Biomass Template Derived Boron/Oxygen Co-Doped Carbon Particles as Advanced Anodes for Potassium-Ion Batteries
Xueyu Lian,Zhongti Sun,Qingqing Mei,Yuyang Yi,Junhua Zhou,Mark H.Rümmeli*,and Jingyu Sun*
Among various anode candidates for potassium-ion batteries,carbonaceous materials have attracted significant attention due to their overwhelming advantages including cost-effectiveness and environmental benignity.However,the inferior specific capacity and the sluggish reaction kinetics hinder the further development in this realm.Herein,we report biomass templated synthesis of boron/oxygen heteroatom co-doped carbon particles(BO-CPs)via direct plasma-enhanced chemical vapor deposition.With the combined advantages of abundant active sites,large accessible surface area,and functional groups,BO-CP anode exhibits high reversible specific capacity(426.5 mAh g-1at 0.1 A g-1)and excellent rate performance(166.5 mAh g-1at 5 A g-1).The K-ion storage mechanism is probed by operando Raman spectroscopy,ex situ X-ray photoelectron spectroscopy/electrochemical impedance spectroscopy,galvanostatic intermittent titration technique measurements,and theoretical simulations.The synergistic effect of boron and oxygen co-doping greatly facilitates the performance of carbon-based anode,wherein boron dopant improves the conductivity of carbon framework and the oxygen dopant affords ample active sites and thus harvests additional specific capacity.This work is anticipated to propel the development of high-performance anode materials for emerging energy storage devices.
Keywords
anode,B/O co-doping,carbon,high capacity,potassium-ion batteries
1.Introduction
Potassium-ion batteries(KIBs)have shown increasingly attractive potential for practical applications in terms of the resource enrichness and cost-effectiveness.[1-3]In comparison with the congener,lithium-ion battery(LIB),one specific merit of KIBs is that K+(3.6˚A)has smaller solvated radius in propylene carbonate electrolytes as compared to Li+(4.8˚A)and lower desolvation energy,which corresponds to higher diffusion kinetics and conductivity of ions.[4,5]In addition,the standard redox potential of K is similar to or even lower than that of Li in nonaqueous electrolyte,consequently guaranteeing advanced energy density.Nevertheless,one of the main challenges for KIBs lies in the much bigger radius of K+(1.38˚A)as compared to Li+(0.76˚A),making it difficult to insert/extract into/from the anodes.[4,6]This would inevitably lead to an irreversible distortion of the electrodes and hence incur the capacity attenuation upon long-term cycling.In this sense,it is a priority to design appropriate anode materials to advance the KIB technology.To date,there has been a plethora of material candidates attempted as anodes for KIBs,including carbonaceous materials,metal alloys,and transition metal oxides/sulfides/selenides/carbides.[2,7]Among these,carbon-based anodes have readily stimulated widespread interests due to its satisfying conductivity and wide availability.[8]Graphite,the commercial anode material for LIB,could also be used as anode for KIB because of their similar cell structure and working mechanism.[9]Despite the fact that graphite has excellent electrochemical activity benefiting from its low and stable voltage plateau,it still suffers from unsatisfactory capacity(with a theoretical value of 279 mAh g-1).Furthermore,inferior cycling stability and poor Coulombic efficiency also hinder the performance of graphite anodes for KIBs.[9,10]
In this sense,designing innovative carbon architectures harnessing heteroatom doping,appropriate surface area,and sufficient active sites are highly desirable for boosting the performance of anodes.[11-15]In particular,the introduction of heteroatom dopants into carbon frameworks has been proven an effective way to modify the crystal structure and intrinsic electronic/ionic states,which is beneficial to enhancing the adsorption of K ions and boosting the electron transport to accelerate reaction kinetics.[16-20]Accordingly,thus-constructed carbon anodes integrate the merits of high capacity,excellent rate capability,and long-life cyclic stability.[21]For instance,boron doping could activate the inert electrons of the carbon skeleton due to the electron-deficient nature of boron atoms,resulting in significant charge transfer from potassium toward substrate.[22-24]Gong et al.initiated the evaluation in the performance of B-doped graphene as an anode material for KIBs throughout theoretical calculations based on density functional theory.[25]The results manifested that B-doped graphene possessed a large specific capacity,a small ion migration barrier,and a moderate potassiation voltage.Meanwhile,oxygen doping could facilitate the contact between electrode and electrolyte,ameliorate interface resistance,and improve ion conductivity.[26,27]In further contexts,dual-doped configurations synergize the merits from both sides of dopants.[28-32]Therefore,boron/oxygen heteroatom co-doping would be expected as an effective solution to boost durable potassium storage.[33]Nevertheless,there has been no report whatsoever on the synthesis of B/O co-doped carbons as anode material targeting high-performance KIBs.
In this contribution,we report controllable preparation of B/O dual-doped carbon particles(BO-CPs)on a biomass template of hydroxyapatite [Ca5(PO4)3(OH)] employing a low-temperature plasma-enhanced chemical vapor deposition(PECVD)route.Thusderived BO-CP,harnessing three-dimensional(3D)carbon framework and heteroatom dual-doped configuration,is tested as an anode material for KIBs.Note that the hydroxyapatite template affords high abundance and low cost,and can be easily removed throughout diluted acid etching,ensuring the scalable production of BO-CPs.[34]Accordingly,our BO-CPs harvesta high reversible capacity(426.5 mAh g-1at 0.1 A g-1)and an excellent rate capability(166 mAh g-1at 5 A g-1).Electroanalytic tools including operando Raman spectroscopy,ex situ X-ray photoelectron spectroscopy(XPS),and galvanostatic intermittent titration technique(GITT)are applied to probe the reversibility and reaction kinetics of BO-CPs with respect to potassium storage.In further contexts,theoretical simulation based upon density functional theory(DFT)calculations is supplementary to unravel the roles of boron and oxygen co-doping.The present study is anticipated to offer insights into the dopant engineering of carbon anodes targeting high-performance KIBs.
2.Results and Discussion
2.1.Material Synthesis and Characterization
Figure 1a shows the schematic illustration of the synthetic process of BO-CPs by a low-temperature PECVD strategy.Commercial Ca5(PO4)3(OH)powders with cost-effectiveness were used as the growth template.Note that ball-milling was applied to decline the particle size of as-received Ca5(PO4)3(OH)powders for facilitating the CVD synthesis(Figure S1,Supporting Information).Trimethyl borate in its liquid form was employed as the carbon precursor,which can be introduced and decomposed into B-and O-containing carbon fragments by the plasma in the PECVD reaction zone.As for the synthesis,the growth temperature at both 700 °C and 800 °C were selected to obtain products with high stability and reproducibility,namely,700 BO-CPs and 800 BO-CPs.Finally,the removal of Ca5(PO4)3(OH)template can be easily realized by HCl solution.

Figure 1.Preparation and characterization of BO-CPs.a)Schematic illustration of the synthetic process of BO-CPs;b)Hydroxyapatite(HA)powder prior to PECVD reaction(left panel)and synthesized BO-CP powder(right panel)after the template removal;c)HRTEM image of 700 BO-CPs;The inset in(c)is the SAED image of 700 BO-CPs;and d)EDS maps of 700 BO-CPs.
Figure 1b displays the digital photo of bare Ca5(PO4)3(OH)powder and the final 700 BO-CPs,indicative of scalable preparation with good uniformity.Accordingly,scanning electron microscopy(SEM)and transmission electron microscopy(TEM)were conducted to investigate the detailed structure of the materials.Upon etching the template,700 BO-CPs display a laminated morphology(Figure S2a,b,Supporting Information).As the growth temperature increases,the carbon particles become textured(Figure S2c,d,Supporting Information).In addition,SEM images of Super P(SP)particles with sphere shapes are also presented(Figure S2e,f,Supporting Information).As shown in Figure S3a,Supporting Information,low-magnification TEM image confirms the flake-like structure of 700 BO-CPs,which would be of benefit to the transportation of electrons and K ions.High-resolution TEM(HRTEM)observations of 700 BO-CPs manifest no obviously long-range-ordered structure(Figure 1c and Figure S3b-d,Supporting Information),highly suggestive of low crystallinity of the products.This is in good agreement with the result shown in the selected area electron diffraction(SAED)pattern(Figure 1c inset,Figure S3e,Supporting Information).Furthermore,elemental mapping of the product clearly exhibit the uniform distribution of B,O,and C elements,verifying the B and O codoping in BO-CPs(Figure 1d and Figure S3f,Supporting Information).
X-ray diffraction(XRD)measurements were carried out to further determine the composition of as-synthesized materials.All the samples show a broad diffraction peak at ca.23°-26°,in relation to the(002)plane of graphitic carbon,again revealing the low crystallinity(Figure 2a).In detail,700 BO-CPs and 800 BO-CPs correspond to 23.3°and 22.5°,which can be ascribed to the interlayer spacing of 0.382 nm and 0.395 nm according to Bragg equation,respectively.Both values are larger than that of graphite(0.335 nm),implying that the co-doping of B and O would cause the expansion of the interlayer spacing,which is beneficial to the uptake/release of K ions.[35]As shown in Figure 2b,Raman spectra were recorded,which present characteristic D(1371 cm-1)and G(1590 cm-1)band signals of carbon species.It is well established that the D band is related to the vibration of disordered carbon and the G band represents the stretching vibration of in-plane C-C bonding.[22]Herein,both 700 BO-CPs and 800 BO-CPs manifest defective features.

Figure 2.Detailed characterizations of BO-CPs and SP.a)XRD patterns and b)Raman spectra of all prepared samples;c)N2adsorption/desorption isotherms of 700 BO-CPs(inset:Corresponding pore size distribution);High-resolution XPS,d)C 1s,e)O 1s,and f)B 1s profiles of 700 BO-CPs.
N2adsorption/desorption measurements were carried out to gain the surface area information of products.Both 700 BO-CPs and 800 BO-CPs display type IV isotherms(Figure 2c).[36]The Brunauer--Emmett-Teller surface area values were measured to be 497 and 842 m2g-1,respectively.It is apparent that BO-CPs generated at a growth temperature of 800°C afford a higher value than its counterpart,which might consume more electrolyte to form the solid electrolyte interface(SEI) film and lead to the increase of irreversible capacity.As depicted in Figure 2c inset and Figure S4,Supporting Information,700 BO-CPs and 800 BO-CPs possess similar pore size distributions that are dominant by micropores,which would be of benefit to the adsorption of K ions.
To further probe the elemental composition and bonding configuration of BO-CPs,X-ray photoelectron spectroscopy(XPS)analysis was performed.As illustrated in Figure 2d-f,700 BO-CPs mainly consist of B,O,and C elements,which account for 1.03% ,20.16% ,and 78.81% ,respectively.The high-resolution C 1s spectrum can be deconvoluted into four contributions:C-B(283.96 eV),C-C/C=C(284.66 eV),C-O(286.06 eV),and O=C-O(288.78 eV)bonding.[35,37]The presence of C-B and C-O bonding imply that the carbon skeleton has readily been incorporated with B and O elements.As shown in Figure 2e,XPS O 1s spectrum of BO-CPs possesses related signals.Similarly,in the highresolution B 1s spectrum(Figure 2f),four peaks at binding energies of 187.09 eV,190.45 eV,191.95 eV,and 193.95 eV are indexed to B4C,BC2O,BCO2,and B2O3bonding,respectively.[23,33]Upon elevating the pyrolysis temperature from 700 to 800°C,the intensity of B4C strengthens and that of BC3declines.It was demonstrated that BC3bonding plays an important role in modifying the valence band structure of carbon and increasing the density of states near the Fermi level,whereas B4C configuration could give rise to a reduction of conductivity.[38]Note that boosting the synthetic temperature(800 BO-CPs)gives rise to the decline of the O content to 12.35% while slight increase of the B content(1.06% )(Figures S5 and S6,Supporting Information).As for the control sample,SP contains 4.43% of O element with no B doping.In this sense,the presence of oxygen would offer a myriad of active sites for K-ion storage.The B doping would enhance the conductivity of the carbon structure.[33]As such,the synergistic effect of the B and O doping is beneficial to K-ion insertion/extraction and electron transportation.
2.2.Potassium Storage Performance
The electrochemical performances of BO-CPs toward K-ion storage were investigated by assembling half cells with the employment of K metal as the counter electrode.Figure 3a presents the first three cycles of cyclic voltammetry(CV)profiles of 700 BO-CPs in the voltage range of 0.01-3 V at a scan rate of 0.5 mV s-1.The first potassiation cycle contains a large and broad cathodic peak at 0.471 V,a small and sharp cathodic peak at 0.016 V,as well as an anodic peak near 0.381 V.The cathodic peak at 0.471 V can be attributed to the formation of SEI film and the occurrence of side reactions.The sharp cathodic peak is pertaining to the insertion of K ions.Accordingly,the anodic peak at 0.381 V corresponds to the extraction of K ions.Obviously,after the initial cycle,the profiles well overlap,verifying the excellent reversibility of the BO-CP electrode.Note that the CV curves of 800 BO-CPs and SP show comparable characteristics(Figure S7,Supporting Information).The galvanostatic charge/discharge curves of BO-CPs and SP are further displayed(Figure 3b,Figure S8,Supporting Information).The initial discharge and charge capacity at a current density of 0.1 A g-1,respectively,reaches 2821.6 and 635.5 mAh g-1for 700 BO-CPs,affording an initial Coulombic efficiency(ICE)of 22.5% .In comparison,the ICE of 800 BO-CPs and SP was calculated to be 17.5% and 22.6% ,respectively.BO-CP electrode suffers from a large irreversible capacity loss and a low ICE value,which can be attributed to the high surface area of the material with exposed defective sites,causing the electrolyte decomposition,SEI formation,and irreversible entrapment of K ions in carbon.
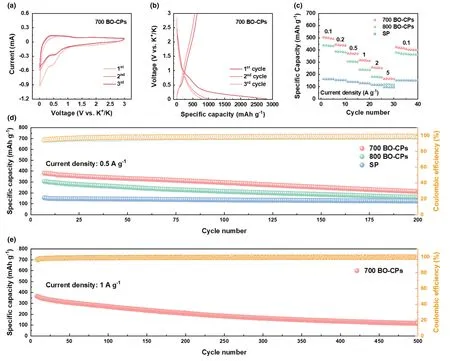
Figure 3.Electrochemical tests of KIB anodes in half-cell configurations.a)CV profiles of 700 BO-CPs in the initial three cycles at a scan rate of 0.5 mV s-1;b)GCD profiles of 700 BO-CPs;c)Rate performances of all tested samples;d)Cycling performances of all tested samples at 0.5 A g-1;and e)Cycling performance of 700 BO-CPs at 1 A g-1.
Figure 3c manifests the rate capabilities of all examined electrodes at diverse current densities,wherein 700 BO-CPs exhibit the best performance.As such,its average capacity value reaches 503.6,441.8,372.5,317.5,256.8,166.9,and 70.8 mAh g-1under the rate of 0.1,0.2,0.5,1,2,5,and 10 A g-1,respectively,showing admirable rate capability even at elevated current densities.Notably,the capacity of such an electrode can be recovered to 426.5 mAh g-1when the current density returns to 0.1 A g-1,demonstrating excellent reversibility(Figure S9,Supporting Information).To evaluate the long-term cycling stability of the materials,the cells were tested at 0.5 and 1 A g-1for various cycles.As depicted in Figure 3d,upon cycling for 200 cycles at 0.5 A g-1,700 BO-CPs still delivers a satisfactory capacity of 215.7 mAh g-1.In addition,it maintains an outstanding reversible capacity of 119.4 mAh g-1after 500 cycles under 1 A g-1(Figure 3e).In contrast,800 BO-CPs and SP electrodes show inferior capacities under both current densities,indicative of poor electrochemical durability.
2.3.Potassium Storage Mechanism and Kinetics Study
To explore the K-ion storage behavior of BO-CPs,operando Raman spectroscopy was employed to observe the evolution of ID/IGratio during the electrochemical process.As shown in Figure 4a,such a ratio continuously increases upon discharge,illustrating the augmented disorder of the carbon layer during the insertion of K ions.At the end of charging process,the ratio returns to 0.928(the initial value:0.903),implying the good reversibility of the electrode material.This serves as a solid evidence for the uptake/release of K ions.Ex situ XPS analysis accords well with the result from operando Raman measurements,fully validating the reversible change of K storage(Figure 4b).In this sense,there is no K signal observed in the pristine electrode.Two featured K 2p peaks locating at 293.2 eV(K 2p3/2)and 296.0 eV(K 2p1/2)show up during potassiation,which is due to the formation of KCxcompound.[39,40]In the subsequent charging step,K ions are reversibly released from the carbon framework,leading to the decline of the K 2p peak intensity.This again verifies the excellent reversibility of BO-CPs for K-ion storage.In further contexts,scanning transmission electron microscopy(STEM)inspection and corresponding elemental mappings of BO-CPs when discharged to 0.01 V indicate the uptake of K ions into carbon upon discharge(Figure S10,Supporting Information).

Figure 4.K-ion storage mechanism and kinetics analysis of BO-CPs.a)Operando Raman spectra of BO-CPs in a discharge/charge cycle and corresponding evolution of ID/IGratio;b)Ex situ XPS K 2p spectra of BO-CPs at representative discharge/charge stages;c)CV profiles at different current densities of the 700 BO-CPs;d)b-value analysis;e)Related capacitive contribution histogram of the 700 BO-CPs at different scan rates;f)Diffusion coefficients of all tested samples calculated from the GITT profiles;and g)Nyquist plots of 700 BO-CPs at different cycles.
To probe the reaction kinetics of BO-CPs toward potassium storage,CV profiles at different scan rates from 0.1 to 2 mV s-1were recorded for all samples.As the scan rate increases,the shapes of all the CV curves are well maintained.In comparison with SP,in the potential range of 1.25-3 V,the quasi-rectangular CV shape of BO-CPs is indicative of double-layer capacitive behavior(Figure 4c).This can be ascribed to the facile capture of K ions via heteroatom doping.In the potential range of 0.01-1.25 V,the obvious redox peaks correspond to the insertion/extraction of K ions(Figures S11 and S12,Supporting Information).The relationship between the current(i)and the scan rate(ν)follows a power-law:

where a and b are the adjustable values.The b-value can be calculated from the slope of log(ν)-log(i)plot.b=0.5 indicates the diffusion-controlled process,while b=1.0 implies a surface-induced capacitive process.[41]As shown in Figure 4d,the b-values for cathodic and anodic peaks are between 0.5 and 1.0(0.911,0.994 for 700 BO-CPs;0.851,0.938 for 800 BO-CPs),suggesting mixed behavior contributed by both diffusive and capacitive process.In this respect,by separating the total current response(i)at a given voltage,the contribution can be quantitatively separated into two parts according to the following equation:i=k1ν+k2ν1/2,where k1ν and k2ν1/2correspond to capacitive-and diffusion-controlled contribution,respectively.The shaded area shows the capacitive contribution at different scan rates at both 0.1 and 1.0 mV s-1(Figure S13,Supporting Information).Throughout simple calculation,the capacitive-dominated contribution at various scan rates from 0.1 to 2 mV s-1can be plotted(Figure 4e).With the increase of the scan rate,the capacitance contribution of BO-CPs prepared at 700°C shifts from 65.5% to 96.8% .At each scan rate,such a contribution is higher than that of 800-CPs,showing boosted reaction kinetics.In further contexts,galvanostatic intermittent titration technique(GITT)was used to analyze the diffusion coefficient(Dk)of K ions in BO-CPs and SP electrodes(Figure S14,Supporting Information).[42]The value of Dkcan be calculated with the aid of Fick’s second law based upon the following equation:

where D50is the average particle size,τ is the duration of the current impulse,ΔEsis the quasi-thermodynamic equilibrium potential difference before and after the current pulse,and ΔEtrepresents the current pulse of the potential difference.It is obvious that BO-CPs harvests a higher Dkas compared to SP(Figure 4f),implying better reaction kinetics due to synergistic effect of B and O dual-doping.The electrochemical impedance spectroscopy(EIS)measurement was carried out from 1 to 200 cycles to further explain the kinetic changes in the K-ion storage.The initial EIS profiles of all samples are shown in Figure S15,Supporting Information.The semicircle obtained in the medium-high frequency range is mainly composed of charge transfer resistance(Rct)and SEI resistance(Rf).[43]The straight line at low frequency reflects the ion diffusion inside the electrode.Obviously,the Rctof 700 BO-CPs is smaller than that of 800 BO-CPs,suggesting superior charge transport kinetics.As shown in Figure 4g,both Rctand Rfof 700 BO-CPs increase at the beginning and then decrease upon further cycles,which can be ascribed to the activation and stabilization of the electrode along with gradual electrolyte penetration.Taken together,the excellent rate performance and outstanding capacity of BO-CPs in terms of potassium storage are attributed to the following aspects:1)The laminated structure with large surface area,ample defects,and B/O co-doped active sites is conducive to the adsorption of K ions;2)The synergistic effect of B/O co-doping boosts the conductivity of the carbon skeleton,increases the surface reaction kinetics,and provides additional capacity;and 3)The B/O co-doping within the carbon network helps enlarge the interlayer spacing and thus contributes to the smooth insertion/extraction of K ions.
2.4.Theoretical Insight into K Storage
To further unravel the roles of B and O co-doped carbon in the potassium storage,DFT simulations were carried out to calculate the K adsorption and the electronic structure of graphitic carbon(G).Different G types with OH-,COOH-,C=O-,BC2O-functional groups,and pristine-G are schematically illustrated(Figure S16,Supporting Information).The optimal adsorption configuration of K atom on these functionalized G are accordingly presented in Figure 5a-e.In this sense,the adsorption energies of K are more negative than pristine-G(-0.60 eV)by-0.64,-1.18,-0.89,and-0.57 eV for OH-,COOH-,C=O-,and BC2O-G,respectively.Moreover,the bond lengths between K and O were 2.81,2.71,and 2.50˚A for OH-,COOH-,and C=O-G,which are also shorter than that of pristine-G(3.03˚A).It is found that the involvement of O-containing functional groups could immensely strengthen the capture of K,especially in the case of implanting COOH-group.Even though the introduction of B into the carbon(BC2O-G)might not contribute further to the increase of K adsorption,it indeed promotes the conductivity in view of the enhancement of density of states around the Fermi level by calculating the band structure(Figure 5f-j).From these theoretical analysis,B-O co-doped carbon could boost the performance of potassium-ion storage,mainly due to enhanced capturing capability of K from O-containing functional groups and increased conductivity by involving B source.
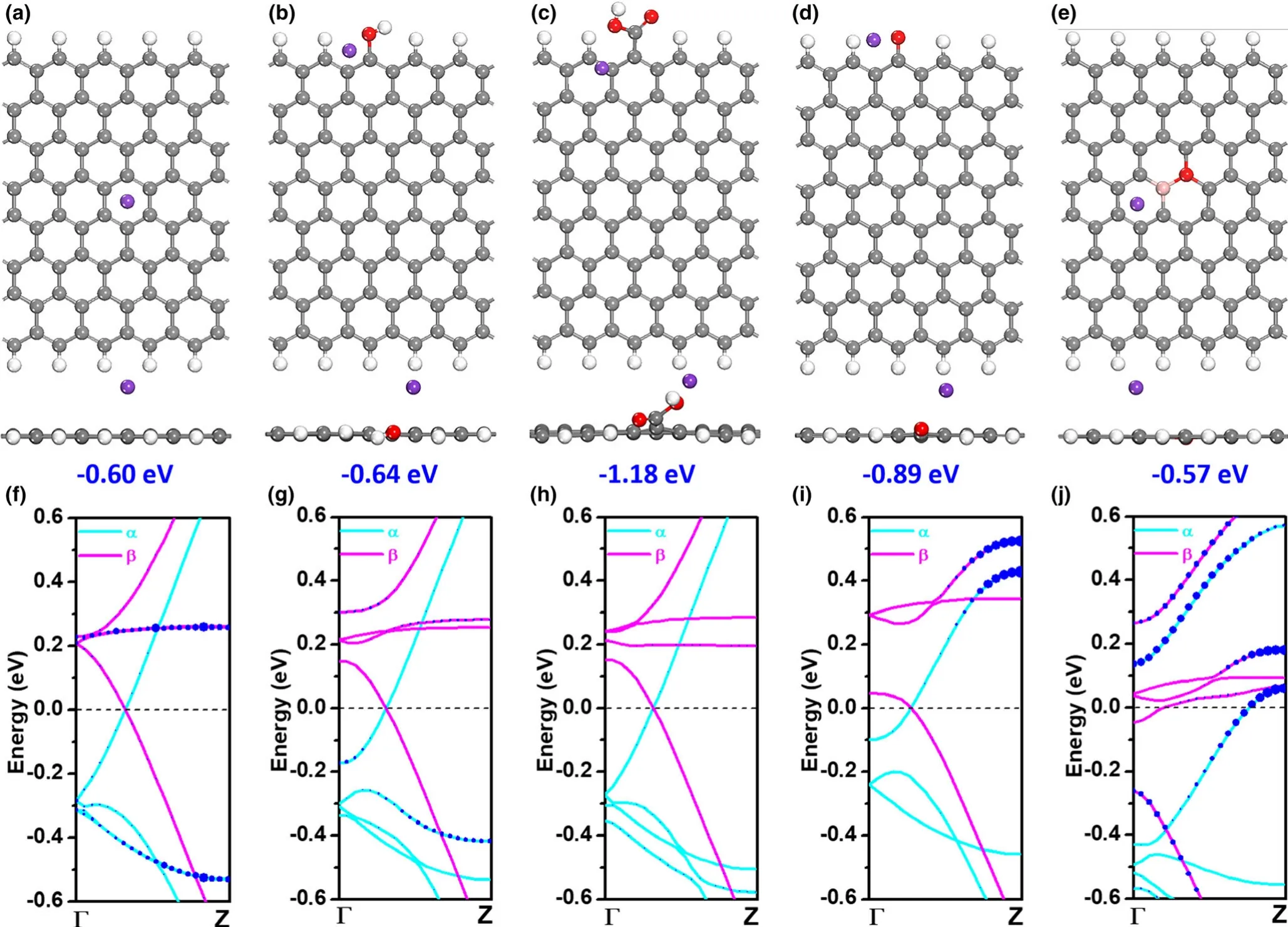
Figure 5.DFT simulations.Optimal adsorption configurations of K atom on the a)Pristine-,b)OH-,c)COOH-,d)C=O-,and e)BC2O-nanoribbon graphene(G);Gray,red,pink,purple,and white ball marks carbon(C),oxygen(O),boron(B),potassium(K),and hydrogen(H)atom,respectively;f-j)Functional group projected band structure graph for f)pristine-,g)OH-,h)COOH-,i)C=O-,and j)BC2O-nanoribbon G;Cyan(α)and pink(β)solid line represents spin-up and spin-down state,respectively;Blue circle indicates functional group:the larger circle size denotes the larger weight in specific energy level and brillouin zone region.
3.Conclusion
In summary,we have reported the synthesis of BO-CPs via a simple,green,and cost-effective PECVD method using hydroxyapatite as growth template and trimethyl borate as the precursor.Upon boron and oxygen dual-doping,the BO-CPs exhibit hierarchical structures with ample defects,expanded interlayer distance,and high surface area.We have investigated the doping influences in which B doping helps improve the conductivity of the carbon network and O doping enhances the adsorption capability of carbon toward potassium ions.When used as KIB anode materials,the BO-CPs display admirable specific capacity(426.5 mAh g-1at 0.1 A g-1),outstanding rate performance(371.4,316.4,252.5 and 166 mAh g-1at 0.5,1,2,and 5 A g-1),and excellent cycling stability(120.0 mAh g-1after 500 cycles at 1 A g-1).This work offers a promising solution for designing advanced carbonaceous materials for next-generation KIBs.
4.Experimental Section
Preparation of BO-CPs:BO-CPs were prepared by a direct PECVD strategy with hydroxyapatite powder served as the sacrificial template.The quartz tube of 4 inch in diameter was used for CVD reaction.Trimethyl borate was used as the carbon source.The hydroxyapatite powder was firstly loaded in a quartz boat,which was subsequently placed at the center of the horizontal quartz tube.The system was pumped to a base pressure of 1 Pa,and Ar gas was then introduced to remove the air.The hydroxyapatite powder was heated to 700°C in 35 min and held at 700°C for 10 min to grow B/O co-doped carbon.Then,the furnace was naturally cooled down to room temperature under Ar.Thus-prepared products were then washed with diluted HCl solution and deionized water for three times to remove the template.Finally,BO-CP products were collected by vacuum filtration.
Characterizations:The morphologies of all samples were observed by SEM(Hitachi,SU-8010).Detailed structures and elemental mappings were detected by TEM(FEI,Titan Themis Cubed G2 300;80-300 kV).The structural information and crystal phases of BO-CPs were probed by XRD(Cu-Kα radiation;D8 Advance).Raman spectroscopy(including operando Raman measurements)was carried out with an excitation wavelength of 532 nm.XPS measurements were performed using an Escalab 250Xi Spectrophotometer.The surface area and pore size distribution were determined by a Micromeritics ASAP 2020 Accelerated Surface Area and Porosimetry System in a N2atmosphere.
Electrochemical measurements:The K-ion storage properties of BO-CPs were investigated based on coin-type cells(2032 type)assembled in an argon- filled glove box([O2]<0.1 ppm,[H2O]<0.1 ppm).K foil was served as the counter electrode.Whatman GF/F glass fiber filter was used as the separator.The electrolyte was composed of 0.8 M KPF6dissolved in 1:1 by volume of EC:DEC with 5% FEC.Galvanostatic charge-discharge profiles were recorded on LAND CT2001A battery testing system between 0.01 and 3.0 V(vs.K+/K).CV curves and EIS profiles were recorded by a CHI660E electrochemistry workstation(Chenhua Instrument).Rate performance and cycling tests were obtained on a LAND CT2001A battery testing system between 0.01 and 3.0 V versus K+/K at room temperature.
Theoretical calculations:All the calculations were performed by spin-polarized density functional theory(DFT),implemented by Vienna Ab-initio Simulation Package (VASP)[44]with projector augmented wave (PAW)[45]pseudopotential.The electronic exchange-correlation interactions were treated by GGA-PBE functional.[46]To consider the van der Waals interaction between K atom and graphene nanoribbon with the width of 5 times graphene unit cell,DFT+D3[47]corrections were involved.The first Brillouin zone integration was selected by 4×1×1 k-point grid for geometrical optimizations and 10×1×1 grid size for static calculations,respectively.The convergence standard for total energy and force per atom was less than respective 10-5eV and-0.02 eV/˚A.The adsorption energy of K atom on the foregoing nanoribbon was computed by the below formula:Eads=Etotal-Enano-EK,where Etotal,Enano,and EKwere the total energy of nanoribbon with and without K adsorption,and chemical potential of bulk K,respectively.
Acknowledgements
X.L,Z.S and Q.M contributed equally to this work.This work was financially supported by the National Natural Science Foundation of China(51702225,51672181, 52071225), the National Key R&D Program of China(2019YFA0708201),the China Post-doctoral Foundation(7131705619),and the Czech Republic from ERDF“Institute of Environmental Technology-Excellent Research”(No.CZ.02.1.01/0.0/0.0/16_019/0000853).M.H.R.thanks the Sino-German Research Institute for support(project:GZ 1400).The authors also acknowledge the support from Suzhou Key Laboratory for Advanced Carbon Materials and Wearable Energy Technologies,Suzhou,China.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Classifying Electrolyte Solutions by Comparing Charge and Mass Transport
- Two-dimensional Boron Nitride for Electronics and Energy Applications
- Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes
- Recent Development in Defects Engineered Photocatalysts:An Overview of the Experimental and Theoretical Strategies
- 2D/2D Heterostructures:Rational Design for Advanced Batteries and Electrocatalysis
- Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries
