Fundamental Understanding and Effect of Anionic Chemistry in Zinc Batteries
Yuxin Gao,Zhexuan Liu,Shan Guo,Xinxin Cao,Guozhao Fang*,Jiang Zhou*,and Shuquan Liang*
With the merit of high capacity,high safety,and low cost,zinc-ion batteries(ZIBs)possess huge application potential in the domain of large-scale energy storage.However,due to the relatively narrow voltage window and large lattice distortion of cationic redox reaction,ZIBs tend to present low energy density,poor kinetics,and unstable cyclic performance.Anion chemistry seems to provide a novel strategy to solve these issues from different aspects,such as enhanced operating voltage,extra capacity contribution,and boosted reaction kinetics.Considering the significance of this theory and the lack of relevant literatures,herein,in-depth comprehension of anionic chemistry and its positive effects on zinc storage performance have been emphasized and summarized.This review aims to present a full scope of anionic chemistry and furnish systematic cognition for rational design of advanced ZIBs with high energy density.Furthermore,insightful analysis and perspectives based on the current research status also have been proposed,which may point out some scientific suggestions and directions for the future research.
Keywords
anionic chemistry,electrode modification,electrolyte regulation,high energy density,zinc-ion batteries
1.Introduction
Zinc-ion batteries(ZIBs),as the most promising candidate for lithiumion batteries(LIBs)in the field of large-scale energy storage,have received widespread attention due to the advantages of environmental friendliness and high safety.[1-6]However,ZIBs still suffer from low energy density,sluggish kinetics and inferior cycling stability owing to the low voltage platform and large lattice distortion based on cationic redox reaction.To address these issues,previous research mainly focused attentions on some conventional strategies:1)promote electrochemical activity through cationic doping;2)enhance the electrical conductivity by conductive surface coating;3)improve the utilization of active material via morphological control;and 4)widen the operating voltage window by lattice structure regulation.These aforesaid strategies did not break the basic principle that the guest cations were intercalated into the crystal structure of electrode along specific diffusion paths,inducing the valence shift of host cations.In sharp contrast,anionic redox reaction,such as the intercalation of anions into the electrode lattice structure and the optimization of electrolyte composition,hold more feasibility for further breakthrough.
Anionic chemistry mainly includes anionic redox,anionic modification of electrode and electrolyte.Different from other strategies,anionic chemistry can endow the battery system with unique features,and simultaneously maintain the pristine state of host material to the greatest extent.[7]For instance,anionic redox reaction occurring at high potential could increase the zinc storage capacity and the corresponding energy density,in which it activates the valence change of O(O2-↔O22-),[8]N(N3-↔N2-),[9]or I(I-↔I2-),[10]and meanwhile keeps the normal reaction of cationic redox.Additionally,the anionic doping also could interact with the nearby cationic lattice framework,and determine the band structure,electronic density,density of state,and coordination environment,thereby affecting the stability of crystal structure,operating voltage,and diffusion kinetics correspondingly.Besides,the anionic modification of electrode could offer extra electrochemically active sites to enhance the ion adsorption and diffusion behavior.Furthermore,the anionic modification of electrolyte could contribute to the ion(de)solvation effect,which helps to strengthen the dynamics of solid-liquid interface reactions.[11-13]
In despite of these remarkable virtues,the further popularization and application of anionic chemistry remain a challenge due to the drawbacks of anion chemistry itself and the lack of systematic theoretical guidance.[14]For instance,anionic framework of electrode crystal lattice is likely to undergo severe irreversible structural transformation and even structural collapse,resulting in the rapid capacity decay.[15-18]In detail,excessive redox of lattice O(O2-↔ O22-→ O2↑)will lead to the formation and release of oxygen,leading to the battery expansion.The residual cations with low valence may lose energy storage capability and even catalyze the electrolyte decomposition.Meanwhile,excessive transfer and reaction of anions in the electrolyte could also cause its uneven distribution and excessive consumption,as well as the formation of by-products,which would accelerate the deterioration of chemical reaction environment and the failure of crystal structure.Therefore,just like the rational design of cationic chemistry-based electrodes for ZIBs,criteria and strategies for the feasibility,reversibility,and stability of anionic chemistry-based electrodes also need to be proposed.
Herein,from the perspectives of electronic structure,chemical reaction thermodynamics and ion diffusion kinetics,the basic principle and understanding of anionic chemistry are proposed and introduced firstly.Referring to the relevant elaborations in other energy storage systems,the fundamental effect and mechanism of the preliminary applications of anionic chemistry in ZIBs are summarized.Furthermore,aiming on the unknown and limit of this strategy,the further reasonable development direction and suggestions are put forward.This review not only provides a promising guideline to design novel anionic chemistrybased electrodes for ZIBs,but also summarizes and explores the basic scientific theories about anionic chemistry.
2.Fundamental Principles and Deep Understanding of Anionic Chemistry
2.1.Anionic Redox Reaction
Generally,the anions with larger ionic radius construct lattice framework by different stacking patterns,in which the cations are in the interstitial sites.During the charge/discharge process,the cation valence mainly depends on the number and location of intercalation sites,being provided by the electronic structure and crystal structure of host materials.After the ligand-hole chemistry was proposed in chalcogenides,[19,20]the slight shortening of O-O interplanar distance was discovered,indicating that the O also participated in the redox reaction at high voltage.[21]The specific operating voltage caused by the valence state is mainly related to the covalency of M-X bond,in which M and X represent d band of metal cations and the p band of anionic elements,respectively.The highest potential represents the higher ionicity.The band structure of M-X consists of low energy band,that is,(M-X)bonding state,and high energy band,that is,(M-X)*anti-bonding state,which is separated by pure non-bonding ligand band.Owing to the high ionicity and the strong binding of anions to electrons in oxides,the density of states(DOS)of the d band of metal cations((M-X)*)is above the p band of anions(Figure 1a).This result means that the metal d band,as the role of redox center,contributes to the electron donation or release.

Figure 1.Fundamental understanding of anionic redox.a)DOS of the d band of metal cations and the p band of anionic elements with polyhedron distortion.b)Effects of anionic chemistry on ion(de)solvation and migration in electrolyte,electrode,and interface between electrolyte and electrode.
However,if two bands are close or overlap,the electrons of the p band can be transferred into the d band,thereby the highly covalent lattice framework could accommodate the redox active anion network.In this framework,the anion ligand O2-can be more electropositive than the central cations.On the other hand,such process will leave holes,and eventually produce and accommodate(O2)n-by changing coordination environment.Therefore,in order to induce more feasible anionic redox,many efforts have been made to improve the covalency of lattice structure,including reducing the electronegativity of anions(replacing O with S,Se,etc.)or increasing the electronegativity of cations(replacing Mn with Fe,Sn,etc.).This could be referred to the reaction mechanism and design principle of electrocatalyst materials for water decomposition.The demanded low decomposition voltage is just opposite to the required stability of batteries.The lattice oxygen can be released into O2at potential higher than 1.23 V vs.RHE(Reversible Hydrogen Electrode)with the activated anionic redox,corresponding to the harmful irreversible electrode decay in batteries.
The band structure analysis provides the anionic redox feasibility from the perspective of reaction thermodynamics,while the consideration of crystal chemistry is also of great importance to evaluate the possibility of ion migration and phase transition kinetics.Layered structures allow the formation of more O-O bonds due to the tunable polyhedrons and lattice parameters,while the spinel,perovskite and Prussian blue analogs with corner-shared octahedrons and rigid 3D frameworks are difficult to change.The lattice distortion lowers the crystal symmetry to allow efficient interactions between(M-X)*and p band of anions,corresponds to the formation of M-(O-O),which opens a gap in the material electronic structure.[12,22,23]In other words,the introduction of crystal defects(such as substitution and disorder)may be a promising method to activate the reversible formation of O-O.For instance,the substitution of M by additional Li in Li-rich phase,such as Li2MnO3or Li2TiO3,[24]results in distortion of the[MO6]coordination environment and lowered symmetry from cubic structure to C2/m or C2/c,which generate the pure non-bonding O 2p states with labile electrons and high energy.
In addition to the electrochemical activation of anions in the electrode lattice,anions in the electrolyte for charge balance can also be introduced into the conversion reaction at the electrode,which delivers a more intuitive reaction mechanism similar to zinc-based flow batteries and Zn-halogen(I/Br)batteries.[25,26]Ideally,in despite of the conventional bulk ion(de)intercalation,the continuous deposition/dissolution of other active phases(such as I-→In-→I2)at the surface of electrode could contribute considerable capacity.However,it should be noted that the lack of flow pump and ion exchange membrane in normal ZIBs means the possible direct contact between anolyte and catholyte,raising the issues of low efficiency and poor reversibility.Taking the Zn-I2flow battery as an example,the Bradditive in electrolyte could stabilize free iodine through anion complexation and unlock the capacity contribution from iodide.[27]The related reaction equations are listed as follows:
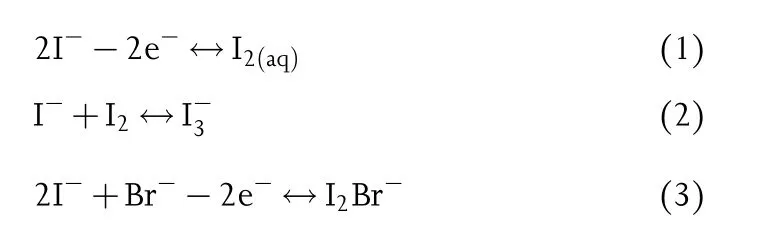
Detailedly,the remaining 1/3 of I-is used to stabilize I2to form I3-without Br-(Equation 2).After the introduction of Br-,the corresponding capacity of zinc-iodine flow battery could be released and increased by at least 1/3(Equation 3),demonstrating that anionic additives hold great potential to upgrade capacity.
2.2.Anionic Modification of Electrode
Based on the above-mentioned anionic lattice framework,the adjustment of lattice framework would also have impact on ion intercalation and ion diffusion.The electrochemical reaction process generally includes two steps,that is,adsorption and desolvation on the electrode surface,as well as migration and diffusion in the bulk phase,which are essentially the competition and change of coordination environment.Therefore,the anionic modification of electrode structure is closely related to the coordination environment in the solid-liquid interface and the bulk lattice framework.
The ions in the electrolyte need to be partially desolvated before the intercalation process,which generally requires high desolvation energy due to the high charge density of Zn2+in aqueous electrolyte environment.[28,29]High(de)solvation energy leads to slow interfacial reaction kinetics and severe polarization,thereby suppressing the electrochemical capacity and cyclic stability.In addition,bulk diffusion barrier of partially desolvated ions would require expanded diffusion channels to accommodate excess solvent molecules,which accelerates the destruction and decay of electrode.[30,31]Hence,the side reaction occurring at the solid-liquid interface conduces to form solid electrolyte interface(SEI) film in the first cycle to buffer the subsequent solvation effect in most nonaqueous secondary batteries.[31]For the batteries without in situ SEI film,the construction of artificial“SEI” film also could effectively maintain the compatibility and stability in solid-liquid interface,[32,33]among which the novel surface modification of anionic groups seems to be more promising.
The ion diffusion process in the electrode lattice can be regarded as the long-range diffusion in the periodic framework.The ion storage from the surface to the interior of active materials is achieved through constant migration among the interstitial sites of stable or metastable coordination polyhedrons.These alternate polyhedrons construct the ion diffusion paths,while the maximum energy required to traverse these paths is the activation energy barrier,which is closely related to the coordination environment around these polyhedrons.In order to evaluate the ion diffusion ability in crystals,the diffusion coefficient(D)is introduced to characterize the ion migration in idealized intercalation-type materials:[34]

where ρ is the geometric factor,which is related to the coordination number(z)and diffusion channel dimension(d)of the interstitial lattice.λ is the migration distance between adjacent interstitial sites.Frequency hopping Γ is described by the following formula:[34]

where v*is the vibration factor,and Eais a barrier to migration.Hence,it is obvious that the anionic framework modification of electrode materials plays an important role in the diffusion kinetics,especially in improving the diffusion dimension(d)and reducing the diffusion barrier(Ea).
The introduction of anion vacancy defects could offer additional diffusion channels,so that it is not difficult to understand the increased diffusion dimension(d).Meanwhile,the diffusion barrier(Ea)is affected by many factors,such as lattice parameters,and element compositions.According to the theory of coordination chemistry,ions in the most suitable coordination state have the lowest thermodynamic energy.The violent change of coordination environment(polyhedron)along diffusion path would lead to the obvious energy difference of each intermediate site,thus generating high energy barrier.Although there have various polyhedrons in different crystal structures,a suitable crystal structure should possess low coordination fluctuation,which is of significance for ion diffusion kinetics.For example,the coordination polyhedron structure of some layered vanadium-based cathodes could be kept stable during the(de)intercalation process of Zn2+,likewise presenting good diffusion kinetic behavior.[35]For the purpose of choosing appropriate crystal structure and anion framework,anion doping may be a feasible strategy.For instance,sulfur holds a larger atomic radius than that of oxygen.When substituting oxygen with sulfur in transition metal oxides,it could effectively enlarge the lattice spacing and reduce the electrostatic repulsion and intercalation energy barrier of Zn2+ions.[36,37]Additionally,the construction of metastable intermediate polyhedrons through reducing the crystallinity and destroying partial anionic framework is also a viable means to buffer the energy fluctuation during ion diffusion process.
2.3.Anionic Regulation of Electrolyte
Different from the anionic redox in electrode,anion chemistry also pays some attentions on the solvation effect in electrolyte.Typically,ZnSO4and Zn(CF3SO3)2are widely used as electrolytes in aqueous ZIBs,in which CF3SO3--based electrolyte shows more stable performance due to its high steric hindrance,high stability,and low desolvation energy.[38,39]Additionally,the presences of SO42-ions in ZIBs tend to induce the formation of hydroxyl sulfate by-products in both side of cathode and anode.[40]As a sharp contrast,ZnCl2and Zn(NO3)2electrolyte always present abnormal zinc storage performance due to their instability and electronegativity.[41]The influences of solvation coordination structure of cations and anions in the electrolyte on the cathode reaction also need to be highlighted.From the perspective of coordination between the ligand anions(L),the reaction is described as follows:[42]

where K is the equilibrium constant,reflecting the stability of the coordinated ion.According to the relationship between standard enthalpy and equilibrium constant

Under certain temperature and pressure conditions,it could be concluded that:

The stability of coordinated ion mainly depends on entropic change and enthalpic change.The decreased energy of metal ions under the influence of the coordination field is attributed to enthalpic factor to affect the stability constant,while for the entropic factor is the change of the molecules number caused by the coordination reaction.
Therefore,the species and concentration of anions in electrolyte could directly determine the coordination ions and Gibbs free energy,which further affect the surface desolvation and ionsolvent molecular cointercalation.Combined with anionic modification of electrode,rapid ion transport,low desolvation energy and stable solid-liquid interface could be theoretically achieved by regulating the change of enthalpy and entropy.In fact,most of the reported literatures pay more attention on the effects of various metal cations and organic additives instead of anions.Therefore,the relevant research about anionic chemical regulation in ZIBs is still in infancy and need to be excavated.[43-45]
3.Positive Effects and Functional Mechanism of Anionic Chemistry
Anionic chemistry has gradually drawn the concerns of researchers due to its guiding significances for the exploitation of high-performance batteries.Herein,positive effects of anionic chemistry on the ZIBs have been summarized and highlighted in Figure 2,which may provide scientific references and guidance for its further research and future applications.

Figure 2.Positive effects of anionic chemistry on ZIBs.a)Elevated voltage platform of Zn/VOPO4batteries.(Reprinted with permission from Wan et al.[46]Copyright 2019 WILEY-VCH.)b)Increased voltage platform and specific capacity of dual-halogen redox couples.(Reprinted with permission from Liu et al.[47]Copyright 2020 Wiley-VCH GmbH.)c)Increased specific capacity by the introduction of oxygen vacancies and surface phosphate ions.(Reprinted with permission from Zeng et al.[48]Copyright 2018 WILEY-VCH Verlag GmbH&Co.KGaA,Weinheim.)d)Ion diffusion paths broaden by oxygen vacancies of Ocu-Mn2O3.(Reprinted with permission from Liu et al.[49]Copyright American Chemical Society.)e)Ion mobility enhanced by anionic substitution.(Reprinted with permission from Wu et al.[50]Copyright 2014 The Royal Society of Chemistry.)f)Fast migration kinetics of Li-S cells by the synergistic effect of cathode design,Se doping and lead electrolyte(Reprinted with permission from Zhao et al.[51]Copyright 2020 WILEY-VCH.)g)Electrochemical stability of both Zn and Li metal batteries endowed by using MXene@Zn paper as current collector.(Reprinted with permission from Tian et al.[52]Copyright 2019 American Chemical Society.)h)Cycling stability of organic electrode.(Reprinted with permission from Wan et al.[53]Copyright 2018 WILEY-VCH Verlag GmbH&Co.KGaA,Weinheim.)i)Mechanical stability enhanced by the insertion of nitrate ion in graphene layers.(Reprinted with permission from Yan et al.[54]Copyright 2018 Macmillan Publishers.)
3.1.Increasement of Energy Density
High energy density could be achieved by the simultaneous cationic and anionic redox reactions of electrode,which mainly depends on whether the non-bonding O 2p band is formed.For example,Li1/3M2/3O2slab in Li-rich layered Li2MO3could cause the distortion of[MO6]polyhedron,in which Li and O atomic orbitals do not overlap effectively and form a non-bonded orbital.[55]As shown in Figure 1a,this non-bonded orbital partially overlaps with the lower-Hubbard bands,which are formed by partially filled splitting(M-O)*bands.On this basis,electrons could be gained and lost from the non-bonded orbital of O 2p,resulting in the reversible redox reaction of oxygen.
The redox reaction of oxygen involves the repetitive migration of transition metals and the continuous formation and rupture of O-O bands.Therefore,the anionic redox requires relatively flexible and weak O bonding environment,otherwise it won’t happen.Taking Na3V2PO4electrode as an example,the internal P-O bonds are so firm and tight that the redox reaction of oxygen could not occur.[56]Meanwhile,for most vanadium-based metal oxides,such as V2O5,V3O7,the lack of PO43-electron group also lead to a lower operating voltage.Benefitting from the oxygen redox reaction,VOPO4cathode could display excellent zinc storage performances due to the high operating voltage, flexible framework of[VOx]polyhedron,as well as structural stability provided by PO43-.[46]Detailedly,the structural distortion of[VOx]polyhedron causes the partial release of oxygen atoms and generate some unbonded electrons.Based on the above principles(Figure 3a),the corresponding cationic and anionic redox of VOPO4cathode could be describedbelow:[46]

Figure 3.Anionic chemistry improves energy density.a)Diagram of energy vs.density of states and b)Comparison of the second charge/discharge curves of VOPO4/SWCNT cathode.(Reprinted with permission from Wan et al.[46]Copyright 2019 WILEY-VCH.)c)Influences of low electronegativity elements on energy band.(Reprinted with permission from Assat et al.[55]Copyright 2018 Springer Nature.)d)The energy(ΔEu)of bond break and combination with Zn in SeS7,Se2S6and S8rings.(Reprinted with permission from Li et al.[58]Copyright 2021 Wiley-VCH GmbH.)e)Schematic diagram of composition and working principle of zinc-dual-halogen batteries.(Reprinted with permission from Liu et al.[47]Copyright 2020 WILEY-VCH.)f)Schematic illustration of the electrolytic-anion-redox adsorption pseudocapacitance mechanism.(Reprinted with permission from Li et al.[61]Copyright 2020 Elsevier Ltd.)

Besides the additional capacity,the average operating voltage of Zn/VOPO4batteries also has been raised to 1.56 V.The reason is that oxygen redox occurs at a high potential,in which O2-is oxidized to O-at 2.1 V in the charge process(Figure 3b).
On basis of theoretical perspective,the increased operating voltage of anionic redox results from the high overlaps of the d orbital and p orbital in its transition metal ion.(M-O)orbitals have strong ligand,while(M-O)*hold metal-like properties.The energy difference between bonding(M-O)and antibonding(M-O)*is huge.Additionally,introducing non-metallic elements with lower electronegativity than O element,such as N,S,Se,could improve the covalency of the bond and reduce the threshold of anionic redox(Figure 3c).[55]In recent report,the O in V2O5was partially replaced by N to prepare the VNxOyelectrode,in which the high covalency of V-N bond reduces the energy difference,and the anionic redox is triggered.[57]Similarly,as shown in Figure 3d,the energy(ΔEu)of S8+Zn(-1.451 eV)was much smaller than that of SeS7+Zn and Se2S6+Zn.The lower ΔEuvalue represented the higher stability and lower reactivity,indicating the introduction of selenium into elemental sulfur could break the structure stability of S8,and enhance the reaction kinetics,which is beneficial to the energy density of batteries.[58]
Conversely,the strongly electronegative anionic element(F)could increase the operating voltage of cations,forming M-F bonds(M represents transition metal element)to provide larger energy difference and higher voltage.[59]Therefore,the introduction of F is also an effective method in modifying the electrode and improving the energy density,especially for fluorophosphates.[60]
In addition to the intrinsic anionic reaction of electrode,the anions in the electrolyte can also provide additional capacity.For example,the I-anion and even the dual halogen anions(Br/Br-and Cl/Cl-)in electrolyte could contribute capacity according to anionic redox reaction(Figure 3e).[27,47]In this zinc-dual halogen battery system with molten hydrate electrolyte,the graphite electrodes can sequentially accommodate intercalated Br-and Cl-,improving the capacity from 78 to 257 mA h g-1compared with the conventional zinc-bromine batteries.There are also applications in aqueous ZIBs,which provide a new avenue for building high voltage batteries through using dual-ion battery system and “water-in-salt”electrolyte.[46,62]
Recent reports have also highlighted the significant importance of electrode-electrolyte interface,which is not limited to the SEI layer with expanded voltage window.[63]For instance,the electrolytic-anionic-redox adsorption pseudocapacitance mechanism improves the capacity via reversible anions adsorption/desorption(such as Mn3O4and PF6-as shown in Figure 3f),which constructs Mn-F bonds for charge transfer and provides fluoride ions as extra redox centers.[61,64]The crucial factors for this mechanism to achieve high capacitance include:1)suitable anions in electrolyte and 2)elaborate electrode with high specific surface area.Hence,in order to further achieve the high compatibility of the electrode-electrolyte interface,anionic groups such as hydroxide,phosphate and oxygen ions are tried to connect to the dangling bonds on the surface of the crystal grains.This mainly includes carbon materials(such as graphene oxide and carbon paper containing oxygen groups on the surface)and MXene with no or low electrochemical activity,[65-67]endowing them surprising performances in ZIBs.[48]Typically,the oxygen-rich groups were connected on carbon paper by using hybrid acid treatment,which significantly improved electrode hydrophilicity,specific capacity and rate performance.[65]For the electrochemical inert material NiCo2O4,the phosphate ion was also tried to anchored on the electrode surface.[48]Compared with O2-,the phosphate ions have a longer bond length and lower electronegativity,which could reduce the energy barrier of electron transport and enhance reaction kinetics.
In short,it can be noted that the increased energy density is usually based on the activation of additional reactions,which requires the construction of thermodynamic metastable state of electrode bulk or electrode-electrolyte interface.These metastable reaction sites are not only conducive to high specific capacity and energy density,[68-71]but also can reduce the barrier during ion diffusion process.The improvement of reaction kinetics is significantly important to the rate performance and output power density,which will be discussed in the bulk anion defect regulation,mainly including anionic doping and vacancy.
3.2.Improvement of Reaction Kinetics
The improvement of reaction kinetics is attributed to the change of crystal coordination environment(dopants,vacancies)to accelerate ion diffusion kinetics.Considering the coordination change between guest ion and lattice framework during diffusion,the polyhedron fluctuation and distortion would break the symmetry of crystal and cause thermodynamic metastability.For example,though the introduction of F can increase the energy density of polyanion-type cathodes,they still suffer from slow ion diffusion kinetics owing to the strong interaction between host and guest ions,thereby resulting in unsatisfactory rate performances.[60]Typically,anionic vacancies can broaden or provide ion diffusion path,such as O,N,S vacancies.[11,16,72,73]In addition to the lattice distortion,anion substitution and doping also can introduce local electric field to accelerate diffusion kinetics.Two principles should be noticed for selecting suitable anionic species:1)replacing anions with low electronegativity to weaken the strong interaction;2)doping anions with low valence to reduce charge interaction.[74]
Generally,the issue of slow ion diffusion kinetics is caused by the limited diffusion path and strong electrostatic repulsion in the host crystal structure.For manganese-based cathode materials,the[MnO6]framework is relatively stable.However,the disproportionation of Mn3+will directly lead to the dissolution and destruction of crystal structure.Therefore,different cations are generally introduced to create oxygen-defects, such as Li(Li1/3Mn2/3)O2,Nax(Mg1/3Mn2/3)O2,in which the disordered atomic arrangement could promote ion migration and ion diffusion behavior.As presented in Figure 4a,[MnO6]polyhedron walls were broken through the introduction of oxygen vacancies in K0.8Mn8O16,and thus extra paths for H+diffusion were opened.These oxygen vacancies guaranteed fast diffusion behavior and high electrochemical reaction activity.[11]Similarly,as shown in Figure 4b,extracting oxygen anions from ZnMn2O4could effectively reduce electrostatic repulsion.[75]Additionally,introducing heterovalent cations into the crystal lattice also could modulate the charge distribution.As displayed in Figure 4c,inducing oxygen vacancies through highvalence Ti doping led to the formation of a local inplane electric field,and unlocked the[MnO6]octahedral walls,availably enhancing ion diffusion/electron transfer behavior.[76]
As similar to the cationic doping,anionic doping also could generate the crystal structural defects and improve the reaction kinetics.Generally,anionic doping elements include N,O,S,Se,and F.According to the density of state results in Figure 4d,the bandgap of N-MnO2-xwas smallest,indicating that electronic conductivity could be greatly improved by N doping and oxygen vacancies.As a result in Figure 4e,N-MnO2-xcathode also presented significantly pseudocapacitive zinc storage capacity,demonstrating that N-doping could enhance the capacitive adsorption behavior.[77]Additionally,doping oxygen atoms into layered MoS2could enlarge the interlayer spacing,modify the hydrophilicity and thus markedly reduce the intercalation energy barrier of Zn2+,guaranteeing fast Zn2+diffusion kinetics(Figure 4f).[78]Additionally,N-doping into V2O5also contributed to form defective disordered rocksalts through charge-compensating function.Consequently,the disordered rocksalts generated lots of active sites for zinc storage,which were conducive to the diffusion kinetics of Zn2+(Figure 4g).[79]Fluorine element with the highest electronegativity,could affect phase and electronic structure of metal oxide/hydroxide deeply,and further determine the reaction kinetics.[80]The fluorine could bond with metal atoms to form firm metal-F bonds,which could prevent electrode dissolution,improve structural stability and reduce polarization effectively.Hence,the F-doped NiCo-CH cathode exhibited significantly improved robustness and conductivity as flexible batteries.[81]
Although the anionic chemistry in electrode is highlighted its profound influence on the reaction kinetics,the anionic effect in the electrolyte has not been studied in detail until now.The anions in the electrolyte usually form special solvation structure and may participate in the reaction at the electrode interface.Therefore,the properties in Zn-Zn symmetrical half-cell could not be applied to the full-cell directly.The compatibility between electrolyte and cathode should be the prime consideration.For instance,the reaction of Zn-MnO2batteries may involve specific solvation sheath and interface reaction,which shows unsatisfactory performance in other organic and ionic liquid electrolytes.[82,83]Moreover,different anions will play different roles even in the same aqueous electrolyte system and the mechanism is not clear,which will be mentioned in next section.
3.3.Enhancement of Battery Stability
Owing to the charge redistribution effect and high electronic conductivity of MXene material,it could guarantee a uniform electric field distribution and a low Zn nucleation energy barrier as the 3D collector,interlayer,or zinc container.As the thin interlayer,the MXene in situ grown on zinc foil could effectively inhibit the formation of zinc dendrites and the corrosion of zinc metal,thereby improving the electrochemical stability.[84]Additionally,MXene could be decorated with different surface terminations,such as O,N,S,Se,Br and Te,through covalent modification method.[85]As the zinc container,Ti3C2TxMXene@Zn paper was employed as anode for ZIBs,[52]which also could suppress the growth of zinc dendrites(Figure 5a).Therefore,MXenemodified zinc anode could display long cycle stability and low voltage hysteresis with dendrites-free on the surface,ensuring high-capacity retention ratio.

Figure 5.Anionic chemistry enhances electrochemical stability.a)Morphology evolution of 2D planar Zn anode and MXene@Zn anode during the stripping/plating process.(Reprinted with permission from Tian et al.[52]Copyright 2019 American Chemical Society.)b)Schematic illustration of Zn-PANI cells during charge/discharge process.(Reprinted with permission from Wan et al.[53]Copyright 2018 WILEY-VCH.)c)Cycling performances of Zn-V2O5cells using different electrolytes,including ZnSO4,Zn(CH3COO)2,Zn(NO3)2and ZnCl2.(Reprinted with permission from Zhou et al.[87]Copyright 2018 The Royal Society of Chemistry.)d)Cyclic voltammograms of Zn anode in aqueous electrolyte of 1 M Zn(CF3SO3)2and 1 M ZnSO4at the scan rate of 0.5 mV s-1 within-0.2 to 2.0 V.(Reprinted with permission from Zhang et al.[88]Copyright 2016 American Chemical Society.)e)Cyclic stability and coulombic efficiency of Zn-MnO2cells in ZnCl2-H2O and ZnCl2-H2O-DMSO electrolytes with 0.1 M MnCl2to suppress Mn2+dissolution at 8 C.(Reprinted with permission from Cao et al.[89]Copyright 2020 American Chemical Society.)f)A schematic two-step synthesis using graphite g)substrate and nitrate precursors(Reprinted with permission from Yan et al.[54]Copyright 2018 Macmillan Publishers.)
Compared with inorganic electrode,organic electrodes hold minor structure changes during the reaction process,and it is also not affected by the ionic radius and the counterion charge.[86]As shown in Figure 5b,the reaction mechanism of Zn-polyaniline(PANI)batteries mainly depended on the redox of electrolyte and Zn foil to ensure electrical neutrality.[53]In this process,the structure of PANI has little change,endowing it with superior cycling stability.Moreover,this supercapacitor-liked mechanism also offers fast ion diffusion dynamics for Zn-organic batteries.
Different zinc salts in aqueous electrolyte usually possess different solvation or desolvation energy between anions and cations,which have significant influences on the cycling performance of ZIBs.Zinc storage performances of zinc salts with the same concentration and different anions,such as Cl-,NO3-,CH3COO-and SO42-,were presented in Figure 5c.As a result,3 M ZnSO4electrolyte hold the highest specific capacity and best cycling stability based on the commercial V2O5cathode.[87]Similar electrochemical behaviors were also detected in Zn-TiN capacitors,in which SO42-anions took part in a two-step adsorption and intercalation process,boosting the anti-self-discharge ability and stable capacitance performance.[90]Furthermore, the electrochemical differences between ZnSO4and Zn(CF3SO3)2electrolyte based on ZnMn2O4cathode have been deeply explored.In consequence,[Zn(N(SO2CF3))3]-and[Zn(SO3CF3)3]-cluster models in Zn(CF3SO3)2electrolyte have a higher Zn-O Mayer bond order values(Figure 5d),which contributes low solvation effect,stable structure,and high dissolution energy.These merits ensure the stable reversibility and fast deposition/dissolution kinetics.[88,91]In addition to the solvation structure,the anions in the electrolyte also could directly participate in the interface reaction between electrode and electrolyte.In Wang’s report,ZnCl2-H2O-dimethyl sulfoxide(DMSO)electrolyte was applied to Zn-MnO2batteries,in which Cl-tightly combined with DMSO molecule to form SEI on the surface of MnO2cathode to maintain the cycling stability(Figure 5e).[89]Similarly,Zn4SO4(OH)6·nH2O product also could be detected on the electrode surface in ZnSO4electrolyte,indicating that SO42-participated in the process of forming SEI.[82,92]
Different from the Zn-I2or Zn-S batteries,the anion intercalation reaction from electrolyte to bulk electrode generally belongs to anionictype or dual-ion batteries.[93,94]As an instance in Figure 5f,intercalating NO3-into the graphite layer could tighten the structure of NiCeOxHyelectrode,which significantly improved the mechanical stability.[54]If there is no intercalation of NO3-,the electrode material would be deposited on the graphite substrate and generate obvious interface gaps,resulting the failure of mechanical properties.
The anionic chemistry shows great potential in improving the electrochemical and mechanical stability of batteries,which will promote the development of novel flexible batteries and wearable batteries.However,for the novel Zn-based batteries,such as quasi-solid and allsolid electrolyte,the role and mechanism of anions still has not been researched.It is also an urgent need to conduct related research in this field to further improve the energy density as well as cycling performance,and promote the actual applications of ZIBs.
4.Issues Facing Promotion and Optimization Strategies
Despite the above-mentioned advantages and positive effects of anionic chemistry,there are still some barriers hindering the practical applications and further popularization of ZIBs.Firstly,the unstable anionic redox reaction may lead to the irreversible structural changes or structural collapse,bringing about the sharp capacity decay of electrode.For instance,excessive redox reaction of lattice-oxygen will result in the release of oxygen from crystal structure,while the residual cations in the crystal lattice may lose energy storage capability.[95,96]Secondly,anionic redox reactions generally occur at higher potential conditions,which easily causes the decomposition of electrolyte.[46]Thirdly,the stability of anion vacancies and surface modifications in the electrode is inferior,[97]and both of them may gradually disappear during the reaction process.For example,sulfur element may precipitate into the electrolyte and passivate the metal anode for the sulfur-containing anion framework.[98,99]Fourthly,excessive anion transfer and reaction in the electrolyte may cause uneven charge distribution,continuous electrolyte consumption as well as the formation of by-products,which will accelerate the deterioration of chemical reaction environment and the failure of crystal structure.In response to these issues,a series of corresponding optimization strategies are listed as follows:
4.1.Optimization of Electrode Structure
The electrode structure directly affects the reaction stability of anionic chemistry.Although the crystal structure constructed by anions is conducive to ion diffusion,it is not always stable.In this case,the introduction of heterogeneous ion doping could effectively support the main structure,and then enhance the stability of electrochemical reaction.Besides,structure induction and antisite occupation induction also could improve the stability and reversibility of anionic redox reaction.For heteroatomic doping,it is of significance to select appropriate doping elements.The anion redox of oxygen element under high potential is prone to undergo irreversible oxygen loss,while the choice of transition metal cations with low mobility as the electrode material could effectively suppress the issue of oxygen loss.Additionally,organic electrodes with strong structural tolerance,are of great benefit for its application in anion chemistry-based batteries.
On the one hand,the stability of anionic defects in suitable transition metal-based materials may be different.For the fixed[MnO6]polyhedron in most manganese-based materials,any oxygen vacancies would cause its distortion and collapse.However,the geometric structure of[VOx]polyhedron varies,[100]indicating that the stability of electrochemical performance and the integrity of lattice structure can be guaranteed during the reaction.The high tunability of polyhedron,especially in vanadium-based materials,also should meet the requirements of rich active sites,spacious diffusion paths and robust framework,generally exhibiting high energy density and long cycle life.[101-103]Therefore,in order to endow other materials with similar properties,such as manganese-based oxides,the introduction of heteroatoms with bonding may be a promising method.K ions were introduced into[MnO6]polyhedron to stabilize the cathode structure(Figure 4a),inducing the formation of oxygenvacancies easier without structure collapse.[11]For the irreversible oxygen loss at high voltage during oxygen redox process,the key factor is the lack of alkali metals,which leads to unbonded oxygen(fewer than 3 cations coordinating oxygen)and the loss of surface oxygen.Thus,introducing alkali metal elements with lower mobility(replacing Li with Mg)could avoid the generation of unbonded oxygen,thereby inhibiting irreversible oxygen loss.[104]Other effective methods also have been taken to suppress the irreversible oxygen release.For instance,both Fe substitution(Figure 6a),[105]and Ti substitution(Figure 4c),[106]could stabilize the oxygen element,thereby inhibiting the release of oxygen.In addition to the heteroatoms doping in the lattice,the synergistic effect between the multiphase and anionic defects also needs to be carefully concerned.Typically,the carbon coating not only provides conductive network and ion transport path,[102]but also inhibits the agglomeration and dissolution of internal material due to the thermodynamic instability of defects to some extent.[75,107]

Figure 6.Issues and strategies of anionic chemistry.a)Effects of Fe substitution on inhibiting oxygen release.(Reprinted with permission from Zhang et al.[105]Copyright 2019 American Chemical Society.)b)First charge/discharge profiles of Zn-VOPO4batteries in 1 M Zn(Tr)2and 21 M LiTFSI+1 M Zn(Tr)2.(Reprinted with permission from Wan et al.[46]Copyright 2019 WILEY-VCH.)c)Schematic illustration of dual-ion battery assembled by PGA cathode,WSOE45-1 electrolyte and Zn/GFF anode.(Reprinted with permission from Cai et al.[117]Copyright 2021 WILEY-VCH.)d)Design strategy for increasing operating voltage of organic electrode.(Reprinted with permission from Tie et al.[118]Copyright 2020 Wiley-VCH.)e)Design strategy of EMC-based electrolyte with a micro-heterogeneous anion solvation network.(Reprinted with permission from Chen et al.[119]Copyright 2020 WILEY-VCH.)
On the other hand,the circumstance that other non-oxygen anions(N,S,Se,P)as well as these anions in organic or polyanionic electrodes,directly involved in the redox reaction,also should be considered.For instance,metal sulfides,including CoS2,[108,109]FeS2,[110,111]and VS4,[112,113]served as sulfur redox-active cathode,could provide high energy density for secondary batteries.Meanwhile,the sulfide ions in the layered sodium chromium sulfide can be reversibly insertion/desorption without destroying the crystal structure or changing the lattice parameters.Furthermore,it will trigger the redox of sulfur,which corresponds to the vacancy antisite of chromium/sodium.Such an inversion induction method enables anion energy storage to be better carried out in layered intercalation compounds.[114]Another special non-oxygen anion is fluorine,which is usually introduced into polyanionic cathodes and widely reported in ZIBs,LIBs,and sodium-ion batteries(SIBs).[115,116]However,there are still some obstacles hindering the practical applications of metal sulfides and polyanionic cathodes,such as low electrical conductivity,poor cycle stability,and inferior rate capability.Based on the previous research,the decorations of conductive polymers or carbon coatings and the constructions of core-shell structure could effectively solve the above problems.[51]
Additionally,compared with inorganic electrode,organic electrode possesses the advantages of wide operating voltage window,stable structure,and molecular-level controllability.These merits endow organic electrode with minor structure changes and long cycle stability during reaction process.Among the organic electrode materials,polyaniline,p-type organic compounds as well as conjugated N-heterocycles could undergo oxidation reaction,bind with anions and present high redox potential in the electrolyte,[86]which could be preferentially considered the applications in ZIBs.
4.2.Modification of Electrolyte Composition
In order to achieve high energy density,zinc storage reaction occurs at higher potential is essential.The optimization of collector and the application of ion selective membrane have made high voltage reaction possible in low concentration electrolyte.[120]However,the high complexity and cost of these methods hinder their practical applications.Usually,oxygen redox occurs at high potential(2.0 V vs.Zn2+/Zn as well as 4.3 V vs.Li+/Li)in both aqueous ZIBs and non-aqueous LIBs.[121,122]Therefore,it cannot be triggered in traditional low concentration electrolyte(such as 1 M Zn(CF3SO3)2)of aqueous ZIBs due to the oxygen evolution reaction occurring at 1.98 V.After adopting“water in salt”electrolyte of 21 M LiTFSI/1 M Zn(CF3SO3)2,its voltage window is much higher than the oxygen evolution reaction voltage(Figure 6b),while the electrolyte decomposition is also effectively suppressed.[46]However,the low solubility of zinc salts(below 21-32 mol kg-1)restricted the concentration of aqueous electrolytes.Recently,a mixed electrolyte of ZnCl2/ZnBr2/Zn(OAc)2with a supersolubility up to 75 M was elaborately prepared.Its record-breaking concentration could be ascribed to the formation of acetate capped watersalt oligomers bridged by Br-/Cl--H and Br-/Cl-/O-Zn2+interactions,demonstrating that the high-performance electrolyte could be obtained by adjusting the proportion of anions in the electrolyte(Figure 6c).[117]In addition to the concentration,the choices of different zinc salts and solvent also have great influence on the properties of electrolyte.[123,124]
4.3.Regulation of Voltage Window
The operating voltage is also an important limiting factor for anionic chemistry reaction.The controllable preparation of high-stability materials and the binding potential can relax the range of conditions for anionic chemistry.[22]The voltage can raised by engineering molecular structures(Figure 6d).[118]The lowest unoccupied molecular orbital(LUMO)energy level can be reduced by introducing electron-withdrawing groups(such as-CN,-F,-Cl and-Br),and then achieve the high operating voltage.[118,125]Additionally,the voltage of Zn/organic batteries can also be affected by the position of active groups.For example,quinone compounds with two carbonyl groups in orthoposition(1,2-NQ and 9,10-PQ)have a higher voltage than the paraposition(1,4-NQ and 9,10-AQ).[126]
The generation of SEI film derived from high concentration electrolyte is also an important approach to broaden the voltage window.[127]As shown in Figure 3e,the voltage window of battery(LiMn2O4cathode∣Mo6S8anode)is significantly widened by the formation of SEI film in high salt concentration.[128]Therefore,the applications of organic electrode and “water-in-salt”electrolyte are valid means to broaden the voltage window and increase the operating voltage platform.However,it still requires further research about the effects of anion groups on the organic electrode and“water-insalt”electrolyte.[129]
Besides,divalent metal ions are more likely to combine with anions to form ion pairs.In recent report,trimethyl phosphate(TMP)solvent with strong electron donation ability is employed to prepare Zn(TFSI)2/EMC electrolyte.These TFSI-anions are tightly confined to the TMP solvation region in the form of associated ion pairs(Figure 6e).[119]Thus,the separation of anions and EMC solvent molecules is realized,which effectively conduces to increase the voltage window and improve the cycle stability.This method provides new reference to widen the voltage window by modifying the electrolyte composition.
Presently,the electrochemical reaction mechanism based on the anionic chemistry is still imperfect and immature.Especially for ZIBs,anions are in an unstable state,easily causing the formation of byproducts and uncontrolled reactions.When the anionic reaction occurs,it is always accompanied by the release of oxygen and irreversible structural collapse.[17,130,131]
These practical barriers seriously hinder the promotion and applications of anionic chemistry.Herein,both the design of electrode structure and the optimization of electrolyte composition could have positive effects on these issues,such as widening the voltage window,increasing the energy density,enhancing the cycling stability,and promoting the reaction kinetics.
5.Summary and Perspectives
In summary,the fundamental principles of anionic chemistry are detailedly illustrated from the basic theories of bonding structure,crystal lattice and coordination environment.Meanwhile,the positive effect and functional mechanism of anionic chemistry for ZIBs are summarized and listed item by item,indicating its potential superiority.In spite of the advantages of anionic chemistry,a series of feasible strategies about electrode modification and electrolyte modulation have been proposed to address the facing issues of anionic chemistry,improve the energy density and facilitate the further development of ZIBs.These critical issues mainly include the irreversible structural changes caused by the unstable anionic redox reaction,the uncontrollable electrolyte decomposition arised from the higher potential conditions of anionic redox reaction,and the inferior stability of anion vacancies and surface modifications in the electrodes.Besides,there are also some other obstacles hindering its further applications,such as the unclear fundamental principle and ambiguous redox reaction mechanism of anionic chemistry.Hence,the outlook and prospective research directions about anionic chemistry may mainly focus on the following aspects:
5.1.In Depth Exploring the Basic Principle of Anionic Chemistry
In addition to the knowledge of band structure,crystallography,and coordination chemistry,the intrinsic relations between fundamental theory of materials science and electrochemical properties needs to be further explored.At present,zinc storage mechanisms depended on the valence change of cations,especially vanadium-based and manganesebased metal oxides,are relatively mature.However,there is still lack of corresponding references to truthfully demonstrate the valence change of anions and reveal the zinc storage mechanism based on anionic chemistry.
5.2.Adopting Advanced Characterization and Theoretical Calculation Techniques for the Measurement of Electrode and Electrolyte
Accurate mechanism analysis always needs to use advanced characterization means.For the electrode material,currently,the valence change of anions only could be qualitatively analyzed by X-ray photoelectron spectroscopy.If the exact valence change of anions could be quantificationally confirmed,the zinc storage mechanism based on anionic chemistry could be easily revealed.For the electrolyte system,both the desolvation and solvation process are very abstract concept,which need to be demonstrated by first principles and molecular dynamics calculation in most cases.However,it is still lack of intuitive characterization methods to observe and reflect the action mechanism of electrolyte on battery performances.Synchrotron radiation X-ray absorption fine structure spectroscopy(XAFS),small angle neutron scattering(SANS)technique and cryo-electron microscopy technique may be potential means to achieve these targets.Therefore,advanced in situ or characterization techniques should be developed to capture the reaction information in real time for both electrode and electrolyte,including qualitative and quantitative information.
5.3.In Detail Elaborating the Solid-liquid Interface Reaction Mechanism
In addition to the exploration of anionic reactions in electrode or electrolyte,the redox reactions occurring at the interface between electrode and electrolyte also should be specially concerned.Owing to the different state of anions in electrolyte and electrode,it is harder to characterize and elaborate the complex chemical reactions at the interface.The detections of interfacial reaction state could be favorable to comprehensively understand the electrochemical behaviors of batteries,such as ion diffusion kinetics,formation of SEI layer,and charge transfer rate.These behaviors have significant effect on the cycling stability,rate capability,and pseudocapacitive contribution of the batteries.Therefore,the interfacial reaction between electrode and electrolyte deserves more attention for the intensive study of anionic chemistry,which is a critical factor for the electrochemical performances of batteries.
5.4.Developing Quasi-solid-state or All-solid-state Electrolytes for ZIBs
Current research on ZIBs have been focused on exploring cathode materials with high capacity and optimizing the cycling stability of zinc anode.However,taking the practical applications of ZIBs into concern,people have long neglected the battery’s shelf life.Unfortunately,ZIBs using aqueous electrolytes exhibit rapidly deteriorating properties due to the high temperature sensitivity of water and the inevitable side reactions between zinc metal and aqueous electrolytes(including corrosion,hydrogen evolution reaction(HER)and passivation).Some general strategies,such as “salt-in-water”electrolytes,hydrate melt electrolytes and hydrogel electrolytes,seem to have certain positive effects on these issues,which can only inhibit but not eliminate HER and the growth of zinc dendrite.Therefore,in order to facilitate the practical application of ZIBs,there is an urgent need to develop alternative electrolyte systems that can significantly extend the shelf life of ZIBs and maintain significant electrochemical properties close to water system electrolytes.Inspired by the research on allsolid-state lithium batteries and the high chemical stability of allsolid-state electrolyte,all-solid-state ZIBs may be a very promising strategy for solving these problems.
Acknowledgements
Y.G.and Z.L.contributed equally to this work.This work was supported by the National Natural Science Foundation of China(Grant no.52072411,51932011,51972346).
Conflict of Interest
The authors declare no conflict of interest.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Classifying Electrolyte Solutions by Comparing Charge and Mass Transport
- Two-dimensional Boron Nitride for Electronics and Energy Applications
- Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes
- Recent Development in Defects Engineered Photocatalysts:An Overview of the Experimental and Theoretical Strategies
- 2D/2D Heterostructures:Rational Design for Advanced Batteries and Electrocatalysis
- Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries
