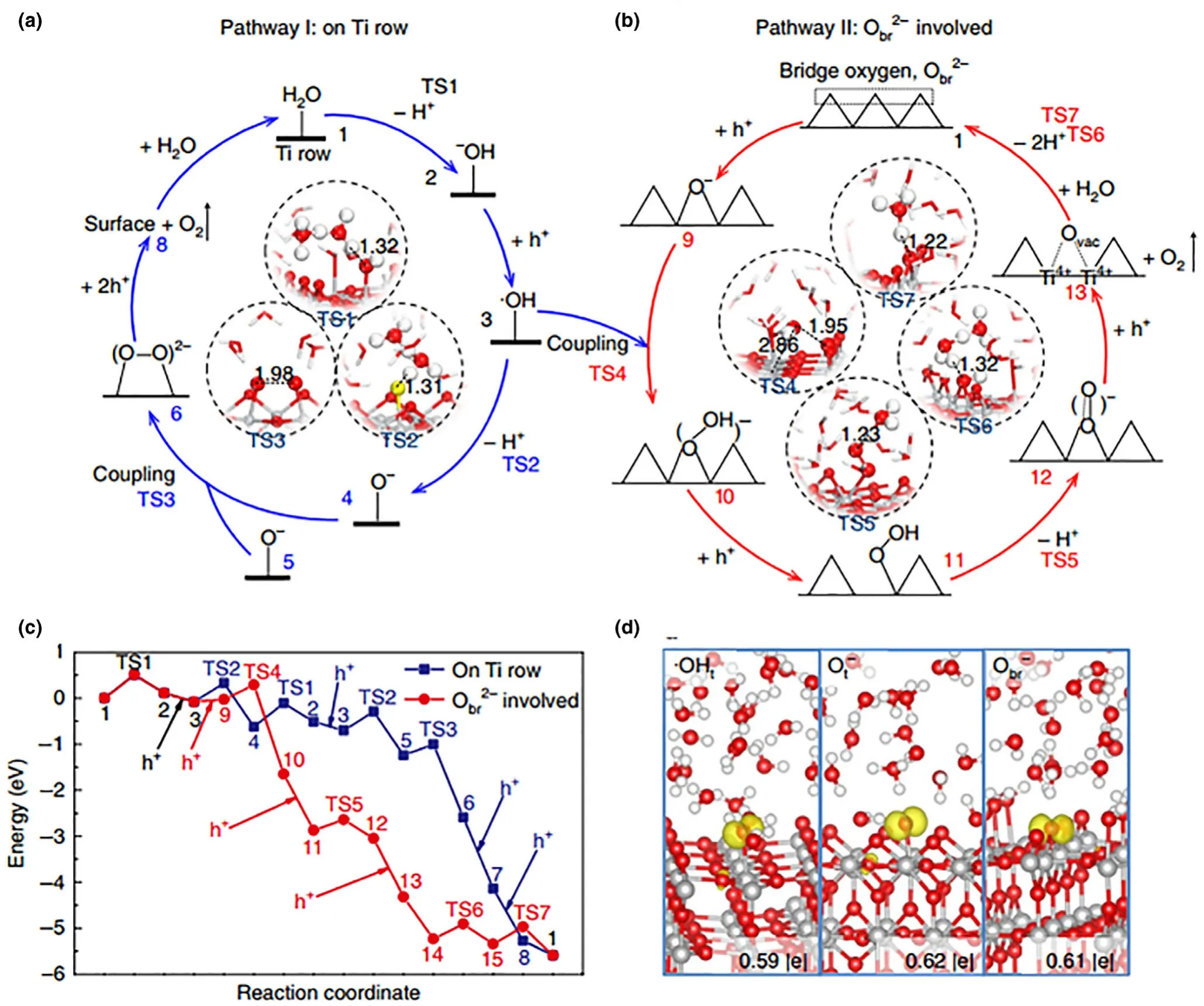
Figure 24.Proposed mechanisms(dual pathways)and energy profiles for the photocatalytic OER.a)One of the dual pathways(pathway I)occurring on the Ti row.b)The other pathway(pathway II)involving bridge oxygen.Insets show the TS structures.c)The energy profiles of pathways i and II,in which states 1,2,15 correspond to the states in a)and b).The elementary steps involving holes are labeled by h+.d)The existence of three key surface radicals in the process.They are illustrated by the spin density plots(dumbbell-shaped O 2p orbital;iso value of 0.005)and the Bader charge difference(~0.6|e|means that those oxygens after trapping h+are positively charged with+0.6|e|with respect to the lattice O in bulk TiO2).Reproduced with permission from Ref.[245]Copyright 2018,Springer Nature Limited.
5.5.Defect States and Optical Property
Defects play a significant role in manipulating the optical properties of the semiconductor materials,where the bound excitons serve as a principle source of defect identification making use of the optical features of the material.[270]For example,TMDs have been extensively researched in regard of their fascinating optical and electronic properties[271,272]including direct band gap[273]and strong electronhole interaction processes.[274]Both neutral and charged excitons in lattice can recombine with charge carriers that result in the formation of bound excitons,showing their characteristic PL spectra peaks with an energy value less than the actual peak of neutral excitons.[275,276]For instance,Carozo et al.systematically investigated defect bound excitons through PL and first principle calculations.[277]The structural differences on the edges and interior of the WS2triangular moieties can be studied via STEM measurements supported on a holy carbon film(Figure 25a).The two types of defects:monosulfur vacancy and occasional WS3vacancies,corresponding to the yellow circles and orange triangle,respectively,with an aggregated vacancy on the edges.However,other vacancies like disulfur vacancies and antisite defects,like S substituted W site or vice versa are very rarely observed.The confirmation of the defect structures can be carried out via comparing the simulated STEM images with that of annular dark- field(ADF)resolution(Figure 25b).
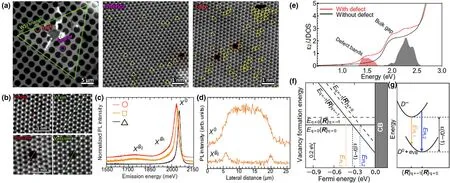
Figure 25.Identification of S vacancies.a)Optical image of transferred WS2triangle onto QUANTIFOIL(left),which is within 1 mm from the edge of the WS2triangle,high magnification ADF images from center part(middle)and edge part(right).Monosulfur vacancies(VS)and one tungsten plus three sulfur vacancies(VWS3)were marked by yellow circles and orange triangles,respectively.b)Comparison of experimental and simulation ADF image of VSand VWS3 vacancies.The line profile was acquired along the line in ADF images.Reproduced with permission Ref.[277]Copyright 2017,American Association for the Advancement of Science.c)PL spectra obtained from the marked regions.d)Un filled curves are the imaginary part of the RPA dielectric function for the 5×5 super cell with(red)and without(black)S vacancies.Red(gray) filled curves are the joint density of states(JDOS)from the three highest VBs to the two defect(six lowest conduction)bands.e)X0and XB2intensity profiles.d)Defect formation energy for S vacancy with q=0 and-1,as a function of the Fermi energy[referenced to the CB minimum].Solid lines are energies of q=0(q=-1)obtained at their respective equilibrium configurations;dashed lines are obtained at the equilibrium configuration of the alternative q=-1(q=0)state.The thermodynamic charge transition level can be found at the crossover between the two solid lines.f)Schematic for the defect energies of neutral and charged defects as a function of the collective coordinates of a system.Optical emission energies can take values between EPL1and EPL2.Reproduced with permission from Ref.[277]Copyright 2017,American Association for the Advancement of Science.
Moreover,in PL spectrum of defective WS2monolayer,the interior triangle has lower defect sites than the exterior edges(Figure 25c,d).[277]Similarly,Figure 25e represents the PL spectra of neutral excitons with normalized intensity taken from the highlighted regions in Figure 25d.The triangular area represents the interior while the squares and circles correspond to the edges.The existence of trains and the degree of n-doping can be extensively confirmed via the X0peak of the interior of the triangle,showing the high degree of doping or the depletion of n-carriers by the S vacancy in close proximity of sites.However,the density of S vacancy is lower at the edges that are not sufficient to compensate for the n-carriers.Therefore,these results in a red shift of neutral exciton peaks in the interior region by 10 and 20 MeV,respectively,which can be ascribed to band gap modulation.[278]The intensity of the bound exciton peak is relatively localized toward the edges as compared to the neutral exciton peak in monolayer WS2,as shown in Figure 25f,g.
Thus,the defect density on the edges and near interior thereof comes out to be 0.33±0.11 and 0.92±0.45 nm-2,respectively.Similarly,the same comparison studies can be carried out for S and WS3vacancies in monolayer MoS2under low-angle ADF(LAADF),where the ADF intensity comprised of both the diffraction intensity and Z contrast.[279]
6.Defect Characterization Techniques
To unveil the role of defects in photocatalysis,proper characterizations are needed to understand the relationship between structure and reactivity for design of high performance photocatalysts.Both quantitative and qualitative techniques have been adopted for defects quantification and identification respectively.The primary identification of defect comes from microscopic evaluation while the structural information is probed from spectroscopic characterizations.The carrier conductivity and defect modulation are,however evaluated by theoretical calculations.
6.1.Microscopic Characterization
Microscopic imaging sets the initial basis of defect exploration of few layered materials quite easier,in case of TMDs,the mono-and few layers can be readily obtained from their bulk crystals.[271,280]The total number of layers can be determined from optical contrast measurement or more accurately through AFM(Figure 26a-h),where each height profile corresponds to the number of layers.[281]Conventionally,in order to estimate atomic locations,electron microscopy is employed,which makes images with elastically scattered electrons.[282,283]For high spatial resolution,EELS is commonly used.[284]However,it commonly suffers from the delocalized EEL signal,weak scattering and lack of the appropriate spectral resolution when high spatial resolution is required.[270]Recently,for atomic level identification specifically in a non-periodic material at high spatial resolution ADF imaging equipped with STEM is utilized where individual B and N atoms in monolayer boron nitride(BN)could be identified clearly via ADF-STEM techniques directly from their intensities(Figure26i).[285]The hexagonal ring marked with green circle represents three N atoms(brighter atoms)which shows the main pattern of the monolayer.However,throughout the image some deviations can be observed,like the hexagonal ring marked with yellow circle that consists of six atoms and relatively low intensity as compared to that of B and N.[270]Moreover,in order to visualize distortions caused by symmetry lowering,TEM equipped with aberration corrector(TEAM)is known to be the main characterization tool for investigation with straightforward insights to a single point defect.[286]
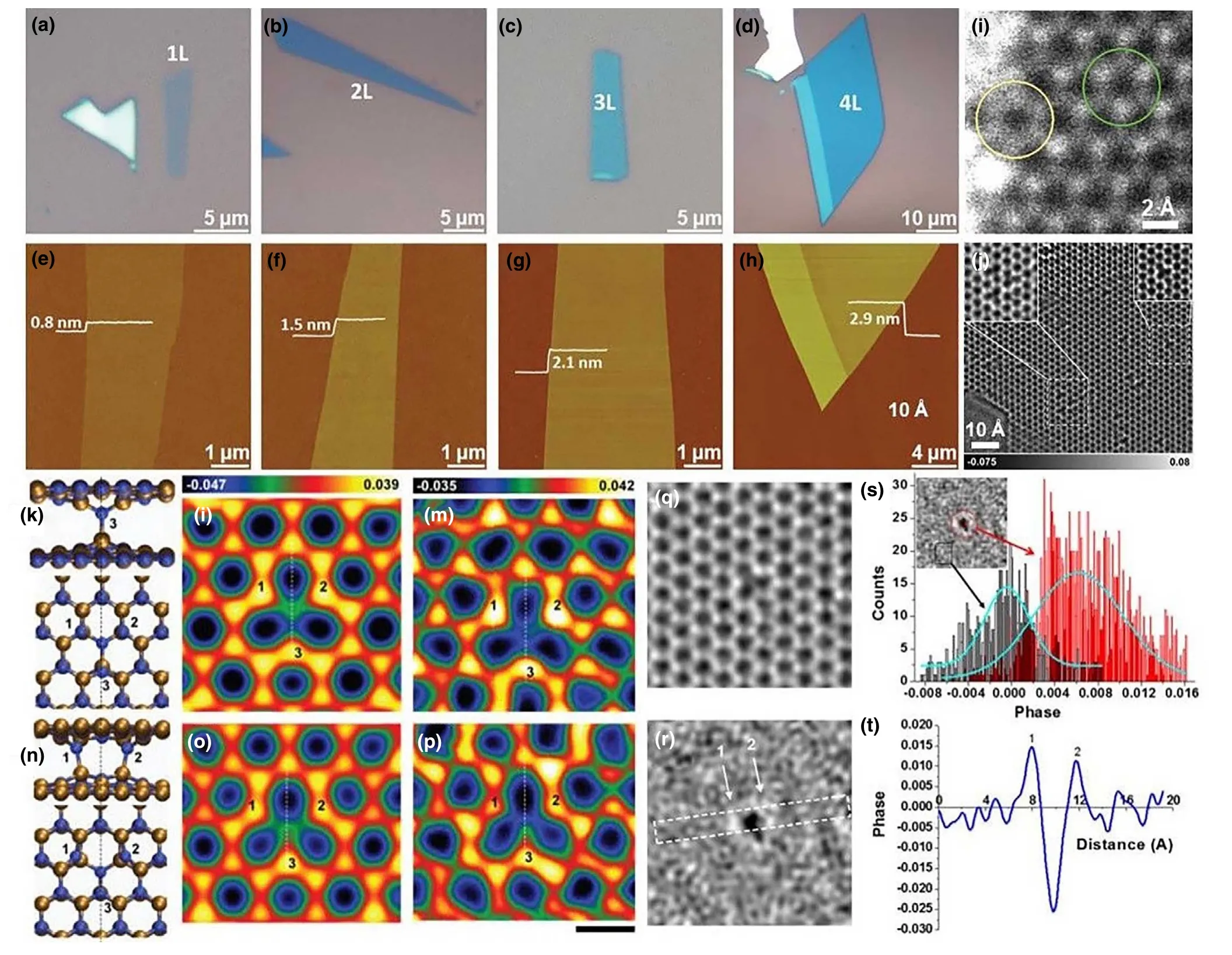
Figure 26.Mechanically exfoliated single-and few-layer MoS2NSs on 300 nm Si/SiO2.Optical microscopy(a-d)and AFM(e-h)images of single-layer(1L,thickness:≈0.8 nm;a,e)double-layer(2L,thickness:≈1.5 nm;b,f)triple-layer(3L,thickness:≈2.1 nm;c,g)and quadruple-layer(4L,thickness:≈2.9 nm;d,h)MoS2NSs.Reproduced with permission from Ref.[280]Copyright 2012,Wiley-VCH.i)ADF-STEM image of monolayer BN.Reproduced with permission from Ref.[270]Copyright 2010,Macmillan Publishers.j)Reconstructed phase of a bilayer h-BN imaged by the TEAM i microscope.The magnified inset images show broken symmetry at the defect sites.Color bar shows the relative phase in radians.k-p)Atomic structure models and phase of distorted boron monovacancies in bilayer h-BN with one and two interlayer bonds.k,n)The top and cross-sectional view of stable defect configurations predicted from first principles showing in-plane and out-of-plane asymmetric distortions at the boron mono-vacancy with one and two B-N interlayer bonds.l,o)A reconstructed phase,simulated from the models shown in k)and n),respectively.m,p)Reconstructed phase of two mono-vacancies in a bilayer h-BN recorded on the TEAM i microscope.Color gradient represents the relative phase of the atoms(yellow indicates individual atoms;black corresponds to the centers of hexagons in the honeycomb lattice).The atoms are numbered for the ease of identification.The single mirror plane across the mono-vacancies is shown by white dashed lines.The scale bar is 2.5˚A.Color bar shows the relative phase in radians.q)Phase of a mono-vacancy and(R)residual phase around the vacancy after the phase from the defect-free region is subtracted.s)Distribution of residual phase showing a phase shift at the vicinity of the vacancy compared to the background.t)Residual phase line profile across the vacancy previously shown in r).Reproduced with permission from Ref.[286]Copyright 2011,American Chemical Society.
The mono-vacancy defects in hexagonal BN(h-BN)induced by symmetry breaking are caused by the bond reformation between the h-BN players(Figure 26j),where the magnified area represents broken symmetry and corresponding defect sites.[286]In addition,Figure 26k-p represents the comparison of the simulated and the reconstructed phase of B vacancy,which reveals that due to the interlayer bonds across theh-BN,the resultant reduced symmetry causes observable gradient contrast and significantly large intensities of the atomic columns at vacancies perimeter.[286]
Furthermore,the former results could be verified by subtracting the phase free region from one consisting vacancy,with an observable phase shift(Figure 26q,r).In addition,the mean shift around the vacancy relative to the background can be shown by two Gaussian curves(Figure 26t).In mechanically exfoliated or vapor deposited MoS2with intrinsic structural defects,the most popular ones are the grain like VMoS3and VMoS6with corresponding removal of one Mo and three neighboring sulfur pairs have also been figured out,as shown in(Figure 26).[287]Similarly,Ru and Au doped MoS2defects could also be observed via STEM studies[288]which is also prominent in the line profile(Figure 26s).[286]Other investigations with most complex defects include TMDs.[289,290]Among other examples in TMDs family,Nb doped WS2with Nb residing at W sites can also be visualized systematically.[291]
6.2.Spectroscopic Characterizations
6.2.1.Raman Spectra
Raman spectroscopy is another effective and powerful tool for detection of impurities and defect states in materials using the fingerprints of vibrational signals.[292]A well-known example of that is the G and D’s bands in Raman spectra of graphene.[293]Similarly,MoS2exhibits two prominent Raman peaks demonstrated as A1′and E′.[292,294,295]Parkin et al.demonstrated the Raman modes with respect to the variable S vacancies created through electron beam irradiation that are accompanied by red and blue shift of their respective E′and A1′peaks,as illustrated in Figure 27a,b.A linear increase in the frequency can be observed between the E′and A1′as the correlates to the Brillouin zone edge phonons(Figure 27c),which are produced by the momentum contribution from the created vacancies.[294,296]
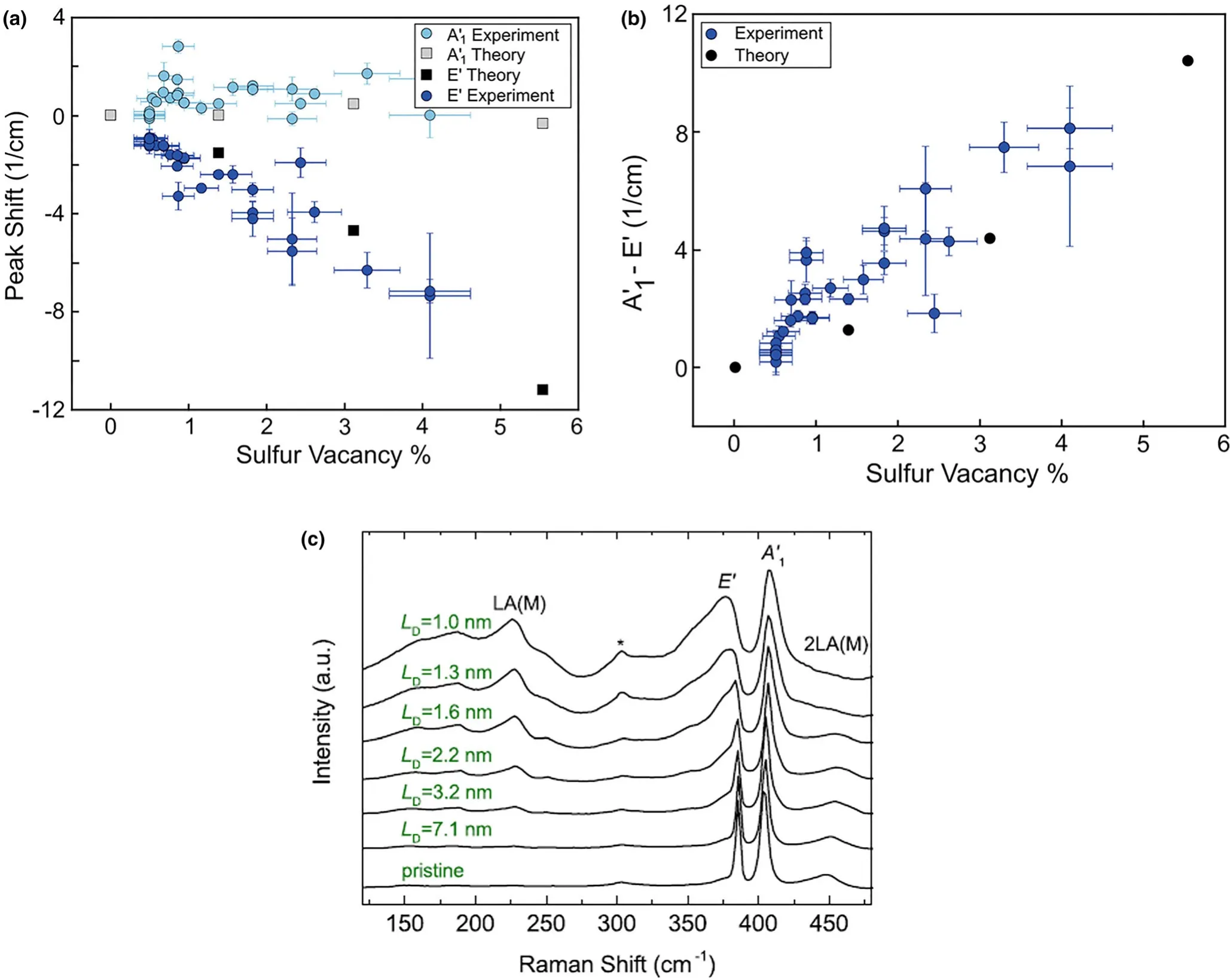
Figure 27.Raman peak shift vs S vacancy:experiment and theory.a)Raman peak shift of E′and A1′modes as a function of sulfur vacancy percentage in monolayer MoS2.b)Change in separation between the E′and A1′Raman modes as a function of defect concentration.Reproduced with permission from Ref.[294]Copyright 2016,American Chemical Society.c)Raman peaks of monolayer MoS2with varying inter-defect distances LD.Reproduced with permission from Ref.[296]Copyright 2015,American Physical Society.
6.2.2.Photoluminescence Spectroscopy
PL spectroscopy also serves as one of the most important tools for defect identification.It is well-known that defects or impurities provide traps for charge carriers or excitons by incorporating mid band gap sates,which significantly enhance the optical and electronic properties of the nanomaterials.[292]The recombination of excitons leads to radiative emission that can be monitored by PL spectroscopy.Such PL emission from both defective and pristine WSe2through electron beam irradiation has been studied to demonstrate defects at a temperature as low as 83 K,as represented in Figure 28a,b.[297]For example,the PL intensity of prepared MoS2is much lower than the direct band gap of MoS2monolayer,due to the formation of trions(negatively charged excitons)and can be enhanced by the electrical gating or molecular adsorption followed by trions to excitons switching.[273,298-301]Nan et al.reported the oxygen plasma irradiated MoS2with monosulfur vacancies.The adsorption of oxygen results in holes doping thereby decreases the hoping of electrons.[301]This can induce the switching of trions to excitons along with the strong PL emission spectra(Figure 28c),confirming the defect localities in the material.
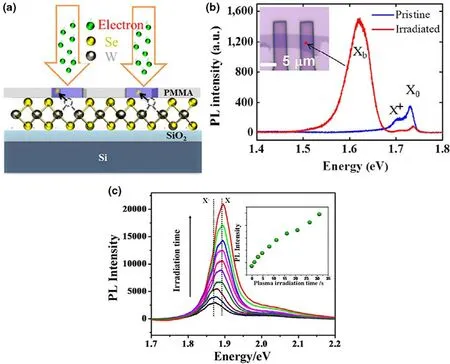
Figure 28.Defect-induced PL emission.a)Schematic diagram of electron beam irradiation on monolayer WSe2sample during the electron beam lithography(BEL)process.b)PL spectroscopy of pristine and irradiated monolayer WSe2.Reproduced with permission from Ref.[297]Copyright 2016,Tsinghua University Press and Springer-Verlag Berlin Heidelberg.c)PL spectra of monolayer MoS2after oxygen plasma irradiation with different durations(inset:the change of PL intensities with plasma irradiation times).Reproduced with permission from Ref.[301]Copyright 2014,American Chemical Society.
6.2.3.Positron Annihilation Spectroscopy
Positron annihilation spectroscopy(PAS)is considered as a sensitive tool for investigation of vacancy type defects and their ratio in photocatalyts.[302]The measurement of positron lifetime provides significant insights to the defects structure in photocatalyst.When thermalized positrons move toward the interstitial sites in lattice upon injection into the bulk of the material,experiences strong coulombic repulsion from the lattice ions and results in annihilation heat due to positron-electron interaction,which causes emission of γ-rays that provides information about positron lifetime.Usually,vacancy defects serves as effective traps for positrons when negatively charged or neutral while the positively charged defects states are considered as ineffective trap sites as the later would result in repulsion of positrons than the former which strongly attracts positrons owing to their long range coulomb potential.[303]The shortest lifetime(τ1)known as the first lifetime of positron is due to the free annihilation of positron in case of defect-free structure.However,in case of vacancies such as mono-vacancies in defective-crystals would result in a decreased average electron density and hence the annihilation,which leads to an extended lifetime of(τ1).Further,the second larger lifetime component(τ2)results from the positron annihilation by the large-sized defects(vacancy clusters)with lower electron density in its surrounding.A typical positron spectrum for defectivephotocatalyst is given as the average lifetime,related to the components(τ1& τ2)and their annihilation rate.[304]Moreover,the third longest lifetime component(τ3)corresponds to the positron annihilation occurred in the voids of photocatalyst.For example,Choudhury et al.synthesized g-C3N4with N defects through calcination and then characterized them with positron annihilation spectroscopy(Figure 29a).It can be seen that at higher calcination temperature,that is,570°C the ratio curve shows a broad dip around the momentum value pL=10×10-3thus representing greater volume defects.[305]Furthermore,the increased defects density can also be depicted from the increase in S-parameter of the system[306]corresponding to the annihilation of low and high momentum positrons(Figure29b),respectively.

Figure 29.a)Ratio of area normalized CDB spectra of calcined C3N4with respect to area normalized CDB spectra of 420 o160 C calcined C3N4.b)Doppler broadening shape parameter,S161 parameter,for differently calcined C3N4samples.Reproduced with permission from Ref.[305]Copyright 2018,American Chemical Society.
6.2.4.X-ray Absorption Spectroscopy
X-ray absorption spectroscopy(XAS)including the X-ray absorption near edge structure(XANES)and extended X-ray absorption fine structure are the most frequently used characterization tools for investigation of the coordination environment and chemical state of the materials with vacancies.For example,Bao et al.utilized XANES for determination of vacancies in TiO2.[307]A significant decrease in absorption intensity can be attributed to the decreased probability of transition to the hybridized orbitals due to the loss of O from TiO2lattice following hydrogenation(Figure 30a).Moreover,the absorption edge remains unchanged for Ti-K-edge XANES during(≈0-4 d)(Figure 30b).However,as the hydrogenation period exceeds beyond(≈0-4 d)the absorption edge shift slightly confirming the creation of vacancies in bulk of TiO2.[308]This observation suggests that in the beginning of hydrogenation process,the vacancies are mainly produced on the surface of H-TiO2which later extend to the bulk of the material after(≈0-4 d).Simultaneously,the EXAFS measurement of the Ti-K-edge revealed notable peak shift for HTiO2relative to that of P-TiO2indicating the distortion in symmetry with hydrogenation(Figure 30c).In addition,it can be observed that both the surface and bulk of TiO2bears distortions with few coordinated O atoms to each Ti after hydrogenation;however,the distances of Ti-O and Ti-Ti are,respectively,shorter than P-TiO2,as shown in Figure 30d.

Figure 30.a)Ti-K-edge XANES spectra(inset shows an enlarged view in the range from approximately 4982.0 to 4983.5 eV)and b)RSFs of the Ti-K-edge obtained from EXAFS spectra of the P-TiO2 and as-prepared H-TiO2 samples.c)Schematic representation of the structure of P-TiO2 before and after hydrogenation.d)Gray balls:Ti,red balls:O,O1:the first shell,Ti1:the second shell,O2and Ti2:the third shell.Reproduced with permission from Ref.[308],Copyright 2016,Wiley-VCH.
6.2.5.Surface Photovoltage Spectroscopy
Surface photovoltaic spectroscopy(SPV)is a spectroscopic technique commonly employed to study the interface and surface defects in semiconductor materials,which probes changes in the contact potential difference(ΔCPD).[309,310]SPV is conventionally used for the detailed investigation of semiconductor potentials(i.e., flat band potential and surface potential),thickness of deposited oxides,charge carriers’diffusion lengths,lifetimes,and doping densities with associated wavelengths.[310]A crystalline semiconductor exhibits a periodic atomic pattern connected via chemical bonds.Introduction of defects either on the surface or bulk results in dangling of bonds,causing unsaturation to reconstruct defects or saturate by adsorbing the impurities to create defects.[311]These defects greatly alter the structure of allowed energetics that mainly depend on the crystal structure.Therefore,change in translational geometry results in localized states in the surface.It has been demonstrated that the energetics of these states are located deep within the band gap,thus creating separate energy states.Therefore,a surface can be thought as a boundary between media and other physical characteristics,where the surface dipole due to disruption can induce localized states within the electronic states of a semiconductor to alter the position of the surface atoms,and minimize the surface energy as a result of the impurity sates.[312]The material evaluated by SPV is need to be oxidized that possesses surface charge(N)along with the surface potential(VS)providing depletion regions.[311]It has been observed from the band diagram that at equilibrium,for n-type and p-type semiconductors,the surface potential is always positive for downward band bending.[312]For example,Zhao et al.synthesized TBAxH(1-x)Ca2Nb3O10with perovskite structure where Ca2+ions fill the octahedral Nb(μ2-O)6voids(Figure 31a)to adopt sheet like morphology and later yield coplanar orientation with respect to the substrate(Figure 31b-d).Furthermore,a typical SPS spectrum of TBAxH(1-x)Ca2Nb3O10film is shown in Figure 31e that comprises mainly of four distinguished regions(I-IV)with negative ΔCPD for the first region(I)that arises at photon energy lower than the band gap of the respective material.This is associated with the excitation of mid-gap defect levels particularly at the surface.In case of direct band gap excitation,the photochemical charge separation is represented through(green region)II with negative ΔCPD signal in Figure 31f,while the other two regions III and IV corresponds to under band gap excitation.In addition,it has been demonstrated that the main photovoltage signals I/II greatly depend on the thickness of the film linearly increases and reaches a constant value beyond 1350 nm.[311]Moreover,it shows that the rate of methanol oxidation increases twice in vacuum through photogenerated holes,with a more efficient electron transport to the gold substrate(Figure 31g),while a new positive signal arises when the spectrum is recorded in air(Figure 31h).It is followed by a prominent reduction in region II and can be attributed to the oxygen reduction by air to cause a low onset of 2.4 eV.This suggests that the corresponding electron transfer processes are associated with these mid-gap surface defects.[311]Furthermore,in case of n-type and p-type semiconductors,the illumination above the band gap results in decreased surface bending.[312]However,if the incident photon energy is equal to the band gap,the SPV will be higher and will be more negative for p-type and more positive for n-type semiconductors.

Figure 31.a)Structural model and b)TEM of TBAxH(1-x)Ca2Nb3O10NSs.c)Top and d)side SEM view of a NS film on a gold substrate.e)Surface photovoltage spectrum of a TBAxH(1-x)Ca2Nb3O10NS film on Au,along with its diffuse reflectance spectrum.f)Photovoltage spectral comparison between low-defect and rich-defect samples.g)Photovoltage spectra of NSs in vacuum before versus after methanol treatment.h)Photovoltage spectra of NSs on an Au substrate with or without 40 nm poly(3,4-ethylenedioxythiophene)poly-(styrene sulfonate)(PEDOT/PSS).Reproduced with permission from Ref.[311]Copyright 2014,American Chemical Society.
The defect-associated surface states can be assumed as trap centers,demonstrating the electronic transitions between the bands and trap states.Photons with energy higher than the CB and trapped charge state can promote electronic transition from CB to the trapped charge state to reduce the band bending with a decrease or increase in SPV signal for both n-and p-type semiconductors.However,on the other hand,electronic transitions to the trap level from the VB would rather results in increase band bending with a corresponding variation of the SPV signal.Thus,for a p-type semiconductor,the positive change in the SPV spectrum that correlates to the electronic transition from occupied defect state to the CB can be identified.But on the other hand,the negative slope change indicates the photoexcitation to the unoccupied defect state from the VB.Like a negative SPV signal has been observed in CdTe and Cd1-xZnxTe(CZT)single crystals,showing the p-type conductivity of these materials.[313-316]As the SPV corresponds to the optical absorption coefficient,the energy gap can be determined from photon energy.However,a hump rather than a peak is observed as a result of electron-hole recombination reducing the SPV.[317]Similarly,in case of CdTe,three major slopes below the band can be observed as an influence of the defect states,indicating the presence of traps.Thus,SPV not only allows the detection of defects,but also provides information about their energy positions within the band gap.[318]
7.Role of Defects in Photocatalysis
Defect engineering plays a crucial role in photocatalysis by controlling the electronic structure,carrier concentration,electrical conductivity and interfacial charge transfer processes.In this section,significant defects with their characteristic influence on photocatalytic processes like,water splitting,CO2reduction,pollutant degradation,organic synthesis,and nitrogen fixation have been discussed.
7.1.Water Splitting
Photocatalytic water splitting provides an efficient and direct route for chemical energy conversion.[319-323]The main feature of efficient solar H2generation technology is the development of one photocatalyst that can work over a broad spectrum of radiation with appropriate redox energy levels.[324,325]Therefore,tuning the rational structure of photocatalyst for solar water splitting is of immense importance.Defect-rich and cost-effective architecture can stand as the most suitable option for water splitting.For example,defect-rich g-C3N4has shown to significantly increase H2evolution activity that can be attributed to the KSCN(potassium thiocyanate)substitution at the surface hydrogen of amino grouping-C3N4to introduce cyanamide defects and provide coordinating sites in the catalytic events.[326]Similarly,nitrogen defects can be introduced to the surface of g-C3N4via chemical reduction using NaBH4as a reducing agent.[91]Further,cyanamide defects can be introduced in g-C3N4at-C-NH2position through alkali-assisted thermal polymerization reaction that undergoes the corresponding band alignment.[327]In an acidic environment,however the hydrolyzation of the cyanamide defects leads to the formation of urea modified g-C3N4.These strategies,therefore,require some post-chemical treatment for defect engineering in the g-C3N4framework.The cyanamide defects and relevant band gaps narrowing can be better interpreted via DFT both for pristine g-C3N4and for CCN(Figure 32a-d),while the latter exhibits a smaller band gap than the former,[328]instead of co-existing in the same unit cell of g-C3N4framework as reported earlier.[329]However,for CCN unit that is attributed to the extended p-conjugated system through electronic redistribution from cyano-terminal groups.[330,331]
The resultant defects have a marked influence on the separation of charge carriers.As expected,CCN-0.03 photocatalyst exhibits high photocatalytic H2evolution activity under visible light irradiation with respect to that of pristine g-C3N4,and the high photochemical stability in a period of long-time irradiation reaction(Figure 32f).Similarly,pit engineered ultrathin BiOCl NSs exhibit much higher O2evolution activity than the bulk specimen.[111]Defects are therefore known to play pivotal role in photocatalytic reaction,for other instances,O vacancies in In2O3,pit built WO3NSs,distortions in ZnSe,SnS2and SnS.[114,124-126]
7.2.CO2Reduction
The conversion of CO2to carbon fuels requires a significant amount of energy to subject the cleavage of highly stable C-O bond.[332]Solar energy,an inexhaustible carbon-free supply,can act as a driving force for this conversion process.Photocatalysis offers the possibility to capture and utilize CO2as an efficient fuel source in the presence of semiconductor catalysts under sunlight irradiation.
Incident energy(hʋ)equals or larger than the band gap of semiconductor causes the surface excitation of an electron from the highest occupied VB to an empty CB to generate electron-hole pairs.[333,334]The reduction and oxidation capacity of a semiconductor is determined by the potentials of photogenerated electrons in CB and holes in VB,respectively.[335]Though,majority of semiconductors bear suitable band gaps for photo reduction,they still suffer from various limitations,for instance metal chalcogenide(PbS,CdSe,and CdS)are being vulnerable to photocorrosion with limited stability in aqueous media.[333,336]Moreover,along with some toxicity,the low adsorption capacity of the respective semiconductors results in low solar fuel conversion.Lee et al.estimated the threshold energy for electron-induced dissociation of CO2adsorbed at a VOsite on the exposed TiO2(110)at single molecule level through STM,which is found to be slightly above the CB minimum of TiO2.[337]Moreover,this VOhas shown to be rebuild again by the oxygen atoms released while CO2dissociation process.A typical illustration of the CO2adsorption process on VOsites of TiO2was shown in Figure 33a.The most stable orientation for CO2adsorption at VOcomes out to be the linear configuration with an energy of 0.44 eV,with a clear bright appearance marked with circles(Figure 33b).[338,339]Furthermore,STM studies revealed that CO2feature to be the most prominent among the bridging(OHb)and VOfeatures with an apparent height profile of 80 pm(Figure 33c,d).However,the later does not correspond to the theoretical adsorption(Figure 33a)due to the fast-thermal motion of CO2at 55K over VO.[340]In addition,the electron tunneling from the EFof STM tip can formin stateonly with the applied bias voltage higher than the threshold voltage,as illustrated in(Figure 33e).After elongation and bending of the C-O bond in the gaseous phase,the O atom released from the dissociation of C-O heals the VOand is substituted in the bridging oxygen(Obr)row(Figure 33f),[341]while CO specie is likely to desorb and inject excess electron into the CB of TiO2as it moves across the surface from the reaction site.[337]Therefore,defect modulation comes out to be one of the most suitable strategies in order to provide abundant chemical adsorption sites for CO2photoreduction.[342,343]Likewise,after introducing O vacancies to ZnAl-LDH ultrathin NSs,the resultant Zn+-VOacts as trapping site for effective adsorption of H2O and CO2molecules to facilitate electron-hole pairs’separation with effective photo-conversion of CO2to CO.[33]
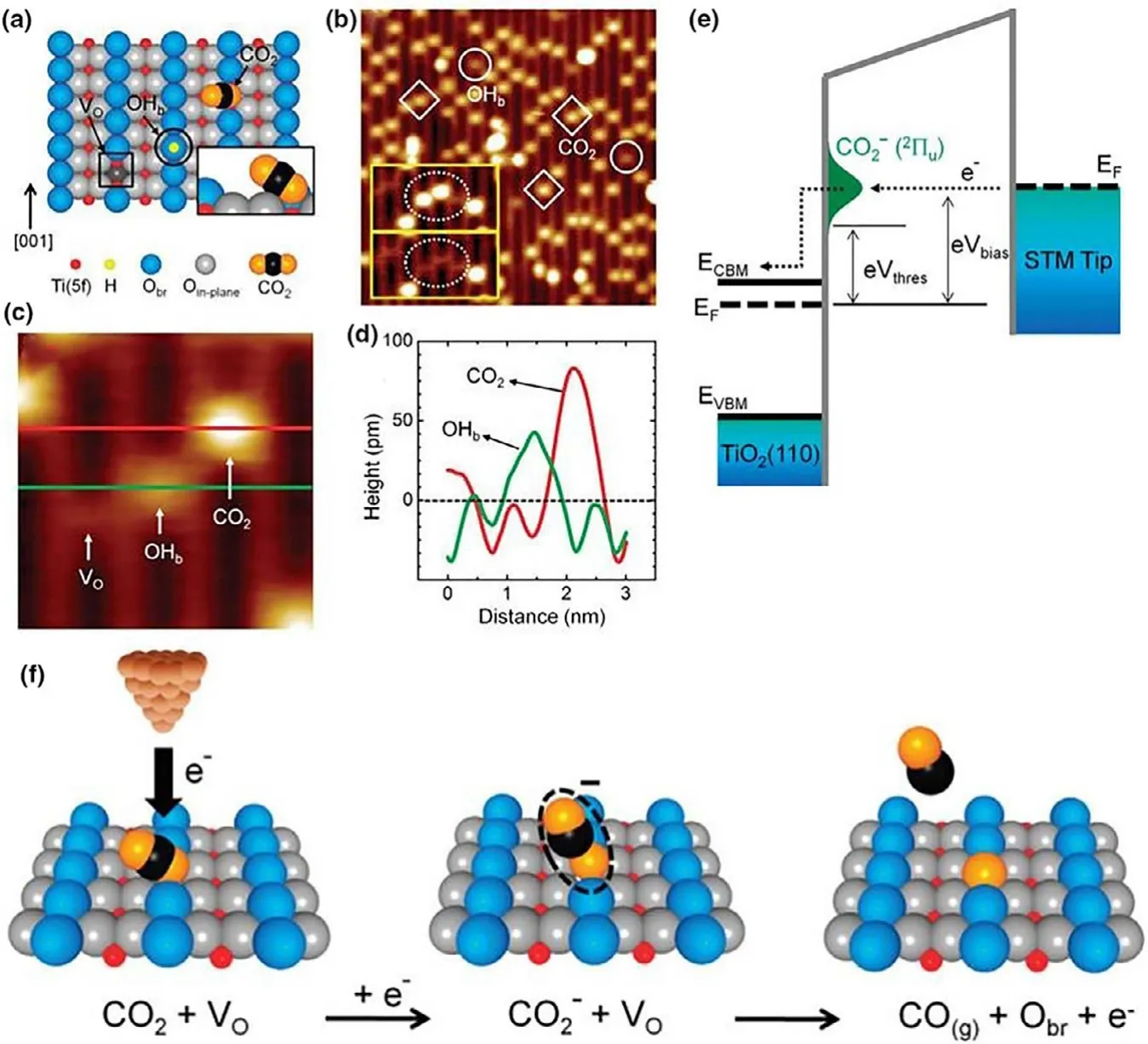
Figure 33.a)Schematic showing a VO(black square),a bridging hydroxyl(OHb)(black circle),and a CO2molecule adsorbed at a VOsite on the reduced TiO2(110)-(1×1)surface.Fivefold coordinated Ti(5f)atoms and bridging oxygens(Obr)are indicated in red and blue,respectively.b)STM image of the TiO2(110)surface after adsorption of CO2at 55 K.All of the VOsites are occupied by CO2.Three CO2 and two OHbfeatures are marked with diamonds and circles,respectively.c)STM image of an area where three different features(CO2,OHb,and VO)are shown for comparison.d)Height profiles along the red(over CO2)and green(over OHb)lines in c).e)The electron transfer process at the STM tip/CO2/TiO2interface.f)Schematics of an electron-induced CO2dissociation process.Reproduced with permission from Ref.[332]Copyright 2011,American Chemical Society.
Besides anion vacancies,cation vacancies also promote the photoreduction of CO2,such as ZnIn2S4unit cell with corresponding Zn vacancies displays effective charge transport with higher separation of charge carriers.[107]Similarly,Xie and co-workers reported high methanol formation relative to the V-vacancies via single-unit cell of BiVO4.[108]Theoretical investigations have revealed that point defects greatly account for the bent conformation of CO2molecule that efficiently stabilizes CO2-intermediate while promoting CO production.[344]STM studies have demonstrated that the strong bonding of CO2with bridging VOand weak adsorptions of CO2has been justified.[345]Pipornpong and coworkers have reported the distinct adsorption behavior of CO2molecules over perfect or O-deficient anatase(001)surface.[346]Yin et al.investigated the adsorption configurations of CO2on rutile(110)surface with VOthat provides excess electrons and adsorption sites.[347]Therefore,most stable configuration in regard of the binding energy calculations iscompared to the most widely reportedconfiguration and other metastableconfigurations with slightly lower binding energy than the former.The adsorption kinetics of water vapors during photoreduction is also of great importance to the scientific community.For instance,the interaction of H2O with both pristine and defective TiO2annealed under O2and ultra-vacuum environment, finding that the facilitative dissociation of adsorbed water could be observed on defective TiO2.[348,349]The dissociation of water at every VOsite results in the removal of H atom,leaving behind an OH group,the O atoms of which come forward to heal the VO.Moreover,according to the insitu diffuse reflectance infrared Fourier transform spectroscopy(DRIFT)analysis,more bridged,and HCOOH species are produced through UV light irradiation from CO2and H2O vapors on defect-rich TiO2.However,in case of Co-doped TiO2,visible light activity of water oxidation by such doped TiO2with selectively controlled products could be carried out through optimal ratio of Co to Ti.[182]Figure 34a represents a typical synthesis illustration of ordered mesoporous Co-TiO2through self-assembly process.It shows significantly enhanced photoreduction of CO2compare to the other visible light-induced photocatalytic systems(Figure 34c),[350-359]owing to the band gap adjustment through Co doping and regular porous network.[174]The possible charge transfer mechanism in defect-rich TiO2summarizing the most acceptable theoretical formulation is illustrated in(Figure 34b).The photocatalytic CO2reduction can be described in two major steps:the first one involves the adsorption of H2O and CO2molecules on catalyst surface,while the second deals activation of excitons through photons for beginning photoreduction.[113]The absorption of light creates charge carriers,with the subsequent migration toward the surface to reduce CO2to the corresponding solar fuels,while the holes left behind in VB oxidizes H2O to O2.[360]The detailed interpretation of the mechanism is quite complicated with very minute details available.However,the two commonly proposed reaction pathways are carbine and formaldehyde pathways.[360-362]The photoreduction of CO2to CO is considered to involve single or most probably two electron transfer pathways,because the former process needs the CB edge to be slightly negative than reference to E(CO/CO2,pH 7)vs NHE,whereas the CB of defect-free TiO2is-0.5 V vs NHE.[363]In principle,the VB of TiO2is located at+2.7 V vs NHE,which theoretically justifies the oxidation of water to O2and H+.[363]For instance,in case of Co-TiO2the CB potential is higher than that of the pristine TiO2,and is enough negative to the reduction potential of CO2to CO(Figure 34d).This is caused by the different ligand field environments due to the difference in ionic radius of CO2+(65 pm)than Ti4+(60.5 pm),that causes distortion and thereby lifting the EFlevel from CB.

Figure 34.a)Schematic illustration of the formation process of ordered mesoporous Co-doped TiO2.b)Schematic illustration of the basic mechanism of the TiO2photocatalytic process.c)Comparison of the photocatalytic activity of the samples with common photocatalysts under visible light calculated according to the amounts evolved in 6 h.d)Schematic illustration of the band structures of the samples.Reproduced with permission from Ref.[174]Copyright 2015,Royal Society of Chemistry.
7.3.Photocatalytic Degradation of Pollutants
Defect-rich photocatalysts are revealed to promote the degradation and removal of pollutants.[364]For example,as compared with pristine g-C3N4,the porous ultrathin g-C3N4NSs show much more effective charge separation and highly enhanced photocatalytic dye degradation efficiency under visible light exposure.[11]As mentioned earlier,TiO2is an intrinsic semiconductor with n-type conductivity.However, in account to VTi,defective TiO2possesses ptype conductivity with increasing concentration of VTi.[94]In addition,the VB of defected TiO2(1.96 eV)is closer to EFin contrast to normal TiO2(2.12 eV),suggesting the p-type conductivity.[24,133]The direct evidence of p-type conductivity comes from Hall effect measurement,with positive Hall coefficient confirming the p-type conductivity of TiO2while the normal TiO2exhibits negative Hall coefficient.[365,366]Furthermore,VTipromotes the carrier mobility in defective TiO2that consequently exhibits higher cathodic currents,with progressive increase in photocurrent along the applied negative potential.[157]This is attributed to the band bending and better charge separation at electrode/electrolyte interface,which results in the higher photocatalytic activity of defectrich TiO2in comparison to the pristine one(Figure 35).Thus,defect engineering offers structural modulation along with other physiochemical properties,producing new types of functional semiconductor materials.[96]Zhang et al.reported the increased photo response of VBi-Oengineered BiO2-xmonolayer owing to the increased DOS with respect to the CB minimum.[116]This effectively reduces the electron-hole recombination and promotes the photocatalytic degradation of phenol and RhB under both ultraviolet and visible light irradiations.In addition,ultrathin BiOCl NSs with controlledvacancy associates exhibit cutoff to offer structural modulation along with other physiochemical properties to produce new types of functional semiconductors that expose the negatively charged(001)facet and hence improves the adsorption capability toward cationic dye.[117]Meanwhile,ultrathin BiOCl experiences the upshift of both VB and CB and ends up the narrowing of band gap,which therefore enhances the separation of electron-hole pairs,causing a significant positive increase in photocatalytic degradation efficiency

Figure 35.EPR spectra of defected and normal TiO2measured at a)low temperature(120 K)and b)room temperature(298 K).Inset:the enlarged EPR spectrum of normal TiO2.c,d)Charge density difference of Ti-defected(Ti15O32)and normal(Ti16O32)TiO2,respectively.e)Density of states of defected and normal TiO2.f)Photodegradation rates of organic pollutants.Reproduced with permission from Ref.[96]Copyright 2015,American Chemical Society.
7.4.Photocatalytic Nitrogen Reduction
In comparison to photocatalytic reduction of CO2,nitrogen fixation is more arduous in regard of the high dissociation energy of N≡N triple bond(962 kJ mol-1)and weak interaction with catalyst surface.[367]The photoreduction of N2to NH3is greatly affected by the abundance of active sites along the defect-rich surface.[368]From previous studies,it is concluded that VOs could act as electron traps due to the localized electrons that helps in activating the inert N2via 1.133˚A.[368]Recently,Shiraishi and co-workers demonstrated the systemic conversion of N2to NH3over the TiO2surface VOstates under ambient condition,where the surface Ti3+sites are the main activity centers for N2reduction reaction.[368]A typical reduction mechanism is illustrated in Figure 36a,which involves the dissociation of N2to Ti3+azo specie after electron donation(process a and b),and later produces Ti4+azo due to interaction of adsorbed N2with surface Ti-OH at adjacent position(process c).These Ti4+azo species upon photoexcitation produces electron-hole pairs(process d),whereas the CB electrons are trapped within the surface defect to rebuild the Ti3+while the VB-holes reside on the surface.[369,370]Further,the dissociation of N2produces Ti4+hydrazo specie(process e)which finally produces NH3and water at room temperature and pressure regenerating Ti3+(process a).[368]Defect-rich TiO2(110)is therefore considered as the most stable photocatalyst for NH3production,with its characteristic alternate rows of(Ob)with fivefold coordination of Ti4+specie(Figure 36b),that acts as the active sites for N2reduction.[371]However,it is noteworthy that in reported systems,O2could be more easily reduced compared to N2which dominates NH3production.For example,by enhancing the rate of N2bubbling,the rate of NH3formation also increases linearly even continues with a longer irradiation time of 48 h(Figure 36c),due to the efficient removal of O2generated from water oxidation.[368]In addition,using the former catalytic system,NH3generated during 100 h of reaction under N2flow of 1.0 L min-1is the highest amount reported.However,among these tested photocatalysts,only rutile-TiO2(JRC-TIO-6)showed the highest NH3conversion activity,with 2.7-fold enhancement upon the addition of a sacrificial electron donor.[372]Similarly,a variety of LDH ultrathin NSs-based photocatalytic system MIIMIII-LDH(MII represents Mg,Cu,Zn,Ni,and MIII denotes Al and Cr,respectively)were synthesized through simple co-precipitation methods with abundant VOshowing predominant photocatalytic N2fixation.[373]One such example is the synthesis of CuCr-NS structure with an overall thickness of 2.5 nm and a lateral size of 20-50 nm(Figure 36d).The induced defects of CuCr-NS can be observed via HRTEM analysis,showing slight disorders(Figure 36e).In addition,Cu(II)ions impart compressive strain and give rise to structural distortion in LDH NSs which increases the interaction of N2with LDH,promoting the photo fixation to NH3.Figure 36f depicts the compressive strain in LDH NSs by the induced VO.[373]Likewise,the NH3formation via photo fixation through CuCr-NS was confirmed by conducting the experiment using Ar as substitute(Figure 36h).Furthermore,it is deduced that the introduction of strain and VOcauses the N-N distance in N2shorten to 1.162˚A in contrast to N2H2(1.268˚A)and N2H4(1.48˚A)with the consequent molecular adsorption on VOsites and strain(Figure 36i).Schrauzer and Guth synthesized Fe-doped TiO2using impregnation technique and reported considerable enhancement in N2reduction under UV light irradiation when compared to pristine TiO2.[374]However,later on they demonstrated that the N2reduction may have been due to the formation of photoassisted organo-Fe compound.[375]Later on,Soria et al.attempted to explain the role of Fe3+and demonstrated that iron could behave as temporary trapping sites for photogenerated electrons and therefore enhances charge separation.[376]Recently,Zhao et al.reported hydrothermal synthesis of Fe-doped TiO2with abundantly exposed(101)-facets via adsorption of Fe3+over hydrogen titanate nanotubes.[377]The higher photoreduction performance of N2can be correlated to the exposed facets of Fe-doped TiO2,behaving as trapping sites to prevent charge recombination via forming Fe2+and Fe4+to transfer electrons and holes to Ti4+,meanwhile generating back the Ti3+.[368]Other examples involving O facets exposure of BiOBr via end on configuration,while the difference in bond length of molecular nitrogen(1.078˚A)to that in diazene(1.201˚A)offer efficient N2fixation.[378]Consequently,the excited electrons in CB of BiOBr are injected evenly to the anti-bonding orbital of N2that bring about reduction of N2to NH3very effectively and hence speeds up the photoreduction activity.However,in some cases such as MoS2with corresponding VSs,the recombination of photogenerated excitons with free electrons produces trions(charged excitons),to donate electrons to the adsorbed N2over VSs,depicting a trion-assisted reduction process of N2to NH3.[103]Zhang et al.demonstrated charge transfer from LDH to N2via VOs in CuCr-LDH with significant adsorption of N2owing to the induced distortions.[373]Thus,CuCr-LDH is reported to exhibit efficient photoreduction power.Moreover,up till now it is the most efficient material reported for NH3synthesis with considerable activity up to five successive cycles,where the optimized concentration reaches to 184.8 and 142.9 μmol L-1at 25°C under visible and UV light irradiation,respectively.

Figure 36.a)Proposed mechanism for photocatalytic fixation of N2around the surface VOof TiO2.b)Proposed photocatalytic cycle for N2 fixation on the rutile TiO2(110)surface.The light blue spheres are the Ob atoms lying in the[001]azimuth.The red and blue spheres are the Ti and bulk O atoms,respectively.The green and yellow spheres are the O and H atoms in the surface-OH.c)Change in the amount of NH3formed by photoreaction on JRCTIO-6(8)under different gas bubbling conditions.Reproduced with permission from Ref.[368]Copyright 2017,American Chemical Society.d)TEM and e)HRTEM images of CuCr-NS.f)Schematic of the in-plane biaxial compressive strain in the as-synthesized LDH NSs.g)Schematic illustrating the photocatalytic N2 fixation process.h)Time course of NH3evolution in the photo fixation of N2or Ar in the presence of water under visible light illumination over CuCr-NS and CuCr-Bulk.i)N-N distance of free N2,N2on CuCr-VO,N2on CuCr-VO-strain,N2H2and N2H4.Reproduced with permission from Ref.[373]Copyright 2017,Wiley-VCH.
7.5.Organic Synthesis
Selective organic synthesis via photocatalysis is one of the most sustainable approaches for chemical synthesis over the last decades utilizing solar light as the redox mediator.[379-381]Photo-redox catalysis has been reported as early as 1990s involving pschorr cyclization.[382-384]Similarly,photocatalytic cycloaddition has been reported by Ganesh Pandy under visible light illumination[385]and many others using photoinduced electron transfer for organic synthesis.[386,387]Up till now,photo-redox organic catalysis has been employed for a wide range of organic transformation,from reduction[388]and oxidation,[389-391]to C-C bond formation from sp3-sp3,sp2-sp2,and sp2-sp3hybridized structures.[392-394]The continuous and ordered fringes in lattice can be found in defect-deficient WO3including C-O,[395]C-S,[396]C-N,[397]and C-P[398]with corresponding cycloaddition.[399]This heteroatom carbon structures approach is the combination of photo-redox catalyst with that of either metal or organocatalyst leading to dual catalysis with some induced defects.[400]VOin defective WO3ultrathin NSs can effectively activate superoxide radicals,which provokes aerobic organic coupling of amines to imines.[401]However,the most suitable(D-WO3)and defect-rich WO3(R-WO3)NSs are accompanied with slight disorders and dislocation(Figure 37a-f).This distinction could be made through HAADF-STEM,due to the Low coordination number of DWO3as compared to the R-WO3NSs,suggesting low O vacancies in D-WO3.[401]These photogenerated electron-hole pairs commonly get distributed over the O atoms bonded with neighboring W atoms and on coordinately unsaturated sites(CUS).In addition,the first principle simulations for chemisorbed O2on a coordinative unsaturated W has been observed to switch to a side-on mode as the WO3is charged with an extra electron(Figure 37g,h).As a consequence,defective WO3will incur 0.72 electrons to adsorbed O2molecules stretching the bond length to 1.47 from 1.22˚A,to promote the formation of O2·-species.Further,these defects can therefore tune the band gap of WO3effectively with the VB maxima of 1.98 and 2.17 eV lower than the EF of R-WO3and D-WO3(Figure 37i),respectively.As expected,R-WO3exhibits significant enhanced activity when even illuminated with NIR(λ> 700 nm)as compared to D-WO3that can be attributed to carrierdriven reactions(Figure 37j).Therefore,the approach provides an effective way of aerobic coupling of amines to the respective imines accompanied with six times improvement in kinetics in contrast to the defect-deficient WO3.[401]
8.Opportunities and Challenges
This review summarizes recent development in defect engineered photocatalysts.Various types of defects such as VOs,metal vacancies,disorders or distortions are discussed for tailoring the electronic and microstructure to improve the conductivity and carrier concentrations to boost up photocatalytic processes.Different effective strategies for defect-controlled photocatalyst synthesis are presented including high temperature treatment,UV irradiation,surface chemical etching,vacuum activation,ball milling,vapor diffusion synthesis,molten salt synthesis,lithium-induced conversion,and plasma etching.Additionally,different characterization techniques for defect identification and quantification in semiconductors have also been demonstrated.Furthermore,the crucial role of defects in photocatalysis is manifested,such as,providing active sites to participate in reaction,enhancing the light harvesting and concentration of the charge carriers with improved charge separation.Finally,several studies highlighted in this review have concluded that defective photocatalysts with unique electronic and structural features greatly favor higher photocatalytic performances for water splitting,CO2photoreduction,nitrogen fixation,organic synthesis and pollutant degradation.Despite the extensive research in defect modulated photocatalyst systems,the research area is still in demand of opportunities to meet the retained challenges in synthesis,unique morphology and stability during photochemical process.For example,the primary challenges associated with defects preparation in metals requires advanced fabrication and systematic identification with mechanistic studies of their role in photocatalysis.Such as,the increased concentration of defects would result in recombination centers.Similarly,calcination at high temperature often demolish chemical bonds of the host material.Further,some of the anion defects,such as VOs are unstable and restored by the ambient O2or H2O environment exhausting the microstructure and unique features of the photocatalysts. In addition, fabrication of metal defects are often random therefore one impurity could lead to the formation of more than one defect.Thus,proper understanding of the correlation between defect engineering and photocatalytic property is of great need to resolve the aforestated limitations.
Hence,alternative strategies to assure the economic advantages of photocatalysis are of great importance.For instance,the relationship between the defect structure and photocatalytic activity must be adequately explored with respect to defect origin,synthesis at elevated temperature,diffusion path,or photocatalytic mechanism.Apart from advanced characterization methods,the in-situ analysis techniques are highly desired to investigate the active sites and activity during photocatalysis.In combination with DFT computations,systemic investigations of various photocatalytic systems involving various defect types or components are of great need to be adopted for the development of novel photocatalysts with efficient computer aided discovery.So far,the current understanding of photocatalysis is concerned;the recent developments in the area demands further discovery of novel materials that might bring the process much closer the ideal system.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China(22002142),China Postdoctoral Science Foundation(2019M652570,2019M650172 and 2020T130605),Support Plan for College Science and Technology Innovation Team of Henan Province(No.16IRTSTHN001),and the Science& Technology Innovation Talent Plan of Henan Province(No.174200510018).
Conflict of Interest
The authors declare no conflict of interests.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Classifying Electrolyte Solutions by Comparing Charge and Mass Transport
- Two-dimensional Boron Nitride for Electronics and Energy Applications
- Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes
- 2D/2D Heterostructures:Rational Design for Advanced Batteries and Electrocatalysis
- Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries
- Density Functional Theory for Electrocatalysis
