Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes
Zhenjiang Cao,Yongzheng Zhang,Yanglansen Cui,Jianan Gu,Zhiguo Du,Yongzheng Shi,Kai Shen,Hao Chen,Bin Li,and Shubin Yang*
Electrochemically active metal anodes,such as lithium,sodium,potassium,and zinc,have attracted great research interests in the advanced rechargeable batteries owing to their superior theoretical energy densities.Unfortunately,the metal anodes suffer from the huge volume changes with loss of active materials during the plating and stripping processes,resulting in fast capacity decay.Moreover,the random growth of dendrites on the metal anodes will penetrate the separator,causing severe safety issues.Engineering metal anodes by introducing the 2D materials are widely investigated to alleviate these issues.Benefitting from the ultrathin structure feature and unique electrical properties,2D materials are regarded as one of the best host of metal anodes.Besides,the tunable active sites on basal plane enable 2D materials to achieve favorable interaction with metal anodes.Moreover,some 2D materials exhibit good mechanical strength and flexibility,serving as building block for the artificial solid electrolyte interphase.In this review,we mainly disclosed the correlations between the intrinsic properties of 2D materials and their functions in guiding uniform nucleation,controlling the growth of metals,and accommodating the volume change.Also,the challenges of 2D materials in metal anodes are well discussed.Finally,the future directions to develop highperformance metal anodes by taking advantage of these unique features of 2D materials are proposed.
Keywords
2D materials,metal anodes,dendrite-free,nucleation,high-energy density
1.Introduction
Portable electronics and electric vehicles have gained ever-increasing attentions in the past decade,which motivate the fast development of advanced energy storage system with high-energy density and safety.Electrochemically active metal anodes such as lithium,sodium,potassium,and zinc are promising candidates for secondary batteries owing to their attractive theoretical capacities as well as the low redox potentials.[1-4]Unlike conventional intercalated anodes,the electrochemistry of metal anodes is more complex,which involves three stages during the plating process:1)the spontaneous formation of the solid electrolyte interphase(SEI) films at the interface of metal anode and electrolyte;2)the nucleation of metals at the initial plating;and 3)the continuous plating/stripping of metals around the nucleus during the charge/discharge processes.[5-8]
Therefore,the complicated electrochemical process upon the metal anodes brings the following major issues,which hindered their practical applications.[9-12]Firstly,most metal anodes are highly chemically active to the electrolyte,forming an inhomogeneous and unstable SEI film on metal anodes during initial plating.[5,13,14]Secondly,the unstable lithium on the surface will gradually pulverize and fall off during cycling,thus losing its electrical activity and producing a large number of dead lithium.Thirdly,the huge volume changes during the continuously plating and stripping process will lead to a porous and loose morphology and structure of the deposited metal on the anode.[15]The continuous volumetric changes also cause the crack and reconstruction of SEI layer on metal anode,resulting in the excessive consumption of electrolyte and active material.Lastly,the numerous metal dendrites originated from the further growth of metals along the nucleation sites can pierce the separator,causing the short circuit and even explosion of the batteries.[16-18]
Recently,great efforts have been devoted to deal with above-mentioned problems.Among which,confining the metal anodes into 3D host is regarded as an effective strategy to alleviate the volume change and suppress the metal dendrites during cycling.[18-24]2D material is an ideal building block for constructing 3D scaffolds.[25-28]Currently,graphene,2D metal carbides,and nitrides(MXenes)have widely applied to build 3D composite metallic lithium,sodium,potassium,and zinc via molten or mechanically rolling-folding approach,which significantly improve the performances of metal anodes.[12,29-38]Additionally,the abundant function groups(-OH,-Cl,-F)on the basal plane of MXenes can induce the formation of initial SEI layer on metal anodes,which homogenizes the electric field/ion flux and induces the homogeneous nucleation of metals.[3,30,39-43]Other 2D materials(MoS2,h-BN,and black phosphorus)with high mechanical strength as well as good flexibility are important ingredients for the artificial SEI layers to suppress the formation of metal dendrites.[29]Moreover,the MXenes,C3N4,and h-BN exhibit metal-philic properties,which can be easily fabricated into various shapes to expand the applications metal anodes in a variety of batteries.[44,45]
To date,a range of 2D materials with various favorable properties have been employed to achieve dendrite-free metal anodes with high reversible capacities,which greatly accelerate the development of next-generation metal-based batteries.[46-50]In this review,we mainly summarized and discussed recent strategies toward dendritefree metal anodes based on the characteristics of 2D materials from the mechanism perspective(Figure 1).The functionalities of 2D materials in metal anodes are closely related to their unique properties,which include the ultrathin 2D feature,high surface area,abundant functional groups,tunable defective sites on basal plane,attractive electrical/thermal features,and the metal-philic properties.Then,the challenges of 2D materials used in metal anodes are well discussed.Finally,we provide some insights into the future development of high-performance dendrite-free metal anodes by taking advantages of the unique structures and properties of 2D materials.

Figure 1.Overall schematic diagram of harnessing the unique properties of 2D materials toward reversible metal anodes.
2.The Unique Features of 2D Materials
The discovery of graphene triggers intensive research interests in 2D materials owing to their unique physical and chemical features.[51-56]A variety of 2D materials have been studied,such as transition metal dichalcogenides(TMDs),[57]MXenes(Mn+1AXn),[58-63]boron nitride(BN),[64,65]black phosphorus,[66-69]and carbon nitride(C3N4).[70-72]The atomic-scale thickness and restricted geometry endow 2D materials with unique properties different from their bulk forms,such as large surface area,[73]favorable electrical/thermal properties,[74,75]tunable surface functional groups,[60]adjustable defective sites on basal plane,good mechanical strength and flexibility,and controllable assembly features(see below),unfolding the great potential in the applications of energy storage and conversion.[54,76-79]
2.1.Ultrathin Layer with High Surface Area
Most 2D nanosheets can be obtained via exfoliation from the layered bulk phase due to the weak Van der Waals between the layers.[80-83]The large lateral size(hundreds of nanometers to several micrometers)and atomic-layer thickness afford 2D materials large surface area and surface-area-to-volume ratio.[79]As the thinnest 2D material,graphene exhibits the thinnest thickness of 0.334 nm as well as the largest theoretical surface area of 2630 m2g-1.MXene also exhibits a thickness of 1-1.5 nm and large surface area ranging from 250 to 1000 m2g-1.[58,60,73,84]In addition,large uniform GO and Ti3C2-MXene monolayer of several square micrometers in lateral size can now be fabricated in high yields.[85]
2.2.Tunable Functional Groups
The functional groups on 2D materials may vary through different synthesis approaches.[86-96]Taking Ti3C2Tx-MXene,for example,in the case of the traditional etching process by HF in aqueous solution,the-OH and-F terminations are commonly found.While the HF is replaced by the LiF/HCl mixture solution,more-O terminations can be introduced.[62,90]To avoid the use of hazardous HF,some green methods were put forward,including Lewis acidic molten salts etching,electrochemical etching,and high-temperature NaOH etching.[91,97,98]For example,Feng et al.obtained F-free Ti3C2with high yield(>90% )by electrochemical etching the Ti3AlC2in binary aqueous electrolyte.[93]However,these etching methods mostly occurred in aqueous solution,which inevitably resulted in-OH termination,restricting the application of MXenes,especially in some water sensitive system.[86,99,100]More recently,polar organic solvents and molten salts are both proved to be feasible for the preparation of MXenes with different terminations.Natu et al.successfully etched Ti3AlC2in polar organic solvents(ammonium bi fluoride)with less-O terminations.[101]By etching the MAX phases in Lewis acidic molten salts,Huang et al.synthesized pure-Cl terminated MXenes.[90,102]Based on molten salt strategy,Talapin et al.precisely controlled the surface group in MXenes by substitution and elimination reactions.MXenes terminated with-O,-NH,-S,-Cl,-Se,-Br,or-Te,even bare surface termination was successfully prepared,which exhibited distinctive physical and chemical properties.[103]As a derivative of graphene,GO can be produced by the modified Hummers method with the assistance of oxidizers,resulting in abundant oxygen-containing functional groups,such as hydroxyl,carboxyl,epoxy,and carbonyl.
2.3.Adjustable Defective Sites on Basal Plane
Owing to the severe etching condition during the preparation and post-treatment process of 2D materials,the defects or vacancies are unavoidable on the basal planes.[90,104,105]Previous works revealed that the defects or vacancies concentrations in Ti3C2were closely related to the concentration of HF during the etching.[91]More defects were generated in higher HF content,while fewer vacancies induced in LiF/HCl etchant due to the milder condition.These atomic defects and vacancies can regulate the electronic structure of 2D materials,which further affect their electrical,optical,magnetic,and catalytic properties.[103]Defect engineering is extensively investigated in 2D materials,and various strategies have been reported,such as electrochemical etching,chemical etching,and heteroatom-doping.For example,single S-vacancies were successfully created in MoS2nanosheet by chemical etching of H2O2.By adjustment of etching time,temperature,and solution concentration,the comprehensive control of S vacancy was realized.[106]In addition,these in situ created vacancies are also reported as effective sites for anchoring single metal atoms catalyst.When Mo2TiC2Txwas electrochemically exfoliated in electrolyte,Mo vacancies were generated and Pt single atoms were simultaneously trapped with Pt foil as the counter electrode.Moreover,single zinc atoms were in situ immobilized on MXene(Ti3C2Clx)layers(Zn-MXene)by molten salts etching strategy,which exhibited excellent behaviors in catalyzing sulfur conversion and guiding Li nucleation.[102,107]Heteroatom-doping is also widely applied to create defects in 2D materials,and different kinds of heteroatoms are introduced into graphene,TMD,and MXene.[38,108-110]Both theoretical and experimental results revealed that N-doped graphene could lower the deposition resistance and increase the nucleation sites of lithium metal,thus suppressing the dendrite growth.[27,111,112]
2.4.Good Mechanical Properties and Flexibility
2D materials also exhibit outstanding mechanical properties and superior flexibility because of their atomic-layer thickness and in-plane covalent bonding.[66,113,114]As the thinnest 2D material,monolayer graphene delivers the strongest intrinsic strength and Young’s modulus of 42 N m-1and 1.0 TPa,respectively.In addition,the calculations also revealed that covalently connected 2D nanomaterials had outstanding mechanical properties.More importantly,the mechanical properties of 2D material have a deep relationship with their thickness,surface functionalization and compositions.In the case of MXenes without functional groups,the Young’s modulus was determined as 597,502,and 534 GPa for Ti2C,Ti3C2,and Ti4C3,respectively.Atomic force microscopy indentation results demonstrated that the Young’s modulus of monolayer Ti3C2Txwas up to 330 GPa,exceeding GO and MoS2.[115,116]Besides,single Ti3C2Txflake also delivered impressive tensile strength of 17.3 GPa.Benefiting from the mechanical feature and flexibility of monolayer 2D materials,freestanding films and fibers with high mechanical strength can be easily assembled by vacuum filtration,spin coating,drop-casting,and electrospinning.By selecting large-size Ti3C2Txflakes(10-40 μm),a flexible and thick(940 nm)Ti3C2-MXene film with recorded tensile strength(570 MPa)was achieved.Even after repeated bending for 500 cycles,the obtained Ti3C2Txfilm also maintained an outstanding flexibility and stable resistance.[115]
2.5.Controllable Assembly Feature
The restacking and aggregation phenomenon commonly occur in aqueous solution of 2D materials owing to the strong hydrogen bonding interaction and Van der Waal force.[66,117]In general,freestanding film of 2D materials can be easily achieved by layer-by-layer assembly,but the densely stacked structure inevitably results in the loss of accessible surface area.[118]To expand the surface utilization,various synthetic strategies have been proposed to construct oriented and disoriented 3D structures.Typically,the strategies can be clarified into cross-linking and template methods.[50,119]For the cross-linking method,chemical cross-linkers(such as divalent metal ions and ammonia)are usually introduced.With the assistance of chemical cross-linkers,3D GO and Ti3C2-MXene aerogels were successfully assembled from GO and Ti3C2Txaqueous dispersion,respectively,in which the pore structures were easily tuned by adjusting the drying process.For the template method,polymer spheres(PMMA and PS)or ice template are usually piling up in the 2D nanosheets to generate porous structure.Using the PMMA spheres as the template,3D porous Ti3C2-MXene films were successfully prepared and the pore size could be well controlled by adjusting the diameter of PMMA sphere.
2.6.Attractive Electrical/Thermal Properties
2D materials also possess outstanding electrical and thermal conductivity.[120]For example,defect-free graphene demonstrates the highest thermal conductivity of 3000-5000 W m-1K-1,which is more than 10 times higher than that of Cu.Calculational results revealed that the thermal conductivity of 2D materials also had a close relationship with the surface terminations,thickness,and defects.[56]For example,Ti3C2FxMXene delivered higher thermal conductivity(108 W m-1K-1)than that of Ti3C2Ox(10 W m-1K-1)and bare Ti3C2MXene(58 W m-1K-1).Besides,the thinner Ti3C2-MXene exhibited more homogeneous heat distribution and the defects as well as the heterogeneities caused severe local heating.Moreover,the electronic properties of 2D materials vary from metallic to semiconducting and even insulator.Graphene delivered the highest electrical conductivity up to 106S m-1,while GO and BN are electrical insulator.[121,122]It is worth noting that the electrical conductivity is also related to composition and phase crystal structure.For example,MXenes containing heavy transition metals(Cr,Mo,and W)are reported to be topological insulators.Research showed that changing the outer M layer could affect the electronic properties of MXenes.And the transport properties of MXenes can also be changed by changing the surface terminations through post-processing.Therefore,the conductivity of Ti3C2Txranges from below 1000 S cm-1(cold pressure HF etching powder with high defects)to 4600 S cm-1(mild etching and layered vacuum filtration)and 6500 S cm-1(mild etching and spin casting thin films).[60,123,124]These values are higher than those of other solution-treated 2D materials.Moreover,superconductivity can be observed in Nb2C-MXene by regulating the surface groups.
2.7.Tunable Metal-Philic Feature
The wetting behavior of alkali metals is a major challenge for the application of secondary batteries.Actually,the pure molten metal anodes always present large surface tension and inferior chemically/electrochemically compatible with many matrix due to the strong internal metal bonds,especially for solid-state electrolytes,resulting in large interface resistance.[125-127]Owing to the existence of-Cl,-F,and-O containing functional groups or heteroatoms on the base planes,2D materials usually display good metal-philic feature with improved the viscosity and surface tension of molten metal.[44,47,117,128-130]Computational results revealed that-F and-O terminated Ti3C2-MXenes also exhibited stronger chemical affinity to metal Li and Na than that of bare Ti3C2-MXene.Experimental results also demonstrated that these-O and-F containing groups of Ti3C2-MXenes were important to enhance the lithiophilicity,leading rapid adsorption of molten lithium into the Ti3C2-MXenes matrix within seconds and generating hybrid metallic lithium anodes(Figure 2a,b).[44]Similar phenomenon was also observed in partially reduced GO by Cui et al.(Figure 2c-f).[130]More importantly,MXenes,BN,and g-C3N4can be also uniformly mixed into liquid Li metal due to the forming of Li3N,generating colloidal gelation with high viscoelastic properties(Figure 3a-g).[128,129]Such unique metal-philicity of 2D materials is originated to the in situ formed Li3N or LiF,which render them promising for developing hybrid metal anodes for high-energy density batteries.

Figure 2.a)Schematic diagram of Li/Na-Ti3C2Tx-rGO preparation and b)the wettability process of Ti3C2Tx-rGO.Reproduced with permission from Fang et al.[44]Copyright 2019,American Chemical Society.c)Schematic of the material design and the consequent synthetic procedures from a GO film(left)to a sparked rGO film(middle)to a layered Li-rGO composite film(right).d-f)Corresponding digital camera images of the GO film d),sparked rGO film e),and layered Li-rGO composite film f).The diameters of the films shown in b-d are~47 mm.Reproduced with permission from Lin et al.[130]Copyright 2016,Nature Publishing Group.

Figure 3.a)The synthesis process of Li-C3N4composite.b)XRD pattern comparison of the as-prepared g-C3N4,Li and Li-C3N4composite,where Li3N is detected in Li-C3N4composite.c)Phase diagram showing the phase equilibrium of the Li-C-N2system.d)High-resolution XPS analysis of N 1s spectra for g-C3N4and Li-C3N4composite.g)Contact angle measurements of Li-C3N4composite droplets with different content of g-C3N4on garnet SSE.Reproduced with permission from Huang et al.[129]Copyright 2019,Wiley-VCH.e)Phase diagram of Li-B-N systems.f)High-resolution XPS spectra of Li 1s and N 1s of the Li-BNNS composite.Reproduced with permission from Wen et al.[128]Copyright 2019,American Chemical Society.
3.Preforming the SEI based on the Functional Groups to Achieve Uniform Nucleation
In the plating process,the Fermi energy levels of almost all electrolytes are higher than the lowest unoccupied molecular orbital,which inevitably cause the formation of heterogeneous organic/inorganic passivated SEI layers on the metal surface,leading to random nucleation and dendrite growth.Taking lithium metal anode,for example,inorganic compounds(LiF,LiNO3,and Li2Sx)with higher interface energy were selected to create stable SEIs on metal anode to inhibit dendrite growth.Among which,LiF showed the highest interface energy to Li(73.28 meV˚A-2),and exhibited the potential change of vertical growth mode.[131-134]
MXene which has abundant surface functional groups,especially fluorinated terminals,tends to generate an uniform,dense,and durable solid electrolyte interface composed of inorganic fluorinated compounds on the MXene nanosheet,effectively homogenizing the electromigration of lithium ions and inducing uniform nucleation on MXenes.[135,136]Our group recently used Langmuir-Blodgett and Marangoni effect to prepare parallelly aligned Ti3C2-MXene layers at the interface of water and air(Figure 4a,e).[135]As in situ covered LiF-containing SEI on the parallel oriented Ti3C2-MXene nansheets,the Li ion flux is equal across the entire parallel Ti3C2-MXene films(Figure 4b).The uniform nucleation of lithium in the whole layer leads to the later uniform growth of lithium,and finally effectively guides the gradual growth of lithium along the horizontal direction,with dense,smooth surface and rich grain boundaries(Figure 4b-d).Typical SEM images showed that when the plating capacity was more than 5 mAh cm-2,lithium grew on the grain surface of a single Li-Ti3C2-MXene,forming cobblestone lithium(Figure 4f-h).In addition,Guo et al.also verified that fluorinated functional groups on Ti3C2-MXenes could induce uniform nucleation of lithium.[137]They constructed an ultrathin, flexible and self-supporting Ti3C2TxMXene/cellulose nanofiber composite paper.The self-assembled microspheres of Ti3C2-MXene nanosheets contributed to the formation of interlocking microstructures during the slide process of the sheets,thus significantly improving the mechanical strength and flexibility of the whole Ti3C2-MXene thin films.The crystal surface was decorated with abundant lithiophilic functional groups,indicating that the strong interaction with lithium can induce uniform nucleation and dendrite growth of lithium metal.As shown in Figure 4i,Ti3C2-MXenes with-F groups showed higher binding energy(-2.70 eV)than pure Ti3C2-MXene(-2.47 eV),indicating stronger interaction between F atoms and Li atoms,so uniform Li nucleation and growth were realized.In addition,this design effectively prevents Ti3C2-MXenes from accumulating repeatedly,thereby widening the voids and accelerating the reaction kinetics,further ensuring a higher plating capacity and superior rate capability.Very recently,Cao and coworkers designed a stable and flexible Ti3C2-MXene modified carbon cloth host with abundant surface functional groups for sodium anodes.Carbon cloth acted as a tenacious matrix to ensure tough metal anodes.And Ti3C2-MXene served as a nucleation center to induce Na aggregate on the sheet with regular configuration,resulting in Na cross growth.It was verified by first-principles calculations;the Ti3C2-MXene performed low adsorption energies for Na(Ti3C2-F at-0.15 eV),indicating a good affinity of Ti3C2-MXenes to Na atoms.

Figure 4.a)Schematic illustration of lithium plating on parallelly aligned MXene(PA-MXene)layers.b)High-resolution F1s XPS spectra of PA-MXene-Cu and bare Cu after initial lithium plating and stripping,showing a F-Li peak at 684.8 eV.c)Voltage profiles of Li plating on PA-MXene-Cu,PA-MXene-O,and Cu foil.d)SEM image of PA-MXene after plating lithium with 5 μAh cm-2,showing lithium is initially nucleated at the edge of the MXene nanosheets.e)SEM images of PA-MXene layers on lithium.Inset in c)is the top view of PA-MXene.SEM images of PA-MXene-lithium with different level lithium plating:f)0.2 mAh cm-2,g)2.5 mAh cm-2,showing gradual growth of lithium in horizontal orientation.SEM images of PA-MXene-Li after plating lithium with capacities h)35 mAh cm-2,clearly showing the formation of cobblestone-like lithium rather than lithium dendrites.Reproduced with permission from Zhang et al.[135]Copyright 2019,Wiley-VCH.i)Binding energy of Li atoms in Ti3C2and Ti3C2F2.Reproduced with permission from Wang et al.[137]Copyright 2020,by Elsevier Ltd.
Therefore,corresponding to the instinct obstacles of metals,2D materials,such as MXene nanosheets with abundant fluorine terminations,have been confirmed to present connatural superiorities on forming the inorganic fluorinated compounds-based SEI which leads to the homogenous nucleation on metal anodes.
4.Constructing the Artificial SEI to Suppress Dendrite Formation based on High Mechanical Strength
Due to high chemical reactivity,metal anodes irretrievably react with liquid electrolyte and form the SEI layers.[1,17]Such SEI layers are easy to crack against the large volume change,then exposing fresh metal to continuously react with more solvent.Seriously,at the crack,the concentration of the electron and ion suffers a sudden increase,resulting in remarkable growth speed of the dendrites.All the above processes finally result in the formation of dendritic and moss metal,the inferior Coulombic efficiency,and even the severe safety issue.Introducing the artificial SEI layer with high mechanical strength and fast ion transport capability is an effective approach to address this issue.Also,the artificial SEI layer should be electrochemically inert in abrasive chemical solvents.2D materials,including MoS2,h-BN,and black phosphorus have been proved to exhibit the good chemical resistance and good mechanical properties at atomic level.For BN nanosheets,the Young’s modulus could approach 1.0 TPa,performed much higher than that of metallic lithium(4.9 GPa)and the inorganic matter in traditional SEI.[138,139]Moreover,despite the high mechanical strength at the atomic level in plane,the thin graphene exhibits its flexibility due to the ultrathin thickness.The good flexibility greatly contributes to accommodate the metal deposition.
Recently,a MoS2film about 10 nm thick was sputtered on metal lithium(Figure 5a--d).[140]The protect MoS2layer could tightly adhere to the metallic lithium even after cycling for 300 h(Figure 5e,f).Different from the ceramic/polymer-based SEI layers,the novel 2D feature and phase-change characteristics allow atomic lithium intercalate in and accelerate the transfer of lithium ions, finally addressing the troubling high interface impedance.More importantly,the improved conductivity of MoS2layers on lithium surface effectively suppresses the dendrite nucleation.Attracted by the unique properties of BN,Cui’s group grew a 2D h-BN on Cu current collectors by CVD method with gaseous ammonia borane(NH3-BH3).[138]The prepared h-BN layer acts as a protective film to form the sandwiched BN-Li-Cu structure,suppressing the formation of dendritic and mossy lithium(Figure 6a-h).The electrochemically active phosphorene can induce the formation of lithium phosphides as protection layer,which not only suppress the decompose of electrolyte but also the dendrite growth(Figure 6i-l).[141]

Figure 5.a)Scheme and preparation of MoS2-coated Li metal.Cross-sectional b)and top-view c)SEM images of the as-deposited MoS2on Li metal.The inset in c is a magnified view.d)Top-view SEM image of the lithiated MoS2on Li metal surface.e)SEM images showing the surface morphological changes between bare and MoS2-coated Li before and after the cycling test at 10 mA cm-2.The insets are the corresponding digital images of the electrodes.f)Cross-sectional SEM image of the MoS2-coated Li metal after 300 h of cycling and the subsequent EDS elemental mapping of Mo and S outlined by the dashed yellow box.Reproduced with permission from Cha et al.[140]Copyright 2018,Nature Publishing Group.

Figure 6.a)Cross-sectional SEM image of deposited Li metal on bare copper.b)Schematic structure of Li metal deposited on copper.c)Cross-sectional SEM image of deposited Li metal protected by h-BN.d)Schematic structure of Li metal with h-BN protection.Li metal might be exposed to electrolyte between patches of h-BN film,resulting in the SEI formation.e,f)Top-view SEM images of the first lithium deposition on bare copper with current rate of 0.5 mA cm-2.g,h)Top-view SEM images of the first lithium deposition on h-BN protected anode with the same condition.Reproduced with permission from Yan et al.[138]Copyright 2014,American Chemical Society.i)Ex situ optical images of electrolytes and Li metal electrodes collected after 100 cycles.Ex situ FE-SEM images of the phosphorene-coated Li metal electrode with 1MLiTFSI in DMA j)before and k)after cycling performed at±0.1 mA cm-2for each 0.5 h cycle(areal capacity:0.05 mA h cm-2)over 100 cycles.l)Ex situ NMR spectra of the electrolyte consisting of 1MLiTFSI in DMA after 100 cycles for bare and phosphorene-coated Li metal symmetric cells.Reproduced with permission from Kim et al.[141]Copyright 2018,American Chemical Society.
5.Controlling the Nucleation and Growth of Metal along the Defective or Heteroatom-doped Sites
Vacancy and atom-level doping as lithiophilic active sites to enhance the Li affinity of substrates are verified to be a vital method to induce the nucleation and growth of metal on 2D substrates.[49,104,108,142]Due to the strong adsorption energy,the initial nucleation of lithium in the lithiphilic region promoted the uniform nucleation behavior of lithium and hindered the growth of lithium dendrites.Wang’s group reported a high-performance potassium anode obtained by restricting the potassium metal in titanium-devoid and nitrogen-containing Ti3CNMXene/carbon nanotubes (CNT) (denoted as DN-MXene)(Figure 7a-d).[104]Theoretical calculation and experimental studies have confirmed that this “potassium-philic”Ti3CN-MXene nanosheet,titanium-devoid and nitrogen-containing,can induce potassium nucleation and guide potassium to be uniformly distributed in the scaffold during the cycling process.Density functional theory was conducted to study the interaction between potassium atoms and different scaffoldings.As shown in Figure 7e,the binding energies absorbed by potassium atoms on Cu foil,Al foil,and CNTs were-0.13,-0.51,and-0.84 eV,respectively.And Ti3CN-MXenes with possible surface terminations(-OH,-O,and-F)were studied.As shown in Figure 7f-h,the binding energies between K-containing Ti3CNTx-MXenes and K atoms were 1.91 eV(Ti3CNO2),1.86 eV(Ti3CN(OH)2),and 1.88 eV(Ti3CNF2),indicating that nitrogen atoms can significantly interact with K atoms.Furthermore,when considering Ti vacancy in N-containing Ti3CN-MXene,the binding energies reached to 2.27(Ti3-xCNO2),2.1(Ti3-xCN(OH)2),and 2.04 eV(Ti3-xCNF2)(Figure 7j-l),indicating that K atoms are more inclined to nucleate on the DN-MXene nanosheets inserted by K than on CNTs or other current collectors(Figure 7i).Therefore,the K-philicity of DN-MXene gave priority to nucleation in the plating process,which made DN-MXene an ideal host for the dendrite metal anodes(Figure 7m).

Figure 7.a)High-magnification HAADF-STEM images of DN-MXene sheets.b)The simulated and c)Corresponding experimental atomic-resolution HAADFSTEM images of the DN-MXene sheets;Ti:yellow atoms,C:gray atoms,N:blue atoms,Ti vacancy:circles,surface groups:red atoms.d)High-resolution XPS spectra of Ti 2p of the DNMXene/CNT scaffold.e)The calculated binding energies of K atoms with different scaffolds or current collectors.The deformation charge density of K atoms absorbed on the defect-free f)Ti3CN(OH)2,g)Ti3CNO2,h)Ti3CNF2MXenes.i)The voltage profiles of K nucleation on MXene/CNT scaffold,CNT scaffold,Cu foil,and Al foil at a current density of 0.05 mA cm-2.The deformation charge density of K atoms absorbed on the N-free defect-free j)Ti3C2(OH)2,k)Ti3C2O2,l)Ti3C2F2MXenes.m)Top-view SEM images of K plated onto DN-MXene/CNT at a current density of 0.5 mA cm-2 with a plating capacity of 5 mA h cm-2in KPF6-based electrolyte.Reproduced with permission from Tang et al.[104]Copyright 2019,Wiley-VCH.
Explained by the concept of definite solubility and solid solution,lithium was verified to nucleate on Au,Ag,Zn,or Mg with no nucleation barriers.[16]Such strategies to induce metal seeds for controlling selective plated behavior of metal are of great significance in achieving dendrite-free metal anodes.Considering this,our groups proposed single zinc atom immobilized Ti3C2Clx(Zn-MXene)to efficiently induce the Li nucleation(Figure 8a).[107]Proverbially,MXenes possess excellent metallic conductivity,chemical stability,hydrophilic properties,and abundant functional groups.These unique properties naturally endowed MXenes as an ideal single-atom matrix.In the initial plating stage,the overpotential is low at 11.3 mV and Li tends to obtain a homogeneous Zn-MXene layer on the nucleation surface due to the presence of large zinc atoms,and then along the vertical site with nuclear growth due to the strong lightning-rod effect,providing bowls without lithium dendrites(Figure 8b).Similarly,Feng and coworkers designed a freestanding Ti3C2TxMXene@Zn paper as Li/Zn metal anode host to induce the nucleation.[108]The Ti3C2TxMXene@Zn paper exhibited superior mechanical flexibility,high electronic conductivity,hydrophilicity,and lithiophilicity(Figure 8c).The freestanding Ti3C2TxMXene@Zn anode exhibited superior advantages in suppressing Li/Zn dendrites.These findings of introducing nucleation sites into the MXene matrix provide new concepts to induce the directional growth of metal anodes.By introducing 2D Si into Ti3C2-MXene,Feng et al.also found that the Si could reacted with Li to form LixSi to act as lithiophilic nuclear sites and enhance the lithium affinity to create an uniform and stable SEI layer for lithium metal anodes(Figure 8d).[143]When the obtained flexible and binder-free Si@MXene paper worked as Li host,the nucleation barriers were effectively reduced and the Li dendrite growth was well controlled.So far,several porous materials that act as hosts for metals have been extensively studied.They can confine metals to pores and inhibit dendritic growth.2D materials with layered structures can not only maintain metallization between layers,but also effectively control and limit nucleation and growth at the nanoscale rather than inhibiting the entry of active materials into pores at the micron scale.[144]The crystal with large and adjustable interlamination is an ideal substrate for metal anodes.Li and colleagues proposed that the interlaminar spacing of Ti3C2-MXene could be adjusted from 1.0 to 2.7 nm by the insertion of cationic surfactants of different sizes,making Ti3C2-MXene an ideal Na metal matrix(Figure 9a,b).[145]However,owing to the high electrical conductivity of Ti3C2-MXenes,Na tends to directly cover the matrix surface of Ti3C2-MXene rather than between the layers.MXenes can be used directly as substrate,thus forming a favorable location for dendrite growth on Ti3C2-MXene(Figure 9b).Therefore,the addition of “seeds”between the Ti3C2-MXene layers is essential for inducing Na nucleation and growth between the Ti3C2-MXene layers(Figure 9b).Moreover,the volume change aroused by the reaction between Na and the “seed” will further expand the interlaminar spacing,enabling Ti3C2-MXenes to accommodate the volume change of Na due to the “pillar effect.”Thus,the easily prepared hexadecyl trimethyl ammonium bromide(CTAB)prepolymerization and subsequent Sn2+columnization resulted in an Sn2+pillared Ti3C2-MXene(denoted as CT-SN(II)@Ti3C2)as a stable substrate for dendrite-free Na metal anodes.When Sn-based nanocomposites were inserted and encapsulated in the Ti3C2-MXene layer as inducers,they served as nucleation sites for the nucleation growth of Na in the intermediate layer,resulting in uniform Na disposition.During Na plating,Na tends to nucleate and grow on Sn(II)seeds to form Na-Sn alloy(Figure 9c,d):1)Sn(II)+Na++e-→Sn+Na(I);2)ySn+xNa++e-→NaxSny.In addition,by combining Na3Ti5O12nanowires with CTAB-treated Ti3C2-MXene as the host of metal Na,uniform Na plating and stable cycling performance were also obtained.[146]The MXenes implanted with nucleation sites provide important insights in constructing dendritefree metal anode for high-performance metal-based batteries.
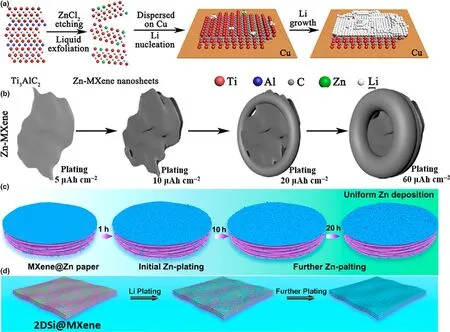
Figure 8.a)The synthesis process of single zinc atoms immobilized on MXene layers(Zn-MXene)for the Li nucleation and growth.b)Schematic illustration of lithium plating Li plating with various capacities of 5,10,20,and 60 μAh cm-2at 80 μA cm-2,respectively.Reproduced with permission from Gu et al.[107]Copyright 2020,American Chemical Society.c)The schematic diagram of fabricating flexible layered Ti3C2TxMXene@Zn paper for uniform Zn plating.Reproduced with permission from Tian et al.[108]Copyright 2019,American Chemical Society.d)Schematic with different Li plating behavior on 2DSi@MXene substrate.Reproduced with permission from An et al.[143]Copyright 2019,American Chemical Society.
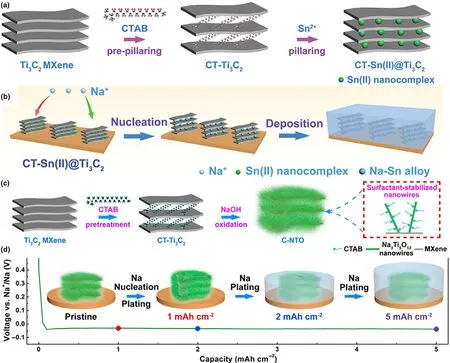
Figure 9.a)Schematic illustration of preparation of CT-Sn(II)@Ti3C2by CTAB prepillaring process followed by a method of Sn2+pillaring.b)Schematic diagrams for the comparison of Na nucleation,plated in Ti3C2and CT-Sn(II)@Ti3C2matrixes.Reproduced with permission from Luo et al.[145]Copyright 2018,Wiley-VCH.c)Schematic illustration of preparation of the CT-Ti3C2-derived 1D/2D Na3Ti5O12-MXene hybrid nanoarchitecture(C-NTO).d)Voltage profile and the corresponding schematic diagrams of Na deposition behavior in the C-NTO matrix at 3 mA cm-2.Reproduced with permission from Luo et al.[146]Copyright 2020,American Chemical Society.
6.Homogenizing the Electric Field and Ion Flux based on the High Electric Conductivity and High Surface Area
The distribution of electric field and ion flux in the electrode greatly affect the metal plated behavior.[147]Therefore,the construction of 3D metal anode with homogeneous ion flow and uniform electric field has a bright future to achieve a dendrite-free metal anode.Based on the Chazalviel model,lithium nucleation begins with a time close to the Sand time,where j is the current density,e is the charge,D is the bipolar diffusion factor,μaand μkare the migration numbers of anions and cations,respectively.In this equation,the transition time τ is directly proportional to the concentration of lithium salt in the electrolyte and inversely proportional to the current density.Therefore,low initial lithium concentration and high local effective current density both can cause the formation of lithium dendrite.[24,117,147]Hence,constructing a 3D host can reduce the local current density in the hybrid anodes due to the increased specific surface area,achieving a dendrite-free metal anode.MXenes are widely acclaimed for having high electric conductivity and high surface area features,and the unique properties give MXenes a unique application value in homogenizing the electric field and ion flux in the 3D hosts,effectively controlling uniform nucleation and metal growth into the 3D MXenes architectures.
Benefiting from the unique ductility of lithium metals and the lubricity of MXene nanosheets,our team first applied Ti3C2-MXene into lithium metal anodes by a simple mechanical rolling method,creating a layered structure with high bending flexibility for Ti3C2-lithium thin film anodes(Figure 10a).[30]In the hybrid anode,Ti3C2-MXene nanosheets were horizontally arranged.After the shortest ion transport path,lithium ions are electrochemically reduced and plated at the top of the Ti3C2-MXene layer.In situ Transmission Electron Microscope(TEM)analysis confirmed that the excellent electrochemical performance of Ti3C2-Li electrode was originated from the high electrical conductivity and the existence of nucleation sites on Ti3C2-MXene nanosheet,which facilitated the lithium plating to enter the gap of Ti3C2-Li nanosheet(Figure 10b).Luo and coworkers prepared a 3D conductive porous Ti3C2-MXene aerogel scaffold for metal Li anode(Figure 10c).[148]In this 3D scaffold,the cross-linking between Ti3C2-MXene sheets can regulate the ion flow for fast Li+transport capability.Furthermore,the Ti3C2-MXene nanosheets with high electric conductivity reduce the current density in the electrode,contributing to favorable conditions for the uniform growth of lithium(Figure 10d,e).[117]

Figure 10.a)Schematic illustration of the synthesis of Ti3C2MXene-lithium films.b)The sectional SEM images of lithiophobic Ti3C2-Li hybrid,disclosing that the atomic layers were uniformly dispersed in hybrid,showing a typical layered structure.Reproduced with permission from Li et al.[30]Copyright 2017,by Elsevier Ltd.c)Schematic showing of the Ti3C2MXene aerogel scaffolds for Li metal anodes.The 3D porous structure lowers the effective current density and accommodates high Li loading.The high electric conductivity and fast Li ion transport ensure uniform Li plating/stripping.Reproduced with permission from Zhang et al.[148]Copyright 2018,Wiley-VCH.d,e)2D transient model of d)the Li ion concentration distribution(CLi)and e)current density distribution of the MXene-melamine foam(MF)electrode in the electrolyte.Inset is the corresponding transport path of Li ions.Reproduced with permission from Shi et al.[117]Copyright 2020,American Chemical Society.
7.Accommodating the Large Volume Change of Metal Anodes via the Controllable Assembly of 2D Materials
To alleviate the volume change of metal anodes,a large number of 3D substrates have been reported involving fiber glass cloth,oxidized polyacrylonitrile nanofiber network,CNT sponges,Cu mesh,and rGO films.[20,21,149-152]However,most of the 3D hosts are usually with disordered pore structure,which is difficult for the metals to plate into the interior spaces,resulting in the increasing thickness of the electrode.More recently,some periodic upright structures are proposed as metal host for effective metal plating to accommodate large volume changes.Thus,the design of a controllable assembled 3D host can realize highly reversible uniform metal transfer in the electrode without any overgrowth of the upper part,which is a promising method to effectively control uniform metal growth in the 3D host and buffer the volume change of metal.
For example,our group developed controllable assembly 3D Ti3C2-MXene-Li arrays with plenty of nanometer-scale and micrometer-scale interspaces(Figure 11a).[153]The 3D perpendicular Ti3C2-MXene-Li arrays could effectively guide the homogeneous lithium plated into the Ti3C2-MXene hosts,reducing the notorious lightning-rod effect owning to the nucleation of Ti3C2-MXene nanosheets(Figure 11a).Moreover,the controlled combination of 3D Ti3C2-MXene arrays effectively reduces the local current density and/or uniform lithium ion flux through periodic intervals.The lithium ions transported through the vertical short channel were uniformly electrochemically plated on the vertical wall,maintaining the effective surface area and homogenizing the current density distribution(Figure 11a).Related to the homogeneous lithium flux,electric field and nucleation of Ti3C2-MXene nanosheet,the controllable assembly 3D perpendicular Ti3C2-MXene-Li arrays can effectively alleviate the volume change of Li.3D printing technology is a newly emerging approach to construct 3D hybrid anode with uniform internal architectures.[154]Yang and coworkers employed the extruded 3D printing technology to prepare a perpendicular aligned array structure with controllable assembly Ti3C2-MXene nanosheets in each array(Figure 11b).[155]These vertically aligned Ti3C2-MXene arrays provided uniform array gaps with large buffer spaces to cope with the infinite volume change.Niu’s group reported an interlayer-calated Li(ILC-Li)electrode by coating non-delaminated 2D Ti3C2Tx-MXene(15 μm)on the surface of metal Li(30 μm)(Figure 11c).[156]A controlled assembly of Ti3C2Tx-MXene array with excellent electrical conductivity and extended interlayer space provides rapid electron transfer,while the layers guide the vertical growth of lithium,thus greatly reducing the volume change of lithium(Figure 11c).Very recently,Zhao and coworkers applied a femtosecond laser to induce lithiophilic TiO2on the channel walls by machining aligned microchannels on controllable assembly Ti3C2-MXene membrane.[157]Under the control of uniform electric field,Li ions can migrate into the micro channel through the exposed Ti3C2-MXene nanoscale edge,and induce Li to be densely plated into the micro channel,effectively alleviating the volume changes of lithium.The controllable assembly feature of Ti3C2-MXene provides guidance for construct 3D composited metal anodes to guide the metal plated inside the 3D hosts, finally accommodating the huge volume change of metal anodes.
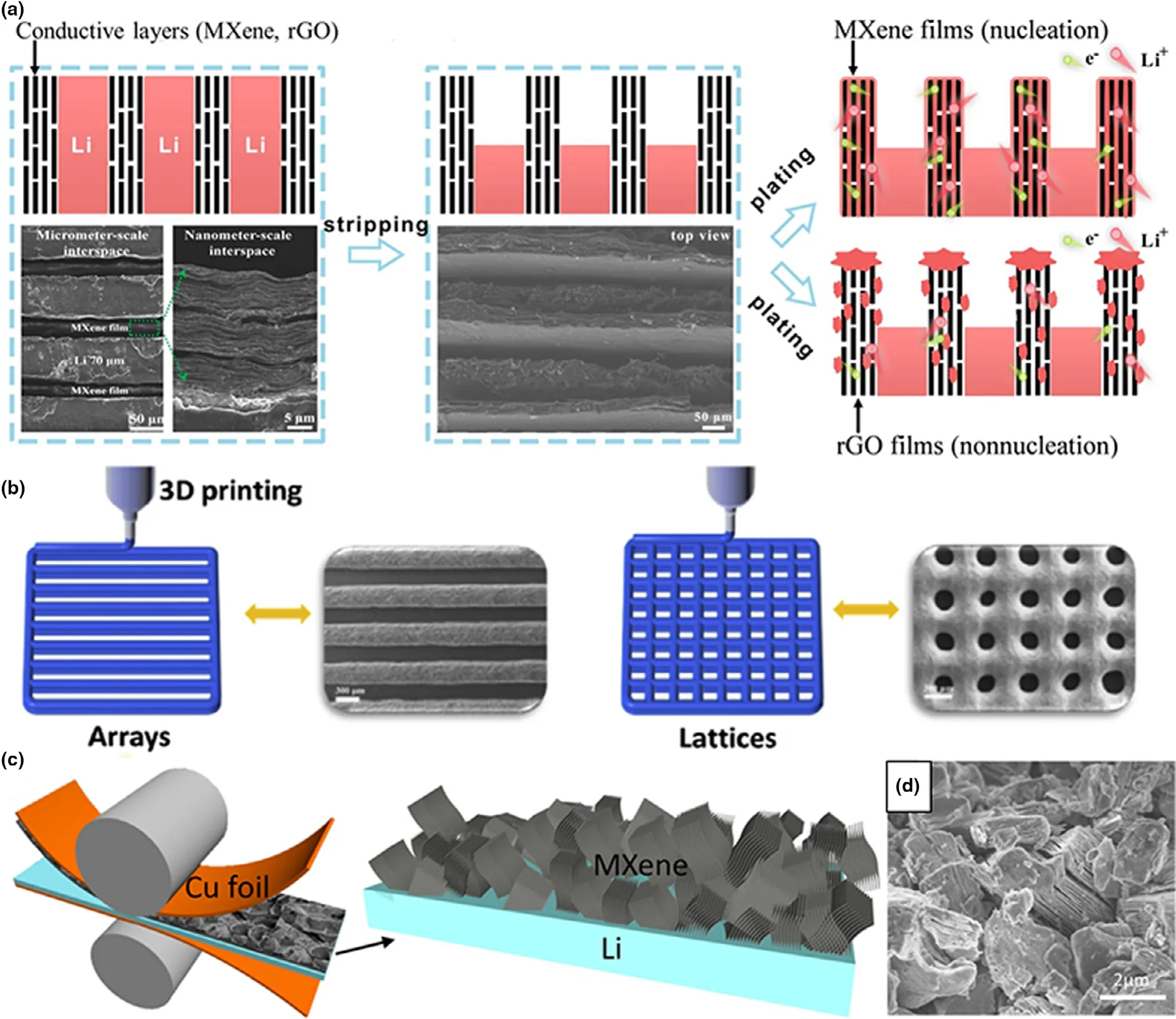
Figure 11.a)Schematic illustration of the stripping and plating states of perpendicular MXene-Li and rGO-Li arrays.The left bottom in a)is the SEM images of as-prepared perpendicular MXene-Li arrays,showing the micrometer-scale(70 μm)and nanometer-scale interspaces.The middle bottom in panel a)is the SEM image of perpendicular MXene-Li arrays after lithium stripping for 20 mAh cm-2,showing the left gaps in between MXene walls.Reproduced with permission from Cao et al.[153]Copyright 2019,Wiley-VCH.b)Scheme of 3D printing MXene arrays and lattices to guide the nucleation and growth of lithium.Reproduced with permission from Shen et al.[155]Copyright 2020,by Elsevier Ltd.c)Two-side press of MXene stacks onto a thin Li host.d)Enlarged SEM image of the cross-section of ILC-Li.Reproduced with permission from Chen et al.[156]Copyright 2020,American Chemical Society.
More recently,Cui et al.proposed that the curvature of the host was a key parameter that affected the deposited morphology of Li and electrochemical properties.[147]The authors designed three kind of rGO hosts with different tortuousities,vertically oriented,horizontally oriented,and random structure(Figure 12a-c).Experimental results demonstrated that the higher tortuousity of the electrode caused higher local current density,giving rise to preferential deposition of Li on the anode surface(Figure 12d-f).On the contrary,the vertically aligned rGO electrode with low tortuousity achieved uniform Li+transport and compact deposition on the whole electrode,thus greatly improving the cycling stability(Figure 12g-i).Based on the principle of low curvature,the electrode has uniform lithium deposition and high Coulombic efficiency up to 99.1% under high current.

Figure 12.Li deposition and cycling behavior in different anodes:a-c)Schematics of Li deposition in HGA a),RGA b),and VGA c).d-f)Cross-sectional SEM images of HGA d),RGA e),and VGA f)after the first Li deposition step.Simulation of Li-concentration distribution in g)low-tortuosity VGA and h)hightortuosity HGA electrode configurations.i)Simulated distributions of Li deposition and stripping current density(stars)and concentrations(lines)at different depths in the VGA and HGA electrodes.The current density through the entire cell was fixed at 3 mA cm-2;the plotted points are the local current density distributed at the electrode interphase at the specified electrode depth.Reproduced with permission from Chen et al.[147]Copyright 2020,Elsevier Inc.
8.Fabricating the Flexible Metal Anodes based on the Good Mechanical Properties and Flexibility
Considering the practical application,the obtained metal anodes must have good mechanical properties in order to meet the various complex working conditions,especially under bending and stretching tests.According to previous report,local plastic deformation and pulverization of pure metal anode caused by bending or stretching will further aggravate dendrite growth.[48,137]Therefore,the construction of flexible scaffold with good mechanical and flexible features to minimize volume fluctuation of metal anodes and increased surface for metal plating during cycling is urgently needed.[9,10,158,159]In general,three practicable preparation methods are widely applied to prepare composited metal anode,including electrodeposition,thermal infusion,and mechanical rolling.[11,12]Among which,thermal infusion is regarded as the most effective one to prepare flexible metal anode.By introducing metalphile additive into molten metal,not only the surface tension between the metal anode and the substrate can be largely reduced,but also the whole mechanical strength and flexibility can be enhanced.[44,160]Moreover,the additive can also provide abundant homogeneous nucleation sites to compensate the negative effects of bending or stretching.[49,117]
2D materials,such as MXenes,are expected to an ideal additive for metal anodes to suppress the lithium dendrite and improve the electrochemical performance.Wu and coworkers proposed a new concept of flexible and bendable Ti3C2-MXene/graphene(MG)framework to achieve a flexible Li anode by thermal infusion strategy(Figure 13ad).[160]Contributing to the good mechanical and flexible features of MG films,the flexible MG-Li anode with high Li content exhibited excellent mechanical properties,long life span,stable cycling performance,and high-rate performance(Figure 13c,d),which enable metal anodes to be applied into flexible wearable energy devices.And then,a lightweight and flexible Ti3C2TXMXene-melamine foam(MXene-MF)hybrid was also constructed for alkali-metal anodes(Li,Na,and K)(Figure 13e).[117]Owing to the high conductivity and lithiophilic property of Ti3C2-MXene as well as the flexible nature of 3D architecture,all the alkali-metal anodes delivered high areal deposition capacity and long life span.By the in situ optical microscopy images during Li plating process,smooth and homogeneous Li deposition morphology without dendrite was observed at different current density,while plenty of Li dendrite,dead Li and large volume change generated in bare copper collector(Figure 13f-i).Impressively,the pouch cells assembled by Ti3C2-MXene-MF-Li anode and Ti3C2-MXene-MF-S cathode exhibited little capacity attenuation under different bending degrees,indicating great application prospects in wearable electronics.

Figure 13.a)SEM image of the MXene/graphene film.Inset is a photograph of the bent MXene/graphene film.b)EDS elemental mapping of carbon(red),titanium(green),and fluorine(blue).c)The photographs of the MXene/graphene-Li anode.d)Photographs(top)and corresponding cross-sectional SEM image(bottom)of the MXene/graphene-Li anode.Reproduced with permission from Shi et al.[160]Copyright 2019,American Chemical Society.e)Schematic illustration of the fabrication process of the 3D MXene-melamine foam(MF)for the alkali-metal anode.f-i)Cross-sectional in situ optical microscopy images of the Li electrodeposition process of MXene-MF.Reproduced with permission from Shi et al.[117]Copyright 2020,American Chemical Society.j)Morphologic and mechanical characterizations of Na-Ti3C2Tx-CC.Reproduced with permission from Fang et al.[159]Copyright 2020,American Chemical Society.Elemental distribution images of k)Li-,l)O-,and m)F-on a MXene@CNF/Li anode(2 mA h cm-2)probed via ToF-SIMS.Reproduced with permission from Wang et al.[137]Copyright 2020,by Elsevier Ltd.
More recently,a Ti3C2-MXene modified carbon cloth(MXene-CC)with ultra-high conductivity and sodiophilic surfaces was prepared as Na host.[159]After thermal infusion treatment,the metal Na composite anode(Na-MXene-CC)exhibited good mechanical properties and could be folded and bent repeatedly(Figure 13j).In addition,the Na-MXene-CC electrode exhibited stable sodium deposition/stripping ability in both ether and carbonate electrolytes.When the Na-MXene-CC anode paired with Na3V2(PO4)3cathode,the obtained full cell delivered stable cycling performance under different bending degree with similar capacity decay rate.These excellent properties provide instruction for the commercialization of metal Na foil and the development of flexible Na-based devices.
Ultrathin metal anode with thickness of 15-30 μm is the key to fulfill high-energy density rechargeable batteries.However,it is still a great challenge to obtain ultrathin metal anode by mechanical rolling method due to the inferior mechanical feature and high viscosity of metallic anode.By designing a flexible and self-supporting Ti3C2-MXene/cellulose nanofiber film as Li host,Guo et al.fabricated an ultrathin composited Li anode with a thickness of 25 μm.[137]The mechanical strength and flexibility of the obtained Ti3C2-MXene@CNF films are greatly enhanced by the micro-topology structure between the Ti3C2-MXene nanosheets and CNF-assisted microspheres.Moreover,Ti3C2-MXene nanosheets with abundant Li nucleation sites showed good affinity to metal Li.The time-off light secondary ion mass spectrometry(ToFSIMS)was further conducted to observe the Li distribution in the Ti3C2-MXene@CNF films,where the elemental results of Li-,O-,and F-were well overlapped,indicating a homogeneous nucleation of metal Li anode(Figure 13k-m).As a result,the flexible,ultra-thin(~25 μm),and self-supporting Ti3C2-MXene@CNF hybrid anode matched with the flexible,self-supported LiFePO4/cellulose nanofibers exhibited high specific capacity and excellent cycling stability,which provided great potential in the application of flexible energy storage devices.
9.Distributing the Heat to Avoid the Hot Spot based on the Good Thermal Conductivity
The hot spot can induce fast growth of lithium metal due to local enhancement of the surface exchange current density compared to the surrounding cooler areas.[161]Note that the high local temperature can lead to short circuit in the battery,thus further raising the temperature and increasing the risk of thermal runaway.Chen et al.reported a fibrous hydroxylated Ti3C2/CNTs(h-Ti3C2/CNTs)composite with good thermal conductivity and high mechanical strength,which can be used as a stable 3D matrix to guide the uniform Na nucleation of dendrite growth.[108]Figure 14a shows the temperature distribution of the scaffold after laser heating.The results demonstrate that the rate of sodium deposition in high-temperature region is much faster than that in other regions.Therefore,the host should have good thermal conductivity to avoid the formation of hot spots and the growth of dendrites.Benefiting from the excellent thermal conductivity of h-Ti3C2/CNTs composite,can distribute the current uniformly,the uniform current distribution and homogeneous Na plating rate were realized(Figure 14b-e).

Figure 14.a)Illustration of the synthesis of h-Ti3C2/CNTs.b)Schematic diagram of temperature distribution of scaffold by laser heating.c and d)The corresponding SEM image(top-down view)of Na plated on CNTs and h-Ti3C2/CNTs films.e)Thermal conductivity of CNTs and h-Ti3C2/CNTs films.Reproduced with permission from He et al.[161]Copyright 2020,Wiley-VCH.
The above-mentioned advantages make 2D materials potential candidates for constructing dendrite-free metal anodes.Recent progresses of 2D materials for dendrite-free metal anodes are summarized based on the functional mechanism in improving the electrochemical properties,which are listed in Tables 1 and 2.

Table 1.A comparison of related parameters for reported 2D materials in metal anodes.

Table 2.A summary of electrochemical performances for metal-based batteries based on the reported 2D materials/metal composite anodes.
10.Conclusion and Perspective
In this review,we summarize the diverse properties of 2D materials which have been used in improving the electrochemical performance in dendrite-free metal anodes.The correlations between the 2D materials’favorable properties and nucleation mechanism are well discussed,including ultrathin 2D structure with high surface area,abundant functional groups,tunable heteroatom-doping and vacancies,good mechanical strength, flexibility,and high electrical/thermal features.Until now,great effects on harnessing the various unique physical or/and chemical properties of 2D materials and the construction of this unique 2D-3D structure have been achieved in improving cycle stability,Coulombic efficiency,and rate capability of the reversible metal anodes.However,the underlying reasons for their inherent roles,such as nucleation and growth of metal ions,are not well understood owing to the currently limited characterization methods.Moreover,the above experimental achievements have not been enough to support the commercial practical application of metal anodes.Therefore,there are still several aspects to explore the underlying reasons and make better use of unique properties of MXenes in improving the electrochemical properties of dendrite-free metal anodes:
10.1.Identifying the Plating Mechanisms of Metals on 2D Materials by in situ Characterization Techniques
Sufficient experimental results have revealed that some 2D materials(graphene and MXenes)can effectively control the nucleation of metal ions and inhibit the dendrite growth.However,there is no direct evidence in revealing the mechanism of nucleation process on 2D materials.More advanced characterization methods,especially in situ characterization techniques,including the in situ transmission electron microscopy and in situ X-ray photoelectron spectroscopy,are needed to visualize the nucleation process on 2D materials and clarify how the various functional groups on 2D materials can control the nucleation of metals.
10.2.Efficiently Controlling the Nucleation and Growth of Metals on 2D Materials by Oriented Atomic Level Modified(Vacancy,Heteroatoms,or Clusters)
Previous experiment result verified that the modification at atomic level(vacancy,heteroatoms,or clusters)could help to introduce nucleation functional sites into the matrix,thus enhancing the metal affinity of substrates.Contributing to the strong adsorption energy,metal ion prefers nucleating at the modified sites,resulting in the uniform nucleation behavior of metal and inhibits the growth of dendrites.Graphene and MXenes possess superior electric conductivity,chemical stability,hydrophilic properties,and sufficient functional groups/active sites.These favorable intrinsic properties make 2D materials as perfect matrix,which also can be facilely modified at atomic level(vacancy,heteroatoms,or clusters).However,the strong interaction between the imperfect sites on 2D materials and nucleation mechanism remains blurred.Therefore,it is urgent to elucidate why the metal ions tend to nucleate at imperfect sites on 2D materials.
10.3.Exploring Self-healing Dendrite-free Metal Anodes via Ferromagnetic Properties of 2D Materials
Over the years,plenty of innovative approaches were applied to suppress the dendrites growth of metal anodes,but complete avoiding of dendrites is a big challenge.Recent works verified that the self-heating at the electrolyte/dendrite interface can cause surface atoms migration away from the tips of dendrite to smooth the surface of dendrite,achieving self-healing of the dendrites.Previous studies have shown that selectively terminated 2D materials can lead to the enhanced performance,including tunable work functions and 2D ferromagnetism.Electromagnetic heating technology adopts the principle of magnetic induction eddy current heating,which could heat the matrix with ferromagnetic.Therefore,designing ferromagnetic 2D materials as the matrix for metal anodes is required.Then,in the following plated process,the self-healing of dendrites can be achieved through electromagnetic heating,resulting in a great breakthrough of dendritesfree metal anodes with high performance.
10.4.Practical Application of 2D Materials in Metal Anodes
Although the new concepts on constructing dendrite-free metal anodes based on 2D materials are booming,the practical application of 2D materials in metal anodes is mainly limited by the complicated synthesis process and high cost of 2D materials.Also,the evaluation of the 2D materials in metal anodes is mostly conducted in the coin cells.The fabrication of 2D materials-based metal anodes at large scale remains challenging.The future study should not only focus on reducing the cost of synthesizing 2D material.More importantly,the manufacturing process the 2D materials-based metal anodes needs to simplified,thus further reducing the cost to meet industrial standard.
Acknowledgements
Z.C.,Y.Z.,and Y.C.contributed equally to this work.This work was financially supported by the National Natural Science Foundation of China(grant number,52072014,52002012).The authors would like to thank for the financial support from China Postdoctoral Science Foundation(2020M670090 and 2020TQ0022)and National Postdoctoral Program for Innovative Talents(BX20200027 and BX20200037).
Conflict of Interest
The authors declare no conflict of interest.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Classifying Electrolyte Solutions by Comparing Charge and Mass Transport
- Two-dimensional Boron Nitride for Electronics and Energy Applications
- Recent Development in Defects Engineered Photocatalysts:An Overview of the Experimental and Theoretical Strategies
- 2D/2D Heterostructures:Rational Design for Advanced Batteries and Electrocatalysis
- Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries
- Density Functional Theory for Electrocatalysis
