Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
Wangwang YE , Guanxiang DU , Honghai ZHANG , Guiling ZHANG ,**
1 Frontiers Science Center for Deep Ocean Multispheres and Earth System, and Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, China
2 Key Laboratory of Global Change and Marine-Atmospheric Chemistry, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen 361005, China
3 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
Abstract The Yellow Sea (YS) and East China Sea (ECS) are important marginal seas of the western Pacif ic. Understanding the dynamics of methane (CH 4) in the YS and ECS are essential to evaluate the role of coastal seas in global warming. We measured dissolved CH 4 at various depths in the water column of the YS and ECS during a cruise from March to April 2017. The concentrations of CH 4 varied greatly in diff erent water masses, suggesting that the hydrographic conditions can substantially aff ect the CH 4 distribution. The CH 4 budget in the shelf of the ECS, which was estimated with a box model, suggests CH 4 consumption in the water column was the major sink (>95%), followed by a loss with a total of 2.2% CH 4 released to the atmosphere. Overall a local CH 4 production of 0.28 nmol/(L·d) was needed to maintain the CH 4 excess. Results from laboratory incubations showed an increase in CH 4 (1.5 times higher than the value of the control) after the addition of dimethylsulfoniopropionate (DMSP). Field incubations result in a CH 4 production rate of 1.2 nmol/(L·d) under a N-stressed conditions (N:P<1), indicates that the DMSPdependent CH 4 production prefer to occur in the oligotrophic seawaters, where nitrogen is depleted. This study demonstrates that the marginal seas of China is a hotspot for CH 4 dynamics, and the cycling of methylated sulfur compounds (such as DMSP) may contribute importantly to locally formed CH 4. This may have further implication to carbon and sulfur biogeochemical cycles in the western Pacif ic.
Keyword: methane; East China Sea; Yellow Sea; aerobic production; dimethylsulf ide (DMS);dimethylsulfoniopropionate (DMSP)
1 INTRODUCTION
Methane (CH4) is an important greenhouse gas that inf luences the global climate (IPCC, 2013). Emissions of individual sources have been poorly quantif ied,even though the total global emissions of CH4into the atmosphere have been reasonably constrained(Saunois et al., 2020). Oceans cover 71% of the planet’s surface and are considered a minor source of atmospheric CH4(Saunois et al., 2020). The CH4concentrations in the surface water of most of the world’s oceans are 5%-75% supersaturated with respect to atmospheric CH4(Karl et al., 2008; Damm et al., 2009), implying a local in-situ methane source.In particular, studies showed that near-shore environment contributes the largest but most uncertain diff usive f lux of the oceanic CH4, but comprise only 3% of ocean surface areas (Weber et al., 2019). This observation can be partially attributed to the high CH4production rates and fast ventilation of the shallow water column (Borges et al., 2018; Schmale et al.,2018), or CH4escape from localized gas seeps via bubbles (Sakai et al., 1990; Judd, 2004; Shakhova et al., 2014; Di et al., 2020).
The Yellow Sea (YS) and East China Sea (ECS)are marginal seas in the northwestern Pacif ic Ocean,with average water depths of 44 m and 350 m,respectively. The distribution of dissolved CH4in the YS and ECS exhibits great temporal and spatial variability, which has been well documented in previous studies (Zhang et al., 2004, 2008a, b; Yang et al., 2010; Ye et al., 2016; Sun et al., 2018). Although several studies have reported that the surface and subsurface layers is supersaturated with CH4with respect to the atmosphere (Zhang et al., 2004; Ye et al., 2016; Sun et al., 2018), the mechanisms are still unclear.
Physical processes can contribute to the CH4accumulation at the ocean surface, including lateral transport from the CH4-rich sources (e.g. estuaries;Borges et al., 2018) or vertical diff usion from subsea CH4-hotspots (e.g. gas hydrates, cold seeps, and pockmarks) (Judd, 2004; Shakhova et al., 2014; Di et al., 2020). In the ECS, CO2-rich f luids with other residual gas (including CH4) were observed to emerge from the sea f loor in the hydrothermal f ield (Sakai et al., 1990), suggesting the gas hydrates can serve as potential source to the aqueous CH4(Luong et al.,2019). Biotic factors contributing to the excess CH4was generally attributed to local production by microorganisms in anoxic microniches (de Angelis and Lee, 1994; Karl and Tilbrook, 1994; Stawiarski et al., 2019; Wäge et al., 2020). A recent mechanism that was proposed for maintaining surface CH4excess is that CH4is released as a byproduct via the decomposition of methylated compounds, such as methylphosphonate (MPn) and dimethylsulfoniopropionate (DMSP), when heterotrophic bacteria compete for nutrients (Repeta et al., 2016; Teikari et al., 2018). Studies for MPn identif ied the gene content and expression and thus conf irmed the possibility of a Carbon-Phosphorus pathway for CH4formation at the biological level(Metcalf et al., 2012; Martínez et al., 2013; Sosa et al.,2017, 2020). Alternatively, DMSP is mainly produced in the cells of phytoplankton and can be transferred to the ambient water by grazing or sloppy feeding, so the yield and metabolism ratio of DMSP might be important for CH4formation. Damm et al. (2008)reported CH4production in the Arctic sea that was associated with the metabolism of DMSP and its degradation products. However, evidence for the formation of CH4from DMSP remains ambiguous and is mainly based on occasional correlations between CH4and DMSP concentrations (Damm et al., 2008; Florez-Leiva et al., 2013; Zindler et al.,2013; Damm et al., 2015; Zhai et al., 2019). In addition, the cleavage of the Carbon-Sulfur bond pathway (DMSP-to-CH4) for CH4production has been rarely reported in other marine regimes, such as the marginal seas of the Pacif ic.
In this study, we examine the excess CH4in the upper water column of the YS and ECS and combine hydrographic conditions to discuss the factors that may inf luence the CH4dynamics in oxygenated waters. Then, we develop a preliminary CH4budget using a box-model to quantify CH4sources and sinks in the shelf of the ECS and evaluate the contribution from local CH4production to maintenance of the water column’s CH4inventory. Finally, in combination with the f ield observation, we conduct laboratory incubation experiments alongside f ield observations to provide evidence that DMSP can serve as a potential precursor of CH4, probably via demethylation in the YS and ECS. Thus, the understanding of CH4dynamics in the YS and ECS was improved by providing new data, conducting incubation experiments, interpreting these data and comparing with previous results (Zhang et al., 2004; Ye et al.,2016; Sun et al., 2018).
2 MATERIAL AND METHOD
2.1 Research area
The YS is a semi-enclosed continental shelf sea and is connected to the ECS and the Bohai Sea. The boundary between the ECS and YS spans from the northern tip of the mouth of the Changjiang (Yangtze)River to Cheju Island (Fig.1a). In winter and early spring, strong northerly winds that accompany a surge of cold, dry continental air tend to vertically homogenize most of the YS (Su, 1998). The coastal current f lows southward near the Shandong Peninsula in the north and along the 50-m isobath in the south(Fig.1a). Water exchange between the YS and ECS mainly occurs near this boundary. Thus, three dominant water masses exist in the YS in spring,namely, the coastal water (CW) in the west, YS Central Water (YSCW) in the central trough, and YSECS Mixed Water (YEMW) near the boundary (Li,1989; Su, 1998; Guan and Fang, 2006).

Fig.1 Schematic diagram of the circulation pattern in spring
In spring, the western YS is mainly inf luenced by the Coastal Water, which is fresh (salinity <32.0) and nutrient poor (nitrate <1.0 μmol/L) (Jin et al., 2013).The coastal water contains two components in the YS: Shandong coastal water forms around the Shandong Peninsula and f lows southward along the 40-50-m isobaths, while the Subei CW lies in the southern, coastal regions, f lowing southeast to the ECS and merging with remnants of Changjiang diluted water (Hickox et al., 2000). The YSCW is one of the main water masses that exist in the central YS throughout the year. This water displays great seasonal diff erences in salinity (31.0-32.0) and temperature(5.0-25.0 °C) because of seasonal stratif ication (Su,1998). The YEMW is present in the southeastern YS,which consists of a branch of warm and saline water(from the ECS) that is mixed with YSCW. Thus, the YEMW exhibits high temperature (>9.0 °C) and salinity (>32.5) in spring.
In the ECS, the Changjiang River introduces freshwater from the west and f lows southward in spring (Guan and Fang, 2006). From the east, the Kuroshio introduces water of high temperature and salinity, f lowing northeast along the edge of the ECS and eventually turning east to the Korea Strait and Japan Sea (Fig.1a) (Tang, 1997; Li et al., 2012; Qi et al., 2014). Water exchange between the ECS and Kuroshio occurs across the shelf break through frontal processes at the surface and upwelling in the subsurface (Yang et al., 2018; Zhou et al., 2018).Importantly, this exchange introduces an intrusion of shelf water to the Kuroshio as turbidity tongue,increasing the exchanges in materials (such as nutrients and rare earth element) (Matsuno et al.,2009; Luong et al., 2018; Wang et al., 2019) and may aff ect the CH4concentrations at the Kuroshio subsurface (Luong et al., 2019). From the south, the Taiwan current f lows to the north throughout the year between the 50- and 100-m isobaths (Su, 1998). In this study, we classif ied four dominant water masses in the ECS, namely, the coastal water (CW), ECS Shelf Water (ESW), Taiwan Warm Current Water(TWCW), and Kuroshio Water (KW) (Qi et al., 2014).The Kuroshio Water is usually considered to consist of four water masses, namely, the Kuroshio Surface Water (KSW), Kuroshio Sub-Surface Water (KSSW),Kuroshio Intermediate Water (KIW), and Kuroshio Deep Water (KDW) (Su, 1998; Zhou et al., 2018).The coastal water in the YS and ECS can be divided into three components: the Shandong, the Subei, and the Zhe-Min CW (Fig.1a). For simplicity, we refer to these three water masses as coastal water in this study.
The Zhe-Min CW (Fig.1a) forms from the mixing of seawater with runoff from the Changjiang River,Minjiang River, and other rivers. From spring to summer, the coastal water expands eastward and retreats northward, becoming warmer and fresher(salinity <32.0; Fig.2b) (Qi et al., 2014). In contrast,the Kuroshio water on the outer shelf of the ECS showed relatively high temperature (>23.0 °C) and salinity (>34.2). The TWCW originates from the Taiwan Strait, bringing warm (>17.0 °C) and saline water (34.0-34.5) to the ECS (Yang et al., 2018; Zhou et al., 2018). This water runs northward along the 60-m isobath parallel to the coastal line and encounters the Subei CW in the eastern region off the Changjiang River (Fig.1a). The water mass in the middle continental shelf consisted of ECS water, TWCW,and a mix of KW and CW. These water masses have similar temperature-salinity (T-S) characteristics (Qi et al., 2014), so we collectively call them ESW.
2.2 Field sampling
In early spring of 2017, a cruise was conducted in the YS and ECS on the R/VDongFangHong2from 27 March to 11 April. A total of 57 stations along 10 lines were investigated during the cruise to obtain the overview distribution of biogenic gases in the study area (Fig.1b). Surface seawater for laboratory incubation experiments was collected from the coastal areas of the YS from November 2017 to March 2018.Seawaters for f ield incubation experiment were collected from the western North Pacif ic in a cruise during October 2018.
Water samples were collected at various depths by using 10-L Niskin bottles that were mounted on a rosette. At each station, we used Sea-Bird conductivity,temperature, and depth (CTD) probes (SBE 911 plus),which were equipped with ancillary sensors, to measure the salinity, temperature, and dissolved oxygen (DO) as functions of depth. Replicate samples for CH4analysis were obtained from the Niskin bottles in 100-mL glass vials by an overf low of approximately 1.5-2 times the bottle volume without bubbles. Afterwards, saturated mercuric chloride(HgCl2) was added into the vials to inhibit microbial activity. Then, the vials were immediately sealed with poly tetra f luoroethylene (PTFE)/silicone septum and aluminum caps to avoid air contamination. Water samples for determining the dimethylsulf ide (DMS)and DMSP concentrations were collected by using acid-cleaned polycarbonate bottles through silicone tubing that was attached to the Niskin bottles.
2.3 Box-model
The CH4budgets for the ECS shelf were evaluated by using a box-model, which was based on the concept of the conservation of water and salt masses(Chen and Wang, 1999; Zhang et al., 2007, 2018;Günthel et al., 2019). Brief ly, we assume that the CH4inventory was temporarily constant in a specif ic water mass. Thus, the CH4concentration in these diff erent water masses was the mean value of the stations thatbelonged to these distinct water masses (classif ied by the salinity and temperature, Fig.2). Hence, the CH4f luxes (Fi, mol/s) during water transport could be obtained simply by the water f lux (Qi, m3/s) times the CH4reservoir (Ci, nmol/L) in a specif ic water mass:∑Qi×Ci=Fi. Then the CH4mass balance in the ECS shelf can be expressed as: ∑Fi+Rair+Rsed+Rcon+Rpro=0,whereFirepresents the water-transported CH4input(+) and outf low (-) over the shelf (Table 1),R(mol/s)indicates the rates of CH4accumulation (+)/elimination (-), including sea-to-air exchange (Rair),sedimentary release (Rsed), microbial consumption(Rcon), and production (Rpro). TheRairandRsedwere computed by the interface-f luxes (μmol/(m2·d)) times the shelf area (~5.1×105km2) as indicated by Ye et al.(2019). TheRconwas calculated by the shelf water volume (~2.7×1014m3) multiplies the CH4oxidation rate (OR, nmol/(L·d)). The OR was calculated using a f irst-order equation (OR=k×CESW) as reported by Rogener et al. (2018, 2019), where k is the turnover rate constant andCESWis the mean CH4concentration in the ESW (Table 1). The net production rate (Rpro)was the balance of other sources and sinks.
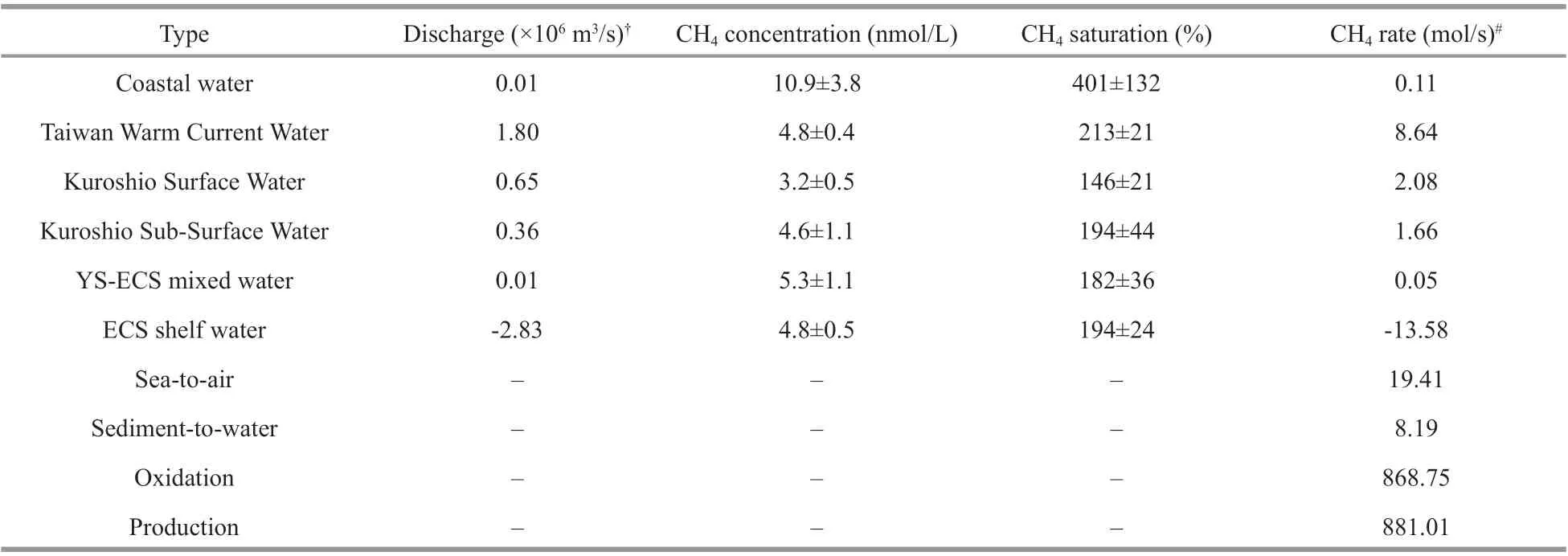
Table 1 CH 4 concentration in water mass and the CH 4 rate used in box-model in the ECS
2.4 Incubation experiment
Four separate incubation experiments were conducted to study the links between DMSP/DMS degradation and CH4production. The laboratory experiments were conducted at the Ocean University of China (Qingdao) and the f ield incubation experiment was conducted onboard. The seawater for these experiments was placed into an acid-cleaned polycarbonate carboy before the start of the experiment. For the f irst experiment, a f inal concentration of 100-μmol/L DMSP was directly added into the carboy. After mixing homogeneously,the seawater in the carboy was transferred into 160-mL glass bottles as the DMSP-amended group.Subsamples were dispensed into the glass bottles prior to the addition of DMSP as the control treatment.This experiment was conducted in November 2017 and repeated in January 2018. The second incubation experiment was set to identify the eff ect of nutrientstressed conditions (in particular, N def iciency) on DMSP-dependent CH4production. Specif ically, the carboy was amended with phosphate (P) to a f inal concentration of 10.0-μmol/L. Subsamples were dispensed into the glass bottles as the control treatment. Afterwards, DMSP was added to the carboy to a f inal concentration of 1.0 mmol/L, and then the water was placed into another group of glass bottles as the (P+DMSP) treatment (N:P<1, N-depleted).Finally, additional subsamples were collected after the carboy was sequentially amended with 50.0-μmol/L and 160.0-μmol/L nitrate, which were the (P+DMSP+N) treatments (5<N:P<16, N-stressed or N-rich). The third incubation experiment was implemented to test the direct eff ect of spiked DMS on CH4production. Similarly, subsamples were sequentially transferred from a carboy that was amended with diff erent nutrients, including glucose(100.0-μmol/L C), nitrate (16.0-μmol/L N), phosphate(1.0-μmol/L P), and DMS (100.0 mmol/L). Then, the bottles were assigned to the control (without addition),the (C+N+P) treatment, and the (C+N+P+DMS)treatment. The fourth incubation was conducted in the western North Pacif ic to test the DMSP-dependent CH4production in the oligotrophic areas (station E24,130°E/5°N, see detail in Ye et al. (2020)). The DMSP(f inal concentration of 10.0 μmol/L) was amended directly into the surface waters without any other additions, which was the DMSP treatment. Natural seawaters incubated under the same conditions then assigned to the control. All the bottles were f illed with 150-mL seawater and 10-mL headspace volume(except for the fourth experiment with 50-mL seawater and 10-mL headspace volume), sealed with PTFE/silicone septum, crimped with aluminum caps,and incubated in a 12-h:12-h light-dark cycle incubator or in the onboard-surface-water-circulationsystem that was maintained at nearly in-situ temperatures (~25 °C). At each time point per day,triplicate bottles per treatment were randomly sacrif iced to obtain the CH4and DO concentrations.The DO was measured by using the Winkler titration method (Bryan et al., 1976).
2.5 Chemical analyses
CH4was determined by the headspace method for the incubation samples (Bange et al., 2010) or the gas-stripping method for the f ield samples (Zhang et al., 2004). The CH4in the headspace was measured by a Shimadzu GC-14B gas chromatograph that was packed with Porapak Q (80-100 mesh) and f itted with a f lame ionization detector (FID). The CH4was quantif ied by calibrating peak areas to the FID’s response to a three-point calibration by using knownvolume injections of CH4standards (CH4:N2mixtures of 2.0×10-6, 4.0×10-6, and 50.0×10-6, China Institute of Metrology). For the f ield samples, seawater was introduced into a stripping chamber and purged with high-purity nitrogen. After bubbling, the dissolved CH4was passed through a desiccant tube to remove water vapor. The CH4was concentrated onto a stainless steel trap that was f illed with 80-100 mesh Porapak Q and then was released into a Shimadzu GC-14B gas chromatograph for separation and quantif ication (Zhang et al., 2004).
A purge-and-trap system was used to analyze the DMS concentration, as previously described by Yang et al. (2008). Dissolved DMSP (DMSPd) was determined by using the small-volume drip f iltration method by Li et al. (2016). Brief ly, a known volume of seawater was gently f iltered by a Whatman GF/F glass f iber f ilter (0.7 μm). The DMSP in the f iltrate was then converted completely to DMS by adding sodium hydroxide (5-mol/L NaOH), and the generated DMS was analyzed by the same technique. The total DMSP (DMSPt) concentrations were directly analyzed from unf iltered alkaline subsamples. The concentrations of particulate DMSP (DMSPp) were calculated by subtracting DMSPdfrom the DMSPtvalue (Li et al., 2016).
The seawater for chlorophylla(chla) analysis was f irst f iltered through a 0.45-μm pore-size f ilter(Whatman GF/F). Then, the chlawas extracted by soaking in 90% acetone and measured by using a f luorescence spectrophotometer (Turner Designs,USA) (Zhai et al., 2019).
2.6 Statistical analysis
Principal component analysis (PCA) is a multivariate statistical analysis technique, in which a group of correlated variables is transformed into a new group of variables that are uncorrelated or orthogonal to each other (Jackson, 1991). The datasets of the f ield cruise were analyzed by using PCA and the correlation matrix. Prior to the statistical analysis,the variables were standardized to eliminate the inf luence of diff erences in the data magnitude and measurement scales (Webster, 2009). A VARIMAX rotation was performed to aid with the interpretation of the results. Factors with eigenvalues of 1.0 or greater were selected and considered signif icant.Factor loading (FL) is def ined as the correlation coeffi cient between the principal component score and each variable (Yamamoto et al., 2014).Additionally, univariate analysis of variance(ANOVA) was used to determine whether the CH4concentrations and production rates varied between treatments with or without the addition of DMS/DMSP. The homogeneity of the variance was checked by using Levene’s test prior to the ANOVA.Diff erences were considered statistically signif icant whenPwas less than 0.05.
3 RESULT AND DISCUSSION
3.1 CH 4 in diff erent water masses in the YS and ECS
The main water masses that existed in the YS during March 2017 were classif ied in theT-Splot to constrain the CH4biogeochemical processes (Fig.2a).The CH4concentrations varied greatly within the diff erent water masses in the shallow YS, ranging from near atmospheric equilibrium (3.3 nmol/L,107%) in the YSCW to a mean value of 4.8±1.1 nmol/L in the coastal water and a peak value of 6.9 nmol/L(241%) in the YEMW (Table 1). This distribution resulted in a north-south concentration gradient on the shelf because the concentrations tended to increase towards the south of the YS. Indeed, the highest CH4concentration (14.8 nmol/L) was observed in the southern YS at station D1 (Fig.1b). This high value could be attributed to the CH4-rich Changjiang diluted water with low salinity (25.8) and density (<23.5 kg/m3). Consequently, the CH4concentration at station D2(mean:6.1±1.5 nmol/L) was the result of mixing with diluted water and Coastal Water, corresponding to relatively high salinity (32.3) and density (24.4 kg/m3). The surface water of the Changjiang River was reported to contain high CH4(e.g., >100.0 nmol/L)(Zhang et al., 2008a; Ye et al., 2016; Sun et al., 2018).Thus, mixing between diluted water and the coastal water near the boundary of the YS could have partially contributed to the north-south CH4gradient. In contrast, lower CH4concentrations (mean:3.9±0.2 nmol/L) were observed in the northern central regime, as inf luenced by YSCW (such as stations A3 and A4; Fig.1b). Station D7 was located in the western area off Jeju Island. Although this area belongs to the YS in this study, the dominant water mass at this station was ECS Water according to theT-Scharacteristics (temperature: 12.2-12.8 °C, salinity:33.8-34.1, density: 25.5-25.8 kg/m3; Fig.2a & b),which implies the exchange of YS and ECS water near the boundary.
The CH4varied greatly in the diff erent water masses across the shelf of the ECS (Fig.2b), ranging from undersaturation (2.2 nmol/L, 66%) to signif icant oversaturation (15.7 nmol/L, 566%) with respect to the atmosphere. In the shallow coastal areas (depth<50 m) and middle continental shelf (depths of 50-100 m), the CH4concentrations generally decreased with increasing longitude, ranging from an average of 10.9±3.8 nmol/L in the CW to 4.8±0.5 nmol/L in the ESW and 4.8±0.4 nmol/L in the TWCW (Table 1).The rapid CH4decrease in the ESW compared to the CW was likely the result of microbial CH4oxidation in the water column and emission into the atmosphere(Zhang et al., 2008b; Ye et al., 2016; Sun et al., 2018).At stations with water depths greater than 100 m, the CH4concentrations were typically less than or near equilibrium to the atmosphere. These areas were essentially inf luenced by the Kuroshio (Fig.1b), and the low measured CH4concentrations in the KIW(mean: 3.9±0.4 nmol/L) and KDW (3.2±0.7 nmol/L)indicated that CH4had been consumed in the water column. An exception occurred at water depths of 200-300 m, where CH4peaks (4.8-8.2 nmol/L) were observed. No other source (such as lateral transport or vertical diff usion) was present in the Kuroshioinf luenced area (Zhang et al., 2004), so we speculate that these maxima were correlated with in-situ CH4production, which will be discussed in the following sections. Thus, the CH4concentrations were believed to be associated with diff erent water masses (e.g., the CH4in the coastal water was more than two times higher than the CH4in the Kuroshio). However, the vertical CH4distribution showed great spatial variations, and the water masses could only generally explain the CH4sources in the YS and ECS.
3.2 Vertical distribution of dissolved CH 4 in the YS and ECS
The depth prof iles of hydrographic features and CH4concentrations varied greatly in the entire study area, and they could be classif ied into diff erent subgroups according to the dominant water masses. We found that stations in the same group had similar features in the vertical prof iles. Thus, the depth prof iles at six representative stations were chosen to show the spatial variability of CCW, YSCW, and YEMW, while stations T2, E4, and F6 in the ECS were dominated by TWCW, ESW, and KW (Fig.1b).
Station C1, which had low salinity (31.1) and temperature (9.4 °C), is located in the western YS,where the coastal water is the dominant water mass.The average water depth was quite shallow (16 m),and the physical parameters were well mixed from the surface to the bottom in early spring (Fig.3a).Because of this strong vertical mixing, the dissolved CH4barely varied in the water column, ranging from 5.2 nmol/L at the surface to 5.0 nmol/L at the bottom.The water is oversaturated in CH4with respect to the atmospheric equilibrium, implies that CH4can be signif icantly released from the water column to the atmosphere. Station B6 is located in the central trough of the YS, where YSCW was the dominant water mass during the investigated period (Fig.3b).The overall CH4concentration at station B6 (mean value of 4.4±0.2 nmol/L) was lower than that at station C1 (mean value of 5.1±0.1 nmol/L); as indicated previously, the CH4had lower concentrations in the YSCW than in the CW (Fig.2a).The input of fresh water (i.e., Sheyang River) near the coast can explain the CH4diff erence between the coastal area (CW) and the central YS (YSCW).However, the CH4concentrations at both stations were 1.5-1.8 times higher than the atmospheric equilibrium and were also higher than the reported values from previous studies for the same season(Zhang et al., 2004). The excess CH4in the wellmixed water could have been released into the atmosphere, with a mean rate of 1.3 μmol/(m2·d)(Zhang et al., 2004). Thus, additional CH4sources should have existed to sustain a stable value of CH4(such as 4.4 nmol/L at station B6) in the water column. Sediment release did not appear to be an important source for the water column’s CH4in the YS and barely contributed to the atmospheric CH4because of the low organic-carbon concentrations(~0.3%) in the sediment and the presence of abundant DO (>9.0 mg/L) in the bottom water (Zhao et al.,2018). Moreover, only 5.4% of the sedimentary organic carbon was degraded in the YS sediments,which was three times lower than that in the ECS(Zhao et al., 2018). The low remineralization rates of organic carbon restrict the upward f lux of CH4in sediments (Valentine, 2002). Consequently, limited CH4could diff use through the sediment-water interface in the YS because of anaerobic/aerobic CH4oxidation (Jørgensen et al., 2001; Treude et al.,2005). In addition, CH4-rich CW could not be transported to the central YS (such as station B6), so the extra CH4source should have been attributed to in-situ CH4production and our incubation experiments suggested that DMSP may have been a potential substrate for CH4production in the study area (see details in Section 3.4).
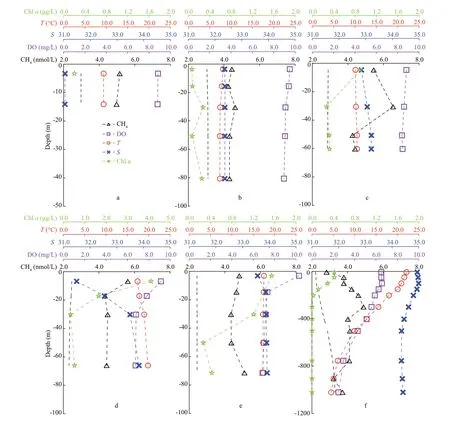
Fig.3 Vertical prof iles for the temperature (°C), salinity, DO (mg/L), chl a (μg/L), and CH 4 (nmol/L) at six representative stations in the YS (a-c) and ECS (d-f)
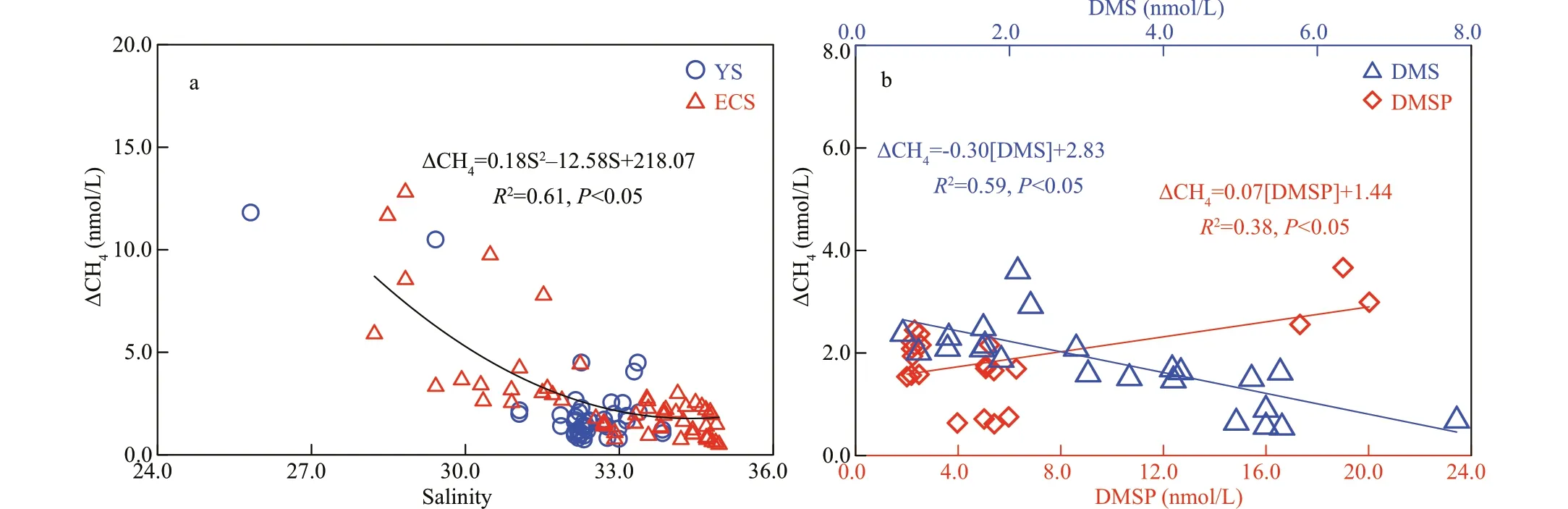
Fig.4 Correlations between ΔCH 4 (nmol/L) and other parameters
At station D6 which is the boundary of ECS and YS (Fig.3c), the surface layers were inf luenced by the YEMW, but the bottom layer was likely a component of the ECS water. CH4concentrations increased from 5.5 nmol/L at the surface to 6.5 nmol/L at a depth of 30 m and then dropped to 4.3 nmol/L at the bottom.Thus, the high CH4concentrations (>5.0 nmol/L) in the upper 30 m were the result of mixing, while the relatively low values (<5.0 nmol/L) below this layer were characteristic of ECS water, further indicating the inf luence of the water masses on the CH4distribution.
Signif icant correlations between excess CH4(ΔCH4,Cin-situ-Cequilibrium) and salinity (P<0.05, Fig.4a)were found in the water columns both in the YS and ECS, suggesting that the mixing with fresh water has important eff ect on CH4distribution in the nearshore regions. In the ECS, however, more complex hydrography inf luences CH4distribution. For example, at station T2 near the coast (Fig.3d), the water layer of the surface 30 m was inf luenced by the coastal water and while water below 30 m could be attributed to the TWCW (Li et al., 2012). Consequently,the chl-aconcentration dropped to <0.5 μg/L below 30 m, and the CH4concentration was higher in the surface layer (5.6 nmol/L) than in the bottom layer(4.4 nmol/L). Hence, we carefully chose stations that could represent the properties of diff erent water masses to calculate the CH4inventories in these waters, and the mean value of CH4in the TWCW was 56% of the mean value in the CW (Table 1). However,the CH4concentrations in the water masses in the ECS exhibited seasonal variations. For example, our observed CH4in the TWCW in early spring was 21%lower than what was reported for summer (Zhang et al., 2008b).
Station E4, which is located in the middle of the continental shelf, had a well-mixed water column below a depth of 15 m (Fig.3e). The CH4only decreased by 0.4 nmol/L within the top 50 m and then reached a maximum of 5.1 nmol/L in the bottom water, suggesting a possible CH4source from sediments. Previous studies showed that the sedimentary release of CH4in the ECS is an important source of dissolved CH4in the water column, and the release rates from the sediment to the water column varied 0.6-2.3 μmol/(m2·d) in season (Zhang et al.,2008a; Sun et al., 2018). Thus, the sediment-water CH4f lux was ~10% of the sea-air CH4f lux (Sun et al.,2018), indicating that sediment was a potential source of the dissolved CH4in the ECS. Our results showed that the CH4in the ESW was comparable to that in the TWCW (Table 1), matching the summer observations by Zhang et al. (2008b).
Station F6 is located at the edge of the Okinawa Trough, where the mainstream of Kuroshio f lows northeastward and intrudes the ECS along the continental slope (Figs.1a & 3f). Thus, the main features of the Kuroshio can be captured from this station. Specif ically, the high temperature (22.1 °C)and salinity (34.8) in the surface layer indicate the existence of KSW. The maximum and minimum salinity at depths of ~150 and ~500 m showed the characteristics of KSSW and KIW, respectively. In addition, the low temperature (<6.0 °C) and high density (>27.0 kg/m3) at depths below 700 m showed the features of KDW. Consequently, the CH4increased in the subsurface layer (50-300 m) and reached a maximum of 4.9 nmol/L. This value subsequently decreased between depths of 300 and 800 m and f luctuated around the atmospheric equilibrium in deep water (800-1 000 m). This CH4prof ile showed the characteristics of the Kuroshio, with relatively rich CH4occurring in the KSSW (4.6±1.1 nmol/L)and KIW (3.9±0.4 nmol/L) and poor CH4occurring in the KSW (3.2±0.5 nmol/L) and KDW(3.2±0.7 nmol/L). The CH4in the KSSW was comparable to that in the ESW, probably resulting from an intrusion of shelf water as a turbidity tongue(Luong et al., 2018, 2019). In contrast, CH4in the KSW and KDW were 33% and 71% lower than that in the ESW and CW, respectively (Table 1). Compared to previous studies, the observed CH4in the KSW in this study was 1.1-1.4 times higher than that in the spring and summer (Zhang et al., 2004, 2008b), but 20% lower than that in the autumn (Sun et al., 2018).The temperature may be a driver of CH4variations in the KSW during diff erent seasons (Sun et al., 2018).Meanwhile, the upward diff usion of CH4in the subsurface layer may be another factor that determines the magnitude of surface CH4. Subsurface CH4maxima at depths between 200 and 300 m were observed at the deep stations (>800 m, stations P7,FJ7, E7, and F6). Consequently, CH4gradients existed within the top 200-300 m, with the intensity ranging from 0.006 nmol/(L·m) to 0.026 nmol/(L·m). The presence of these gradients implies that excess CH4formed locally in the subsurface water, subsequently diff used to the surface, and eventually entered the atmosphere.
3.3 CH 4 budget in the shelf of the ECS
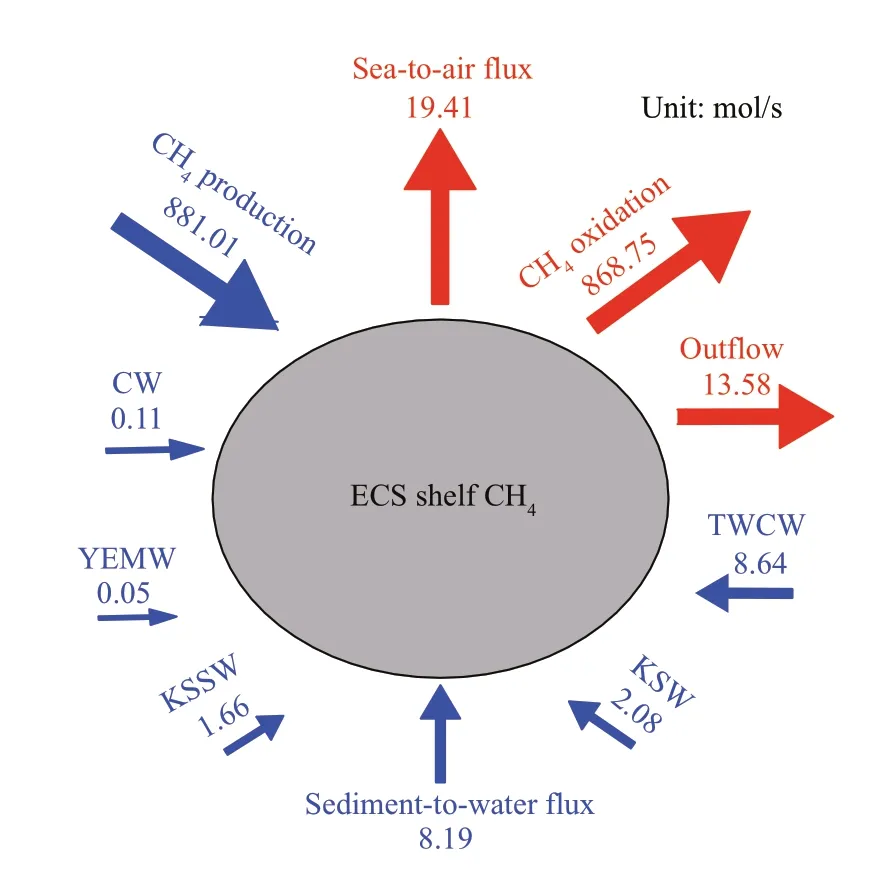
Fig.5 Preliminary CH 4 budget in the shelf of the ECS
The ECS shelf is likely a transition zone between the coast and open ocean. The exchanging of water masses, heat, nutrients, and other materials occur on this shelf, which has far-reaching eff ects on the northern Pacif ic (Jiang et al., 2018). Understanding the CH4dynamics in the shelf water, therefore, is crucial for investigating the CH4biogeochemical processes in the ECS. In early spring, the Changjiang f lows southward and becomes the coastal water by mixing with shelf water, whereas the Kuroshio f lows northeastward along the edge of the ECS and intrudes the shelf mainly through the KSW and KSSW (more than 80%) (Su, 1998; Zhou et al., 2018). When examining the water budgets of the ECS, the discharges of KSW and KSSW inf lows into the shelf were two orders of magnitude higher than that of the coastal water (Table 1). However, the TWCW comprised ~64% of the total water inf low in spring,and the ECS received ~0.01×106m3/s inf lux from the YS. Consequently, the outf lows through the Tsushima Strait and Japan Sea were estimated as 2.83×106m3/s.We estimated the CH4that was carried by the coastal water to be only 0.11 mol/s, which was 15-19 times lower than what was transported via the KSW and KSSW but comparable with the input through the YS(0.05 mol/s), suggesting that the inf lux of freshwater was a minor source (0.01%) of shelf CH4in early spring because of the low water discharge (Table 1;Fig.5). The gas hydrates located at the Okinawa Trough may play an important role in contribution of high CH4to the Kuroshio and the CH4concentrations venting from the hydrothermal f ield were recorded at millimole level (Sakai et al., 1990; Luong et al.,2019), raising the concerns of gas hydrates in contribution to the shelf CH4. However, the f luids were diluted quickly and diff used northwest carried by the Kuroshio, resulting in a relatively low CH4inventory (2.2-5.3 nmol/L) (Luong et al., 2019) that matches with our observation at the KSSW(4.6±1.1 nmol/L, Table 1). This means the gas hydrates located at the Okinawa Trough (water depth>800 m) would have small impact on the CH4distribution in the ECS shelf (<200 m) due to diluting and consuming. The TWCW brought the most CH4to the shelf in spring (8.64 mol/s). Thus, the TWCW could be considered an important source of the shelf’s CH4inventory. The total out-f lux was 8% higher than the total input, implying that the ECS shelf was a net CH4source to the open ocean. The diff erences between the total inf low and outf low also suggested the existence of other CH4sources in the ECS (e.g.,in-situ production, groundwater, and sedimentary release). However, these CH4f luxes may exhibit seasonal variations. For example, Zhang et al. (2008b)reported that the net CH4out-f lux from the shelf in summer was more than f ive times higher than our results in early spring. The diff erence in freshwater discharge, which was more than three times higher in summer than in winter (Zhang et al., 2007), was the main reason for these seasonal CH4changes. In addition, the net CH4outf low in this study was 56% of what was previously reported in the same season(Zhang et al., 2004). The estimation of the net outf lux in this previous study did not consider TWCW,which is believed to be an important CH4source according to our study. Thus, our data showed a reasonable CH4budget in the shelf.
The sedimentary release of CH4was proven a potential source of dissolved CH4in the ECS shelf(Fig.3e) (Zhang et al., 2008a; Sun et al., 2018). The quantity of CH4that was emitted from sediments showed great seasonal variations, presumably lower in winter and higher in summer, especially under hypoxia conditions off the Changjiang River estuary(Zhang et al., 2008a; Ye et al., 2016; Sun et al., 2018).The CH4f luxes from sediment to the water in the ECS were documented in previous studies, such as 0.8 μmol/(m2·d) in April/May 2002 (Zhang et al.,2008a) and 1.7-2.2 μmol/(m2·d) in March 2011 (Sun et al., 2018). We used the average of these studies to represent the mean sediment-water CH4f lux in the ECS in spring since we did not measure the sedimentto-water f lux in our study. Thus, the sediment-water CH4f lux was estimated as 8.19 mol/s based on the shelf area. This result indicates that the sediment in the ECS was a comparable source to the TWCW for the shelf CH4. From literatures, we found benthic CH4f luxes varied spatially. Fox example, in the Baltic Sea, the f luxes ranged from 100 μmol/(m2·d) in coastal area to 26 000 μmol/(m2·d) in the inner eutrophic estuary (Sawicka and Brüchert, 2017).Another study reported the CH4f lux at the sedimentwater interface was estimated 20.9-25.1 μmol/(m2·d)in the Godavari and Krishna estuaries (Rao and Sarma, 2016). Our results were comparable with that reported in the west coast of India (4.71 μmol/(m2·d))(Araujo et al., 2018) and in the continental shelf of the Gulf of Cádiz (0.9-24 μmol/(m2·d)) (Ferrón et al.,2009). With respect to the CH4emissions to the atmosphere, we used the mean value of 3.3 μmol/(m2·d) that was calculated by Du et al. (unpublished data) for this cruise to represent the sea-air CH4f lux in the ECS in spring. Thus, the sea-air CH4f lux(19.41 mol/s) was one of the major CH4sinks in the ECS shelf and was more than ~1.5 times higher than what was transported by water masses, suggesting that the ECS is a net source of atmospheric CH4. The CH4oxidation rate (OR) can be measured by radiotracer techniques using tritiated CH4according to the method described by Bussmann et al. (2017).Here, we used the turnover rate constant (k) in the North Sea, with a mean value of 0.058/d, reported by Osudar et al. (2015), to calculate the CH4oxidation rate in the ECS shelf in our study because the environmental conditions in the North Sea were similar to that in the ECS. Thus, the CH4oxidation rate was computed as 0.278 nmol/(L·d) when considering the mean CH4concentration of 4.8 nmol/L in the ECS shelf (Table 1). Meanwhile, a comparable CH4oxidation rate (0.265 nmol/(L·d)) was measured by a13C-tracking method deployed in this survey. The consistency of CH4oxidation rate from diff erent methods indicates that the result we used in the CH4budget is reasonable. Consequently, theRconin the shelf water was calculated as 868.75 mol/s by multiplying the shelf water volume by the CH4oxidation rate. Taken together, a CH4production rate of 881.01 mol/s was calculated after balance of other sources and sinks in spring (Fig.5). Considerable CH4should be produced in the water column to sustain the CH4def icit. According to the shelf water volume, our mass balance indicated that a local CH4production rate of 0.28 nmol/(L·d) was required to maintain the CH4loss. This value is in the range of the reported CH4production rates from Mexico Bay (-0.04-2.00 nmol/(L·d)) (Bange et al., 1994) but much lower than what was reported for oligotrophic lakes(~50.00 nmol/(L·d)) (Grossart et al., 2011). Hence,our results showed that local production/consumption is the major source/sink (>95%) of CH4in the ECS shelf, following by a total of 2.2% CH4diff used to the atmosphere. It should be noted that the estimation contains great uncertainties due to insuffi cient f ield data (e.g. gas f luxes at seep sites) and the source f lux was presumably underestimated without consideration of the contribution of the gas hydrates. It is hard to quantify because rare data was published to constrain the size, area, and gas spreading velocity in various seeps. This means the local CH4production rate was overestimated to some extent but would not aff ect the fact that the ECS was a hotspot for CH4dynamics(production, consumption, and emission).
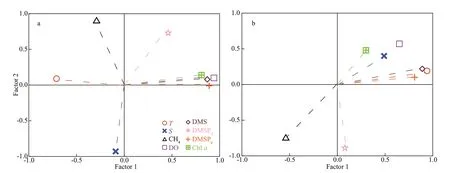
Fig.6 Loading plots that correspond to the f irst two factors following the VARIMAX rotation of the principal components
3.4 CH 4 formation via DMSP/DMS degradation
Field observations suggested the presence of excess CH4in the subsurface layers and the box model indicated the signif icant potential for CH4production in the study area. As DMSP has been recently suggested as the possible substrate for CH4production(Damm et al., 2015; Stawiarski et al., 2019), we measured the dissolved and particulate fractions(DMSPdand DMSPp) (data not shown) in the f ield survey. No correlations between DMSP/DMS and CH4were found when all the stations in the ECS were considered. However, a negative correlation between DMS and ΔCH4(R2=0.59,P<0.01) and positive correlation between DMSPdand ΔCH4(R2=0.38,P<0.05) were found at off shore stations (water depth>800 m) within the water column of 0-300 m (Fig.4b).Further, to weaken the inf luence of multiple factors,the parameters at deep-water stations (>800 m,stations P7, FJ7, E7, and F6) were analyzed by using PCA to determine potential correlations between the Carbon-Sulfur bonded compounds and CH4distribution (Fig.6). Weak correlations between CH4and DMSP/DMS were found in the YS (Supplementary Tables S1-S2). In contrast, two factors were extracted in the ECS, explaining a cumulative variance in the data of 69% (Supplementary Tables S3-S4). Thus,the f irst factor was associated with the features of dissolved gases because the temperature (FL=0.94)and dissolved gas content (DO: FL=0.65, DMS:FL=0.89, CH4: FL=-0.54) were strongly loaded(Fig.6b). The second factor, however, was signif icantly loaded by DMSPd(FL=-0.89), indicating that this factor was related to the cycling of methylated sulfur compounds. CH4(FL=-0.74) was strongly loaded in the second factor, suggesting correlations between CH4and DMSPd. Thus, we speculate that DMSPdand its degradation product (DMS) may serve as CH4precursors in the study area, particularly in oligotrophic areas.
To assess this hypothesis, incubation experiments were conducted in the laboratory as well as in the f ield. In our f irst experiment, spiking treatments with DMSPdsignif icantly increased the CH4concentration compared to the control groups (Fig.7a, ANOVA,P<0.01 and Fig.7c, ANOVA,P<0.05). The oxygen content in the glass bottles was constantly above 5.0 mg/L, illustrating the well-oxygenated conditions during the incubation period (Fig.7b & d). Thus, these results showed that the amendment with DMSP induced CH4production, and DMSP can act as a precursor for CH4formation in oxic water.Furthermore, diff erent N:P ratios were set in the second experiment to test the eff ect of N def iciency on DMSP-dependent CH4production, as indicated by Damm et al. (2009). In particular, the addition of DMSP increased the CH4to ~15.0 nmol/L in less than a week, 1.5 times higher than the value of the control(Fig.8a). The DO in the control was almost constant during the entire incubation, whereas the DO in the DMSP treatments rapidly depleted over the f irst three days and gradually decreased to approximately 5.0 mg/L after seven days of incubation, suggesting strong respiration (Fig.8b). The eff ect of N def iciency on the microbial utilization of DMSP was ambiguous in our experiments, as ref lected in the insignif icant diff erence between the rates under N-rich treatment(5<N:P<16) and N-starved treatment (N:P<1) (Fig.8a,ANOVA,P=0.65; Supplementary Fig.S1, ANOVA,P=0.89), suggesting that N-stressed conditions may not stimulate the transformation of DMSP to CH4in the coastal waters. In the third experiment (seawater incubation with direct addition of DMS, see Section 2.4) CH4production was observed in both the positive control (amended with C+N+P) and DMS-added treatment within the f irst three days, but no CH4accumulations were observed during the last seven days in all the treatments (Fig.8c). Meanwhile, the water in the incubation bottles never became hypoxic or anoxic during this experiment (Fig.8d). The insignif icant diff erence between the concentrations in the (C+N+P) treatment and DMS-added treatment suggested that the addition of DMS did not induce CH4production (ANOVA,P=0.98).
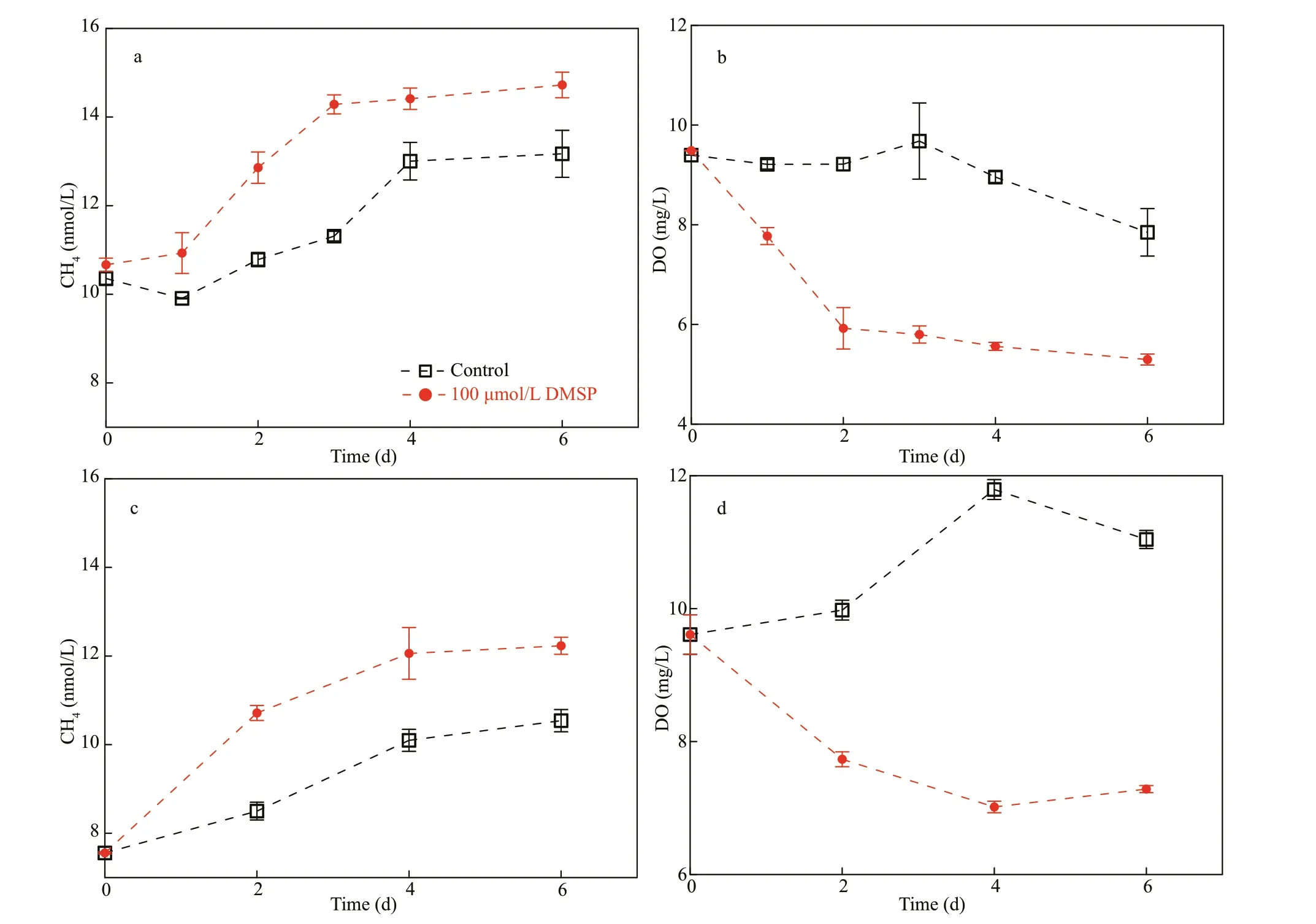
Fig.7 CH 4 production and DO consumption during DMSP-amended incubation experiments
CH4signif icantly accumulated with the addition of DMSP in the f irst seven days compared to the control(Figs.7 & 8a), indicating that the catabolism of DMSP can contribute to the dissolved CH4in the presence of oxygen. The evidence for the formation of CH4from DMSP/DMS remains circumstantial and is mainly based on correlations between the CH4and DMSP/DMS concentrations (Borges et al., 2018). Two mechanisms of DMSP degradation exist in marine systems. First, the cleavage of DMSP results in DMS and acrylate, which is called dissimilation (Kiene et al., 1986; Visscher et al., 1994). Second, DMSP is demethylated to 3-mercaptopropionate (MPA), with 3-methiolpropionate (MMPA) as a possible intermediate (Kiene and Taylor, 1988); MMPA can also degrade with demethylation, yielding methanethiol (MeSH) (Fig.9) (van der Maarel and Hansen, 1997). Thus, CH4is known to be produced through three possible pathways: 1) Methanogenic archaea can decompose DMS to CH4via demethylation if the DMS concentration is high enough that sulfatereducing bacteria do not compete with methanogens(Kiene and Taylor, 1988); 2) Microbes can utilize MeSH (demethylation) to produce CH4under anoxic environments (Tallant and Krzycki, 1997); 3) Acrylate can decompose into fatty acids (such as acetate and propionate, Fig.9), which are the precursors for methanogenesis, so CH4can form indirectly from acrylate degradation. These possible mechanisms for the transformation of DMSP to CH4are thought to occur in the absence of oxygen. However, reducing conditions can form in micro-niches or inside bacterial cells (Jørgensen, 1977) despite the presence of oxygen in the ambient water. A theoretical model was recently built to explain the maintenance of anaerobic conditions for CH4production inside bacterial cells in association with the transformation of DMSP (Damm et al., 2015). In addition, such organisms would metabolize MeSH in cells because they must rid themselves of MeSH before it accumulates to toxic levels (Kiene et al., 1986). Thus, CH4may form inside cells and then be released into the oxygenated water via cell lysis (Reisch et al., 2011; Damm et al., 2015).
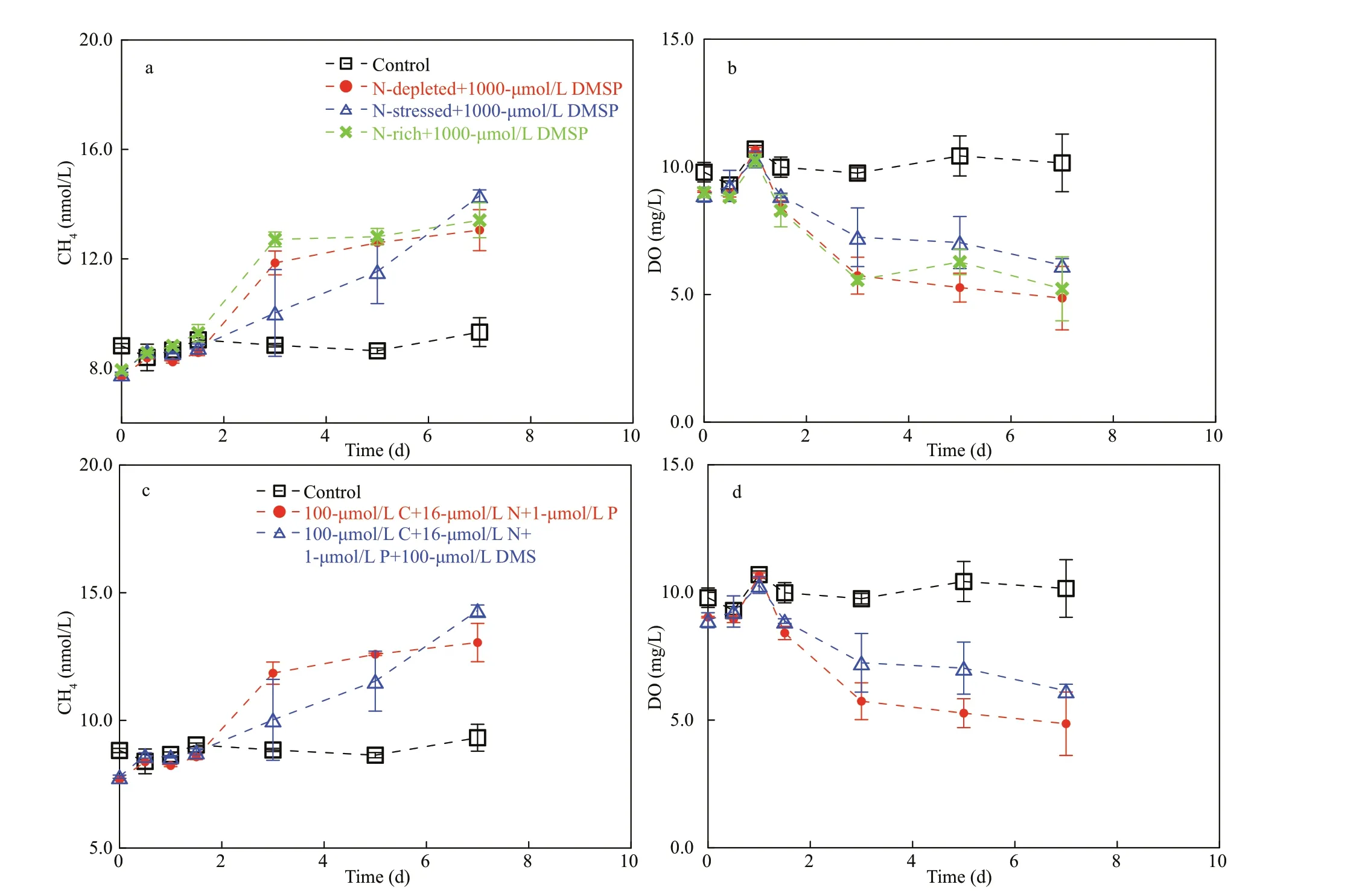
Fig.8 CH 4 and DO concentrations in surface waters incubated over time
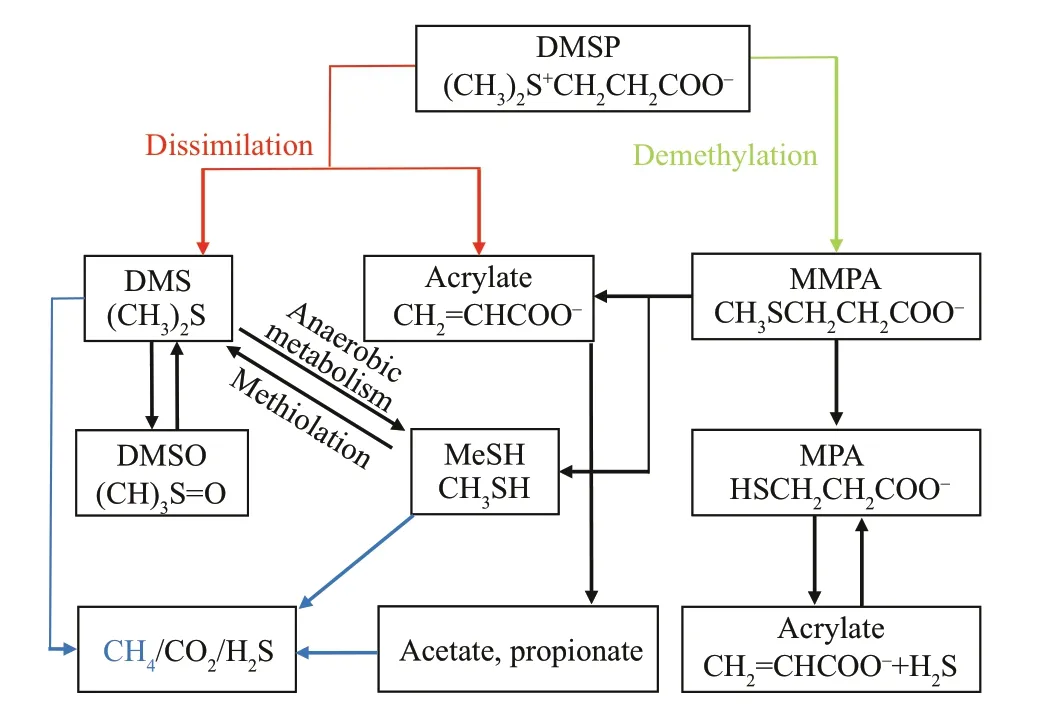
Fig.9 Schematic diagram for the conversion of DMSP and the connection with the production of CH 4
One concern is that the transformation effi ciency from DMSP to CH4in our study was low; the addition of DMSP was 100-1 000 μmol/L and the CH4concentration only increased by 2.0-5.0 nmol/L(Fig.8a). We suppose that this result was associated with the mechanisms of DMSP degradation. Previous studies showed that the dissimilation pathway comprises 30% of DMSP metabolism (Kiene and Service, 1991; Kiene et al., 2000; Reisch et al., 2011;Damm et al., 2015) and only 6%-17% in surface seawater (Archer et al., 2002), which means that DMS is a minor product of DMSP metabolism.However, the f irst and second pathways of CH4formation were directly connected to the degradation of DMS (Fig.9). If limited DMS was produced from DMSP, then the yielding of CH4would also have been limited. Moreover, over 80% of DMS in the marine system is known to be bacterially and photochemically oxidized to dimethylsulfoxide (DMSO) (Davis et al.,1998; Nowak et al., 2001), which further reduces the conversion effi ciency from DMSP to CH4. In combination with the results of the second incubation,where CH4did not obviously accumulate when the treatment was directly amended with DMS (Fig.8c,ANOVA,P=0.98), we speculate that CH4somehow has diffi culty forming directly from DMS, at least in environments such as the YS and ECS. The exact mechanism is unknown; one possibility is that CH4-producing organisms prefer substrates other than DMS under environments where abundant carbon and sulfur sources are present (Lin et al., 2000, 2002).This observation is consistent with the negative correlations between DMS and ΔCH4in the ECS(Fig.4b), which means that the conversion of DMSP to CH4was restricted if the DMSP preferred to dissimilate to DMS. Thus, DMS accumulated in the ambient seawater but CH4production was limited because of the low effi ciency of the DMS-to-CH4process. On the other hand, DMS production would be limited if the DMSP mainly degraded via demethylation (to form MPA and acrylate), whereas CH4would accumulate via the second and third pathways (Fig.9). Consequently, in either case, we could expect to observe positive correlations between the accumulated DMSP and ΔCH4and negative correlations between DMS and ΔCH4, as we did in the f ield observations (Fig.4b). Another independent study that was conducted in the ECS also showed a positive correlation between DMSP and CH4(Zhai et al., 2019). Thus, the CH4in the study area may prefer to be produced via the demethylation of MeSH and/or degradation of acrylate rather than the decomposition of DMS.
Another concern is that DMSPdcan be low in seawater (e.g., ~5.5 nmol/L in the YS and ECS in this study), and its contribution to the dissolved CH4concentration can be negligible. However, studies showed that the intracellular concentration of DMSP could reach millimolar levels (Reisch et al., 2011).Thus, the potential importance of DMSP for CH4production should be brought to the forefront if sulfur compounds transform in cells, as speculated above.According to the incubation experiments in the coastal waters, the DMSP-to-CH4production rates were estimated as an average of 0.17 nmol/(L·d)(after subtracting the rate of 0.48 nmol/(L·d) in the control) with the addition of 100-μmol/L DMSP(Fig.7a & c) and ~0.80 nmol/(L·d) with the addition of 1 000-μmol/L DMSP (Fig.8a). Our results were lower than the recently reported production rate of~1.8 nmol/(L·d) in the central ECS with the addition of 5-10-μmol/L DMSP (Zhai et al., 2019). Damm et al. (2009) suggested that a low N:P ratio in oligotrophic areas may enhance the microbial utilization of DMSP as a C source, with CH4released as a by-product.However, our experiments conducted in coastal waters may weaken the eff ect of DMSP on CH4concentrations, where abundant C sources (e.g.,dissolved organic carbon: 3.0 mg/L) were present for microbial assimilation (Liu et al., 2015). Thus, DMSP may be out-competed by those substrates in coastal areas because the Gibbs free energy for DMSPdependent CH4production was low (-35.7 kJ/mol)(Damm et al., 2009). Consequently, even N def iciency could not trigger DMSP-dependent CH4production in CW (Fig.8a) because the low N:P ratio probably could not eff ectively stimulate the microbial utilization of DMSP in the presence of other competitive energy sources. In contrast, the experiment conducted in the oligotrophic surface seawaters of the western North Pacif ic (N:P<1)showed that directly addition of DMSP (10 μmol/L)induced signif icantly increase in CH4(Fig.10,ANOVA,P<0.01) and the CH4production rate(1.2 nmol/(L·d)) is comparable to that reported in the central ECS (Zhai et al., 2019). This suggests that the DMSP-dependent CH4production prefer to occur in the oligotrophic seawaters, where nitrogen is depleted.
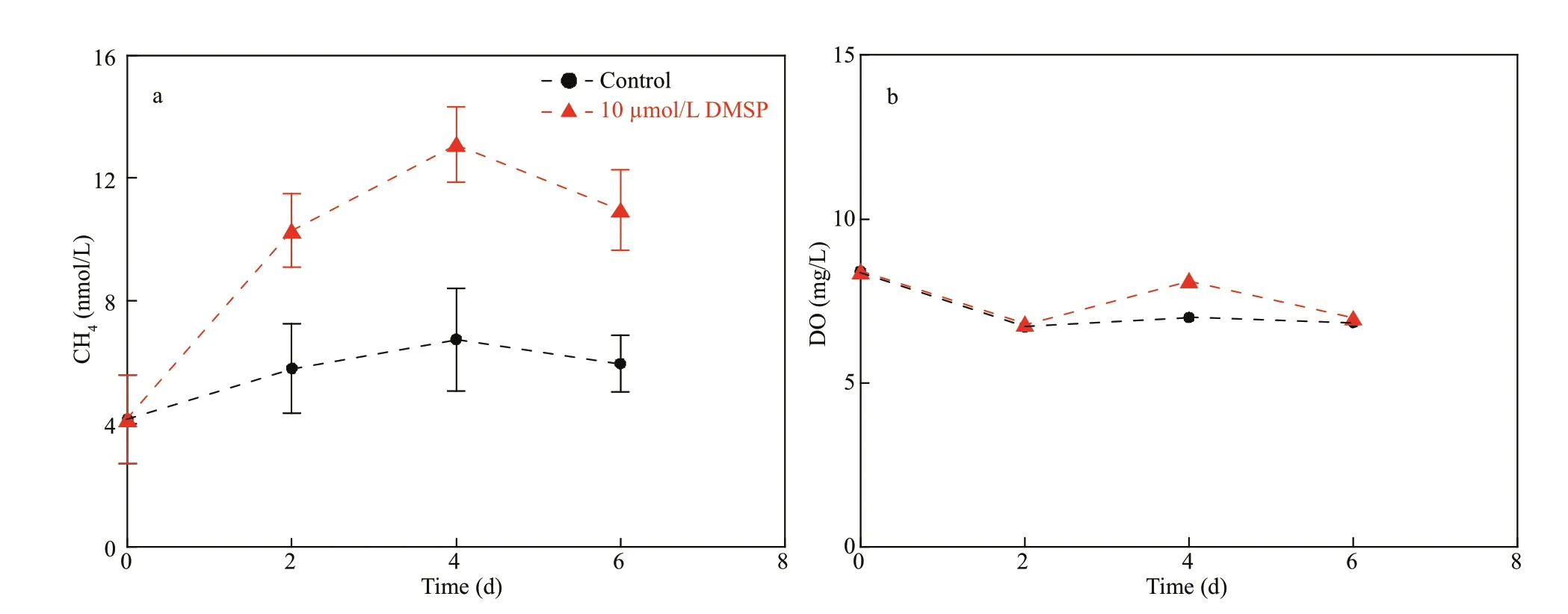
Fig.10 Incubation experiment for seawaters directly amended with DMSP in the oligotrophic Western North Pacif ic
In addition, studies showed that the DMSPdconcentration in the ECS can exceed 40.0 nmol/L in summer (Yang et al., 2011) and exceed 250.0 nmol/L during blooms (Matrai and Keller, 1993), which increases the possibility of a DMSP-dependent pathway for CH4production in the water column.Moreover, additional CH4sources (such as anaerobic methanogenesis in micro-niches and the degradation of Carbon-Phosphorus/Carbon-Nitrogen bonded compounds) alongside DMSP pathways should exist in the oxygenated water column to maintain the loss of CH4into the atmosphere. Recently, CH4production from MPn was attributed to microbial activity in P-starved oceanic areas (Karl et al., 2008; Metcalf et al., 2012; Repeta et al., 2016). Our recent experiments in the YS also indicated the possibility of a Carbon-Phosphorus cleavage pathway for the enrichment of CH4in oxic water (Ye et al., 2020). Hence, the CH4dynamics in the YS and ECS are complicated, and CH4production in well-oxygenated water may be attributed to several mechanisms. According to our f ield observations and incubation experiments, the Carbon-Sulfur cleavage pathway for CH4production in well-oxygenated water is a potential mechanism for the production of excess CH4in the western North Pacif ic. Although the exact molecular mechanism is unclear and no direct evidence exists of carbon transfer from DMSP to CH4, the implications for the biogeochemical cycling of carbon and sulfur in the marine system is essential to future work, especially when considering the eff ect of CH4and DMS on global warming and atmospheric chemistry.
4 CONCLUSION
The CH4concentrations in the ECS and YS showed great variations among diff erent water masses, which profoundly aff ected the CH4distribution. In spring,the vertical prof iles of CH4were homogenous in the YS but varied greatly in the ECS because of the latter’s more complicated hydrological conditions.The surface and subsurface waters are oversaturated in CH4indicated that the YS and ECS are the net sources of atmospheric CH4in spring. A box model was used to evaluate the CH4dynamics in the ECS.The results suggested that the coastal water plays an important role in distributing CH4among the nearshore areas, while it was a minor source (0.01%) for CH4in the continental shelf because of the low water discharge. Over 95% CH4was considered to be produced and consumed in the shelf water,subsequently with 2.2% CH4diff used to the atmosphere, suggesting ECS is a hotspot for CH4dynamics. Further incubation experiments were carried out to f igure out the source of this hotspot.Results show that DMSP can be a potential CH4precursor in oxygenated coastal water and DMSPdependent CH4production has potential to be enhanced in oligotrophic areas.
This study demonstrates that the in-situ production could be a major source of dissolved CH4in the marginal seas of China and its correlation with cleaving of Carbon-Sulfur bonded compounds (such as DMSP and MeSH) may have further implications for carbon and sulfur biogeochemical processes in the western Pacif ic. Further work is required to conf irm the intrinsic connections between methylated sulfur compounds and CH4, presumably by molecularbiology techniques.
5 DATA AVAILABILITY STATEMENT
All data included in this study are available upon request by contact with the corresponding author.
6 ACKNOWLEDGMENT
The authors wish to thank the crews of the R/VDongFangHong2and colleagues from the Laboratory of Marine Biogeochemistry at the Ocean University of China for their assistance with the collection of f ield samples. We also thank Haibing DING from the Ocean University of China for providing CH4oxidation data.
7 CONFLICT OF INTEREST
The authors declare that they have no conf lict of interest.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
- The phycocyanin-chlorophyll-protein complexes isolated from Chroomonas placoidea*
