Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
Ying JIANG , Lian-Gang LÜ , Zongwei LIU , Chunmei YANG , Jingsong GUO ,**
1 First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China
2 Laboratory for Regional Oceanography and Numerical Modeling, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 Key Laboratory of Marine Science and Numerical Modeling, Ministry of Natural Resources, Qingdao 266061, China
Abstract Although four species of odontocete and four species of baleen whale have been recorded in Prydz Bay, their vocalizations have been rarely investigated. Underwater vocalizations were recorded during March 2017 in Prydz Bay, Antarctica. Bio-duck sounds, downsweeps, inverted “u” shape signals, whistles,pulsed sounds, and broadband clicks were recorded. Bio-duck sounds and downsweeps were associated with Antarctic minke whales ( Balaenoptera bonaerensis) based on visual observations. Similarities between inverted “u” shape signals, biphonic calls, and clicks with vocalizations previously described for killer whales( Orcinus orca) lead us believe the presence of Antarctic killer whales. According to sound structures, signal characteristics, and recording location, Antarctic type C killer whales were the most probable candidates to produce these detected calls. These represent the f irst detection of inverted “u” shape signals in Antarctic waters, and the f irst report of Antarctic killer whale in Prydz Bay based on passive acoustic monitoring.The co-existence of Antarctic minke and killer whales may imply that minke whales can detect diff erences between the sounds of mammal-eating and f ish-eating killer whales. Our descriptions of these underwater vocalizations contribute to the limited body of information regarding the distribution and acoustic behavior of cetaceans in Prydz Bay.
Keyword: passive acoustic monitoring; inverted “u” shaped signal; killer whale; Antarctic minke whales;Prydz Bay; type C
1 INTRODUCTION
The highly generative waters around Antarctica attract or are home to many marine mammal species,such as pinnipeds and cetaceans. Because marine mammals produce various sounds for communication,prey and navigation, passive acoustic methods provide powerful tools to study their behavior,monitor their presence, and investigate their abundance and distribution.
Recently, passive acoustic monitoring (PAM) of aquatic mammals has been used widely to observe the presence, movement, and behavior of target species(reviewed by Mellinger et al., 2007). In the wild,PAM can be divided roughly into stationary and towed detection methods (Mellinger et al., 2007).Using stationary sensor, acoustic-based surveys can be used as a monitoring technique for surveying cetaceans in remote locations and adverse weather conditions. Towed hydrophone array on a shipboard line transect survey can be used to obtain locations of repeatedly vocalizing animals. PAM allows researchers to examine the variability in calling activities over a range of spatial and temporal scales.The ability to monitor throughout the night facilitates analyses of the acoustic activity of dolphins, which is not possible with visual monitoring and might allow the sampling of animals that cannot be observed in visual surveys (Soldevilla et al., 2010).
Identifying underwater sounds to the species level is an important step when processing passive acoustic recordings obtained in a marine environment. Various mysticete calls have been successfully identif ied and their signals have been detected automatically in passive acoustic recordings (e.g., Širović et al., 2004,2009; Oleson et al., 2007; Munger et al., 2008), as well as a few odontocete calls (e.g., Mellinger et al.,2004; Verfuß et al., 2007; Soldevilla et al., 2010), but the discrimination of most odontocete calls remains diffi cult.
Prydz Bay is a deep embayment off Antarctica between the Lars Christensen Coast and Ingrid Christensen Coast. Prydz Bay is located at the downstream end of a giant glacial drainage system that originates in the East Antarctic interior. The Lambert Glacier f lows from Lambert Graben into the Amery Ice Shelf on the southwest side of Prydz Bay.Other major glaciers drain into the southern end of the Amery Ice Shelf at 73°S where the marine part of the system starts at the modern grounding zone.Australia’s Davis, Russia’s Progress, Romania’s Law-Racoviţă, and China’s Zhongshan Stations are located on the southern side of Prydz Bay. Thus, Prydz Bay is a region of active oceanographic research.
The odontocete species detected in Prydz Bay include four species: beaked whales, sperm whales(Physetermacrocephalus), killer whales (Orcinusorca), and long-f inned pilot whales (Globicephalamelas) (Kasamatsu and Joyce, 1995). Beaked whales can produce stereotypical echolocation clicks with unique temporal and spectral characteristics(Baumann-Pickering et al., 2013). Sperm whales produce loud impulsive vocalizations and clicks(Douglas et al., 2005; Miller et al., 2008). The repertoire of long-f inned pilot whales comprises a variety of clicks and buzzes, broadband nonharmonic calls, and diff erent types of whistles, as well as diff erent types of pulsed calls ranging from simple calls with single segment to complex calls with up to six segments (Nemiroff and Whitehead, 2009; Vester et al., 2017). Killer whales are known to produce three main sound categories: echolocation clicks used for foraging and navigation, and pulsed sounds and whistles used for social activities (Ford, 1989). In recent years, high-frequency modulated signals have been described in several populations (Samarra et al.,2010; Filatova et al., 2012; Simonis et al., 2012;Andriolo et al., 2015). In addition, it has been reported that low-frequency (below 300 Hz) signals with an inverted “u” shape are produced by northeast Atlantic killer whales in Icelandic waters (Samarra et al.,2016).
Among the 13 species of baleen whale that are currently recognized globally, six can be def ined as true Antarctic whales: humpback (Megapteranovaeangeliae), blue (Balaenopteramusculus),minke (Balaenopterabonaerensis), f in (Balaenopteraphysalus), sei (Balaenopteraborealis), and southern right whales (Eubalaenaaustralis) (Leaper and Miller, 2011). Humpback, blue, f in, and Antarctic minke whales have been recorded in Prydz Bay(Leaper and Miller, 2011).
Although underwater vocalizations of cetaceans have been investigated in some locations, such as Ross Sea, Weddell Sea, and areas off Antarctic Peninsula (e.g., Širović et al., 2004, 2009; Richlen and Thomas, 2008; Van Opzeeland, 2010; Pitman et al., 2011; Risch et al., 2014; Dominello and Širović,2016; Reyes Reyes et al., 2017; Schall and Van Opzeeland, 2017), their vocalizations in Prydz Bay, a deep embayment off Antarctica between the Lars Christensen Coast and Ingrid Christensen Coast, to our knowledge were rare. Furthermore, low frequency inverted “u” signals also have not been previously described from Antarctic waters. Herein we report underwater marine mammal vocalizations recorded in Prydz Bay with a stationary sensor, and identify lowfrequency signals with an inverted “u” shape, whistles,pulsed sounds, clicks, bio-duck sounds, and downsweeps. To investigate relationships between vocalizations and mammal species, especially Antarctic killer whales, statistical analyses of call acoustic parameters are given.
2 METHOD
2.1 Data collection
Underwater sound recordings were acquired at two sites (S1 and S2) in Prydz Bay (Fig.1). Observations were conducted at S1 (69°03′S, 76°13′E) on March 5,2017 between 22:00 and 24:00 (local time). The sound data were collected at night so no visual observations of marine mammals were available at S1. Sound recordings were obtained at S2 (68°55′S,75°58′E) only in the presence of Antarctic minke whales on March 6, 2017 between 06:00 and 07:30(local time). During this period, researchers saw several Antarctic minke whales (6-8 individuals)swimming around the survey ship at a distance of no more than a few hundred meters.
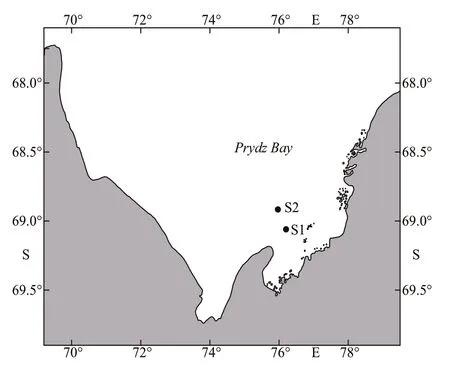
Fig.1 Locations where underwater sound observations were conducted in Prydz Bay
Recordings were acquired using a multi-channel recorder (LTT 186/16, Integrated System Ltd.) with a sampling frequency of 520.8 kHz and 16-bit resolution, and an omnidirectional hydrophone with a sensitivity of -170.1 dB re 1 V/μPa and a f lat frequency response from 10 Hz to 100 kHz (±2 dB). Sound data were stored on a computer hard disk connected to the recorder. The hydrophone was deployed at a water depth of 10 m from the Chinese icebreaker,Xue Long,(Snow Dragon in Chinese).
2.2 Acoustic and statistical analyses
Acoustic recordings were downsampled to 192 kHz and initially inspected using Adobe Audition 4.0 (Adobe Systems Inc., San Jose CA). Only recordings with a good signal-to-noise ratio (SNR)were included in the aural and visual inspections of the sounds and spectrogram (Rendell et al., 1999).The quality of the recordings was assessed based on two criteria: (1) clear contours with respect to the SNR; and (2) no overlap with more than one other call of the same type.
Vocalizations were divided into six acoustics categories: bio-duck calls, downsweep calls, inverted“u” shaped calls, whistles, pulsed calls, and click trains. Bio-duck calls are characterized by their repetitive nature, where most of the energy is located in the range of 50-300 Hz and with higher intensity harmonics up to 1 kHz (Van Opzeeland, 2010; Risch et al., 2014). A bio-duck call comprises several series where a series is def ined as a cluster of downswept pulses separated by less than 1 s (Dominello and Širović, 2016). Inverted “u” shaped calls are characterized by an inverted “u” contour with an increase in frequency followed by a decrease, ranging below 500 Hz, and with one or more harmonics(Samarra et al., 2016). Clicks are pulses of sound,typically in a series, and they are generally used as echolocation signals by odontocetes (Ford, 1989).Whistles have a nonpulsed or continuous waveform,which appears as a single narrow-band tone in the spectrogram with little or no harmonic or side-band structure (Ford, 1989). Pulsed calls are sounds made up of pulses generated at a high rate, and they are resolved as a sideband equivalent to the pulse repetition rate (Ford, 1989). According to Filatova et al. (2009), the pulsed sounds were divided into monophonic call types without an overlapping highfrequency component (HFC) and biphonic call types containing an overlapping HFC.
Initially, the vocalizations were qualitatively categorized using a triple blind, independent observer method (Soto et al., 2014). The vocalizations were originally categorized by a primary observer, before the initial categorizations of the vocalizations were then tested and verif ied by two other independent observers. The sound f iles were randomly sorted and relabeled. The other two observers had no information about the vocalization types. The same acoustics software (Adobe Audition 4.0) and spectrogram parameters were employed by the other two observers.The classif ications were compared to determine the number of f inal vocalization types determined by all observers (Rehn et al., 2011).
Various acoustic variables were measured for diff erent vocalization categories (Table 1). A custom code written in MATLAB R2016a (Mathworks,Natick, MA) was also used to obtain acoustic parameters. The mean and standard deviation of each parameter were calculated. Four primary acoustic variables were measured for each bio-duck and downsweep: (1) start frequency (Hz); (2) end frequency (Hz); (3) peak frequency (Hz); and(4) duration (s). Two additional values were determined from each bio-duck call: (1) inter-series interval and(2) inter-pulse interval. Five acoustic variables were measured for inverted “u” shaped calls, whistles, and pulsed calls: (1) start frequency (kHz); (2) end frequency (kHz); (3) minimum frequency (kHz); (4)maximum frequency (kHz); and (5) duration (s).Clicks with a SNR higher than 20 dB were selected to measure acoustic parameters, where the SNR was calculated by computing the root mean squared sound pressure level in a window that covered 95% of the relative energy (Madsen and Wahlberg, 2007). Four variables were obtained for each click: -3-dB and -10-dB durations, peak frequency, and -10-dB bandwidth.
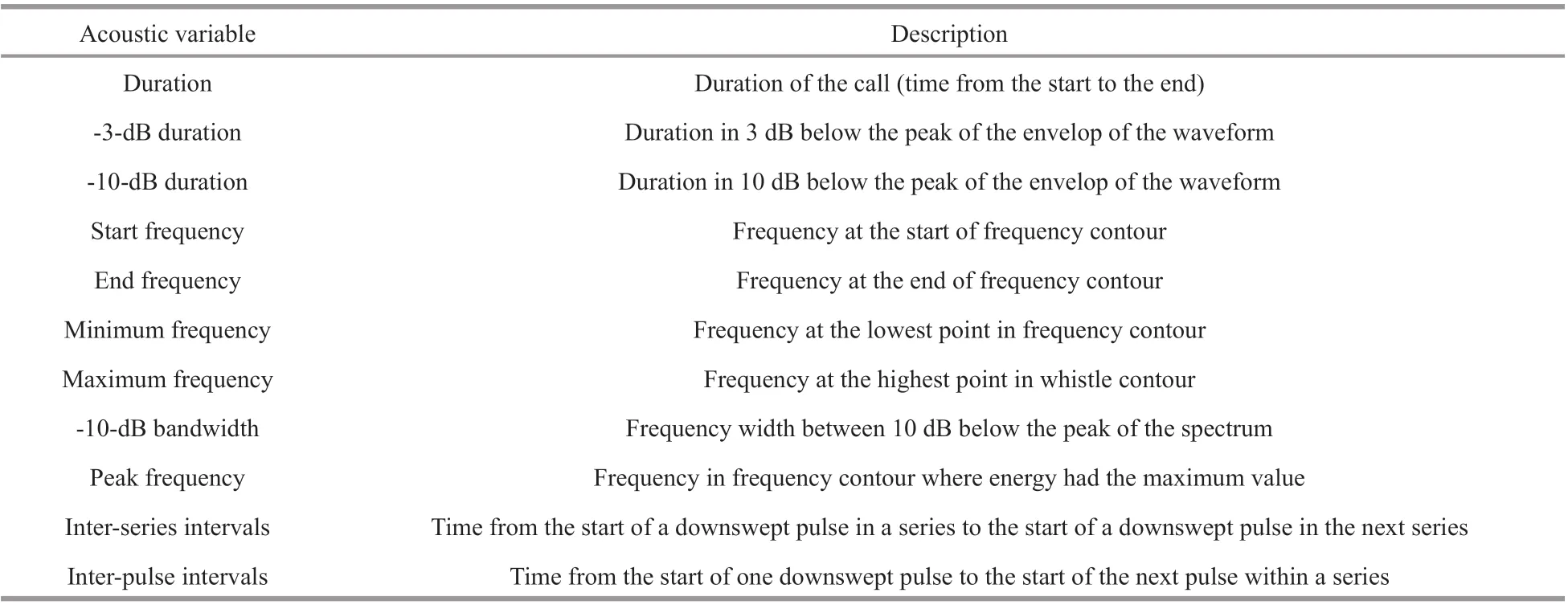
Table 1 Descriptions of acoustic variables
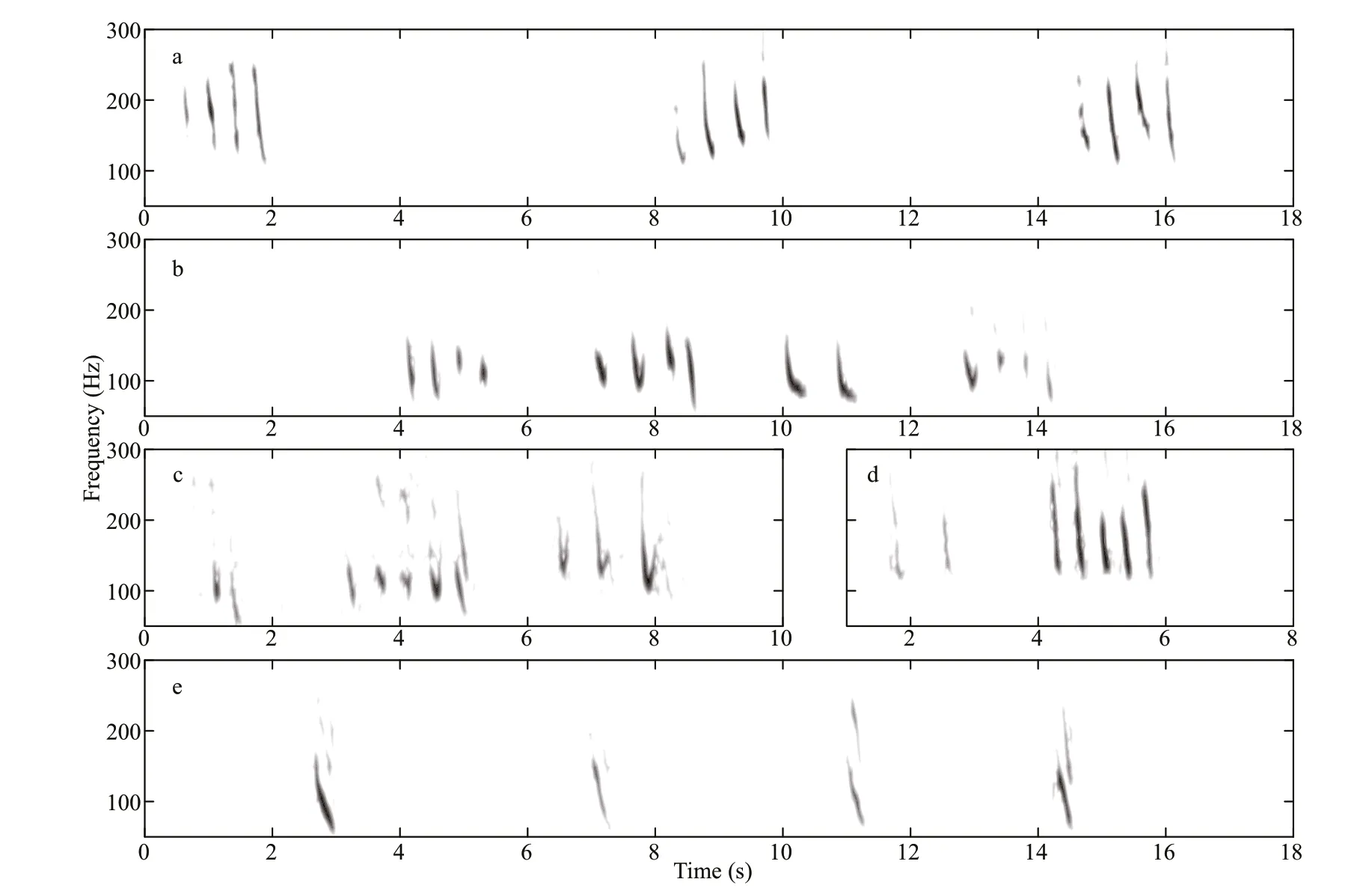
Fig.2 Representative spectrograms of bio-duck sounds (a-d) and four concatenated downsweeps (e) (512 point fast Fourier transform (FFT); 90% overlap; Hann window)
3 RESULT
Whistles, pulsed sounds, clicks, inverted “u”shaped calls, bio-duck sounds, and downsweeps were detected in the recordings obtained from S1, but only bio-duck and downsweep sounds were identif ied in the recordings acquired from S2.
3.1 Bio-duck calls and downsweeps
The bio-duck calls comprised a series of 2-5 downswept pulses with a mean peak frequency at 138 Hz (Fig.2a-d; Table 2). The individual downswept pulses had a mean duration of 0.25 s. This calls had similarities in terms of the frequency range and pulse number with previously described calls (Risch et al.,2014; Dominello and Širović, 2016). Only eight bioduck calls were detected in the recordings obtained from S1 and one in the recordings acquired from S2.One call had a consistent number (four) of pulses in a series (Fig.2a), whereas the others comprised series with a variable number (2-5) of pulses (Fig.2b-d).The inter-series interval had a mean±standard deviation of 4.9±2.5 s, and the inter-pulse interval had a mean±standard deviation of 0.46±0.12 s. All of the bio-duck calls belonged to type A, which is a common Antarctic minke whale call (Dominello and Širović,2016).
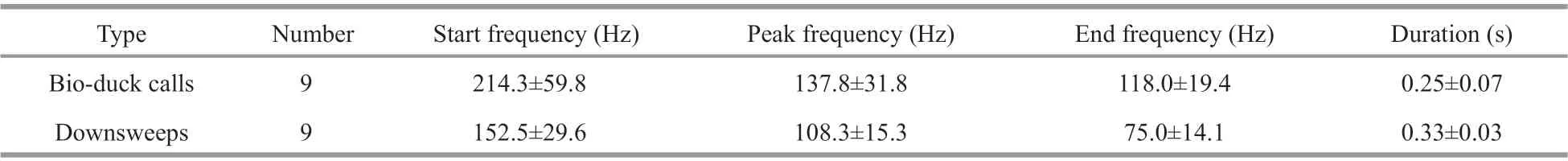
Table 2 Acoustic parameters (mean±standard deviation) determined for bio-duck and downsweep calls

Table 3 Acoustic parameters (mean±standard deviation) obtained for whistles
Nine individually occurring downsweeps (Fig.2e)were identif ied in our recordings (eight from S1 and one from S2). Their spectrograms were similar to those obtained previously for the downsweeps from Antarctic minke whales (see Fig.2d in Risch et al.,2014; and Fig.2f in Dominello and Širović, 2016).The frequency and temporal parameters measured in the present study (Table 2) were also similar to those reported previously (Risch et al., 2014; Dominello and Širović, 2016).
3.2 Inverted “u” shaped calls, whistles, pulsed sounds, and click trains
Low frequency calls were characterized by an inverted “u” shape, an increase followed by a decrease in frequency, with at least one partially visible harmonic (Fig.3). The inverted “u” shape calls had an average maximum frequency of 128.8±14.7 Hz, an average minimum frequency of 64.3±8.8 Hz, and an average duration of 0.33±0.03 s (n=22). These calls were characterized by a start frequency of 113.1±20.5 Hz and an end frequency of 62.6±5.4 Hz.Calls were generally asymmetrical, i.e., higher start frequency and relatively lower end frequency(Samarra et al., 2016), and very similar (in terms of the spectrogram shape, frequency range, and oscillogram) to the low frequency signals measured in Icelandic waters, described as an inverted “u”shape and related to killer whales (see Fig.1 in Samarra et al., 2016).
Whistles displayed much higher frequencies than inverted “u” shaped calls, with 94 signals detected and 85 of whose parameters were calculated (Table 3).Their frequencies ranged about from 5.56 kHz to 7.53 kHz. The mean of duration was usually 0.54 s.81.2% of whistles were with no harmonic. The basic fundamental contour shapes of these signals were shown as constant frequency (Fig.4a), downsweep(Fig.4b), and scarce as convex (Fig.4c). The most common shape was downsweep, occupying 70.6%.Whistles with no harmonic usually had higher frequencies than those with harmonics.
With 38 calls detected, pulsed sounds were common in our recording. Such calls were complex and composed of several call parts. The pulsed sounds included monophonic call types (Fig.5a), and biphonic call types (Fig.5b-c). Only one monophonic call was detected. The biphonic call types comprising overlapping lower-frequency component (LFC) and HFC were rich in harmonics, but highly variable in the extent of frequency modulation; for example,despite similarities in the biphonic calls depicted in Fig.5b-c, the LFC was highly frequency-modulated(indicated by several inf lection points) and the HFC frequency greater in the latter than former.
Biphonic calls with clear spectrogram signatures that did not overlap with other calls (n=37) were selected for measuring the acoustic parameters. The acoustic parameters were measured separately for both LFC and HFC structures in biphonic calls(Table 4). The HFC duration was slightly longer than that of the LFC. The LFC frequency was typically below 6 kHz, and the HFC frequency was typically centered above 6 kHz. It seemed like a frequency boundary existed between LFCs and HFCs.
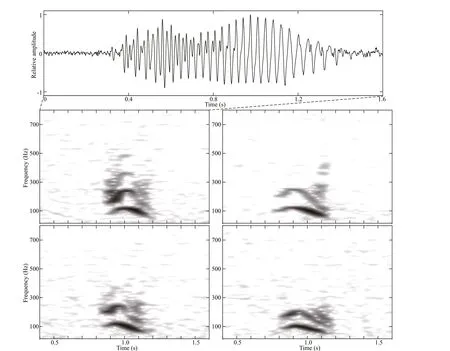
Fig.3 Representative spectrograms of inverted “u” shaped calls with the waveform for one example shown at the top (1 024 point FFT; 90% overlap; Hann window)
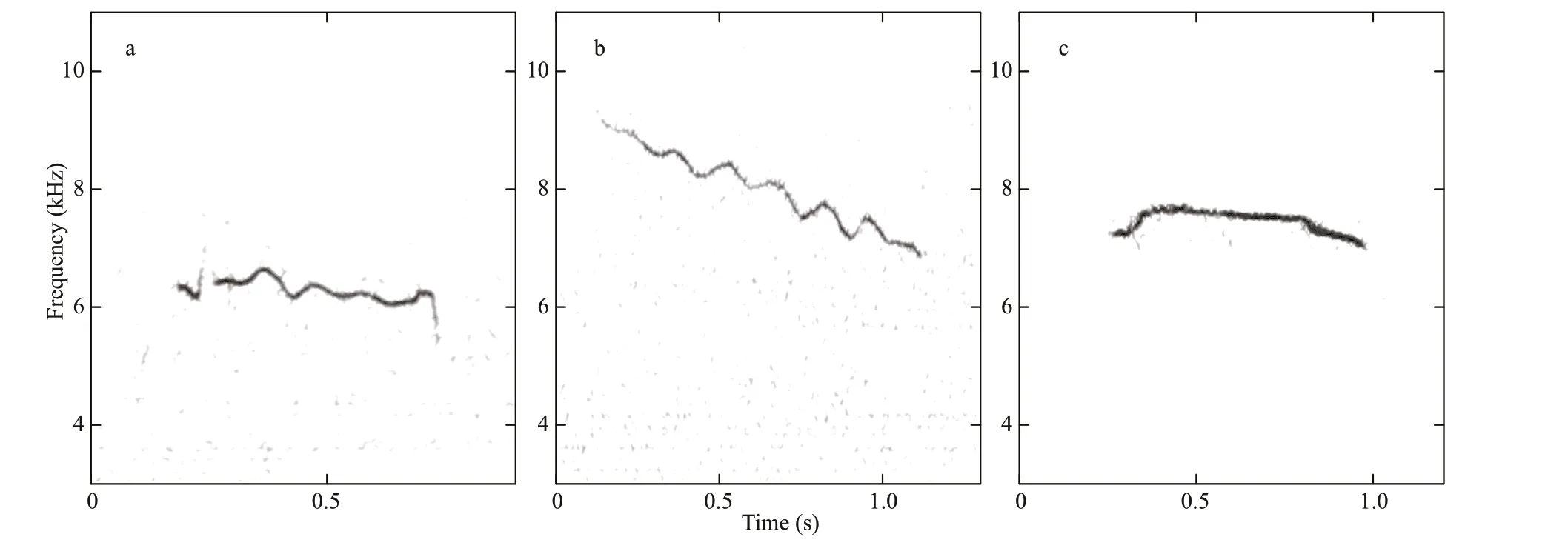
Fig.4 Examples of the spectrograms obtained for whistles lumped according to the basic fundamental contour shapes (4 096 point FFT; 90% overlap; Hann window)
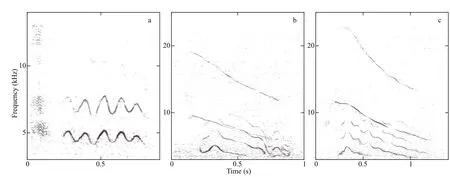
Fig.5 Representative spectrograms of pulsed sounds (4 096 point FFT; 90% overlap; Hann window)

Table 4 Acoustic parameters (mean±standard deviation) obtained for whistles and pulsed sounds
With 281 click trains detected, clicks were the prevalent call type in our recording. A total of 717 clicks with SNR higher than 20 dB were selected to parameter measurement. The clicks were broadband pulses (-10-dB bandwidth of 16 kHz) with a -3-dB duration of 58 μs and a -10-dB duration of 125 μs.The peak frequency ranged from 12 to 44 kHz with a mean±standard deviation of 17±4 kHz. Measurable energy usually extended to 50 kHz and occasionally to 80 kHz.
4 DISCUSSION
4.1 Presence of Antarctic minke whales
The bio-duck and downsweep sounds recorded at S2 in the presence of Antarctic minke whales which were identif ied in visual supported the attribution of these sounds to Antarctic minke whales. Thus,although no concurrent visual observations were available at S1 (our recordings were made at night),the occurrence of bio-duck calls and downsweeps suggested that Antarctic minke whales were also around site S1.
Bio-duck calls have been recorded at various locations in the Southern Ocean, but the origin of these signals remained unknown for many years (Van Opzeeland, 2010). Evidence that this sound is produced by Antarctic minke whales was only obtained recently by tag deployment on an individual in Wilhelmina Bay off the western Antarctic Peninsula(Risch et al., 2014). Antarctic minke whales also produce low frequency downsweeps (Risch et al.,2014). Therefore, bio-duck calls and downsweeps can be used to investigate the seasonal trends and diel patterns in Antarctic minke whale call production,and their relationships with sea ice cover (Dominello and Širović, 2016). The bio-duck and downsweep call rates should be low during this season because of the extensive sea ice over (Dominello and Širović, 2016),which is consistent with our results.
4.2 Presence of killer whales
The relative time of occurrence for each signal type during our entire recording at S1 is depicted in Fig.6. The signals containing clicks, pulsed calls,whistles, and inverted “u” shaped calls had strong temporal correlations. Among the odontocete species reported in Prydz Bay, only killer whales and long f inned pilot whales have been shown to produce all of the recorded sound types comprising whistles,echolocation clicks and pulsed sounds (Reyes Reyes et al., 2017).
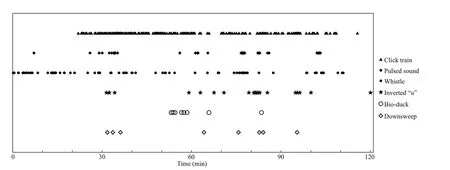
Fig.6 Relative timings of call types during acoustic recordings obtained at site S1
Only killer whales, false killer whales, and both species of pilot whales have been reported to produce calls as a lower component of biphonic sounds(Filatova et al., 2016), and the frequency features of biphonic calls in our recordings, such as the apparent frequency boundary between the LFCs and HFCs,linked our pulsed calls with those made by killer whales (Filatova et al., 2016).
Only killer whales and long f inned pilot whales produce whistles in Prydz Bay. The sound structures of the whistles determined in the present study (Fig.4)were similar to those made by killer whales (Richlen and Thomas, 2008; Schall and Van Opzeeland, 2017).
Odontocetes are known to produce echolocation click signals, thereby making killer whales, beaked whales, sperm whales, and long-f inned whales possible candidates for the production of the clicks in our recordings. The echolocation clicks produced by beaked whales comprise 250-μs transients with a peak energy in the 30-40-kHz band (Madsen et al.,2005). Sperm whale clicks have been described as multipulsed, long duration signals with a moderate intensity, and a spectrum peaking below 13 kHz(Møhl et al., 2003). The echolocation clicks from long-f inned pilot whales have higher peak frequencies than those produced by killer whales, with a mean peak frequency at 50 kHz (Eskesen et al., 2011). In addition, the echolocation clicks with a peak frequency around 17 kHz and duration of 125 μs in our recordings were similar to the clicks described for populations of killer whales in the northwestern Antarctic Peninsula(Reyes Reyes et al., 2017) and southern California Bight (Gassmann et al., 2013). These similarities suggest that our click signals were produced by Antarctic killer whales.
Inverted “u” shaped calls were f irst reported in Iceland and conclusively (by tagging) attributed to killer whales (Samarra et al., 2016). Similarities in oscillogram, spectrogram shape, and frequency range of our Antarctic sounds and the Icelandic inverted “u”shaped calls (Samarra et al., 2016) suggest a conspecif ic origin. However, it is possible that the inverted “u” shaped calls could have been produced by another species, e.g., humpback whale (Helble et al., 2015), and thus visual conf irmation of the presence of killer whales during sound production will be needed in the future.
These reasons lead us believe that our clicks,pulsed sounds, whistles, and inverted “u” shape signals were produced by Antarctic killer whales.These signals are also appropriate targets for PAM methods for killer whales.
The underwater vocalizations of Antarctic killer whales have been investigated in locations such as Ross Sea, Weddell Sea, and areas off the Antarctic Peninsula, but to the best of our knowledge, the vocalizations of killer whales have not been reported previously in Prydz Bay. Furthermore, there are few descriptions of the acoustic repertoires of Antarctic killer whales. Clicks, whistles, pulsed signals, and buzzes have been recorded in Ross Sea (possibly type C), but the clicks have not been analyzed due to the frequency limits of the recordings (Richlen and Thomas, 2008). Additional signals produced by Antarctic killer whales have been described recently,especially high-frequency modulated signals from killer whales (possibly type A) in the northwestern Antarctic Peninsula (Reyes Reyes et al., 2017), and calls produced by type C killer whales in the eastern Weddell Sea coast (Schall and Van Opzeeland, 2017).Low frequency inverted “u” shaped calls have not been previously described from Antarctic waters.Thus, our descriptions of inverted “u” shaped calls,whistles, pulsed sounds, and clicks could help to understand the call repertoires of Antarctic killer whales.
4.3 Type C killer whales
Fish-eating killer whales in the Northern Hemisphere are known to frequently produce sounds(Filatova et al., 2013), whereas mammal-eating killer whales generally restrict sound production to avoid alerting their acoustically sensitive prey (Deecke et al., 2005). Additionally, Icelandic killer whales that produce low-frequency inverted “u” signals are also f ish-eaters (Samarra et al., 2016). Accordingly, we believe that the high whistle and click call rate, and low-frequency inverted “u” signals in our recording are indicative of the occurrence of f ish-eating killer whales around our recording site.
The sound structures of the whistles (Fig.4) were similar to the sounds from type C killer whales(Richlen and Thomas, 2008; Schall and Van Opzeeland, 2017). The similarity between the calls depicted in Fig.5 of this study and those in call type AM1 and AM7 (Richlen and Thomas, 2008) suggest the potential presence of type C killer whales.Furthermore, 76.3% of the pulsed calls in our recording had similar sound structures to the call segments of type C killer whales (Schall and Van Opzeeland, 2017).
Type C and D killer whales consume f ish in the waters around Antarctica (Pitman and Ensor, 2003;Pitman et al., 2011). Type C occurs mostly off East Antarctica, whereas type D has a distribution in sub-Antarctic waters. Given the locations of our study sites in Prydz Bay, the killer whales around the recording sites were possibly type C. Furthermore,the vocal activities of the killer whales also supported this conclusion (Barrett-Lennard et al., 1996; Van Opzeeland et al., 2005; Filatova et al., 2013).
Therefore, although no visual observations were made in the area, we suggest that the clicks, pulsed sounds, whistles, and inverted “u” shaped calls in our recording were probably produced by Antarctic type C killer whales.
The occurrence times shown in Fig.6 suggest that Antarctic minke and killer whales probably co-existed around site S1, thereby indicating that Antarctic minke whales were aware that they could vocalize while killer whales were also present in the area. That implies Antarctic minke whales may be able to detect diff erences between the sounds of mammal-eating versus f ish-eating killer whales.
5 CONCLUSION
We reported minke whale sounds (bio-duck and downsweep calls) and the calls of killer whales(clicks, pulsed calls, whistles, and inverted “u” shape calls) in Prydz Bay. The whistles and pulsed calls resembled those described previously for type C killer whales, thereby suggesting the presence of this type of killer whale, even without visual conf irmation. The co-occurrence of calls made by Antarctic minke and killer whales may imply that minke whales can detect diff erences between the sounds of mammal-eating and f ish-eating killer whales.
Inverted “u” shape calls were f irst found in Antarctic waters. Their similar sound structures to the previous reported signals of killer whales and cooccurrence with other killer whale sounds suggest that these signals were produced by type C killer whales. Further research is needed to conf irm whether Antarctic type C killer whales produce inverted “u”shape calls, and what the social function(s) of these sounds may be. From the perspective of killer whale evolution, the bipolar occurrence of inverted “u”shape signals warrants additional study.
Our descriptions and interpretations of underwater vocalizations contribute to the limited body of information regarding the distribution and acoustic behavior of cetaceans in Prydz Bay, and they might be important for PAM studies in Antarctic waters.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENT
The authors thank the crew of the Chinese icebreakerXue Longfor support in data collection.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
- The phycocyanin-chlorophyll-protein complexes isolated from Chroomonas placoidea*
