Replacing f ishmeal with soybean meal aff ects survival,antioxidant capacity, intestinal microbiota, and mRNA expression of TOR and S6K1 in Macrobrachium rosenbergii*
Zhili DING , Dongsheng ZHOU , Jinxian ZHENG , Xuefeng CHEN , Youqin KONG ,Changle QI , Yan LIU , Qiongying TANG , Guoliang YANG , Jinyun YE
1 Zhejiang Provincial Key Laboratory of Aquatic Resources Conservation and Development, College of Life Science, Huzhou University, Huzhou 313000, China
2 Agriculture Ministry Key Laboratory of Healthy Freshwater Aquaculture, Key Laboratory of Freshwater Aquaculture Genetic and Breeding of Zhejiang Province, Zhejiang Institute of Freshwater Fisheries, Huzhou 313001, China
Abstract Using alternative plant-derived dietary protein to replace f ishmeal, combined with practical evaluation indexes, is a recent focus for aquaculture practices. An 8-week feeding experiment with giant freshwater prawn Macrobrachium rosenbergii post-larvae was conducted to determine the eff ects of replacing f ishmeal (FM) with soybean meal in the feed, in terms of growth performance, antioxidant capacity, intestinal microbiota, and mRNA expression of target of rapamycin (TOR) and ribosomal protein S6 kinase B1 (S6K1). Four isonitrogenous diets with isocaloric value were prepared to contain 100%, 75%,50%, or 25% FM as the protein source (dietary treatments FM100, FM75, FM50, and FM25, respectively).Each diet was fed to post-larval prawns (mean weight 0.045±0.002 g) twice a day in four replicates. No signif icant diff erence in weight gain was observed among all groups, but the survival rate of prawns fed the FM50 and FM25 diets was signif icantly lower than that of prawns fed the FM diet. The mRNA expression of both TOR and S6K1 were the lowest in hepatopancreas of prawns fed the FM25 diet. Superoxide dismutase activity of prawns fed the FM25 diet was signif icantly lower than that of prawns fed FM50. In contrast,the malondialdehyde content was signif icantly higher in prawns fed FM25 as compared with those fed FM75. The proportion of f ishmeal in the diet did not aff ect the composition of core (phylum-level) intestinal microbiota, but greater f ishmeal replacement with soybean meal had a potential risk to increase the relative abundance of opportunistic pathogens in the gut when considered at the genus level. These results suggest that f ishmeal replacement with soybean meal should not exceed 50% in a diet for post-larval M. rosenbergii.
Keyword: animal protein; plant protein; replacement; protein synthesis; health; crustacean
1 INTRODUCTION
Somatic growth and metabolism involve complex physiological processes, governed primarily by nutritional status and hormones (Fuentes et al., 2013).Proteins are of great nutritional value and are directly involved in the chemical processes essential for life.In aquaculture, f ishmeal is used as a major protein source in aquaculture feeds owing to its high nutritional value and palatability (Ding et al., 2015;Anderson et al., 2016). However, increasing prices and a shortage of f ishmeal supplies because of declines in wild f isheries populations are problematic(Hardy, 2010). Thus, plant-derived protein ingredients with wider availability and at competitive costs are sought as alternative sources for aquaculture feeds(Gatlin III et al., 2007; Hu et al., 2019).
The growth performance of aquatic animals is usually the f irst parameter to be considered after substituting f ishmeal with a plant-derived protein in feedstuff s. However, the health of aquatic animals should not be ignored considering the potential for immune damage from amino acid prof ile imbalances or anti-nutritional factors that accompany many plantbased feeds. The intestine is an extremely complex ecosystem, with extensive interactions occurring among the gut microbiota, nutrients, and host cells(Bourlioux et al., 2003). Especially, bacterial imbalances in the gut can lead to disease (Bourlioux et al., 2003; Hooper and Macpherson, 2010), and several studies have found that plant-based diets could induce changes in intestinal microbiota (Green et al., 2013; Reveco et al., 2014; Li et al., 2020; Shen et al., 2020; Ye et al., 2020b). For example, in f ish,feeding soybean protein concentrate caused intestinal disorders by altering the intestinal microbiome in Atlantic salmonSalmosalar(Green et al., 2013),Dietary conditions highly aff ected the intestinal bacterial population in Atlantic salmon, and inclusion of soybean meal could induce enteritis (Reveco et al.,2014). Adding soybean meal or fermented soybean meal modulated the intestinal microbiota in turbotScophthalmusmaximus(Li et al., 2020). Replacing f ishmeal with peanut meal of up to 50% changed obviously the intestinal microbiota of juvenile hybrid grouper (Epinephelusfuscoguttatus♀ ×Epinepheluslanceolatus♂) (Ye et al., 2020a). Replacing f ishmeal with cottonseed protein concentrate decreased the abundance and diversity of intestinal f lora (Shen et al., 2020). However, in crustaceans, diff erent levels of fermented soybean meal in the diet did not signif icantly inf luence the composition, diversity, and functions of the intestinal microbiota in white shrimpLitopenaeusvannamei(Shao et al., 2019). Together, these results demonstrate diff erent responses of the intestinal microbiota among aquatic animals fed plant-derived proteins.
The quality and quantity of protein and amino acids in feeds consumed by aquatic animals correlate closely with the amount of protein synthesis in the body (Dumas et al., 2007; Wu et al., 2017). The target of rapamycin (TOR) signaling is a vital pathway that can sense protein sources or protein levels (Jiang et al., 2017). In mammals, TOR is a serine/threonine protein kinase which has two distinct protein complexes, known as mTOR Complex 1 (mTORC1)and mTOR Complex 2 (mTORC2) (Blenis, 2017).mTORC1 includes TOR, mammalian lethal with SEC13 protein 8 (mLST8) and regulatory-associated protein of mTOR (Raptor) (Saxton and Sabatini,2017). Once TOR is activated it regulates two downstream targets—eukaryotic translation initiation factor 4E-binding proteins (4E-BPs) and ribosomal protein S6 kinases (S6Ks)—and then controls mRNA translation initiation (Corradetti and Guan, 2006).Therefore, the mTORC1 signaling pathways can control protein synthesis.
The giant freshwater prawnMacrobrachiumrosenbergiiis an economically important crustacean,cultured on a large scale worldwide, owing to its rapid growth, relatively large size, good meat quality, and delicious taste (Muralisankar et al., 2015). Several studies have considered substituting f ishmeal with other protein sources in diets for this species (Tidwell et al., 1993; Zhu and Yang, 1995; Du and Niu, 2003;Anh et al., 2009; Bijoy et al., 2018). Among the alternative protein sources, soybean meal is considered to be a suitable ingredient for replacing f ishmeal used in aquafeeds because of its high protein content, a balanced amino acid prof ile and relatively low cost (Gatlin III et al., 2007; Ding et al., 2015).Tidwell et al. (1993) demonstrated that f ishmeal could be partly or completely replaced with soybean meal in a feed for prawns (initial weight 0.51 g) raised in temperate pond. Zhu and Yang (1995) found that prawns (initial weight 3.6 g) fed a diet with soybean meal as the sole protein source showed growth as good as when fed with f ishmeal. Both studies examined the eff ects of substituting soybean meal with f ishmeal as the dietary protein source only in terms of growth performance. Du and Niu (2003)indicated that soybean meal as a major protein source is not suitable in a prawn diet based on growth and metabolism. Thus, diff erent evaluation indexes can aff ect conclusions about the use of soybean meal.However, the replacement of f ishmeal by soybean meal onM.rosenbergiipost-larvae have not been studied, and the current evaluation indexes have also not been involved in antioxidant capacity, intestinal microbiota, and TOR signaling pathways.Furthermore, it should be noted that soy antinutritional factors (ANFs) in soybean meal could limit replacement level of f ishmeal by soybean in aquatic animals (Francis et al., 2001; Tibaldi et al.,2006). Therefore, we hypothesized that there might be a proper replacement level for f ishmeal by soybean meal forM.nipponensepost-larvae based on above systematic indexes. Therefore, the present study aimed to evaluate the response of giant freshwater prawnM.rosenbergii, reared from post-larvae to juveniles, to diff erent replacement ratios of f ishmeal by soybean meal, according to the growthperformance, antioxidant capacity, intestinal microbiota, and mRNA expression of TOR and S6K1.
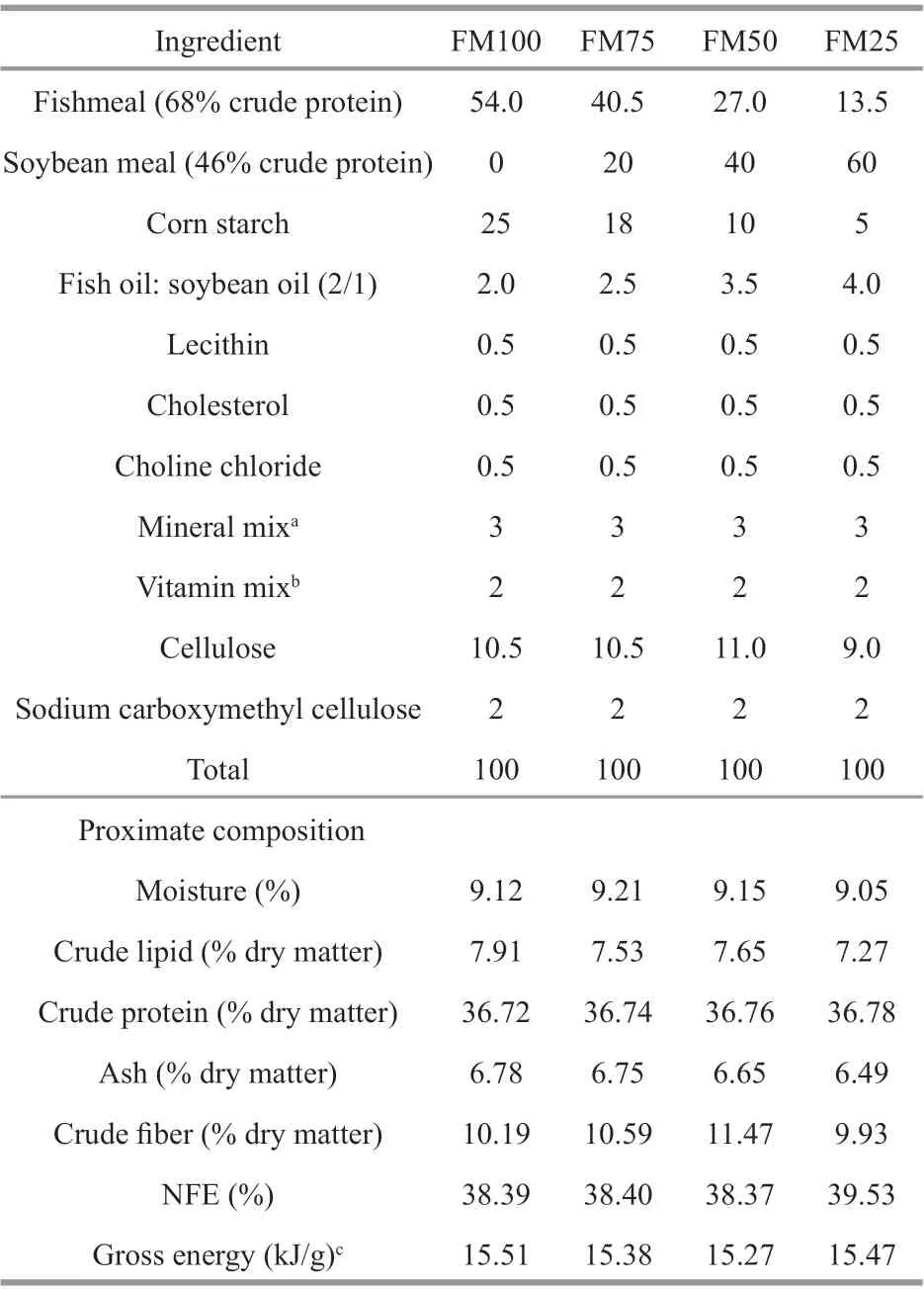
Table 1 Ingredients and nutrient composition (% dry matter) of the test diets (formulated with 100%,75%, 50%, or 25% f ishmeal (FM) as the protein source) for giant freshwater prawn Macrobrachium rosenbergii post-larvae
2 MATERIAL AND METHOD
2.1 Experimental diet
A basal diet containing 54% f ishmeal (FM, 68%crude protein) was used as the FM-based diet (no replacement). Soybean meal (46% crude protein) was used to replace f ishmeal at 0%, 25%, 50%, and 75%.Thus, four isonitrogenous diets (~36.7% crude protein)with isocaloric value (~15 kJ/g) were formulated with 100%, 75%, 50%, and 25% FM as the protein source(dietary treatments FM100, FM75, FM50, and FM25,respectively; Table 1). The dietary protein and energy levels of the experimental diets were designed according to Habashy (2009) and Du and Niu (2003).
All dry ingredients of each diet were passed through a 212-μm mesh and then were thoroughly mixed with 30% distilled water. This mixture was extruded through a 1.5-mm die with a pelleting machine (F-26, South China University of Technology,Guangzhou, China), dried in a forced-air oven at 40 °C, to achieve about 10% moisture. In order to prevent oxidation of dietary lipids by high temperature in summer, the prepared diets were then stored at-20 °C in airtight plastic containers until use.
2.2 Experimental animals and feeding trials
Post-larvae ofM.rosenbergiiwere purchased from a breeding base (Jiangsu, China). Before the feeding experiment, prawns were fed a commercial diet for 2 weeks to acclimate the experimental conditions. After acclimation, healthy prawns (initial weight 0.045±0.002 g) were randomly distributed in 16 tanks(4 groups), with 300 L in volume. Each group had 4 replicates and each tank had 50 prawns. Prawns were fed two times (08:00 and 16:30) every day to apparent satiation and the experiment period lasted for 8 weeks.A few nylon f ishing nets were put in each tank, which can reduce animal cannibalization as an artif icial shelter. The f ishing nets were cleaned once per week.During the feeding trial, the water-quality parameters were monitored to maintain a temperature range of 25-28 °C, dissolved oxygen at >6.5 mg/L, and ammonia and nitrate levels of <0.1 mg/L under natural photoperiod conditions. One-third of the water in each tank was replaced daily and the unused food/wastes were removed every day.
2.3 Sample collection
Feeding was stopped 24 h prior to sampling. The prawns in each tank were counted and weighed to determine survival rate, weight gain, and specif ic growth rate. The hepatopancreas were dissected from the cephalothorax and stored at -80 °C for subsequent analyses. To compare the gut microbial composition between treatment groups, the guts of 10 prawns from each tank (12 tanks in all, 3 parallel tanks from each treatment FM100, FM75, FM50, and FM25) were collected under sterile conditions. Owing to lower survival rate of FM25 group, only three parallels in every group were selected for gut microbial composition analysis.
2.4 Growth performance and biochemical measurement
Weight gain, specif ic growth rate, and survival rate were calculated as: weight gain (%)=100×(mean f inal weight-mean initial weight)/mean initial weight;specif ic growth rate (%)=100×[(ln(mean f inal weight)-ln(mean initial weight)]/days of the experiment; survival rate (%)=(f inal number of prawns/initial number of prawns)×100. The f inal stocking density (inds./m3)=f inal number of prawns/water volume.
The moisture, crude lipid, and crude protein in feed were carried out in standard procedures (AOAC International and Horwitz, 2005). Ash content was determined after combustion in a muffl e furnace at 550 °C for 6 h. Crude f iber content was measured by Filter Bag Technology using an automatic analyzer(ANKOM A200i, Ankom Technology, NY, USA). The nitrogen-free extract (NFE) was calculated by diff erence. The hepatopancreas samples (about 0.08 g)were homogenized in nine volumes of 0.86% (w/v)precooled sodium chloride (720 mL), and homogenates were centrifuged at 5 000×gat 4 °C for 20 min. The supernatants were employed for measurements of all enzyme activities. Superoxide dismutase (SOD)activity and the malondialdehyde (MDA) content in the hepatopancreas were determined using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Alkaline phosphatase (AKP) activity in the hepatopancreas was likewise quantif ied using an assay kit (Nanjing Jiancheng Bioengineering Institute).
2.5 qRT-PCR analysis of gene expression
Relative expression of mTOR signaling genes was determined using qRT-PCR analysis as described in our previous study (Ding et al., 2017). Brief ly, total RNA was extracted from the hepatopancreas of prawns using an RNA extraction kit (Aidlab Biotech,Beijing, China). Complementary DNA (cDNA) was synthesized using a PrimeScript™ RT-PCR Kit(TaKaRa, Japan). The qRT-PCR analysis used a SYBR®Premix Ex Taq™ Kit (TaKaRa) in the CFX96™ Touch Real-Time PCR System (Bio-Rad,Hercules, CA, USA). The gene-specif ic primers were designed based on the cDNA sequences in GenBank(target of rapamycin (TOR); ribosomal protein S6 kinase B1 (S6K1)) (Table 2).
2.6 Bacterial genomic DNA extraction
No signif icant diff erences of growth performance and antioxidant indexes between FM25 and FM50 group according to later analysis, so we just selected one of the two groups to detect intestinal microbiota.Therefore, total bacterial community DNA was extracted from the intestinal contents of 10 prawns inno replacement group (FM100), medium replacement group (FM75), and high replacement group (FM25),using a TIANamp Micro DNA Purif ication Kit(Tiangen, Beijing, China). After measuring the quantity and quality, the DNA were submitted to the Origingene Bio-pharm Technology Co. (Shanghai,China) for 16S rRNA sequencing.
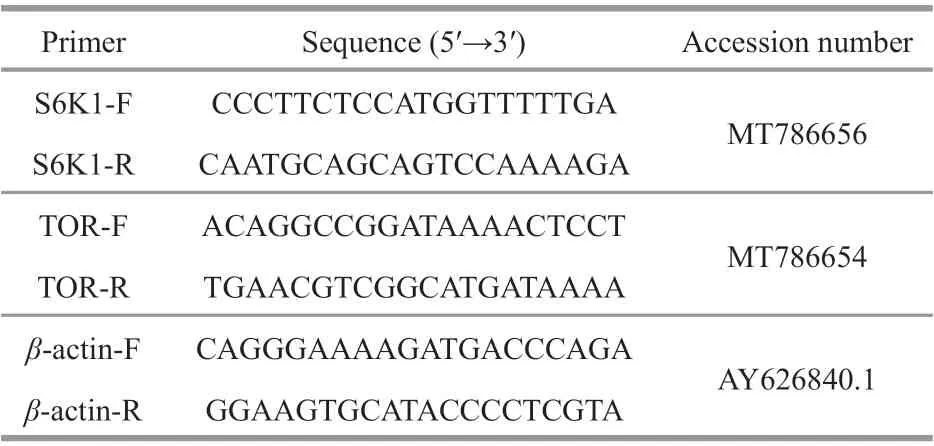
Table 2 Primers used in this study
2.7 Intestinal microbiota analysis
The V3-V4 region of the bacterial 16S rRNA gene was amplif ied with the universal prokaryotic primers F341 (5′-CCTAYGGGRBGCASCAG-3′)and R806 (5′-GGACTACHVGGGTWTCTAAT-3′).The polymerase chain reaction (PCR) system and procedures were performed as described in Ding et al. (2020). All amplicon PCR products were purif ied and subjected to Illumina-based high-throughput sequencing. The nine sequences have been submitted to GenBank, and the SRA submission accession numbers are SRR12400109-SRR12400117.
Raw reads were analyzed and f iltered using QIIME(version 1.17). Qualif ied reads with ≥97% similarity were clustered to generate the same operational taxonomic units (OTUs) using USEARCH (version 7.1). The phylogenetic affi liation for each OTU was annotated by searching the SILVA database applying a conf idence threshold of 70% (Pruesse et al., 2007).Taxonomic richness estimators and community diversity (alpha diversity and beta diversity estimation) were determined in Mothur (version v.1.30.1). Principal coordinates analysis (PCoA) on the weighted UniFrac distance matrices was performed to visualize diff erences in bacterial community composition and structure.
2.8 Data analysis
All data are expressed as mean±standard deviation.Statistical analyses were performed using SPSS 17.0(IBM SPSS, Armonk, NY, USA). Statistical signif icance was determined using one-way ANOVA and Tukey’s post hoc multiple comparisons test after normality test and homogeneity of variance.P<0.05 was considered signif icant in statistics.

Fig.1 Relative mRNA expression of TOR (a) and S6K1 (b) in Macrobrachium rosenbergii post-larvae that were fed test diets with diff erent ratios of replacement of f ishmeal with soybean meal for 8 weeks
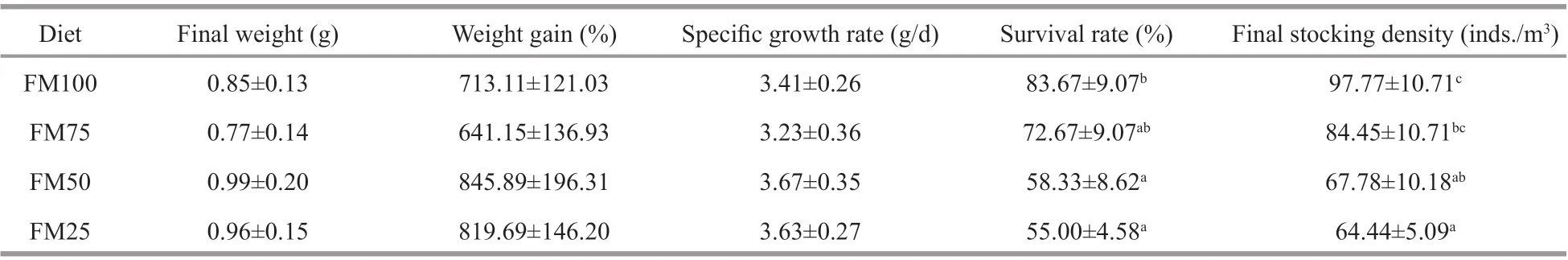
Table 3 Growth performance of Macrobrachium rosenbergii post-larvae fed test diets with diff erent replacement ratios of f ishmeal with soybean meal for 8 weeks (mean±SD, n=4)
3 RESULT
3.1 Growth performance
Measures of growth performance for theM.rosenbergiiraised from post-larvae to juveniles are shown in Table 3. The results show no signif icant diff erences in f inal weight, weight gain, and specif ic growth rate between the dietary treatment groups.Even so, when the FM replacement level was more than 50%, the survival rate decreased signif icantly,and the survival rate in groups FM50 and FM25 was signif icantly lower than that in the FM100 (no replacement) group (P<0.05). The f inal stocking density in FM25 group was signif icantly lower than that in FM100 and FM75 groups (P<0.05).
3.2 mRNA expression of mTOR signaling
TOR and S6K1 gene transcripts were detected in all three groups examined (Fig.1). TOR mRNA expression in hepatopancreas was lowest in prawns fed the FM25 diet, which was lower than that in theother three treatments (P<0.05). Similarly, prawns fed the FM25 diet also had the lowest S6K1 mRNA expression.
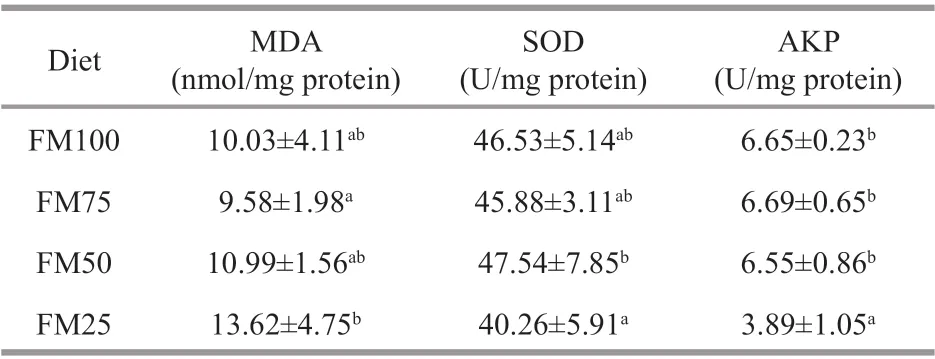
Table 4 The antioxidant activities of malondialdehyde(MDA), superoxide dismutase (SOD), and alkaline phosphatase (AKP) in Macrobrachium rosenbergii post-larvae fed test diets with diff erent replacement ratios of f ishmeal with soybean meal for 8 weeks(mean±SD, n=4)
3.3 Hepatopancreas lipid oxidation and antioxidant enzyme activities
The activity of SOD in hepatopancreas of the FM25 group was signif icantly lower than that of the FM50 group (P<0.05) (Table 4). At the same time, theactivity of AKP in hepatopancreas was signif icantly lower in the FM25 group than that in the other three groups (P<0.05). In contrast, MDA content in the FM25 group was signif icantly higher than that in the FM75 group (P<0.05).
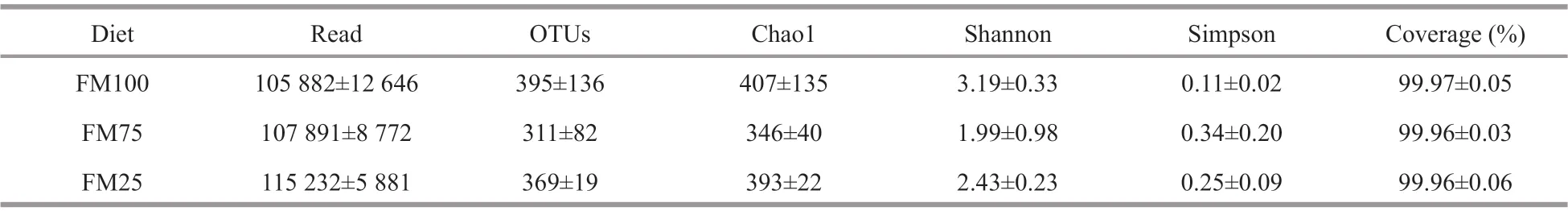
Table 5 Alpha diversity of intestinal microbiota in Macrobrachium rosenbergii post-larvae fed test diets with diff erent replacement ratios of f ishmeal with soybean meal for 8 weeks
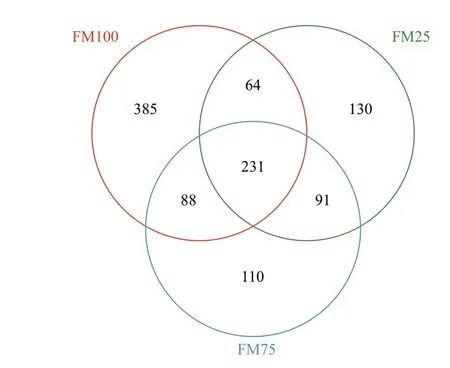
Fig.2 Venn diagram demonstrating the distribution of the unique and shared operational taxonomic units in the three groups: FM100 (100% FM), FM75 (75%FM), and FM25 (25% FM)
3.4 Analysis of intestinal microbiota
To study the eff ects of diff erent dietary treatments on the intestinal microbiota of prawns, the ecological characteristics of intestinal bacteria in 100% FM(FM100 group), 75% FM (FM75 group), and 25%FM (FM25 group) were evaluated (Table 5). A total of 987 016 high-quality reads from the three intestinal samples was obtained, with an average of 109 668 sequences per sample. The coverage rates were more than 99% in each sample, demonstrating that the 16S rRNA gene sequences identif ied in each group represent the most of bacteria present in the samples.The number of OTUs observed at a 97% taxonomic cutoff . Suffi cient sampling depth was obtained for each sample in the study according to the rarefaction analysis. Before subsampling, there were 1 101 OTUs with a clear taxonomic level. After subsampling, a total of 1 099 OTUs were left, of which 231 OTUs were shared among the three samples. There were 385, 110, and 130 unique OTUs for FM100, FM75,and FM25 treatment groups, respectively (Fig.2). No signif icant diff erences were observed in the number of OTUs, Shannon and Simpson diversity indexes,and the Chao1 richness estimators (Table 5).
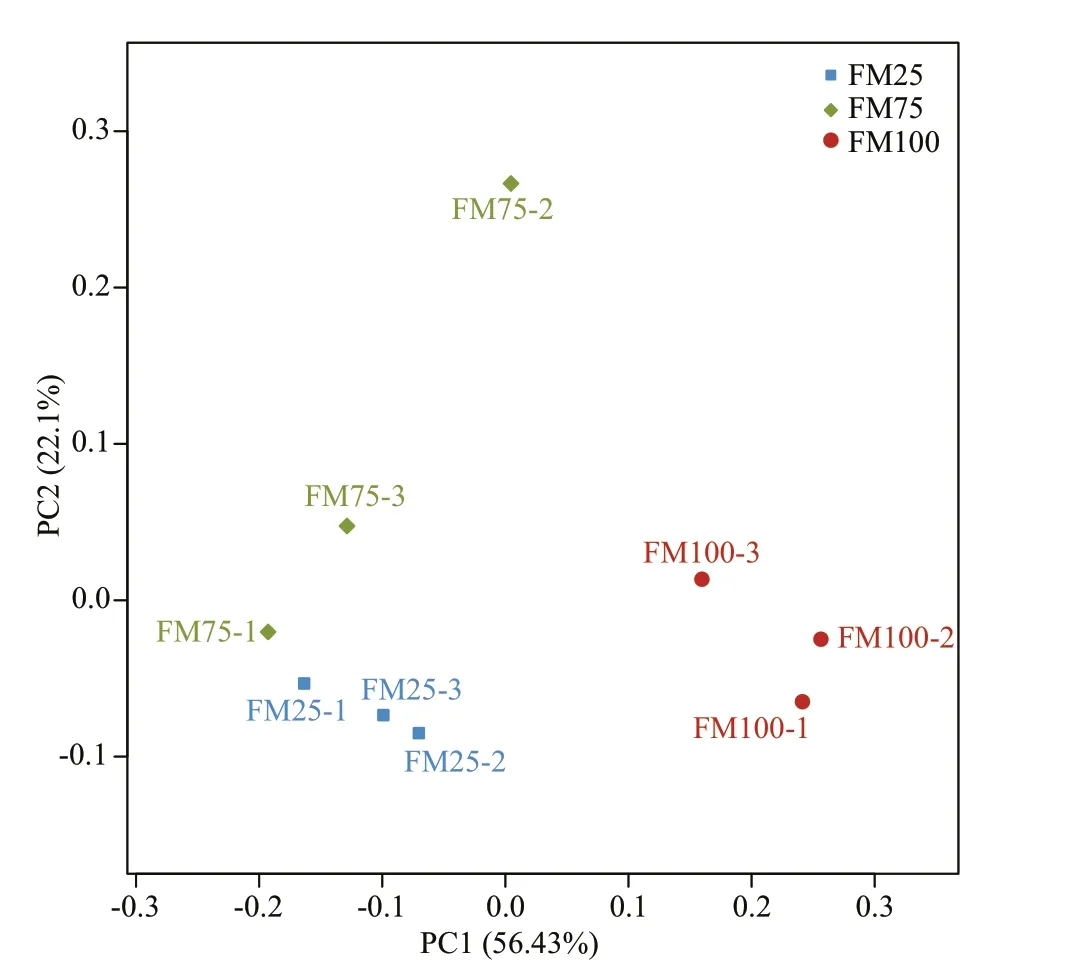
Fig.3 Principal coordinates analysis (PCoA) of weighted UniFrac distances, for the intestinal bacterial communities in Macrobrachium rosenbergii postlarvae that were fed diets containing diff erent ratios of f ishmeal (FM) as the protein source
The UniFrac distance was visualized by a PCoA plot, which showed the bacterial communities in prawns fed diff erent diets (Fig.3). The groups FM75(replicates FM75-1, FM75-2, FM75-3) and FM25(FM25-1, FM25-2, FM25-3) showed clear and distinct patterns in bacterial community composition along PC1 as compared with the FM100 group(FM100-1, FM100-2, FM100-3).
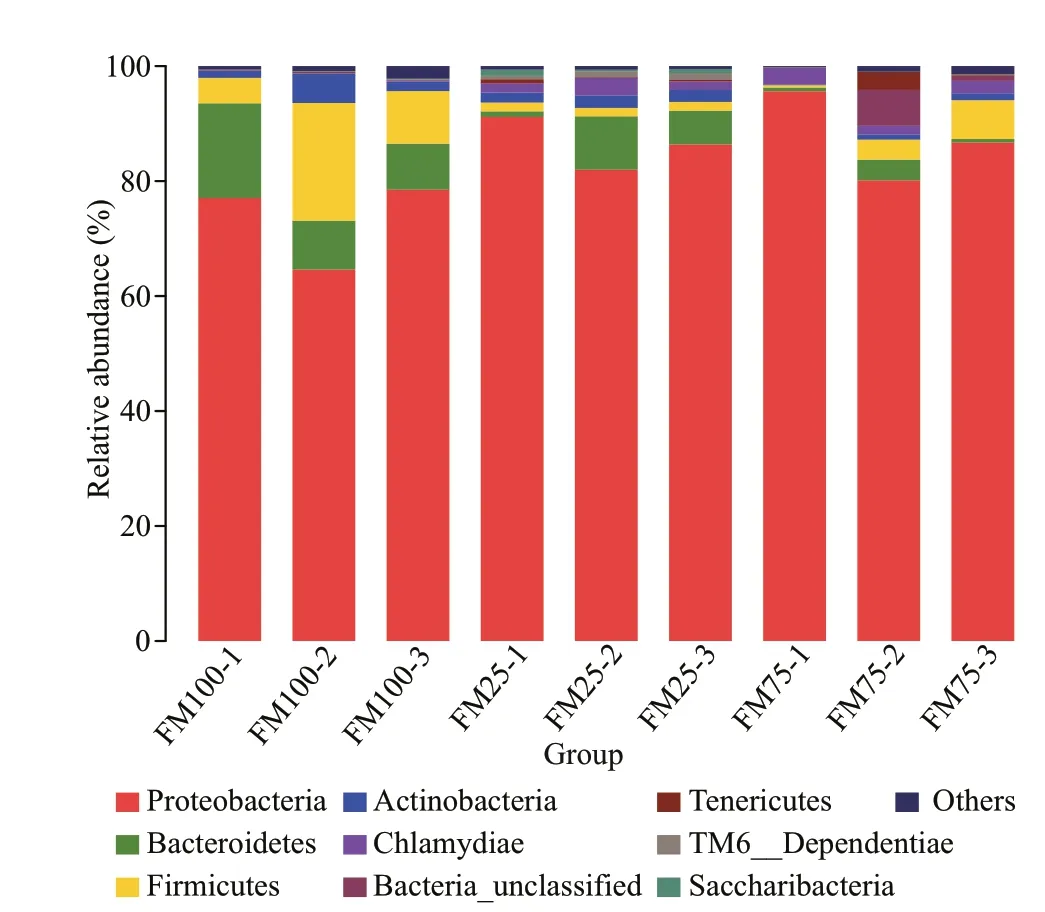
Fig.4 Relative abundance (%) of the bacterial phyla in the intestine of Macrobrachium rosenbergii post-larvae that were fed diets containing 100%, 75%, or 25%f ishmeal (FM) as the protein source (FM100 group,FM75 group, and FM25 group, respectively)
Figure 4 shows the relative abundance of the gut microbiota in each sample at the phylum level.Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria were the most abundant phyla in the three dietary treatment groups. The relative abundances of these four phyla were: 73.39%±7.63%,10.99%±4.74%, 11.36%±8.23%, and 2.66%±2.11%,respectively, in group FM100; 87.49%±7.77%,1.61%±1.71%, 3.55%±3.17%, and 0.72%±0.57%,respectively, in group FM75; and 86.50%±4.59%,5.38%±4.18%, 1.51%±0.03%, and 1.98%±2.10%,respectively, in group FM25. At the phylum level, the diff erences among the three dietary treatment groups of prawns were not signif icant (P>0.05) (Table 6).However, at the genus level, the relative abundances diff ered signif icantly, that ofAeromonaswas lower in the FM100 group than in groups FM75 and FM25(P<0.05) (Table 6); the relative abundances ofPseudomonasandStenotrophomonaswere lower in groups FM75 and FM25 than in the FM100 group(P<0.05); and the relative abundance ofChryseobacteriumwas higher in the FM100 group than in the FM25 group (P<0.05).
4 DISCUSSION
Soybean meal is the most common alternative protein feedstuff to replace f ishmeal in formulated feeds for f ish and shrimp because of its high protein content and excellent amino acid prof ile (Gatlin III et al., 2007). Our results indicate that replacing f ishmeal by 0-75% with soybean meal in the diet did not negatively impact the growth performance ofM.rosenbergii, with an average initial weight of 0.045 g,as compared with animals fed the f ish meal-based diet(i.e. with no replacement). Although there is adef iciency in the essential amino acids (methionine,lysine, and tryptophan) of soybean meal (Floreto et al., 2000), the giant freshwater prawn seemed to can tolerate the diet with 75% f ishmeal replacement by soybean meal from the index of growth performance.Similar results were observed by Zhu and Yang (1995)who found that prawns (initial weight 3.6 g) fed a diet with raw soybean meal as the sole protein source showed no poorer growth than prawns given a feed with f ish meal. Tidwell et al. (1993) demonstrated that f ishmeal could be partly or completely replaced with raw soybean meal in a feed for prawns (initial weight 0.51 g) raised in temperate pond. However, the results of our present study partially diff er with Hasanuzzaman et al. (2009) and Du and Niu (2003). Hasanuzzaman et al. (2009) found that replacing 80% of f ishmeal protein by extracted soybean meal protein was the most favorable option, based on growth performance,inM.rosenbergiiwith an initial average weight of 2.51-5.19 g. Du and Niu (2003) reported that defatted soybean meal is not suitable as a major protein source for this freshwater prawn, based on growth performance and standard metabolic rate indexes on juveniles ofM.rosenbergii(0.32 g) fed diets with 0%,20%, 50%, 75%, and 100% of the f ishmeal replacement with soybean meal. The diff erence between these reported results may be related to the developmental stages of the experimental prawns and the evaluation indexes chosen to measure the eff ects of f ishmeal replacement in an aquafeed.
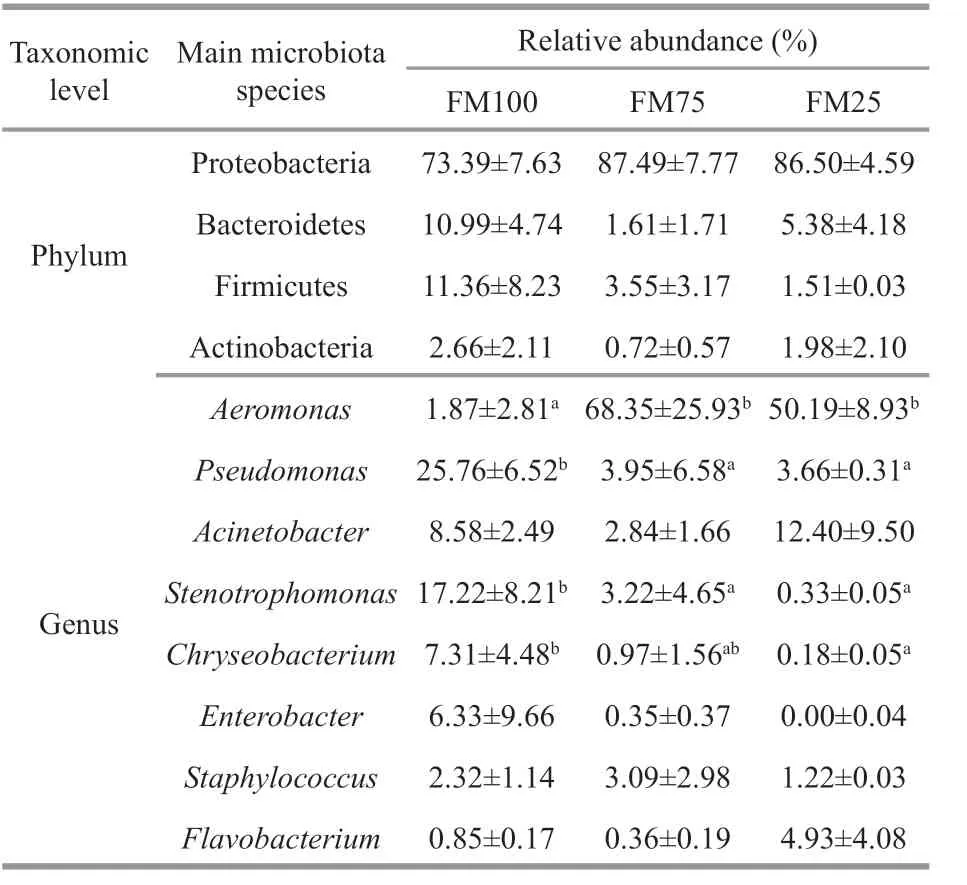
Table 6 Main gut microbiota species in Macrobrachium rosenbergii post larvae fed test diets with diff erent ratio replacement of f ishmeal with soybean meal for 8 weeks
The prawns in our study were raised from postlarvae to juveniles, and indeed underwent two developmental stages. Furthermore, we could not ignore the fact that although we found no signif icant diff erences in weight gain among all groups, a signif icantly decreased survival rate was observed in our study when f ishmeal replacement by soybean meal exceeded 50%. The decreased survival rate may be becauseM.rosenbergiipost-larvae are intolerant of a high proportion of soybean meal in the diet.During the experiment, the density of the prawns in the tanks changed as mortalities ensued in the FM50 and FM25 groups. Therefore, the lack of signif icant diff erences in growth performance among all groups does not exclude the possibility that diminishing densities inf luenced the outcome for groups FM50 and FM25 when compared with groups FM100 and FM75 with their higher survival rates. Many studies have proved that stocking density is an environmental factor that will aff ect the growth of crustaceans, and lower stocking densities could improve growth performance (Li et al., 2006; Sun et al., 2016).
The mTOR signaling pathway can be involved in cell growth and proliferation by regulating protein synthesis (Hay and Sonenberg, 2004). TOR and S6K1 are important upstream and downstream regulators of mTOR signaling pathway, respectively. TOR can incorporate signals from nutrients to control protein translation (Saxton and Sabatini, 2017), and S6K1 controls protein synthesis and growth by phosphorylating ribosomal protein S6 and the translation initiation factor eIF4B (Adegooke and Samimi-Seisan, 2009). In the present study, there were no signif icant diff erences in TOR and S6K1 mRNA expression among groups FM100, FM75, and FM50, but consuming a diet with a greater proportion of soybean meal (group FM25) resulted in signif icant decreases of TOR and S6K1 mRNA expression.mTOR signaling activation is manifested as an increase in the expression of S6K1 (Sonenberg and Hinnebusch, 2009); in this study, less than 50%replacement of f ishmeal with soybean meal was not demonstrably detrimental to protein synthesis, but signif icantly decreased the rate of protein synthesis with 75% replacement of f ishmeal with soybean meal.The inhibiting protein synthesis of prawns fed FM25 did not result in retarded growth of prawns, which may be due to the lower stocking density of prawns mentioned above. Xu et al. (2020) found that 10% or 15% replacement of f ishmeal with soybean meal increased the expression of S6K1 in hepatopancreas of Chinese mitten crabEriocheirsinensis, indicating that crabs that consumed some amount of soybean meal had more-active protein metabolism. Similar results were reported for f ish, in terms of including other alternative sources of dietary protein. In juvenile blackhead seabreamAcanthopagrusschlegelii, when f ishmeal was replaced by poultry byproduct meal from 0% to 30%, the TOR and S6K1 relative expressions were signif icantly increased, while the expression levels of the two genes signif icantly decreased with the increase of poultry byproduct (Irm et al., 2020). In blunt snout breamMegalobramaamblycephala, TOR mRNA expression in liver and gut were not aff ected by 1% or 3% cottonseed meal protein hydrolysate in the feed, but the expression levels signif icantly decreased as the supplementation was increased (Yuan et al., 2019).
To further understand the mechanism of a decreased survival rate when f ishmeal replacement with soybean meal was more than 50%, we examined the hepatopancreas for antioxidant abilities and intestinal microbiota populations.
In aquaculture, replacing f ishmeal with too high a proportion of a plant-based protein in a feed often results in oxidative stress to the animals (Ding et al.,2015; Kokou et al., 2015; Li et al., 2020). Oxidative stress is caused by excess production of reactive oxygen species (ROS) relative to the antioxidant defense (Shankar and Mehendale, 2014). SOD is usually considered the f irst line of defense for removing ROS, the activity of which can ref lect the antioxidant status of an organism (Wu et al., 2013).MDA is a f inal product of lipid peroxidation, which ref lects the degree of cell damage (Livingstone,2013). In this study, decreased SOD activity and increased MDA content were determined in the FM25 group, which may be linked to ROS generation,leading to exhaustion of the antioxidant defense,which ultimately results in oxidative damage to the cell membrane, as indicated by enhanced MDA levels.It is believed that plant-based feedstuff s can trigger oxidative stress in animals as a result of their antinutritional content (Sitjà-Bobadilla et al., 2005;Rahimnejad et al., 2019). Hence, too much soybean meal (i.e. with more anti-nutritional factors) in the FM25 diet could have induced oxidative stress among theM.rosenbergiipost-larvae. AKP is thought to act as an antibacterial agent because of its hydrolytic activity, which has long been recognized as an important component of innate immunity (Qin et al.,2012; Roosta et al., 2014). Here, lower AKP activity in hepatopancreas of the FM25 group might be attributable to a poorer immune response in postlarvalM.rosenbergii. This is in line with research on oriental river prawnMacrobrachiumnipponense(Ding et al., 2015), which correspondingly indicated the nutrient limitations of soybean meal in diets forM.rosenbergiipost-larvae.
Determination of the intestinal microbiota community in aquatic animals provides additional insight into the eff ects of f ishmeal replacement. In the present study, the composition of intestinal microbiota inM.rosenbergiiwas analyzed by sequencing the 16S rRNA gene amplicons. In accordance with our analyses of the Chao1 estimator and the Shannon and Simpson indices, rearing on a feed with less f ishmeal did not aff ect the richness and diversity of the intestinal microbiota in this prawn species; this is similar to results with white shrimpLitopenaeusvannameithat were fed fermented soybean meal to replace f ishmeal (Shao et al., 2019). However,Siriyappagouder et al. (2018) suggest that the presence or enrichment of certain benef icial genera/species in the community has a more benef icial eff ect than the bacterial diversity itself, although diversity plays an important role in maintaining the gut ecological function. The unique OTUs and the consistent PCoA results in this study suggest that the diff erent dietary treatments led to unique microbial populations in the prawn intestines.
The dominant phyla of gut microbiota identif ied were the Proteobacteria, Bacteroidetes, and Firmicutes, regardless of the level of f ishmeal contained in the prawns’ diet; this f inding indicates that populations of these core taxa could tolerate soybean meal as the dietary protein source. These data are in general agreement with descriptions of these same phyla in the microbial community in the intestines of many aquatic taxa, such as f ish (Shao et al., 2019; Ye et al., 2020b), prawns (Ding et al., 2020),and crabs (Sun et al., 2018). Although not statistically signif icant, the abundance of Proteobacteria in the intestine ofM.rosenbergiishowed an increasing trend with increased replacement with soybean meal protein. Previously, it was proposed that a greater abundance of Proteobacteria is a potential risk for disease (Shin et al., 2015; Rizzatti et al., 2017),therefore we further analyzed the intestinal microbial populations at the genus level.
We found that the relative abundance of 4 of the 8 dominant genera diff ered signif icantly among the three dietary treatment groups examined. Species ofAeromonasexist predominantly in aquatic environments and are recognized as opportunistic pathogens that cause diseases in humans and animals(Pessoa et al., 2020). Furthermore,Aeromonascan cause intestinal damage through the secretion of lipases, which can hydrolyze the lipids of the outermost cell layers of the intestinal tract (Beaz-Hidalgo and Figueras, 2013). In the present study, the relative abundance ofAeromonassignif icantly increased when f ishmeal was replaced with soybean meal by more than 50%, implying that excessive soybean meal in diets forM.rosenbergiimight aff ect the health state of the prawns.
Pseudomonads are common inhabitants of the aquatic environment, including culture ponds for shrimp (Otta and Karunasagar, 1999). The benef icial eff ects ofPseudomonashave been investigated,including reduced mortality from stress-induced furunculosis (Smith and Devey, 1993) and an antagonistic activity against pathogens (Chythanya et al., 2002). Our data showed lower relative abundance ofPseudomonasin groups FM75 and FM25 compared with in the FM100 group, indicating that increasing the proportion of soybean meal in the feed might lessen the anti-disease ability of the prawns. So decreased f ishmeal ratio in diet may potentially decrease the abundance of intestinal benef icial microbiota, aff ecting health and production of the prawns.
The genusStenotrophomonasof family Lysobacteraceae is common in natural environments(Zhang et al., 2020). In the aquatic environment,Stenotrophomonasspecies may function as acceptors,stable reservoirs, and potential vectors of antimicrobial resistance (Mançano et al., 2020). However, few studies have examined intestinal microbiota in cultured animals in relation to the use of f ishmeal replacement in their diet. The underlying reasons for the higher abundance ofStenotrophomonasin the FM group in the present study merits future research.Shao et al. (2019) reported that replacement of f ishmeal by fermented soybean meal did not signif icantly inf luence the intestinal microbiota of white shrimp; however, that study analyzed the intestinal microbiota only at the phylum level, and not genus level.
5 CONCLUSION
The soybean meal replacement to f ishmeal inM.rosenbergiipost-larvae was proved feasible, which will promote the healthy farming of the prawns. The replacement of dietary f ishmeal by soybean meal by up to 75% had no adverse eff ects on the growth ofM.rosenbergiipost-larvae, but the expression of TOR and S6K1 involved in mTOR signaling pathway was inhibited in prawns given a diet with 75% soybean meal. More than 50% replacement with soybean meal decreased survival and induced oxidant stress. The dominant phyla in the gut were Proteobacteria,followed by Firmicutes and Bacteroidetes, regardless of the level of f ishmeal in the prawns’ diet. A high level of f ishmeal replacement with soybean meal had a potential risk to increase relative abundances of several opportunistic pathogens in the gut when considered at the genus level.
6 DATA AVAILABILITY STATEMENT
Bacterial 16S ribosomal RNA gene sequences have been submitted to GenBank, and the SRA submission accession numbers are SRR12400109-SRR12400117.
The cDNA sequence data that support the f indings of this study have been deposited to NCBI with the accession codes of MT786656, MT786654, and AY626840.1.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
