Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries
Jie Lin ,Liang Lin,Shasha Qu,Dongyuan Deng,Yunfan Wu,Xiaolin Yan,Qingshui Xie*,Laisen Wang,and Dongliang Peng*
All-solid-state thin- film lithium batteries(TFLBs)are the ideal wireless power sources for on-chip micro/nanodevices due to the significant advantages of safety,portability,and integration.As the bottleneck for increasing the energy density of TFLBs,the key components of cathode,electrolyte,and anode are still underway to be improved.In this review,a brief history of TFLBs is first outlined by presenting several TFLB configurations.Based on the state-of-the-art materials developed for lithium-ion batteries(LIBs),the challenges and related strategies for the application of those potential electrode and electrolyte materials in TFLBs are discussed.Given the advanced manufacture and characterization techniques,the recent advances of TFLBs are reviewed for pursuing the high-energy-density and long-termdurability demands,which could guide the development of future TFLBs and analogous all-solid-state lithium batteries.
Keywords
all-solid-state,high-energy-density,lithium battery,physical vapor deposition,thin film
1.Introduction
Globally,the increasing races of semiconductor industry have triggered the development of on-chip systems.[1,2]The associated uninterruptible power supply(UPS)for dynamic random-access memory(DRAM),micro-electromechanical system(MEMS),radio frequency identification(RFID),and related devices require integrated power sources to realize the continuous working demand.[3]All-solid-state thin- film lithium batteries(TFLBs)[4]are the cells using thin- film electrodes and solidstate electrolytes with a microscale thickness.The key components of TFLBs are similar to those of lithium-ion batteries(LIBs),which are composed of the current collector,cathode,electrolyte,and anode materials.As illustrated in Figure 1,the microscale, flexible,and threedimensional(3D)TFLBs are promising candidates in the above applications due to the high safety and flexible structure,which are mainly fabricated by physical vapor deposition(PVD)methods,including magnetron sputtering,thermal evaporation,pulsed laser deposition(PLD),atomic layer deposition(ALD),etc.Herein,we first outline the development of PVD-fabricated TFLBs and then discuss the potential electrode and electrolyte materials to be applied in future high-energydensity TFLBs.

Figure 1.Photographs of microscale, flexible,and 3D TFLBs.a)Parylene coated LiCoO2|Li TFLBs fabricated at ORNL.Reproduced with permission.[4]Copyright 2017,Elsevier.b)Different sizes of microscale TFLBs(25 and 7 mm2)on Si wafer.Reproduced with permission.[256]Copyright 2011,Elsevier.c)Microscale TFLB array using Cu2ZnSnS4thin- film anode.Reproduced with permission.[10]Copyright 2016,American Chemical Society.d)Flexible TFLBs bent over sample holder.Reproduced with permission.[11]Copyright 2017,Elsevier.e)Flexible TFLB array with small dots of individually defined Si/Cu electrodes.Reproduced with permission.[12]Copyright 2017,American Chemical Society.f)SEM images of Si microtubes after ALD of Pt and TiO2.Reproduced with permission.[15]Copyright 2014,Wiley-VCH.
In 1969,Liang et al.developed the first TFLBs of AgI/LiI/Li by spray and vacuum deposition.In 1983,Kanehori et al.reported the secondary TFLBs of TiS2/Li3.6Si0.6P0.4O4/Li by CVD and PVD methods.In 1992,Bates et al.[5]first prepared the thin- film electrolyte of lithium phosphorus oxynitride (LiPON), which remains the most commonly used electrolyte film for TFLBs thus far.Afterward,a series of microscale TFLBs(Figure 1a)are fabricated.In 2002,West et al.[6]reported the microscale TFLBs(LiCoO2|LiPON|Li)with an area of 50 μm×50 μm by a micro-fabrication process for their application in National Aeronautics and Space Administration(NASA).The TFLBs operated at 3.9 V can stably deliver a high volumetric capacity of 40 μAh cm-2μm-1.In 2007,Notten et al.[7]proposed the 3D integrated TFLBs(LiCoO2|LiPON|Si)based on etched Si wafers.The 3D structure design could boost the areal capacity and be combined with the Si-based solar cells.In 2015,Cras et al.[8]presented the microscale TFLBs(Li1.2TiO0.5S2.1|LiPON|Si)(Figure 1b)by using the land grid array(LGA)packages,which deliver a stable volumetric capacity of ~38 μAh cm-2μm-1after 740 cycles.In 2016,we applied the high-performance Cu2ZnSnS4film[9]in the microscale TFLBs(Figure 1c)and highlighted the importance of simultaneously regulating the voltage profiles of half-cells and full cells.[10]In 2017,utilizing the mechanical flexibility of LiPON[11](Figure 1d),Rubloff et al.[12]fabricated the ultrathin TFLBs(LiCoO2|LiPON|Si)(Figure 1e)by ALD in the whole process.The thickness of LiCoO2,LiPON,and Si is only 500,90,and 80 nm,respectively.The TFLBs can retain a stable volumetric capacity of~30 μAh cm-2μm-1after 150 cycles.In 2018,Rupp et al.[13]deposited the Li7La3Zr2O12electrolyte film by PLD,and improved the ionic conductivity by changing the heat temperature,which is a promising solid electrolyte film for the future TFLBs.In 2016,CYMBET corporation presented the solidstate TFLBs with a unit capacity of 50 μAh for over 5000 cycles.In 2017,STMicroelectronics proposed the TFLBs of LiCoO2|LiPON|Li with a unit capacity of 1 mAh and a nominal voltage of 3.9 V.In 2020,TDK Electronics launched the all-ceramic-structure TFLBs with a unit capacity of 100 μAh and a nominal voltage of 1.5 V.The above commercial TFLBs are expected to be applied in the real-time clock,internet of things,energy harvesting,and wearables.
The performance parameters of some reported representative TFLBs are listed in Table 1.The working potential of oxide cathodes is higher than other Li-free cathodes.At present,the most common TFLB battery system is LiCoO2|LiPON|Li due to the high energy density.Despite the excellent durability of TFLBs over 30000 cycles,[14]the limited areal capacity hinders their widespread application.There are two effective pathways:1)Constructing 3D structures to increase the space utilization;and 2)Developing high-capacity electrodes and highly ionconductive electrolytes to improve the intrinsic capacity and cycling performance of TFLBs.

Table 1.Electrochemical performance of reported thin- film lithium batteries
Various types of 3D substrates(Figure 1f)are exploited in TFLBs,such as the etched Si wafer,[15]self-assembled virus,[16]and 3D printed micro-battery.[17]Chiang et al.[18]applied a densely sintered pellet of~400 μm LiCoO2into the micro-batteries(3 mm × 3 mm ×0.7 mm)by an electroformed packaging approach.For the charge voltage to 4.6 V,the cells deliver an ultrahigh energy density of 675 Wh L-1,realizing the small-volume and low-cost energy device demands by the unique 3D architecture.Pearse et al.[19]deposited all of the active components(LiV2O5|Li2PO2N|SnNx)by ALD on the Si wafers with deep pore arrays.The 2D TFLBs deliver a volumetric capacity of 37 μAh cm-2·μm-1after 100 cycles,while the 3D TFLBs increase the areal capacity by an order of magnitude,demonstrating the effective strategy of 3D structural design for increasing the power and energy density of TFLBs.
The current challenges of TFLBs are mainly the low capacity of cathodes,low conductivity of electrolytes,huge volume change of anodes,and unstable interfaces of full batteries.The possible solutions could be the utilization of Li-free cathodes,crystallized bulk electrolytes,zerostrained anodes,and all-in-one TFLBs which have been applied in practical lithium-ion batteries.Fundamentally,the bottleneck for improving the electrochemical performance of TFLBs lies in material development.High-capacity cathodes(high-voltage LiCoO2,Li-rich Mn-based layered oxide,Ni-rich layered oxide,high-voltage spinel,etc.),high-capacity anodes(lithium,silicon,etc.),and highly ion-conductive electrolytes(glass,oxide,sulfide,etc.)are the promising candidates to be applied in future high-energy-density TFLBs.As shown in Figure 2,this review will discuss the key problems and corresponding solutions for the potential application of novel materials in TFLBs,and describe the recent advances of state-of-the-art TFLBs about the challenging issues of interface reaction,material development,and device integration.

Figure 2.Main challenges of cathode,electrolyte,and anode materials to be applied in future high-energydensity TFLBs.
2.Cathode Materials
Considering the high-voltage and inherent Li source of lithium transition metal oxide materials,the corresponding thin- film materials fabricated by PVD methods are the most common cathodes for TFLBs.As listed in Table 2,high-voltage LCO cathode outperforms due to the high theoretical capacity and high material density.[20,21]LRLO cathode has a low cost and can deliver a high capacity within a wide voltage window up to 4.8 V.[22]Ni-rich layered oxide has been applied in electrical vehicles due to the high specific capacity and favorable cycling performance.[23]High-voltage spinel is compatible with the solid electrolyte film of TFLBs.[24]Therefore,the above cathode materials are all promising cathode candidates for TFLBs and will be discussed below.

Table 2.Electrochemical performance of reported thin- film cathode materials
2.1.Lithium Cobalt Oxide(LCO)
LCO is one of the most commonly used cathodes in the present computer,communication,and consumer electronics(3C products).[25,26]Although the theoretical capacity of LCO is 274 mAh g-1,the achieved practical capacity is only~140 mAh g-1while the reversible deintercalation of Li ion is 0.5 for per LCO.[27,28]To satisfy the increasing requirements of 3C products toward intelligent,long standby,and lightweight,the energy density of LCO needs to be further elevated by raising the charge voltage to extract more Li ions.The reason for limiting the charge voltage of layer-structured LMO2(M=metal)is that the redox couple pins at the top of anion p-band,in which Co3+/Co4+redox couple at the top of O band determines the voltage limit of LCO.[29]High-voltage LCO remains one of the most promising cathodes thus far.The performance parameters of some reported highvoltage LCO cathodes are summarized in Table 3.Surface degradation,phase transformation,and inhomogeneous reaction of LCO could aggravate at high voltages(>4.2 V vs Li/Li+).[25]The most effective strategies are mainly bulk-doping and surface coating.Most of the PVD-fabricated LCO films are prepared at low temperatures(<200 °C),in which the insufficient diffusion kinetics of nucleation and growth leads to the lower crystallinity compared to that of bulk LCO materials.[30]Consequently,the structural instability of LCO films is the key issue to be addressed for their application in TFLBs.
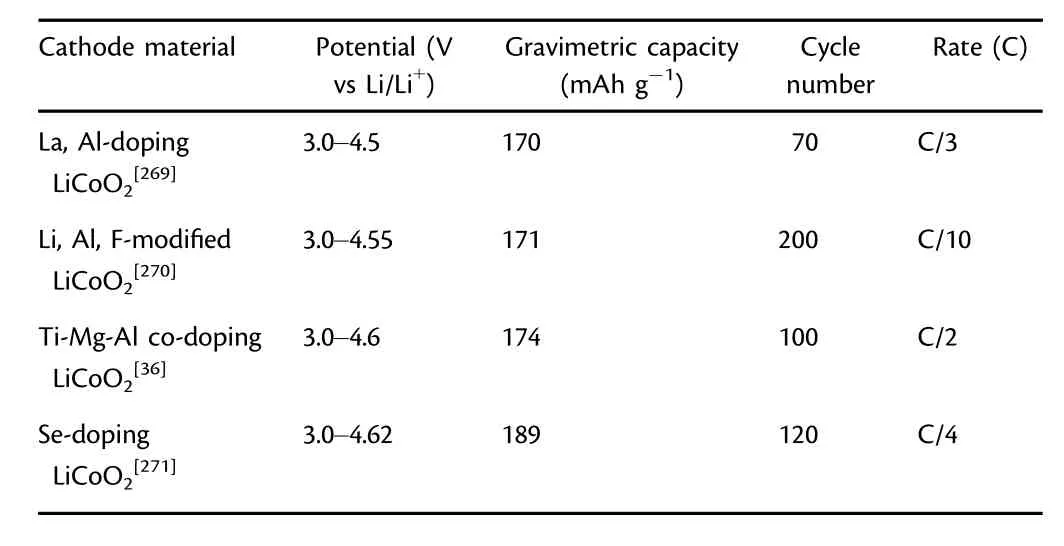
Table 3.Performance parameters of reported high-voltage LiCoO2cathodes.(1C=274 mA g-1)
2.1.1.Structural Instability Mechanism
LCO has the α-NaFeO2structure,in which oxygen is arranged in a cubic close-packed(ccp)framework and alternated with Co3+and Li+in the(111)plane.The LCO crystallizes in the R-3m space group with the cell parameters of a=2.816 °A and c=14.08 °A.Metal ions occupying octahedral sites form continuous Li2O and MO2layers.[31-33]As shown in Figure 3a,with the increasing amount of Li ions extracted from LCO,the oxygen layers rearrange,causing the lattice to be distorted,and LCO undergoes the phase transition from O3 to H1-3 and O1 phases.[28,34]If the Li ions are completely removed,the irreversible hexagonal close packing of oxygen is formed through an O-Co-O motif.When the charge voltage reaches 4.55 V,the O-Co-O plates rearrange with Li ions and the crystal cell shrinks leading to stress accumulation and grain breakage.[27,35,36]Meanwhile,excessive delithiation through partial dislocation slips promotes the migration of transition metal into octahedral sites forming spinel structure,[32]and leads to fast capacity degradation and poor rate performance.[37]

Figure 3.a)Differential capacity vs Li concentration in LixCoO2.Reproduced with permission.[27]Copyright 2004,Elsevier.b)Cross-sectional SEM image of TFLBs showing crack in middle of LCO film.Reproduced with permission.[41]Copyright 2018,Elsevier.c)Schematics of intercalation pathways of Li ions in different lattice layers of LCO cathode.Reproduced with permission.[43]Copyright 2013,Elsevier.d)Supercell of Li45[Li9Ni6Co6Mn24]O90and its atomic arrangement in transition metal layer of LRLO cathode.Reproduced with permission.[50]Copyright 2014,Wiley-VCH.e)Schematics of structural changes of LRLO cathode from layered(R-3m)to spinel-like(Fd-3m)and rock-salt(Fm-3m)phases.Reproduced with permission.[57]Copyright 2014,Wiley-VCH.f)Schematics of protective mechanism of LRLO cathode treated by atomic surface reduction method upon cycling.Reproduced with permission.[65]Copyright 2019,American Chemical Society.
2.1.2.Regulating Preferred Orientation
Post-annealing in air or oxygen can improve the crystallinity and release the residual stress of thin films,[38]as confirmed by the well-crystallized LCO film annealed at 700°C in air.[39]However,due to the different thermal expansion coefficients of deposit and substrate,[40]the PVD-fabricated films without binders are easy to crack and exfoliate from the substrate,as shown in Figure 3b.[41]Therefore,it is necessary to adjust the deposition and post-annealing parameters to balance the crystallinity and accumulated stress of LCO film.Kim et al.[42]performed rapid thermal annealing of the sputtered LCO film at 700°C in oxygen inducing the smooth and crack-free morphology.Yoon et al.[38]also prepared the crack-free LCO film by reducing the residual stress through the two-step heat treatments of substrate heating and rapid thermal annealing.
The nanoscale grains deposited at room temperature grow parallel to the substrate because of the minimum surface energy in the preferred(003)plane.With the increase of substrate temperature,the surface energy of atomic planes decreases,and its influence on nucleation becomes weaker.[43]Figure 3c shows the intercalation pathways of Li ions in different lattice layers of LCO films.The(003)plane of LCO can stabilize the structure upon intercalation,but the blocked Li-ion pathways could increase the resistance.[44]The(101)and(104)planes which are vertical to the substrate can enhance the Li-ion deintercalation kinetic and reduce the polarization voltage.[36,37,45]Yoon et al.[43]used the Li2O buffer layer to prevent the loss of Li ions,and the Li2O/Al/Si substrate can facilitate the formation of(110)facet of LCO.Bates et al.[46]found that when the film thickness is larger than 1 μm,LCO islands tend to grow in the(101)and(104)facets.In contrast,the(003)facet is preferentially formed during the fabrication of nanoscale LCO films.
The high-voltage LCO cathode is a promising candidate to fabricate high-energy-density TFLBs.However,the high cutoff voltage causes the irreversible collapse of LCO structure,resulting in severe capacity decay and poor rate capability.[26]How to constrain the structural change of LCO is the key problem to be solved for high-voltage application.Improving crystallinity and regulating orientation could induce higher-quality LCO thin films,[21,47]which may improve the durability of present TFLBs.
2.2.Li-rich Manganese-Based Layered Oxide(LRLO)
LRLO cathode is formed by adding more Li ions into the layered oxides and partially replacing the transition metal cations,delivering a capacity of>250 mAh g-1.[48-50]LRLO is regarded as a solid solution of hexagonal LiMO2(M=Mn,Ni,or Co)(R-3m)and monoclinic Li2MO3(C2/m).For example,0.6Li[Li1/3Mn2/3]O2·0.4Li[Ni1/3Co1/3Mn1/3]O2can be expressed as Li[Li0.2Ni0.13Co0.13Mn0.54]O2,and the atomic arrangement in the transition metal layer is[Li3Ni2Co2Mn8](Figure 3d).[50]The main challenges of LRLO are continuous voltage fading,rapid capacity decay,poor rate capability,and low initial Coulombic efficiency( first-cycle irreversible capacity loss).[51]The voltage fading of LRLO is closely related to transition metal migration,oxygen release,and phase transition,[52]especially when it is applied in TFLBs.
2.2.1.Voltage Fading Mechanism
The charge-discharge mechanism of LRLO can be divided into two steps.In the first step,with the de-intercalation of Li ions from the Li layer,Li ions in the octahedral position of the transition metal layer diffuse into the tetrahedral position of the Li layer.[53]In the second step,when the voltage is higher than 4.5 V,the Li layer and transition metal layer both extract a Li-ion accompanied by one oxygen,equaling to the formation of Li2O.After the first charge,the generated Li2O cannot return to the crystal lattice,leading to the large irreversible capacity and voltage fading in the following process.Density functional theory(DFT)calculations of LRLO have proved that the Mg and Ti elements with high oxygen affinities can increase the energy barrier of oxygen release reaction,thereby stabilizing the surface structure of LRLO.[54]
The main bottleneck for the application of LRLO in TFLBs is the severe voltage fading.[55]With the extraction of Li ions,the transition metal ions migrate from the octahedral to tetrahedral position and Li octahedral sites,leading to the formation of the spinel structure.[56,57]Figure 3e shows the schematics of structural change from the layered(R-3m)to spinel-like(Fd-3m)and rock-salt(Fm-3m)structures upon the cycling of LRLO.In addition to the Ni2+/Ni4+and O2-/O-redox pairs,the Mn3+/Mn4+and Co2+/Co3+redox pairs with lower voltage are activated,[58]and the polarization voltage is increased.[59]Reducing the Li or Co amount and increasing the Ni content can shorten the charge plateau of oxygen release and mitigate the voltage fading.[49]
2.2.2.Mitigating Voltage Fading
For the redox reaction of LRLO cathode,the surface is more sensitive than the internal structure.[60]The irreversible oxygen loss on the surface leads to the transition metal migration and phase transition,resulting in the drastic voltage fading.[61,62]Surface coating can protect the electrode and inhibit the irreversible loss of oxygen.[63]Martha et al.[64]sputtered a LiPON film on the surface of LRLO particles to prevent the corrosion of electrolyte.When the LiPON-coated LRLO electrode is charged to 4.9 V,it exhibits a stable capacity of~275 mAh g-1over 300 cycles at the cost of increased resistance.Rosy et al.[65]exploited an atomic surface reduction method to prepare the LRLO cathode as shown in Figure 3f.The oxygen release and transition metal dissolution are reduced and the structural stability of LRLO is improved because the modification changes the electronic structures of Mn and Ni on the surface of LRLO.Zeng et al.[66]coated the highly conductive sulfide electrolyte and Al2O3layers on the surface of LRLO,greatly reducing the interface resistance and irreversible oxygen loss.Wang et al.[67]deposited a ZnO/TiO2coating(~1.7 nm)on the surface of LRLO by ALD to mitigate the structural rearrangement and phase transition from layered to spinel structures,reducing the voltage fading and capacity decay.The above results imply that reasonable surface modifications are advantageous for mitigating the voltage fading issues of LRLO in TFLBs.
Bulk doping is also effective for alleviating the voltage fading of LRLO.Vanaphuti et al.utilized in situ XRD tests to confirm that the codoping of Na and F decreases the Li+/Ni2+mixing degree of LRLO.The Na doping enhances the cation ordering through its substitution of Ni 2b,and the F doping decreases the oxygen loss due to the compensation of charge balance.The synergetic doping of cation and anion both increases the average voltage,[68]which could inspire the co-deposition of LRLO and dopants for addressing the voltage fading issue in TFLBs.
The LRLO cathode is a potential cathode for TFLBs due to the high capacity and wide working potential.However,it is difficult to prepare the stoichiometric LRLO films using PVD methods.[69-71]Due to the lack of conductive agents,the voltage fading of LRLO may be more serious in TFLBs.Once the fabrication of well-crystallized LRLO is realized,its application in TFLBs will become competitive.[60]
2.3.Ni-Rich Layered Oxide
The LiMO2(M=metal)cathode materials have the α-NaFeO2structure of R-3m symmetry.[72]Partial substitution of Ni by transition metal(Co,Mn)or non-transition metal(Al,Mg)can stabilize the layered structure of LiMO2upon lithiation/delithiation.[73]The LiNi0.8-Co0.1Mn0.1O2(NCM811)and LiNi0.8Co0.15Al0.05O2(NCA)can deliver a high capacity of>200 mAh g-1,being the potential cathodefilms for high-energy-density TFLBs.[74,75]Ni-rich cathodes face many structural problems,including side reactions with electrolytes,intergranular crack of grains,reconstruction of surface structure,and disintegration of secondary particles.
2.3.1.Cation Mixture Mechanism
The ionic radius of Li+(0.076 nm)is similar to that of Ni2+(0.068 nm),and the Li+/Ni2+disorder[76]renders it difficult to prepare stoichiometric LiNiO2.[73]Figure 4a displays the atomic configurations of LiMO2and their Li+/Ni2+disordered structures.Due to the cation mixing,[77]phase transition occurs from layered to spinel and rock-salt phases enlarging the plate gaps.[62,78]Hence,higher activation energy is required to drive the Li diffusion in the pathways.The phase transition is also accompanied by oxygen release,[79,80]and thus,the damage is more serious on the surface than in the bulk materials.[81]

Figure 4.a)Atomic configurations of a1)LiMO2and schematic structures for different defects including b1)Li vacancy,c1)oxygen vacancy,d1)excess Ni,e1)Li/Ni exchange,and f1)Ni migration.Reproduced with permission.[76]Copyright 2014,Royal Society of Chemistry.b)Schematics of degradation process of secondary NCA particles.The arrow in each primary particle represents the different crystal orientations within aggregate.Reproduced with permission.[82]Copyright 2017,American Chemical Society.c)Schematics of different microstructural and interfacial evolutions in pristine NCA and FCG-NCA electrodes in all-solid-state cells.Reproduced with permission.[81]Copyright 2019,Wiley-VCH.d)Atomic structures of cation disordered(Fd-3m)and ordered(P4332)LNMO cathodes.Reproduced with permission.[91]Copyright 2012,Royal Society of Chemistry.e)Schematic of Mn and Ni dissolution process in LNMO/graphite full cells.Reproduced with permission.[257]Copyright 2013,American Chemical Society.f)Dissolved metal-ion concentration of Ni and Mn in LNMO|Li2TiO3full cells.Reproduced with permission.[98]Copyright 2020,Elsevier.
2.3.2.Preventing Intergranular Crack
The commercial Ni-rich cathodes are mostly polycrystalline particles,in which the submicron primary particles aggregate into micron secondary particles.[82]During the Li+insertion and extraction,the anisotropic change in lattice dimension causes the intergranular cracks between the primary Ni-rich particles.[83,84]The structural degradation process of NCA is shown in Figure 4b.This crack could lead to the contact loss between the primary particles,resulting in a slow and incomplete reaction process.[85]Therefore,suppressing the formation of microcrack is important for extending the cycling life of Ni-rich cathodes.[82]Structural modification and surface coating are beneficial for reducing intergranular cracks.[77]
Jung et al.[81]found that by adjusting the concentration gradient,the high-aspect-ratio rod-shaped particles can be obtained with released volume change and without intergranular cracks(Figure 4c).Yan et al.[86]injected Li3PO4into the grain boundary of LiNi0.76Mn0.14Co0.10O2to prevent the rupture of secondary particles and irreversible phase transition.Zhang et al.[87]coated Li3PO4on the surface of NCM811 to suppress the formation and growth of intergranular crack.Therefore,the combination of Ni-rich cathode and solid electrolyte in TFLBs[88]could simultaneously address the key problems of Li+/Ni2+disorder and intergranular crack.
Although the Ni-rich materials have been widely applied in practice,there are few reports about Ni-rich thin films due to the difficulty in the synthesis of defective-free and well-crystallized Ni-rich thin films.The intergranular crack and crystalline degree of Ni-rich cathode is a trade-off point,[85]which should be considered when applied in TFLBs.
2.4.High-Voltage Spinel LiNi0.5Mn1.5O4(LNMO)
High-voltage spinel LNMO is a typical low-cost,high-voltage,and Cofree cathode material,[89]which has been applied in some TFLBs.[90]The high-voltage spinel LNMO has two different crystal structures of the ordered P4332 and disordered Fd-3m space groups as shown in Figure 4d.[91]The Mn-ions are all Mn4+in P4332,while a small amount of Mn3+coexists with Mn4+in Fd-3m.However,due to the high operating voltage of>4.7 V,the practical application of LNMO is mainly hindered by the poor structural stability and Mn dissolution.[89,92]
2.4.1.Enhancing Structural Stability
For the LNMO films fabricated by PVD methods,oxygen vacancies and impurity phases tend to form on the film surface,resulting in the lowcrystallized thin films.[93,94]Su et al.[95]employed a biased sputtering method to decrease the particle size of LNMO films and obtain a crystalline spinel structure of Fd-3m LNMO.Among the modified samples,the LNMO film fabricated at a substrate bias of-30 V exhibits a higher discharge capacity of~103 mAh g-1at 0.2C and two distinct discharge plateaus at ~4.7 and ~4.0 V.Wang et al.[94]deposited the wellcrystallized LNMO films by using Li-rich samples for PLD at 750°C,which retains a high capacity of~117 mAh g-1at 0.5C after 100 cycles.The aforementioned reports could improve the structural stability of LNMO thin- film cathodes in high-voltage TFLBs.[40,93]
2.4.2.Inhibiting Mn Dissolution
For the stoichiometric LNMO cathode,Mn should exist as Mn4+to achieve charge balance.[93]Normally,the LNMO thin film deposited by PVD methods have a small amount of Mn3+due to the existence of oxygen defects and impurity phases.[96]Moreover,the increase in the thickness of LNMO film will further reduce the Mn valence.[97]Upon cycling,the Mn change of Mn3+to Mn2+and Mn4+generates byproducts at the interface and hinders the Li-ion transport in the LNMO film.[96]Figure 4e shows the schematic of Mn dissolution in the LNMO/graphite full cell.Chu et al.[98]confirmed that the AlF3coating(~5.2 nm)greatly inhibits the Mn dissolution(Figure 4f)and increases the capacity retention of LNMO film.Wang et al.[99]also increased the capacity retention of LNMO by coating the similar MgAl2O4spinel(~10nm)on the surface of LNMO powder.As a result,synergetically coating and compositing LNMO film is an effective strategy to improve the lithiation performance of LNMO in high-voltage TFLBs.
The crystallinity of LNMO thin film can be increased by adjusting the deposition and heating conditions.[94]Surface modification can also suppress the side reactions including Mn dissolution.As a result,the simultaneous regulation of multiple deposition parameters upon the PVD process could advance the application of LNMO cathode in TFLBs.
3.Solid-State Electrolyte(SSE)
The SSE film is the most important component that limits the Li-ion transport of TFLBs.Highly ion-conductive SSE films can be fabricated by various techniques,including magnetron sputtering,CVD,ALD,PLD,and thermal evaporation.[100]As listed in Table 4,the SSE films of LiPON,Na superionic conductor(NASICON),Li superionic conductor(LISICON),perovskite,anti-perovskite,and garnet structures have been applied in all-solid-state batteries due to their high ionic conductivity,good stability,and facile synthesis.The main challenges and key solutions to apply the SSE films in TFLBs will be discussed in the following.The ionic conductivity of SSE noted below refers to the value obtained at room temperature(RT)unless otherwise specified.

Table 4.Parameters of reported thin- film solid-state electrolyte materials
3.1.Lithium Phosphorous Oxynitride(LiPON)
LiPON is an amorphous oxide electrolyte material,which is firstly prepared by sputtering the Li3PO4crystalline materials in the N2atmosphere.[5]Compared with Li3PO4,LiPON delivers higher ionic conductivity and better interface compatibility with Li metal in TFLBs.The N/O substitution enhances the crosslink between tetrahedral units,provoking a decrease of phosphate chain length and an electronic effect by the strong covalence of P-N bonds.[101,102]Specifically,the P-N bonds involve two kinds of N atoms,including the double-coordinate nitrogen bonds(=N-)and triple-coordinate nitrogen bonds(-N<)as shown in Figure 5a,b.The substitution of bridging oxygen bonds(-O-)and non-bridging oxygen bonds(=O)by N atoms reinforces the bonds to Li+and reduces the mobile charge density,leading to the enhanced ionic conductivity of LiPON.[102-104]Lacivita et al.[105]provided the atomic models of LiPON(Figure 5c)in which N atoms are in two different configurations as tested by the neutron scattering(Figure 5d):1)apical N(Na)atoms are in isolated P(O,N)4tetrahedra;and 2)bridging N(Nd)atoms are between two phosphate groups.Moreover,LiPON film exhibits good interface compatibility with Li metal anode,making it a promising electrolyte film for Li-based TFLBs.However,the low ionic conductivity(<10-6S cm-1)of LiPON renders it difficult to be applied in high-energy-density TFLBs.

Figure 5.a)Partial structure of nitride Li3PO4glass with the incorporation of(-N<)and b)partial structure of nitride Li3PO4glass with the incorporation of(=N-).[102]Copyright 2011,Elsevier.c)Schematic of simulated Li2.94PO3.50N0.31structure from Ab initio molecular dynamics(AIMD).Atomic coloring:O,red;N,blue;Li,green;P,light gray.[105]d)Simulated and experimental neutron data and inset shows neutron scattering schematic.[105]Copyright 2018,American Chemical Society.
3.1.1.Versatile Fabrication Process
Inspired by the reactive sputtering of LiPON film,[5]some modern techniques are adopted to change the chemical composition to increase the ionic conductivity of LiPON,including ion-beam sputtering,[106]ALD,[107]PLD,[108]and metal-organic chemical vapor deposition(MOCVD).[109]By those specific methods,the thickness of LiPON can be controlled from microscale to nanoscale.Nowak et al.[106]reported the preparation of LiPON film by ion-beam sputtering as shown in Figure 6a.Because the ion-beam sputtering provides spatial separation between the sputter plasma and substrate,it results in a low thermal stress and avoids the formation of interface reaction layers.Kim et al.[109]deposited the Li0.36P0.117O0.474N0.049film by MOCVD as shown in Figure 6b.The raised substrate temperature increases the ionic conductivity due to the elevated ratio of triple-coordinated nitrogen bonds(Figure 6c).Nisula et al.[107]deposited the Li0.95PO3.00N0.60film by ALD and investigated the influence of substrate temperature on the content of triple-coordinated nitrogen bonds,increasing the ionic conductivity of LiPON film to 6.6×10-7S cm-1.Yu et al.[110]deposited the LiPON thin film by RF magnetron sputtering,the average ionic conductivity of samples is 2.3×10-6S cm-1at room temperature(RT),and the average activation energy is~0.55 eV.By the same methods,Su et al.[111]deposited the Li3.13PO1.69N1.39thin film with ionic conductivity of 4.9×10-6S cm-1and the activation energy is~0.55 eV.Xiao et al.[112]reported a Li-compensated LiPON(Li-LiPON)SSE film,which was deposited by sputtering a sintered Li-rich Li3.3PO4target instead of the normally Li3PO4target,showing an increased ionic conductivity of 3.2×10-6S cm-1.Fleutot et al.[101,102]increased the ionic conductivity of LiPON from 3.0×10-6S cm-1to 7.2×10-6S cm-1through the thermal treatment of LiPON film at 260°C,but the treatment causes an increase of activation energy from 0.57 to 0.80 eV.

Figure 6.a)Schematic of ion-beam sputtering for LiPON film.Reproduced with permission.[106]Copyright 2015,Elsevier.b)Schematic of MOCVD for LiPON film and c)FT-IR spectra of LiPON films deposited at different substrate temperatures,and inset shows deconvoluted FT-IR peaks of LiPON.Reproduced with permission.[109]Copyright 2013,Elsevier.d)Photographs of LATP powder,mold,target,and sputtering flame and e)cross-sectional SEM image of LATP film on stainless steel.Reproduced with permission.[127]Copyright 2016,Elsevier.
3.1.2.Regulating Film Composition
The Li-ion diffusion kinetic of LiPON film is closely related to its chemical composition.Suzuki et al.[113]prepared the Li3.3PO2.9N0.83film by sputtering an unsintered Li3PO4target in an N2atmosphere.The Li content of LiPON is only~60% of the other film sputtered from a Li3PO4target sintered at 700°C.After adding Li2O in the target to compensate the Li loss,the ionic conductivity of LiPON film is increased to 3.1×10-6S cm-1.The triple-coordinated nitrogen bonds are dominant in the modified films while the double-coordinated nitrogen bonds are dominant in the control sample,leading to the higher ionic conductivity of modified LiPON film.Yoon et al.[114]reported the Li2.65B0.11P0.89O3.00N0.15(LiBPON)electrolyte film by co-sputtering the Li3PO4and Li3BO4targets.The triple-coordinated nitrogen bonds increase the number of mobile Li ions and improve the ionic conductivity to 6.88×10-7S cm-1.Since the B atom is a strong glass network former of rigid solid electrolyte,the air stability of LiBPON film is also improved.The TFLBs using the electrolyte film(LCO|LiBPON|Li)exhibits an initial charge capacity of 34.5 Ah cm-2and a high initial CE of 95.3% .Famprikis et al.[115]sputtered the Li1.35Si0.79P0.21O1.98N0.98(LiSiPON)electrolyte film from the single-phase ceramic target of Li3+xSixP1-xO4.The high ionic conductivity of 2.06×10-5S cm-1is ascribed to the combination of N-doping and mixed-former effects.
3.1.3.Chemical and Electrochemical Stability
The chemical and electrochemical stability are the key factors for applying SSEs.Reaction layers are often generated at the electrode/electrolyte interface,reducing the durability of TFLBs.LiPON is reported to decompose into Li3P,Li3N,and Li2O when it is in contact with Li metal.[116,117]The products are stable against Li metal and form a passivation layer which shields the electrolyte from further decomposing.[118]Li et al.[119]reported a protective effect of inhibiting the electrolyte decomposition by coating the Si anodes with a 50 nm or thicker LiPON film.Richards et al.[120]informed that the thiophosphate electrolyte has high reactivity with the high-voltage cathode(i.e.,a narrow voltage window for electrochemical stability)by DFT calculation.Put et al.[121]found the decomposition of LiPON occurs at~4.3 V,which proceeds in a diffusion-limited way.The LiPON layer decomposes into Li+,O2,N2,and phosphate-rich compounds,forming metallic Li filaments that result in the breakdown of SSEs.
The LiPON film is still the most commonly used electrolyte film for TFLBs.In recent years,many reports are focused on the optimization of LiPON films,especially for the improvement of ionic conductivity,including the study of deposition conditions and the optimal thickness.[111,112]The quality of LiPON film is highly sensitive to sputtering power,pressure,nitrogen atmosphere,and deposition rate.[112]The ionic conductivity of state-of-the-art LiPON is close to 10-5S cm-1[101]and the studies on structural models and interfacial stability of LiPON have been improved gradually.[102,105,122]It can be predicted that increasing the ionic conductivity and clarifying the structure change could further broaden the application of LiPON film.
3.2.NASICON Electrolyte
NASICON electrolytes can be written as AB(PO4)3(A=Li,Na,etc.;B=Ge,Zr,Ti,etc.),and the framework is composed of B2P3O12polyhedrons in which two BO6and three PO4are connected by sharing the corner oxygen atoms.[123]Hence,the large gaps can support the Li-ion transport,but the ionic conductivity of NASICON is insufficient to be applied in TFLBs.By doping Al atoms in the LiTi2(PO4)3and LiGe2(PO4)3phases,the solid electrolytes of Li1+xAlxTi2-x(PO4)3(LATP,x=0.3-0.5)and Li1+xAlxGe2-x(PO4)3(LAGP,x=0.3-0.5)become the most popular NASICON electrolytes due to the elevated ionic conductivity.The Ab initio molecular dynamics(AIMD)and DFT results have indicated that the interstitial Li ions introduced by Al dopants can activate the neighboring occupied intrinsic Li ions to induce long-range Li ion mobility.[124]However,the LATP and LAGP electrolytes have poor interfacial compatibility with both cathode and anode materials.[125]Moreover,the Ti4+of LATP is easy to be reduced,resulting in the collapsed crystal structure and increased interfacial resistance.[126]
3.2.1.Improving Morphology by Substrate
Although lapping the solid electrolyte into a thin film can improve interface contact with Li metal,it is easy to cause cracks or pores during the annealing process of SSE films on different substrates.Ling et al.[127]sputtered the Li1.3Al0.3Ti1.7(PO4)3film on stainless steels(Figure 6d,e)with post-annealing at 500°C,which is composed of fine particles on the dense surface without obvious cracks or pinholes.The LATP thin film with a thickness of~926 nm has high ionic conductivity of 6.47×10-6S cm-1and low electronic conductivity of 2.34×10-14S cm-1.Chen et al.[128]sputtered the Li1.3Al0.3Ti1.7(PO4)3thin film on the conductive glass substrate.The thin film becomes denser,smoother,and more uniform at the substrate temperature of 300°C,leading to the high ionic conductivity of 2.46×10-5S cm-1.Sun et al.[129]sputtered the Li1.5Al0.5Ge1.5(PO4)3thin film on Si substrate,and the crystalline and non-crystalline films are obtained at different substrate temperatures.The conductivity of the amorphous film(1.29×10-6S cm-1)is higher than that of the crystalline film.The low growth temperature ensures a smooth surface and uniform thickness of the amorphous film.Zhang et al.[130]prepared the Li1.4Al0.4-Ge1.6(PO4)3film with the ionic conductivity of 3.38×10-4S cm-1by tape casting on the polyethylene-coated Si substrate,and the film thickness is reduced to ~75 μm.
3.2.2.Protecting Interface by Coating
To suppress the interfacial side reactions of LATP with Li metal,Hao et al.[131]sputtered a lithiophilic ZnO layer(~200 nm)on the surface of Li1.4Al0.4Ti1.6(PO4)3pellets as shown in Figure 7a.The dense ZnO layer prevents the electron transport from Li metal to the electrolyte as confirmed by the unchanged Ti XPS peaks.The Li|ZnO@LATP|LiFePO4full cell delivers an excellent cycling performance of 167.3 mAh g-1after 200 cycles.Liu et al.[132]deposited an Al2O3film on the surface of Li1.3Al0.3Ti1.7(PO4)3electrolyte by ALD to reduce the overpotential and interfacial resistance.The Al2O3coating(~15 nm)improves the contact of LATP and Li metal,leading to the stable cycling of the coated Li symmetric cells(Figure 7b).Bai et al.[133]sputtered the Al2O3/ZnO(AZO) film(~50 nm)on the surface of Li1.3Al0.3Ti1.7(PO4)3to improve the stability against Li metal and promote the ionic transport.The Li symmetric cell exhibits high stability when cycled for 960 h at 100 μA cm-2,and the full cell of Li/AZO coated LATP/LiFePO4delivers an initial capacity of 149 mAh/g and high Columbic efficiency of~99.5% after 50 cycles.

Figure 7.a)Interface evolution between Li metal and LATP with/without ultrathin ZnO layer.Reproduced with permission.[131]Copyright 2019,Wiley-VCH.b)Electrochemical performance of Li|LATP|Li symmetrical cells with/without Al2O3coating.Reproduced with permission.[132]Copyright 2018,American Chemical Society.c)Schematic of co-sputtering and post-annealing process for forming Ga-doped LLZO multilayer film.[146]Copyright 2019,American Chemical Society.d)Cross-sectional image of half-cell(Si/MgO/Ni-Al-Cr/LiCoO2/LiNbO3/Al-doped LLZO)and schematic of co-sputtering method for making Al-doped LLZO film.[148]Copyright 2019,American Chemical Society.
The LATP films are deposited on different substrates to achieve compact,uniform,and smooth morphology for increasing the ionic conductivity.Surface coating is an effective approach to prevent the valence change of Ti and block the side reactions of LATP.The modification could improve the compatibility of LATP films with cathode and anode materials,which are advantageous for their future application in TFLBs.
3.3.Perovskite Electrolyte
The titanate-containing alkaline earth metals are generally regarded as the perovskite materials.Their general formula can be written as ABO3(A=Ba,Ca,Li,Na,etc.)within the Pnma space group,in which A ions are at the top,B ions are in the body-center,and oxygen atoms are in the face-center surrounded by the B ions to form an octahedral structure.The multiple octahedral BO3shares the oxygen vertex and A ions are in the gaps of octahedrons.Brous et al.[134]firstly replaced the A sites with Li and La to create the Li3xLa2/3-x[]1/3-2xTiO3(LLTO,[]means the vacancy).[135]The replacement of La5+generates vacancies in the A sites,and with the increase of vacancy concentration,the ionic conductivity of LLTO is improved.There are some challenges of LLTO for their application in TFLBs.The interface instability results in a large resistance when contacted with cathode and anode films.For example,the reduction of Ti4+at the interface of La0.50Li0.37TiO2.94elevates the electronic conductivity which could lead to the penetration of Li dendrites.[136]
3.3.1.Various Fabrication Techniques
Z.Lee et al.[137]prepared an amorphous Li0.5La0.5TiO3film by PLD method.By controlling the background pressure and temperature,the uniform and well-grown LLTO film(1.2 μm)delivers the ionic conductivity of 3×10-4S cm-1.The amorphous LLTO film is deposited on the LiNi0.5Mn1.5O4cathode film to assemble the LNMO/LLTO/Li cell with a capacity retention of 98% after 50 cycles.Li et al.[138]fabricated the amorphous Li0.50La0.40TiO3electrolyte film by e-beam evaporation.The elevated e-beam power reduces the activation energy of deposits leading to the high ionic conductivity of 1.8×10-7S cm-1.The all-solid-state battery of LCO|LLTO|Li exhibits a high capacity of~50 μAh cm-2μm-1after 100 cycles at 7 μA cm-2.Tape casting is a simple but effective method to prepare SSE films for mass production.Jiang et al.[139]fabricated the Li0.34La0.56TiO3electrolyte film with a thickness of 25 μm by a scalable tape-casting method.The dense surface morphology and closely connected grain boundary lead to the high ionic conductivity of 2.0×10-5S cm-1.The all-solid-state battery of LiFePO4|LLTO|Li delivers an initial discharge capacity of 145 mAh g-1and a highcapacity retention of 86.2% after 50 cycles at 0.1C.
3.3.2.Improving Crystallinity and Morphology
Furusawa et al.[140]prepared the dense and uniform LixLa(2-x)/3TiO3(LLTO)thin film by PLD.The ionic conductivity of the LLTO film(4.43×10-4S cm-1)is an order of magnitude higher than that of its polycrystalline precursor,which is mainly due to the grain boundary-free and disordered structure.The activation energy of LLTO film at the temperatures of 300-475 K is 0.35 eV.After heated at high temperatures(>1350 °C),the film starts to crystallize and the ionic conductivity is affected.The annealing effects become remarkable with the increased temperature,but the ionic conductivity may not increase at some points,which could be due to the aging-induced defects or onset of crystallization.Xiong et al.[141]sputtered the Li0.31La0.41TiO3film on ITO/glass substrates.Though the film is annealed at temperature up to 300°C,the annealed film is still amorphous.However,the annealed film presents a dense,smooth,and uniform morphology,leading to the high ionic conductivity of 5.25×10-5S cm-1.Although it is believed that the ionic conductivity of SSE films can be improved by annealing,[141-143]highly ion-conductive SSE films could still be obtained in the dense and homogeneous films without a high degree of crystallinity.
The LLTO films can be fabricated by various methods,but they also face the similar problem of valence change as LATP films.The side reactions could be retarded by surface coating.Meanwhile,reducing the film cracks or grain boundaries is also effective in elevating the ionic conductivity of LLTO films.As a result,the multilayer and dense SSE film could be an ideal candidate for TFLBs.
3.4.Garnet Electrolyte
The molecular formula of traditional garnet structure can be written as A3B2(XO4)3(A=Ca,Mg,Y,La,etc.;B=Al,Fe,Ga,Ge,Mn,Ni,V,etc.;X=Si,Ge,Al,etc.),which belongs to the face-centered cubic Ia3¯d space group,and the A,B,and X ions are 8,6,and 4 coordinated cations,respectively.Thangadurai et al.[144]have demonstrated that the Li5La3M2O12(M=Ta,Nb)garnet has high ionic conductivity and a wide electrochemical window.Murugan et al.[145]found that replacing Ta with Zr could get the cubic Li7La3Zr2O12(LLZO)with high ionic conductivity of>10-4S cm-1.Afterward,the LLZO SSEs composed of LaO8dodecahedron and ZrO6octahedron are extensively studied.The LLZO SSEs have two crystal structures of tetragonal and cubic phases.The cubic phase can be easily transformed into the tetragonal phase,but the ionic conductivity of the cubic phase is higher than that of the tetragonal phase.The Li arrangement in the four directions of the tetragonal phase is relatively ordered leading to the sluggish Li-ion diffusion kinetic.
3.4.1.Different Fabrication Process
Rawlence et al.[146]prepared the cubic phase of Li7-3xGaxLa3Zr2O12thin film with the high ionic conductivity of 1.6×10-5S cm-1by multilayer co-sputtering and post-annealing(Figure 7c),which allows the Li compensation and the comparison of different phase stabilizing elements.The results indicate that the cubic phase starts to crystallize at~500 °C.Pfenninger et al.[147]reported a low-temperature approach for making the desired cubic LLZO film,and the highest ionic conductivity is~2.9× 10-5S cm-1.This film is achieved by combining multilayer LLZO and Li3N films using the PLD process followed by post-annealing in which the Li3N reservoirs decompose and lithiate the garnet phase.Sastre et al.[148]used the co-sputtering method(Figure 7d)to fabricate a 400 nm Al-substituted LLZO thin film with the ionic conductivity of 1.9×10-5S cm-1.By sputtering the Li2O and LLZO targets simultaneously and annealing at 700°C in oxygen,the adjusted Li amount induces the formation of cubic-phased Gasubstituted submicron LLZO thin film with high ionic conductivity of 1.9×10-4S cm-1.[149]By sputtering ultrathin layers of LLZO,Li2CO3,and Ga2O3sequentially on polished MgO(100)substrates in Ar atmosphere,Zhu et al.[150]obtained a multilayer LLZO thin film with cubic phase and amorphous domains between the crystalline grains,achieving the high ionic conductivity of 6.36×10-4S cm-1.The amorphous domains provide additional Li+vacant sites around grain boundary and lower the energy barriers for Li+transport across grain boundary via the released space charge effects.
3.4.2.Improving Interfacial Contact
Despite LLZO film has high ionic conductivity and a wide electrochemical stability window,it is crucial to deal with the garnet/electrode interface instability.The poor air stability of LLZO renders it sensitive to humidity and CO2,forming a Li2CO3layer which remarkably increases the interfacial impedance.By surface modification,the interfacial impedance can be greatly decreased on the anode side.[151-153]On the other side,the reactions between cathode and electrolyte are always inevitable.Zarabian et al.[154]studied the chemical reactions of LLZO/LCO interfaces by XPS and EIS tests.The results show that the reaction products containing Co with a variety of oxidation states are formed at the interfaces even at a low temperature of 400°C,demonstrating the need to add a protective layer between cathode and electrolyte.Uhlenbruck et al.[155]found that a LaCoO3layer is formed between LiCoO2and Li6.6La3Zr1.6Ta0.4O12at 700°C.
In order to obtain stable interfaces,ensuring enough activation energy for crystallization and creating a tight bonding are necessary.Sastre et al.[156]designed a simple all-thin- film architecture to check the LLZO/LCO interface.By introducing an in situ-lithiated Nb2O5interlayer by ALD,a low charge transfer resistance of 53.5 Ω cm2is obtained at the solid-solid interface.Delluva et al.[157]studied the influence of polarization and temperature on interfacial stability between LiMn2O4and Li7La3Zr2O12.The Mn-rich region is detected throughout the interface,which becomes wider after cycling at higher temperatures,indicating that the metal ions migrate from the cathode to LLZO.Lobe et al.[158]showed that the Li5La3Ta2O12electrolyte has good thermal stability with the spinel LiCoMnO4cathode at~600 °C.The garnet phase can be crystallized at 400°C,but the ionic conductivity is too low for practical application.
The LLZO films have been prepared by sputtering,[146,159]PLD,[147,160]ALD,[161]and CVD[162]methods,and the ionic conductivity is close to that of bulk LLZO powder.So far,the highest ionic conductivity of thin- film LLZO is 6.36×10-4S cm-1,[150]which is two orders of magnitude higher than that of the most advanced LiPON.Upon the fabrication,a deposition temperature of 700°C is required to obtain a well-crystallized LLZO thin film with a favorable ionic conductivity,and the high temperature also leads to additional by-products.Therefore,how to reduce the processing temperature is a key point to advance the application of LLZO thin film for TFLBs.
3.5.Sulfide SSEs
Sulfide SSEs hold the dominant superiority in ionic conductivity among various SSEs.Sulfide SSEs are derived from oxide SSEs by replacing the oxygen with sulfur,including Li-P-S-based glass ceramics,Li6PS5X(X=Cl,Br,or I)argyrodites,thio-LISICONs,and Li11-xM2-xP1+xS12(M=Ge,Sn,and Si)compounds,etc.[163-165]Compared with oxides,sulfides exhibit lower electronegativity and weaker binding strength to Li+,providing more free-moving Li+.Meanwhile,the larger radius of S2-enables the wider ionic migration channels and facilitates fast Li-ion diffusion.[166]Therefore,sulfide SSEs have reached the ionic conductivities of>10-2S cm-1,some of which even compass the organic liquid electrolytes(Table 4).Consequently,the utilization of sulfide SSEs in TFLBs could greatly improve ionic transport,but the interfacial instability still needs to be addressed.[167]
3.5.1.Interfacial Instability Mechanism
The poor interfacial stability between sulfide SSEs and high-voltage cathodes(such as LiCoO2and LiMn2O4)remains the most challenging problem.In early 2012,Ogawa et al.pointed out the large interface resistance is formed between the commonly used LiCoO2cathode and Li2S-P2S5SSE in TFLBs,which would induce negative effects on cycling performance.It is ascribed to the formation of Co and S containing interfaces(Figure 8a-d)and the diffusion of Co,P,and S into electrolyte and cathode.[168,169]The interface layer with low ionic conductivity leads to the high interface resistance between LiCoO2cathode and sulfide SSEs.Moreover,the oxide anions in the cathode materials have a stronger electrostatic attraction to Li-ion than the sulfur anions due to the hard-soft acid-base theory,inducing a large difference in Li chemical potential between electrode and electrolyte.Therefore,the Li ions immigrate from the sulfide SSEs to the cathode,and a specific-charge layer(SCL)is formed at the interface(Figure 7e-f)greatly increasing the interfacial resistance.[170]

Figure 8.a)Cross-sectional high-angle annular dark- field(HAADF)TEM images of interface between LiCoO2electrode and Li2S-P2S5solid electrolyte.b)Magnified TEM image of area described by square in a).Observations of LiCoO2/Li2S-P2S5interface after initial charging to 3.6 V vs Li-In.c)Cross-sectional HAADF-STEM image and d)corresponding elemental mapping for Co element near LiCoO2/Li2S-P2S5interface after initial charging.Reproduced with permission.[169]Copyright 2010,American Chemical Society.e)Interfacial atomic structure of LiCoO2/β-Li3PS4contact and f)Calculated interfacial Li-ion equilibrium concentration of LiCoO2/β-Li3PS4interface.Reproduced with permission.[170]Copyright 2014,American Chemical Society.
3.5.2.Restraining Interfacial Reactions
Surface coatings including LiSiO3,[171]LiAlO2,[172]LiNbO3,[173]Li3PO4,[92]and Al2O3[174]could suppress the side reactions and the SCL formation between cathode and sulfide SSEs.Normally,these oxides mostly have good chemical compatibility and a wide electrochemical window with both cathode and electrolyte,which can work as buffer layers to physically isolate cathode and electrolyte.Ogawa et al.proposed that a 10 nm LiNbO3layer forbids the mutual diffusion because of the strong bonding between Nb and P,and thus,the resistance of TFLBs is decreased by two orders of magnitudes.Tatsumisago et al.inserted a Li3PO4thin film between 5V LiNi0.5Mn1.5O4cathode and Li2S-P2S5SSE by PLD.[92]Since the Li-ion diffusion is charge-balanced by the formed SCL,the Li3PO4thin film exhibits electronic insulation but a similar potential with the cathode,restraining the undesirable Li-ion diffusion.The total resistance of batteries is reduced from 15000 Ω to 350 Ω,showing application prospects in highvoltage TFLBs.
Sulfide SSEs also suffer from severe side reactions with Li metal.For instance, the Li10GeP2S12with high ionic conductivity of 1.2×10-2S cm-1transforms to the electron-conductive Li15Ge4,insulating Li3P and Li2S after contacting Li metal.[175,176]One solution is to use the Li-alloy anodes,including Li-Si,Li-Sn,and Li-In alloys.The alloy anodes have less reactivity compared with Li metal,which can improve the interfacial stability and suppress the Li dendrite growth.[173,177]
Furthermore,sulfide SSEs should be synthesized in the inert atmosphere,and otherwise,the humid air could spontaneously react with them to generate H2S gas and decrease the ionic conductivity.[178,179]Adding MxOy(Fe2O3,ZnO,and Bi2O3)nanoparticles into the sulfide SSEs could inhibit the H2S release because MxOycan absorb the H2S in the hydrolysis process.Among these oxides,it is reported that the utilization of Bi2O3provides a more negative Gibbs energy change for clearing up H2S in SSEs.However,with the increasing content of MxOy,the ionic conductivity of SSEs is reduced due to the blocking effects of the oxide filler.[180]
The sulfide SSEs fabricated by solid-state synthesis or PVD methods still face the interfacial issues which impede their application in TFLBs.The bulk-doping and surface-coating methods for the bulk targets or precursors are still under research to realize the combination of various electrodes and sulfide SSEs,and the sulfide SSEs-based TFLBs[173,181]could outperform the oxide-based TFLBs due to the intrinsic higher ionic conductivity.
4.Anode Materials
Recently,some researchers start to highlight the importance of Li metal anode in all-solid-state batteries.[182-184]The possible configurations of TFLBs include anode-free batteries,lithium metal batteries(LMBs),and lithium-ion batteries(LIBs).Batas et al.reported the Cu|LiPON|LiCoO2anode-free TFLBs with a volumetric capacity of 62.5 μAh cm-2μm-1and a capacity retention of 80% after 1000 cycles.[185]The uneven local current of anode-free batteries causes irregular Li dendrite growth,leading to mechanical damage and capacity degradation of TFLBs.The widely reported anodes based on alloying and conversion reactions are listed in Table 5.Among them,Li and Si anodes are regarded as promising anode materials for TFLBs due to the high theoretical capacity,and Li4Ti5O12(LTO)outperforms owing to the negligible volume expansion and stable cycling performance.

Table 5.Electrochemical performance of reported thin- film anode materials
4.1.Li Metal Anode
The high-capacity(3861 mAh g-1)and low working potential(-3.04 V vs standard hydrogen electrode)of Li metal endow it as the ultimate anode for LIBs.[186]However,the high chemical activity of Li metal leads to dendrite growth,interface instability,and package restriction.Low-content impurity can generate high local activity and inhomogeneous current, accelerating the Li dendrite growth.[187]The TFLBs fabricated by vacuum deposition are encapsulated in an inert atmosphere and the contamination from the air could be inhibited.As a result,Li metal is the most widely used anode for TFLBs so far.
4.1.1.Main Challenges
The improved bulk modulus and increased interface energy of Li metal anode can impede the Li dendrite growth.[188]The deformation mechanism of Li metal obeys the power-law creep,[189]and the yield strength against the plastic deformation greatly affects the mechanical stability.[190]However,the DFT calculation results inform that soft Li metal can tolerate plastic deformation.[191]The local mechanical response of Li metal depends on the viscous flow out of the crack and the stress development at the crack tip(Figure 9a,b),[189]which simultaneously determine the Li dendrite growth degree.

Figure 9.a)Schematics of stress accumulated at Li tip as a function of current density and b)viscous flow with a boundary layer away from Li tip.Reproduced with permission.[189]Copyright 2019,The Electrochemical Society.c)Electrochemical window of solid-state electrolytes with Li metal.Different oxidation potential to fully delithiated electrolytes is marked by a dashed line.Reproduced with permission.[116]Copyright 2015,American Chemical Society.d)Schematics of interfacial contact for bulk-type and film-type batteries observed at different length scales.Reproduced with permission.[194]Copyright 2017,The Electrochemical Society.e)Reactive and mixed conducting interphase(MCI).f)Reactive and metastable solid-electrolyte interphase(SEI).196Copyright 2015,Elsevier.
The low chemical potential of Li metal(Figure 9c)leads to the formation of a high-impedance interlayer when contacted with most SSEs.[116]Based on the failure model of the Na β-alumina system and Griffith’s theory,the critical current density depends on the aspect ratio of surface defects,wettability to alkali metal,and mechanical properties.[192]The interface in the PVD-fabricated TFLBs cannot prevent the cracking and delamination,[193]leading to the contact loss of active materials(Figure 9d)and capacity degradation of TFLBs.[194]The Li lattice defects and voids at the interface are the main locations for the redistribution of Li wrinkling,generating residual stress[195]and unstable interfaces(Figure 9e-f).[196]Introducing metal/metalloid and coating interfacial layers are important strategies to improve the thermodynamic stability of Li metal in TFLBs.
4.1.2.Suppressing Dendrite Growth
The electrochemical potential of Li alloys(LiC6,Li22Si5,Li9Al4,etc.)is higher than that of pure Li metal,inducing more stable interface with SSEs.Meanwhile,morphological integrity,mechanical strength,and lithiophilic wettability can be improved simultaneously.For example,an interface layer of 20 nm Si is already enough to weaken the reduction of Li anode in TFLBs,[173]and the sputtered metallic Bi can further decrease the interfacial resistance.[197]The Li-Si and Li-Bi alloys with high Li-ion diffusion coefficients can lower the local current concentration and facilitate the fast Li-ion transport across the interface.
The electron-isolative films(Li3N,LiPON,ZnO,SnO2)can improve the thermodynamic stability of Li metal.For example,the Li3N-coated LLZO has good wettability to Li metal,and the full cell of Litype="In-Basic_Latin">|Li3N-LLZO|LiFePO4delivers a stable capacity over 300 cycles at 40°C.[198]The mixed ion/electronic conductors(MIEC)that do not electrochemically react with Li metal are ideal coatings.The Li ions diffuse along the MIEC/metal phase boundary by the Coble creep mechanism,which can release stress,maintain electronic/ionic contacts,and eliminate surface defects.[199]The MIEC layer can be in situ formed on the surface of garnet SSEs by the deposition of CuN and the following lithiation,in which the Cu nanoparticles are embedded in the Li3N network.The MIEC layer homogenizes the electric field and weakens the electronic attack,enabling the LiCoO2/LLZTO/MIEC/Li cells to show a high capacity retention of 81.1% after 300 cycles under 0.2 C.[200]
4.2.Si-Based Anode
Si-based materials have attracted great attention due to the high capacity and good compatibility with on-chip devices.The active materials of Si thin films are limited,which is advantageous to improve the capacity and cycling performance compared with bulk Si materials.[201]However,the huge volume change and low electronic/ionic conductivity of Si-based thin films still restrict their practical application in TFLBs.
4.2.1.Main Challenges
The lithiation mechanism of Si anode is based on the alloying reaction,where the Li-Si alloy is initially formed at~0.4 V(vs Li/Li+).The Li1.7Si and Li3.25Si alloys are generated at~0.3 and 0.2 V,respectively.Finally,the highly lithiated phases(LixSi,x=3.75,4.2,and 4.4)are produced when the voltage is lower than 0.1 V.[202]The linear relationship between the volume change and Li content of Li-Si alloys is shown in Figure 10a,[203]and the accumulated stress in Si film changes at different lithiation states.The compressive stress appears at~0.3 V and increases below 0.3 V,which is accompanied by the formation of Li2Si.The highly lithiated Si alloys have a large volume expansion of>400% which leads to many troublesome phenomena[204]including mechanical degradation,voltage hysteresis,and interfacial diffusion,decreasing the electrochemical performance of Si films upon cycling.
Thin films tend to expand along the direction vertical to the current collector for releasing stress.However,the complicated lithiation mechanism of Si film in liquid electrolyte is proposed as shown in Figure 10b.The characterization results show that the Li ions initially diffuse to the bottom of Si film,indicating an internal rapid diffusion path as the second driving force.The Li ions insert through Si defects and diffuse along the direction parallel to the surface.[205]Similar rapid diffusion channels could exist in TFLBs due to the low diffusion kinetic at the interface,but the complex lithiation process could produce multi-directional stress and cause mechanical degradation of Si films.
Ionic diffusion and atomic migration at the interface of Si/SSE are influenced by the volumetric strain-induced stress.The voltage hysteresis of Si film is associated with the compressive stress and migration energy barrier of Li ions,leading to the Li accumulation and potential drop.[206]The extreme distortion of Si films increases the ionic mobility upon lithiation,further aggravating the migration of Si compounds to liquid or solid electrolytes.[207,208]
4.2.2.Releasing Volume Expansion
Stacking planar thick films in the limited area to increase the space utilization could raise the compressive stress and lower the critical fracture stress of Si films,which hinders the Li-ion insertion and accelerates the mechanical crumbling.[209,210]In comparison,the 3D-structured thin films provide enlarged buffer space,additional active sites,and strong mechanical properties for Si anodes.
Various 3D Si anodes have been reported,including Si-Cu helical arrays,[211]Si nanowires,[212,213]nanocolumnar Cu-Si films.[214]The amorphous Si thin films with high porosity can provide enough space for releasing the volume expansion and mechanical stress.Sakabe et al.[215]prepared the porous thick Si film(~3 μm)by sputtering the Si target in a He atmosphere.When it is assembled with the sulfide SSE and Li-In anode,the full cell delivers a high capacity of 2962 mAh g-1after 100 cycles.Another porous Si film prepared by spay-deposition can form a continuous and compact component after the initial lithiation(Figure 10c),facilitating the high capacity of 2655 mAh g-1at 24C.[216]The TFLBs based on high-aspect-ratio arrays can simultaneously increase the area capacity and energy density.Benefiting from the mechanical stability,the 3D micro-battery using the 10% -lithiated Si array anode delivers a high areal capacity of 1.8 mAh cm-2at 0.66 mA cm-2after 100 cycles.[217]
To maintain structural integrity and endow fast Li-ion diffusion transport,the P element is introduced into Si films to avoid the formation of highly lithiated Li-Si alloys.The P-doped nano-crystalline Si has an amorphous buffer matrix and delivers a high capacity retention of 70% after 250 cycles at 1C.[218]The n-type Si films sputtered on the 3D porous Cu can also alleviate the emerging stress at the interface.[219]The protective effect of LiPON film as an artificial SEI film is demonstrated in Si anodes.[220-222]The Si films capped with the electrospraying polyimide can overcome the mechanical instability and retain a high capacity of 2610 mAh g-1at 100 mA g-1after 50 cycles.[223]We have prepared the LiF-coated Si nanocolumns by the glancing-angle e-beam evaporation,and the 3D LiF-coated Si film exhibits superior rate capability and higher cycling stability than the pristine Si nanocolumns(Figure 10d).[224]
Thick Si films can be fabricated by simply stacking several thin- film layers with different compounds to form multilayer films.[225]High ionic/electric conductivity and low volume change are the most important demands for the inserted films.The multilayer Si-C films including Si-C-Si,[226]C/Si/C/Si/C,[227]and Si/C/Si/C/Si/C/Si[228]are fabricated by alternately depositing Si and C films.The carbon films on the surface and in the interlayers of Si films can suppress phase transition and accelerate electron transport.[226]We have deposited the porous Cu-Si multilayer(Figure 10e)based on the porous Cu nanoparticles which provide buffer space for the huge volume expansion of Si films.With the increase in the layer number of Si/Cu films(1-4),a high and stable capacity is maintained after hundreds of cycles.[229]
4.3.Li4Ti5O12Anode
The theoretical capacity of LTO anode is 175 mAh g-1and the voltage plateau is 1.55 V vs Li/Li+.The Li-insertion reaction of LTO anode in liquid electrolyte is based on the two-phase transformation of disordered spinel phase(Li4Ti5O12)to rock-salt phase(Li7Ti5O12).[230]The poor Li-ion mobility of Li7Ti5O12results from the synergetic occupation of face-sharing 8a and 16c by the intermediate states of Li4+x-Ti5O12(0≤x≤3)[231]and the kinetic pathways of distorted Li polyhedra in metastable intermediates along the two-phase boundaries.[232]
The electrical conductivity and Li-ion diffusion kinetics can be improved when applied in TFLBs.The LTO anode can accommodate the Li solid solution transformation in the LiPON-based TFLBs.[233]It is concluded that the ionic conduction of LTO rather than the charge transfer resistance between LTO and LiPON is the major rate-limiting factor.The activation energy and Li-ion diffusion coefficients of LTO thin films are 40% and 30% higher than those of LTO powders,respectively.[234]The improved Li-ion diffusion kinetics have been demonstrated in the epitaxial LTO thin films prepared by PLD.The epitaxial LTO thin films with different surface morphology are deposited on SrTiO3single-crystal substrates with(111),(110),and(100)orientations,and the reversible intercalation mechanism through the diffusion pathway of lattice planes is confirmed.[235]Hereafter,the similar epitaxial LTO film exhibits superior electrochemical performance than the polycrystalline LTO film.The established phase- field models indicate that the formed Li4Ti5O12/Li7Ti5O12interfaces can accelerate the Li-ion diffusion.[236]However,the epitaxial LTO film deposited on MgO(111)single-crystal substrate shows higher ionic conductivity of 2.5× 10-5S cm-1at 230°C and higher activation energy of 0.79 eV compared to polycrystalline LTO film,which is attributed to the elimination of grain boundaries.[237]
LTO films have been made by various routes including electrostatic spray,[238]electrophoretic deposition,[239]metal-organic chemical vapor deposition,[240]flame spray pyrolysis technique,[241]3D printing,[17]and sol-gel methods,[242,243]providing the potential application of LTO film anode in TFLBs through different pathways.
To solve the key problems of Li,Si,and LTO anodes to be applied in TFLBs,some strategies have been proposed so far.Li-containing composites and interface coatings are effective methods for suppressing Li dendrite growth.Structural design,defect engineering,and stacked multilayer structure are used for releasing the volume expansion of Si anode.Epitaxial growth is a main method to enhance the ionic conductivity of LTO anode.In nowadays,the multiple modifications of composite anode materials could be the key solution to improve the overall performance of TFLBs.
5.State-of-the-Art Thin-Film Lithium Batteries
In modern times,coupling advanced manufacture and integrated techniques,some novel TFLBs have been proposed for approaching their practical application(Table 1).[244]Among them,Dudney et al.[245]demonstrated the stable cycling of high-voltage TFLBs(LiNi0.5Mn1.5O4|LiPON|Li)with a highcapacity retention of 90% over 10000 cycles.The high performance is ascribed to the suppressed dissolution of transition metals,prevented roughening of Li metal,and improved stability of electrolyte.Recently,to shed light on the hindered understanding for this TFLB,Meng et al.[246]employed cryogenic electron microscopy(cryo-EM)to probe the multilayer-mosaic SEI structure including Li2O,Li3N,and Li3PO4as shown in Figure 11a-i.The Li3N compound is the ideal SEI component in TFLBs due to the high ionic conductivity and low electronic conductivity(Table 6).The decomposed dense crystallite within an amorphous matrix is the key component for stabilizing Li metal batteries.
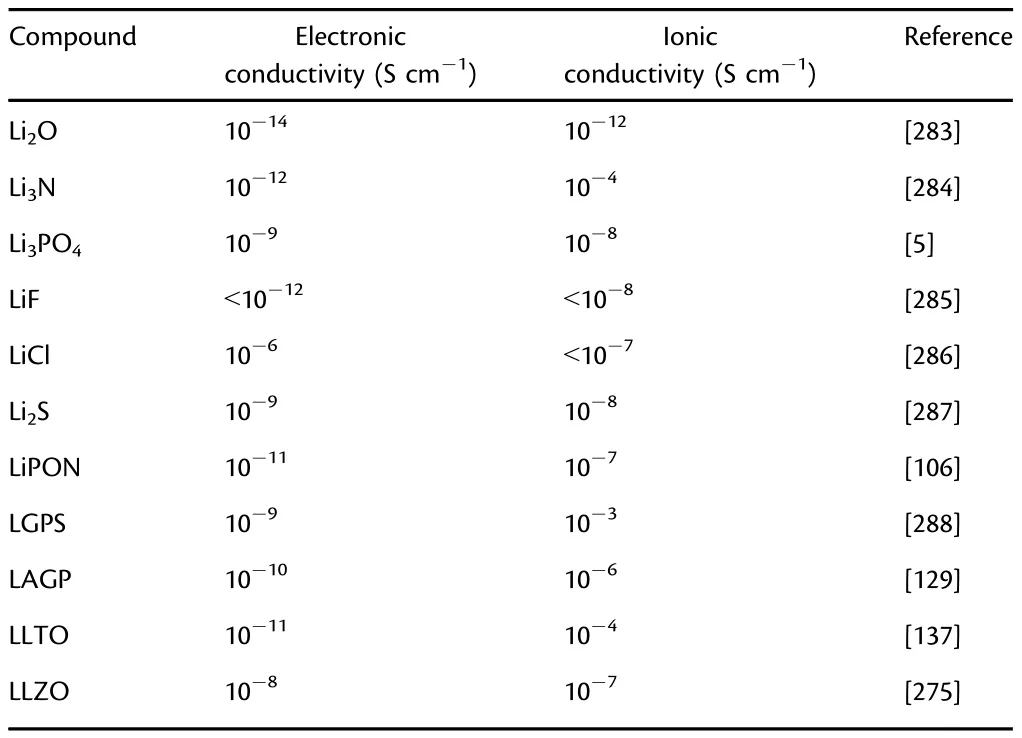
Table 6.Electronic and ionic conductivities of different Li-containing compounds

Figure 11.a)HRTEM image of Li/LiPON interface where four regions 1-4 are highlighted by orange squares to indicate different stages of multilayer structure.b,d,f,and h)FFT patterns corresponding to regions 1-4 as highlighted in a).c,e,g,and i)Nanostructure schematic overlaying HRTEM images that correspond to regions 1-4 as highlighted in a).Reproduced with permission.[246]Copyright 2020,Elsevier.j)HAADF image of nanobattery stack along with Li K-edge concentration mapping of pristine,ex situ,and in situ samples.Reproduced with permission.[247]Copyright 2016,American Chemical Society.k)Development of in operando spatial difference energy spectra during first two charging and discharging cycles with measured battery voltage curves at the left-hand side.Reproduced with permission.[207]Copyright 2018,Wiley-VCH.l)Optical micrograph of Cu current collectors and Li tree structures and m)cross-sectional back-scattered SEM image of LCO|LiPON|Cu|LiPON TFLBs.Reproduced with permission.[248]Copyright 2019,American Chemical Society.n)Galvanostatic cycling of Li1.2TiO0.5S2.1thin films in Li-Ti-O-S|LiPON|Li TFLBs at various current densities and o)cycling volumetric capacity and their Coulombic efficiency.Reproduced with permission.[252]Copyright 2017,American Chemical Society.p)Schematic illustration of the printing-based stepwise fabrication of solid-state bipolar LIB cell on Si photovoltaic(SiPV)module and cross-sectional SEM images of 2-stack bipolar LIB cell.q)Galvanostatic charge/discharge profiles of 1-stack(gray lines)and 2-stack(blue lines)LIB cells.r)open-circuit-voltage profiles of SiPV-LIB device and bare bipolar LIB cell as a function of elapsed time.s)Galvanostatic charge/discharge profiles of bipolar LIB cell in SiPV-LIB device as a function of cycle number at charge/discharge current density of 1.0C/1.0C.Reproduced with permission.[255]Copyright 2017,Royal Society of Chemistry.
5.1.Interfacial Diffusion
Thus far,the interfacial phenomena of TFLBs are the most challenging issues to be addressed.For the cathode interface,Wang et al.[247]conducted in situ TEM to check the interfacial phenomena of TFLBs(LCO|LiPON|Si).It is uncovered that the disordered interfacial layer between LCO and LiPON(Figure 11j)contains highly oxidized Co ion species along with lithium oxide and lithium peroxide species,suggesting the chemical changes could be the main cause for the elevated interfacial impedance of TFLBs.For the anode interface,Notten et al.[207]developed an in operando neutron depth pro filing(NDP)method to investigate the interfacial degradation of TFLBs(LCO|Li3PO4|Si).It is unraveled that the Li immobilization at the Si/Li3PO4interface(Figure 11k)could be the main origin for the capacity loss of Si-based TFLBs.Westover et al.[248]used a new TFLB structure of LCO|LiPON|Cu|LiPON to explore the root cause for the inhibition of Li dendrite growth by LiPON film.The penetration of Li metal along the 2D LiPON|LiPON interface(Figure 11l-m)confirms that the homogeneous and pore-free morphology could be the key solution to suppress Li penetration.Gong et al.[249]investigated the interface/surface effects between the current collector and anode in the TFLBs of LCO|LiPON|Al.The Li-Al-O alloys formed at the anode/collector interface lead to the rapid capacity degradation of TFLBs,which could be restrained by replacing the Al anode with Si anode.
Except for the chemical changes,in practice,if the TFLBs are exposed to repeated external load and mechanical fatigue,the contact loss is highly prone to occur at the current collector and electrode interfaces upon dynamic bending,also greatly affecting the bulk resistance.[250,251]Moreover,the adhesion between the films and substrate is another important issue that limits the durability of TFLBs,as demonstrated by the superior performance of TFLBs fabricated on Pt/Cr/Al2O3compared to those on Pt/Al2O3substrates.[40]
5.2.Advanced Components and Devices
Consequently,some novel electrode materials are proposed and applied in TFLBs to optimize the interface issues of TFLBs.To eliminate the requirements of anneal and control of preferred crystal orientation for cathode,Cras et al.[252]fabricated a Li-Ti-O-S cathode thin film based on the dual cation-and anion-based redox process,delivering a high capacity of 85 μAh cm-2μm-1for hundreds of cycles in the TFLBs of Li-Ti-O-S|LiPON|Li(Figure 11n-o).Tan et al.sputtered a Li-Al-Ti-P-ON electrolyte film[253]on a Li-Co-Ni-Mn-O cathode film with in situ post-annealing.[254]The balance between the surface energy and volume strain energy enables the cathode film to exhibit a stable capacity of 127.2 mAh g-1after 100 cycles in the assembled TFLBs.
Based on the development of durable TFLBs,energy generation devices could be integrated with energy storage systems for minimizing power sources.For example,Um et al.[255]demonstrated a combination of crystalline photovoltaics and printed TFLBs in portable power sources.The stacked TFLBs are composed of LCO cathode,polymer electrolyte,and LTO anode,delivering a stable capacity of~0.5 mAh cm-2for over 100 cycles,opening a facile and scalable route for the development of integrated TFLBs(Figure 11p-s).To be integrated into optoelectronic devices(such as the above solar cells),the transparent TFLBs(LiCoO2|LiPON|Si)with a grid-structured design have been fabricated on glass substrates.For the highest UV-vis transmittances of~60% ,the discharge capacity of the transparent TFLBs is 0.15 mAh within 3-4.2 V at 0.5 C.
6.Summary and Outlook
Pursuing the rapid development of integrated circuits and on-chip systems,high-energy-density TFLBs as the power source devices are urgently required.Up to now,many advanced approaches have been applied to manufacture the relevant electrode and electrolyte films.To increase the reversible capacity and cycling durability,designing 3D structures and developing novel high-performance components are the most effective approaches for TFLBs.In this review,following a brief historical context of TFLBs,the potential cathode,electrolyte,and anode materials are highlighted to fulfill the high-performance demands in the practical application of TFLBs.To address the main challenges of the superior electrode and electrolyte materials when applied in TFLBs,the dominant strategies and recent advances of TFLBs are discussed.
For cathode films,post-annealing or in situ heating could tune the preferred facets and improve the structural instability of LCO films.The voltage fading of LRLO renders it difficult to be applied in conventional LIBs,but solid electrolyte coatings or co-deposition doping methods may mitigate the intractable problems in TFLBs.Since the solid electrolyte film could enhance the structural stability and protect the surface of Ni-rich layered oxide,the cation disorder and intergranular crack issues may be simultaneously alleviated in TFLBs.Similarly,the intrinsic interface contact of LNMO/SSE could inhibit the Mn dissolution and realize the long-term cycling of high-voltage TFLBs.Utilizing the techniques of glancing-angle deposition,nanoparticle assembly,and multilayer deposition could greatly increase the cathode loading and boost the energy density of TFLBs.
For solid electrolyte films,LiPON is the most widely used electrolyte for TFLBs thus far,and various deposition techniques and doping targets are exploited to increase the low ionic conductivity.The crystal oxide electrolytes are just starting to be explored and applied in TFLBs due to the inferior crystallinity of PVD-fabricated films.By changing substrates and surface coatings,the interfacial reactions of LATP and LAGP could be restrained in Li-based TFLBs.The scalable fabrication of LLTO films has been developed to explore the potential mass production of future TFLBs.Despite the various fabrication of LLZO films,the double-edged sword of high stability and poor wettability against Li metal requires surface modification to enable its application in Li-based TFLBs.Sulfide electrolytes have the highest ionic conductivity among SSEs,but the high interfacial activity claims for coating or interlayer to suppress the side reactions in different TFLB systems.Realizing the lowtemperature deposition of highly crystallized films is the bottleneck for the widespread application of novel highly ion-conductive electrolyte materials in TFLBs.
For anode films,Li metal is the ultimate anode for LIB,and current methodologies of alloying,coating,interlayer,etc.,have been used for enhancing the mechanical properties and reducing the interface resistance,which could inhibit its dendrite growth in Li-based TFLBs.The huge volume expansion of the Si anode leads to mechanical degradation,voltage hysteresis,and interface diffusion,which could be buffered by 3D construction,bulk-doping,surface coating,and multilayer structure.The Li-ion mobility of LTO anode could be improved by various epitaxial growth.The development of multiple-compound anodes including complex alloy,pre-lithiated anode,and in situ deposited Li anode are promising directions for future TFLB anodes.
Though long-term cycling TFLBs have been fabricated,the underlined mechanisms of solid-solid contact reactions are still unclear.The interfacial evolutions between the active components of TFLBs before,upon,and after cycling need to be discovered by combining various in situ/ex situ advanced characterization techniques and delicate micro/-nano operations.For the future high-energy-density TFLBs,the cathode films need to be operated at high potential with high and stable capacity.The electrolyte films should be highly ion-conductive,lithiophilic,and compatible with the electrodes.The anode films should be operated at low potential with limited volume change,low interface resistance,and stable capacity.Based on the development of high-safety,high-energydensity,and high-durability TFLBs,the energy storage devices may be combined with the energy conversion systems such as solar cells and fuelcells, or even integrated into a whole system on one chip.
Acknowledgements
J.L.,L.L.,S.S.Q.,and D.Y.D.contributed equally to this work.The authors gratefully acknowledge financial support from the National Key R&D Program of China(Grant No.2016YFA0202602),the National Natural Science Foundation of China(Grant Nos.51931006,51871188,and 51701169),the Natural Science Foundation of Fujian Province of China(No.2019J06003 and 2020J05014),the Fundamental Research Funds for the Central Universities of China(Xiamen University:Nos.20720200080,20720200068,and 20720190007),and the “Double-First Class”Foundation of Materials Intelligent Manufacturing Discipline of Xiamen University.
Conflict of Interest
The authors declare no conflict of interest.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Classifying Electrolyte Solutions by Comparing Charge and Mass Transport
- Two-dimensional Boron Nitride for Electronics and Energy Applications
- Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes
- Recent Development in Defects Engineered Photocatalysts:An Overview of the Experimental and Theoretical Strategies
- 2D/2D Heterostructures:Rational Design for Advanced Batteries and Electrocatalysis
- Density Functional Theory for Electrocatalysis
