Two bicistronic DNA vaccines against Vibrio anguillarum and the immune eff ects on f lounder Paralichthys olivaceus*
Hanlin LI , Jing XING ,2,**, Xiaoqian TANG ,2, Xiuzhen SHENG , Heng CHI , Wenbin ZHAN ,2
1 Laboratory of Pathology and Immunology of Aquatic Animals, KLMME, Ocean University of China, Qingdao 266003, China
2 Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071, China
Abstract Chemokines are cytokines that can promote the activation and migration of immune cells, and increase the recognition of antigen by antigen-presenting cells (APC). Previous studies showed that a DNA vaccine can induce humoral and cellular immune responses of f lounder after immunization. To explore the improvement of chemokines on the effi ciency of OmpK vaccine, two bicistronic DNA candidate vaccines were constructed and the immune responses they induced in the f lounder were investigated by reverse transcription polymerase chain reaction (RT-PCR), indirect immunof luorescent assay (IFA), H&E staining, f low cytometry(FCM), and quantif icational real-time polymerase chain reaction (qRT-PCR). pBudCE4.1 plasmid as an expression vector, bicistronic DNA vaccines encoding OmpK gene and CC-motif ligand 4 gene (p-OmpKCCL4), or Ompk gene and CC-motif ligand 19 gene (p-OmpK-CCL19) were successfully constructed. The results showed that two bicistronic DNA vaccines expressed Ompk protein of Vibrio anguillarum and CCL4/CCL19 proteins of f lounder both in vitro and in vivo. After immunization, a large number of leucocytes in muscle were recruited at the injection site in treatment groups. The constructed vaccines induced signif icant increases in CD4-1 + and CD4-2 + T lymphocytes, and sIgM + B lymphocytes in peripheral blood, spleen, and head kidney. The percentage of T lymphocytes peaked on the 14 th post-vaccination day whereas that of B lymphocytes peaked in the 6 th post-vaccination week. Moreover, the expression prof iles of 10 immune-related genes increased in muscles around the injection site, spleen, and head kidney. After the challenge, p-OmpKCCL4 and p-OmpK-CCL19 conferred a relative percentage survival (RPS) of 74.1% and 63.3%, respectively,higher than p-OmpK alone (40.8%). In conclusion, both CCL4 and CCL19 can improve the protection of p-OmpK via evoking local immune response and then humoral and cellular immunity. CCL4 and CCL19 will be potential molecular adjuvants for use in DNA vaccines.
Keyword: Vibrio anguillarum; outer membrane protein K; bicistronic DNA vaccines; CC-motif ligand 4;CC-motif ligand 19; immune response
1 INTRODUCTION
DNA vaccines can provide good protection after immunization because its ability to induce humoral and cellular immune responses, therefore, it has been proven to be a very promising strategy in disease prevention, especially against viral diseases (Cui,2005; Chang, 2020). Using adjuvants is one of the most eff ective ways to increase the eff ectiveness of DNA vaccines, including IL-2, IL-6, IFN-γ, TNF-α,and other cytokines (Lu et al., 2008; Su et al., 2008;Gaertner et al., 2009; Tang et al., 2020).
Vibrioanguillarumis a gram-negative pathogen in aquaculture, which threatens aquatic animals seriously. A large number of studies on the vaccine againstV.anguillarumhave been performed to prevent f ish disease caused byV.anguillarum.Inactivated vaccines, live attenuated vaccines, subunit vaccines, and DNA vaccines againstV.anguillarumwere reported previously (Hamod et al., 2012; Xing et al., 2018; Xu et al., 2019a, b, c; Li et al., 2020). The advantages of the DNA vaccines include its versatility,safety, ease of production, and low cost compared to traditional vaccines (Klinman et al., 2010). In a previous study, a DNA vaccine encoding the OmpK gene ofV.anguillarumwas constructed successfully,it can induce humoral and cellular immune responses in f lounder by intramuscular injection (Xu et al.,2019a).
Chemokines are inf lammatory molecules with high biological activity, and they can bind to specif ic receptors and play an important role in the immune response, such as activating diff erent types of lymphocytes and inducing the migration of lymphocytes to inf lammatory foic (Kim et al., 2000).Inf lammatory chemokines are thought to recruit neutrophils, monocytes/macrophages, dendritic cells(DC), and natural killer cells (NK). Therefore, they play an important role in mediating a variety of immune and inf lammatory responses (Esche et al.,2005). According to the number and position of the f irst two cysteine residues in their sequence,chemokines can be classif ied into four subfamilies,including CXC, CC, CX3C, and XC (Zou et al., 2014).Chemokines bind to their receptors causes the recruitment and activation of intracellular G-proteins that initiate a variety of signaling pathways (Bonecchi and Graham, 2016). They can chemotactic on monocytes/macrophages, T cells, B cells, eosinophils,dendritic cells, mast cells, natural killer cells, etc., and participate in the inf lammatory response and the pathogenesis of various diseases (Kono et al., 2003).Chemokine CC-motif ligand 4 (CCL4) and chemokine CC-motif ligand 19 (CCL19) are members of the CCchemokine subfamily, and their specif ic receptors are chemokine receptor 5 (CCR5) and chemokine receptor 7 (CCR7), respectively (Bonecchi et al., 2009). CCL4 is also known as macrophage inf lammatory protein-1β(MIP-1β) and it is a member of the inf lammatory chemokine family, which binds to its specif ic receptor CCR5 and acts on a variety of immune eff ector cells,including NK, T cells, DC, and monocytes (Alejo and Tafalla, 2011; Li et al., 2011). CCL19, also known as macrophage inf lammatory protein-3β (MIP-3β) or EBI1 ligand chemokine (ELC), which binds to its specif ic receptor CCR7 and induces DC maturation and increases DC co-stimulatory molecules and proinf lammatory cells factor production to promote T cell proliferation (Kutzler and Weiner, 2004; Maurer and von Stebut, 2004). Many kinds of research on chemokines as vaccine adjuvants have been performed in mammals and f ish (Yamano et al., 2006; Kutzler et al., 2010; Matsuo et al., 2018; Choi et al., 2019; Kamei et al., 2020).
In this study, CCL4 and CCL19 were coupled into OmpK-based DNA vaccine, respectively. Two bicistronic DNA vaccines were constructed. The transcription and expression of these bicistronic DNA vaccines were verif ied in vivo and in vitro. Then immune responses they induced in f lounder after immunization were investigated. The purpose of this paper is to explore the adjuvant potential of CCL4 and CCL19 on the OmpK-based DNA vaccine againstV.anguillarum.
2 MATERIAL AND METHOD
2.1 Fish
Flounder (Paralichthysolivaceus, 35±5 g) were obtained from a f ish farm (Rizhao, Shandong, China),and all of the f ish were held in the wet lab at 21±0.5 °C for two weeks before the experiments for acclimatization and evaluation of overall f ish health.Fish was randomly sampled before the experiment.General appearance, level of activity, bacteria isolation, and molecular biological detection (PCR)were performed to conf irmV.anguillarumfree. Then only healthy f ish were used in the following experiments. Anesthetized the f ish with tricaine methanesulfonate (MS222, Sigma, USA), and the subsequent operation was performed. All eff orts were dedicated to minimizing suff ering.
Investigations were performed strictly according to the ethical standards and the Guidelines of“Regulations for the Administration of Aff airs Concerning Experimental Animals” documented by the State Science and Technology Commission of Shandong Province. These studies were also approved by the Committee of the Ethics on Animal Care and Experiments in Ocean University of China.
2.2 Bacteria, plasmids, antibodies, and cells
The pathogenicV.anguillarumSJ060621 was stored in our lab (Tang et al., 2008). The strain was cultured in 2216E marine medium at 28 °C for 24 h and diluted with phosphate buff ered solution (PBS) tothe f inal concentration of 1.0×108CFU/mL for challenge.
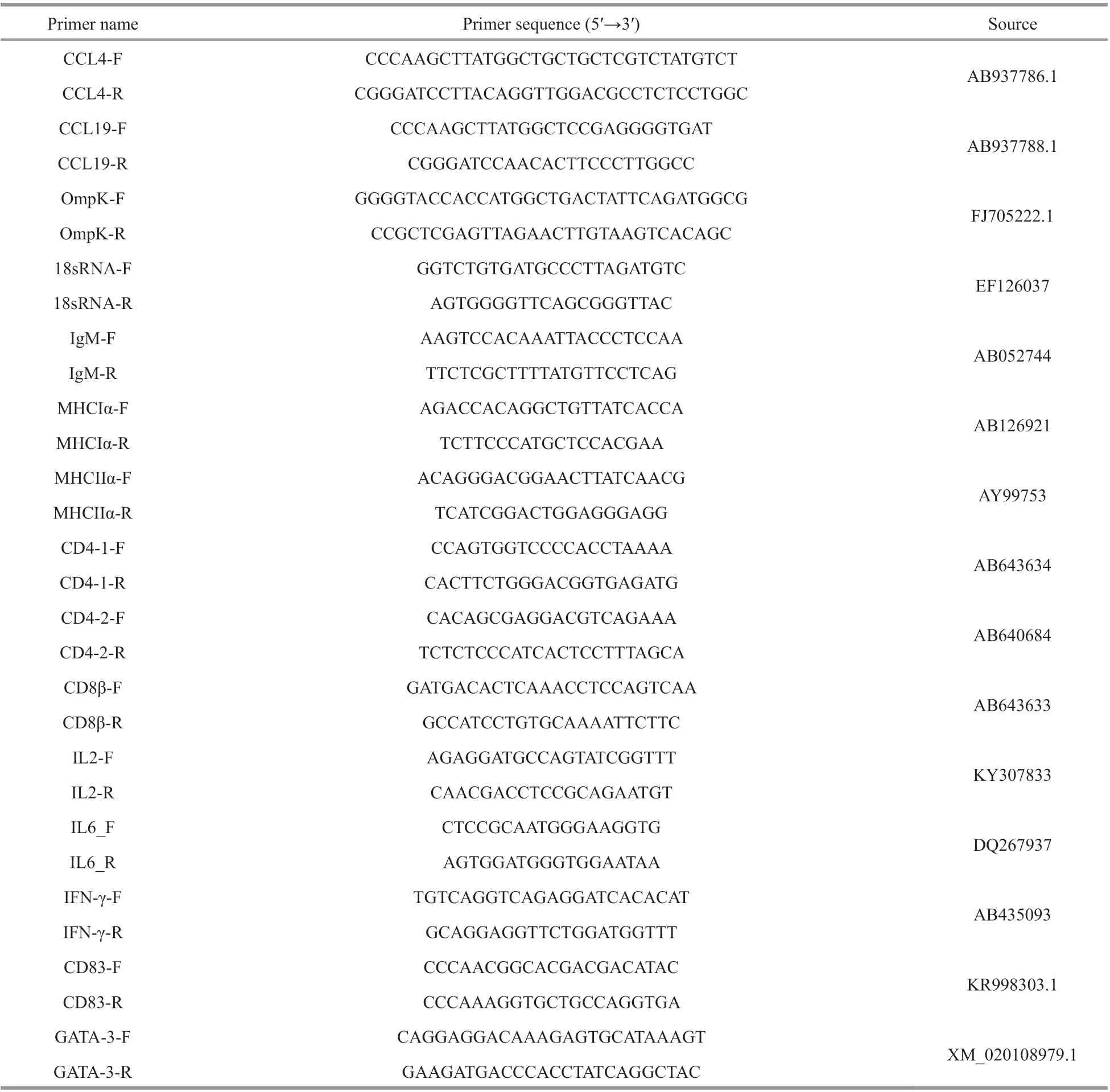
Table 1 The primers used in this study
The eukaryotic plasmid pBudCE4.1 (Invitrogen,Carlsbad, CA, USA) contains two multiple cloning sites, which are located downstream of the human cytomegalovirus (CMV) immediate-early promoter and the human elongation factor 1α subunit (EF-1α)promoter. There is 6×His-tag on the downstream of multiple cloning sites in pBudCE4.1, and the terminator is downstream of the 6×His-tag. Specif ic primers without terminator were designed for amplif ication of CCL4 and CCL19 genes (Table 1) to enable the recombinant plasmids to express CCL4/CCL19 with 6×His-tag. Therefore, the expression of CCL4 and CCL19 proteins can be detected by mouseanti His-tag monoclonal antibodies, and the expression of OmpK protein can be detected by rabbit-anti OmpK polyclonal antibodies.
Recombinant OmpK protein with GST tag (rOmpKGST) was expressed and purif ied inEscherichiacoli,and used for the preparation of rabbit anti-rOmpK polyclonal antibody according to previous reports(Ahmadivand et al., 2018; Jung et al., 2018). Western blot was used to detect the specif icity of the polyclonal antibodies, and it was found that the antiserum could specif ically bind to purif ied rOmpK-GST and OmpK protein fromV.anguillarum(Supplementary Material). Mouse-anti His-tag monoclonal antibodies were purchased from Biyuntian, China. Mouse antif lounder IgM monoclonal antibodies (FIgM-Mab) and mouse anti-f lounder CD4-1, CD4-2 monoclonal antibodies (FCD4-1-Pab, FCD4-2-Pab) were produced previously in our laboratory (Li et al., 2007; Tian et al.,2019). In this study, mouse-anti His-tag monoclonal antibodies and rabbit-anti rOmpK-GST polyclonal antibodies were diluted into 1:200 and 1:1 000 respectively with PBS for indirect immunof luorescent assay (IFA). The ascites f luids of FIgM-Mab, FCD4-1-Pab, and FCD4-2-Pab were diluted into 1:1 000 and were used in f low cytometry (FCM).
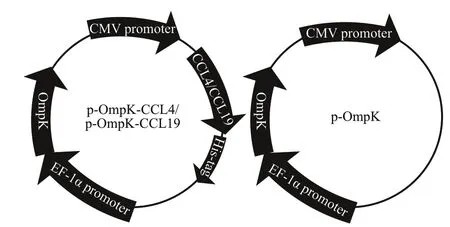
Fig.1 The diagram of p-OmpK-CCL4/p-OmpK-CCL19 and p-OmpK
Hirame natural embryo (HINAE) cells were kindly provided by Dr. Ikuo Hirono, the professor of Tokyo University of Marine Science and Technology and stored in our lab (Liu et al., 2016b). The cells were plated in 6-well plates, cultured with Leibovitz’s L-15 medium (Thermo Fisher, MA, USA) containing 20%fetal calf serum (FBS), 100-IU/mL penicillin, and 100-μg/mL streptomycin, and used for transfection to analyze the expression of OmpK and CCL4/CCL19 proteins.
2.3 Plasmids construction and preparation
The pathogenicV.anguillarumSJ060621 was cultured in 2216E marine medium at 28 °C for 8 h,and the bacteria suspensions were used as the template for amplif ication of OmpK gene with specif ic primers(Table 1). According to the instructions, the PCR products were purif ied using TIANgel Midi Purif ication Kit (Tiangen, China) and digested with KpnI and XhoI, and then the digested PCR products were inserted into the multiple cloning sites under the control of the EF-1α promoter in pBudCE4.1 to construct the p-OmpK plasmid. According to the manufacturer’s instructions, total RNA from spleen of the f lounder was extracted using TRIZOL reagent(Bao Sheng, Dalian, China) and measured the concentration by Nanodrop 8000 spectrophotometer(Thermo Fisher, MA, USA). The f irst strand of complementary DNA (cDNA) was synthesized by the reverse transcriptase M-MLV kit (TaKaRa, Dalian,China). Refer to the sequences from NCBI (Gene bank accession Nos. AB937786.1 and AB937788.1),the CCL4 and CCL19 genes of f lounder were amplif ied using the designed specif ic primers,respectively (Table 1). The PCR products of CCL4 and CCL9 were digested with HindIII and BamHI,and then were inserted multiple cloning sites on the downstream of CMV promoter of p-OmpK plasmid respectively to construct p-OmpK-CCL4 and p-OmpK-CCL19. The diagram of p-OmpK-CCL4/p-OmpK-CCL19 and p-OmpK is shown in Fig.1.
To prove the accuracy of the transgene, these recombinant plasmids (p-OmpK, p-OmpK-CCL4,and p-OmpK-CCL19) were subjected to DNA sequencing (Tsingke, Qingdao, China), moreover,they were determined by double digestion to detect the foreign genes that have been successfully inserted into pBudCE4.1 plasmid. According to the manufacturer’s instructions, endotoxin-free recombinant plasmids were purif ied using Endo Free Plasmid Kit (Tiangen, China), and the concentration was quantif ied using Nanodrop 8000 spectrophotometer. These plasmids are used for subsequent transfection and injection.
2.4 Expression of plasmids in vitro
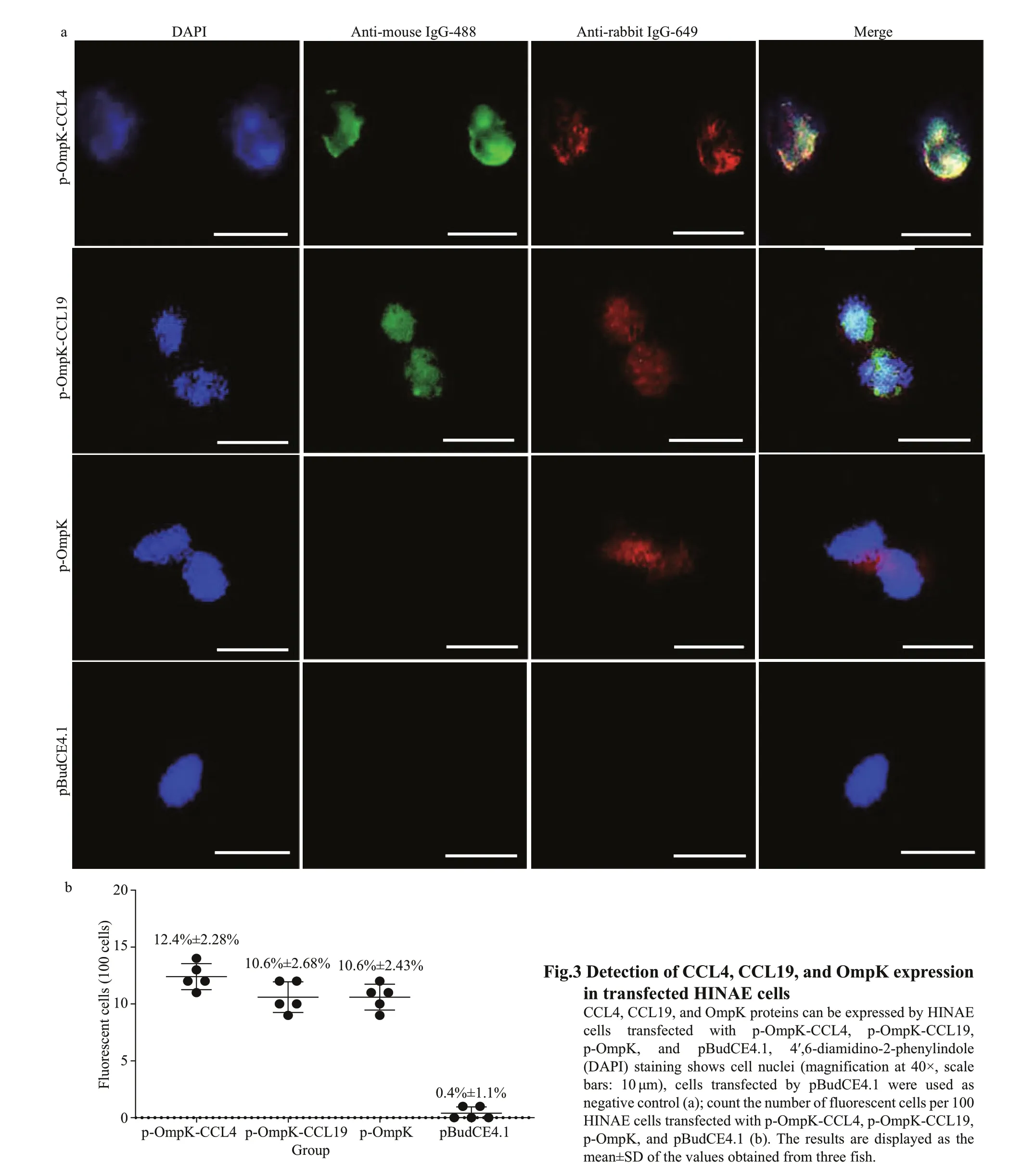
According to the manufacturer’s instructions of Lipofectamine®3000 (Thermo Fisher, MA, USA),approximately 70%-80% conf luent HINAE cells were transfected with 500-ng p-OmpK-CCL4,p-OmpK-CCL19, p-OmpK, and pBudCE4.1,respectively. The cells were cultured at 24 °C for 48 h and then digested with pancreatin and resuspended with PBS. After that, the cells were dropped on adherent glass slides, left at room temperature for 2 h,and then f ixed with 4% (w/v) paraformaldehyde for 15 min for IFA. Brief ly, transfected cells were inoculated with rabbit-anti rOmpK-GST polyclonal antibody (1:1 000) and mouse-anti His-tag monoclonal antibody (1:200, Biyuntian, China) for 1 h at 37 °C,and then goat-anti-rabbit Ig-Alexa Fluor®649 and goat-anti-mouse Ig-Alexa Fluor®488 (1:1 000,Thermo Fisher Scientif ic, USA) were inoculated with the transfected cells for 45 min at 37 °C. Finally, cells were stained with nuclei of 4′, 6-diamidino-2-phenylindole (DAPI, 1:1 000, Invitrogen, Carlsbad,USA) for 15 min at room temperature, and then specif ic f luorescence was imaged by epi-f luorescence microscope IX71 (Olympus, Japan). The observation f ield was randomly selected and the number of cells with f luorescence per 100 cells was counted in p-OmpK-CCL4, p-OmpK-CCL19, and p-OmpK groups. This operation was repeated f ive times. Cells transfected with pBudCE4.1 plasmid served as a negative control.
2.5 Transcription and expression of plasmids in vivo
RT-PCR and IFA were performed to analyze the transcription and expression of p-OmpK-CCL4,p-OmpK-CCL19, and p-OmpK in muscles on the 3thday after immunization. Total RNA was exacted by TRIZOL reagent (Bao Sheng, Dalian, China) and cDNA was generated by Reverse Transcriptase M-MLV kit (TaKaRa), the template of PCR amplif ication was the cDNA. The specif ic primers of the study are shown in Table 1.
Muscle tissue cryosections were prepared according to the method previously (Wu et al, 2009).The frozen sections of muscle were washed with PBST (PBS containing 0.05% Tween 20) for 5 min and blocked with 5% bovine serum albumin (BSA) in PBS for 1 h at 37 °C, then these sections incubate with rabbit-anti rOmpK-GST polyclonal antibodies(1:1 000) and mouse-anti His-Tag monoclonal antibodies (1:200) for 1 h at 37 °C, goat-anti-rabbit Ig-Alexa Fluor®649 (1:1 000) and goat-anti-mouse Ig-Alexa Fluor®488 (1:1 000) as secondary antibodies for 45 min at 37 °C and then stained with DAPI in a moisture chamber for 15 min at room temperature.Finally, these sections were washed three times by PBST and mounted in buff ered glycerin for observation with f luorescence microscopy IX71(Olympus, Japan). Wash three times with PBST after each step. The frozen sections from pBudCE4.1 group were used as the negative control.
2.6 Fish vaccination and sampling
Healthy individuals were divided into 4 groups(120 f ish per group), and the 120 f ish were evenly divided into 5 tanks. Fish were sampled randomly and RT-PCR was conducted to detect the level of CCL4 and CCL19 in healthy f lounder, and the results showed that the level of CCL4 and CCL19 in healthy f lounder was no signif icant diff erence compared with the f lounder injected with pBudCE4.1 (data not shown). The f ish were injected with 20 μg of pBudCE4.1, p-OmpK, p-OmpK-CCL4, and p-OmpKCCL19, respectively (100 μL per individual). After injection, samples were randomly taken from each group to detect the immune response of the f lounder.
The percentage of CD4-1+and CD4-2+T lymphocytes was detected by FCM after immunization. Three individuals were randomly selected in each group, and then the peripheral blood(PBL), spleen, and head kidney were sampled on the 3rd, 5th, 7th, 14th, 21st, and 28thday, respectively. The isolation protocol of lymphocytes of PBL, spleen, and head kidney was performed according to a previously reported method (Li et al., 2007). Then, the isolated lymphocytes were adjusted to 1.0×107cells/mL, and were used to analyze the percentage of CD4-1+and CD4-2+T lymphocytes. Similarly, three individuals were randomly selected in each group after immunization, and then the PBL, spleen, and head kidney were sampled in the 1st, 2nd, 3rd, 4th, 5th, 6th, and 7thweek, respectively. The lymphocytes were collected as described above and then were used to analyze the percentage of sIgM+B lymphocytes.
The injection sites were collected from three individuals in each group on the 5thday after immunization for detecting the recruitment of leucocytes by H&E staining.
The muscles, spleen, and head kidney were dissected from three individuals from each group on the 7thday after immunization and stored in RNA Later reagent (TaKaRa), were used to detect the expression prof iles of immune-related genes by qRTPCR.
2.7 H&E staining
The muscles at the injection site were f ixed with Bonn’s f luid for 20 h, and all samples were dehydrated and embedded in paraffi n wax after rinsed with 75%alcohol, then 7-μm sections were cut using a rotary microtome (Leica, Heidelberg, Germany) and mounted on pretreated slides. After deparaffi nized with xylene and 50% xylene/ethanol solution, these sections were rehydrated by successive immersion in 95%, 80%, 70%, 50%, and 30% ethanol for 5 min each step. These sections were stained with hematoxylin for 20 min, and after diff erentiated in 1% acidic alcohol for 30 s, the sections were washed with water for 30 min. Next, after stained with eosin for 10 s and diff erentiated in 95% ethanol for 30 s,the sections were dehydrated through a series of ethanol solutions (30%, 50%, 70%, 80%, and 95%).Finally, the sections were dehydrated in 100%ethanol and cleared in xylene. Subsequently, they were f ixed with neutral balsam and examined for histological changes by a light microscope (Olympus DP70, Japan).
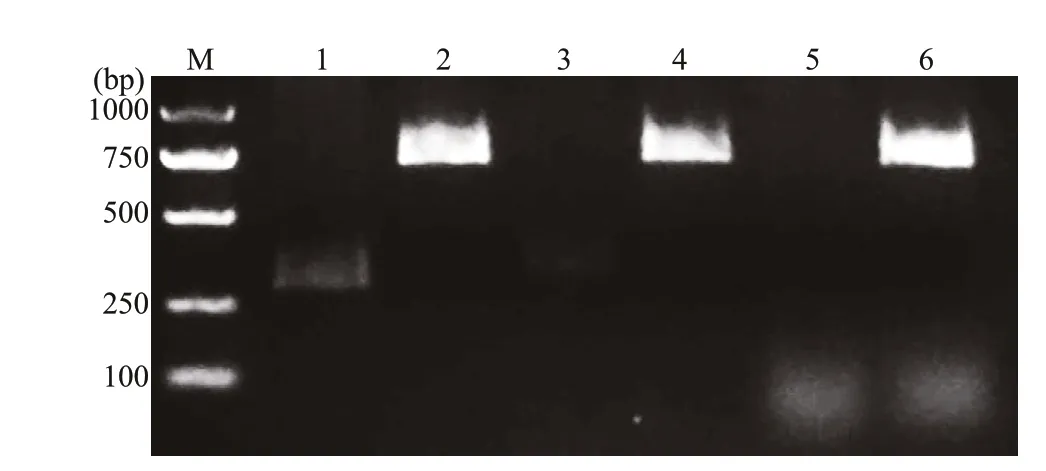
Fig.2 Digestion of recombinant plasmids
2.8 Flow cytometry
For CD4-1+and CD4-2+T lymphocytes detection after immunization, the lymphocytes were incubated with FCD4-1-Pab or FCD4-2-Pab for 1 h at 37 °C,after washing with PBS for three times, incubated goat-anti-mouse Ig-Alexa Fluor®488 (1:1 000,Thermo Fisher Scientif ic, USA) for 1 h in the dark at 37 °C, and then the lymphocytes were washed three times with PBS and the cell suspensions were analyzed by Accuri C6 cytometer (BD Accuri., USA).For detecting sIgM+B lymphocytes after vaccination,the lymphocytes were incubated with FIgM-Mab for 1 h at 37 °C, and then incubated goat-anti-mouse Ig-Alexa Fluor®649 (1:1 000, Thermo Fisher Scientif ic,USA) for 1 h in the dark at 37 °C after washing by PBS for three times. And then the cell suspensions were analyzed by Accuri C6 cytometer. Fluorescent light (FL)-1 and FL-4 were used to determine Alexa Fluor®488 labeled cells and Alexa Fluor®649 labeled cells, respectively. The myeloma culture supernatant instead of FIgM-Mab, and mouse negative serum instead of FCD4-1-Pab or FCD4-2-Pab were used as negative controls.
2.9 qRT-PCR
The immune response of T, B lymphocytes in f lounder was signif icantly enhanced on the 7thday after immunization with DNA vaccines based on our previous researches (Liu et al., 2017b; Xu et al.,2019a), therefore, the expression of genes related to T, B lymphocytes immune response was detected(IgM, MHC I, MHC II, CD8β, CD4-1, CD4-2, IFN-γ,IL-2, IL-6, CD83, and GATA-3). Three individuals were randomly killed from each group on the 7thday after immunization, total RNA was extracted from muscles, spleen, and head kidney, and then cDNA was generated by Reverse Transcriptase M-MLV kit(TaKaRa) following the manufacturer’s instructions.qRT-PCR was performed by SYBR Green I Master Mix (Roche, Basel, Switzerland) in Light Cycle®48ⅡReal-Time PCR System (Roche, Basel, Switzerland).The specif ic primers are shown in Table 1. The thermal cycling prof ile consisted of an initial denaturation at 95 °C for 30 s, followed by 45 cycles of denaturation at 95 °C for 5 s, and extension at 60 °C for 30 s. An additional temperature ramping step was utilized to produce melting curves of the reaction from 65 °C to 95 °C. The expression prof iles in blank control individuals were def ined as 1. Each assay was performed in triplicate and the 18S gene as an internal control. All data were analyzed using the 2-ΔΔCtmethod according to a previous report (Livak and Schmittgen, 2001).
2.10 Challenge
Seven weeks after vaccination, 30 individuals were randomly selected from each group and injected with 1.0×107CFU (10×LD50) live virulentV.anguillarum.The f ish were also maintained in the oxygen-supplying laboratory water system and the water temperature was 21±0.5 °C. Mortality was monitored over for 14 d after challenge, and the relative percentage survival (RPS) was calculated as described previously after determining that the death of f lounder was caused byV.anguillarum(Amend, 1981).
2.11 Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc. San Diego, CA,USA). The diff erences were determined using a oneway analysis of variance (ANOVA). In all cases, the results were expressed as means±SD (standard deviation), and the signif icance level was def ined asP<0.05.
3 RESULT
3.1 Identif ication of constructed bicistronic DNA vaccines
As shown in Fig.2, the OmpK, CCL4, and CCL19 genes were successfully inserted into the pBudCE4.1 under the control of EF-1α promoter and CMV promoter, respectively.
After double digestion, the results of p-OmpKCCL4 and p-OmpK-CCL19 (HindⅢ/BamHI, KpnI/XhoI) showed that the specif ic CCL4 and CCL19 bands were observed in lane 1 and 3, and OmpK bands were observed in lane 2 and 4. For p-OmpK plasmid,after digestion with KpnI and XhoI and agarose gel electrophoresis, a specif ic OmpK gene band was observed in lane 6, however, there is no specif ic gene band after digested by HindⅢ and BamHI. Moreover,the results of sequencing show that the foreign genes inserted into the plasmid are correct (data not shown).The above results showed that the CCL4, CCL19, and OmpK genes were inserted into the expected sites, and p-OmpK-CCL4 and p-OmpK-CCL19 plasmids were prepared successfully.
3.2 Expression of bicistronic DNA vaccines in vitro
The results of IFA showed that HINAE transfected with p-OmpK-CCL4 and p-OmpK-CCL19 plasmids can express OmpK, CCL4, and CCL19 proteins, and HINAE transfected with p-OmpK can only express OmpK protein, but f luorescence was not observed in the pBudCE4.1 group (Fig.3a). Green f luorescence represented CCL4 and CCL19 proteins, and red f luorescence represented OmpK protein. Moreover,the effi ciency of transfection in p-OmpK-CCL4,p-OmpK-CCL19, and p-OmpK groups was 12.4%±2.28%, 10.6%±2.68%, and 10.6%±2.43%,respectively. However, for the pBudCE4.1 group,there were almost no f luorescent cells (Fig.3b).
3.3 Transcription and expression of bicistronic DNA vaccines in vivo
In Fig.4, bright and clear DNA bands are observed in lanes 1, 3, 5, and 6, representing CCL4, OmpK,CCL19, and OmpK genes, respectively, which proved that p-OmpK-CCL4 and p-OmpK-CCL19 can be transcribed in the muscles of f lounder. Similarly, a bright DNA band of OmpK was observed in lane 9,demonstrating that p-OmpK can be transcribed in f lounder muscles, but not in the pBudCE4.1-injected f ish. Notably, the specif ic CCL4 gene bands were shown in lanes 2, 7, and 10, and the specif ic CCL19 gen bands were shown in lanes 4, 8, and 11, but they were not as clear and bright as those in the p-OmpKCCL4 and p-OmpK-CCL19 groups. This probably because the muscles contain a small amount of CCL4 and CCL19 genes rather than transcribed by recombinant plasmids.
The IFA results showed that the specif ic green and red f luorescence can be observed in muscles of p-OmpK-CCL4- and p-OmpK-CCL19-injected f ish,which revealed that OmpK, CCL4, and CCL19 proteins can be expressed in f lounder. In the p-OmpK group, only red f luorescence was observed, indicating that the recombinant plasmid can only express OmpK protein in f lounder. And there is no specif ic f luorescence was detected in muscles of the f lounder from pBudCE4.1 group (Fig.5).
3.4 Increase of leukocytes at injection site
H&E staining was performed to investigate the recruitment of leukocytes after immunization. The results of H&E staining showed that a large number of leukocytes were recruited at the injection site of f lounder in p-OmpK-CCL4 and p-OmpK-CCL19 groups, followed by the pBudCE4.1-OmpK(p-OmpK) group. But the result of the pBudCE4.1 group showed that little leukocytes were recruited to the injection site (Fig.6).
3.5 Variations on T lymphocytes in peripheral blood, spleen, and head kidney after immunization
The percentage of CD4-1+and CD4-2+T lymphocytes in PBL, spleen, and head kidney for all groups was showed in Fig.7.
In the control group, CD4-1+and CD4-2+T lymphocytes maintained relative invariant during the experimental period. In p-OmpK, p-OmpK-CCL4,and p-OmpK-CCL19 groups, CD4-1+and CD4-2+T lymphocytes increased signif icantly on the 7thday(P<0.05), and their peak levels occurred on the 14thday, then they reduced slowly. In PBL, spleen, and head kidney, the percentage of CD4-1+T lymphocytes were 10.5%±0.64% and 9.4%±0.29%, 11.9%±0.71%and 9.4%±0.74%, 19.2%±0.50% and 19.6%±0.88%on the 7thday after immunization, respectively. And the percentage of CD4-2+T lymphocytes were 11%±0.67% and 11.1%±0.68%, 10.8%±0.55% and 9.8%±0.58%, 23.1%±0.32%, and 23.8%±0.68%,respectively. Moreover, it was higher in both p-OmpK-CCL4 and p-OmpK-CCL19 groups than those in the p-OmpK group (P<0.05).
The above results indicated that CCL4 and CCL19 can further increase the percentage of CD4-1+and CD4-2+T lymphocytes based on the OmpK DNA vaccine.
3.6 Variations on sIgM + B lymphocytes in peripheral blood, spleen, and head kidney after immunization
In pBudCE4.1-OmpK, pBudCE4.1-OmpK-CCL4,and pBudCE4.1-OmpK-CCL19 (p-OmpK, p-OmpKCCL4, and p-OmpK-CCL19) groups, the results showed that the percentage of sIgM+B lymphocytes in PBL, spleen, and head kidney increased signif icantly in 2ndor 3rdweek, peaked in the 6thweek, and then began to decline (Fig.8).
In PBL, spleen, and head kidney of p-OmpK-CCL4 and p-OmpK-CCL19 groups, the percentage of sIgM+B lymphocytes was 23.9%±0.62% and 23.9%±0.98%,14.8%±0.46% and 15.9%±0.60%, 23.4%±0.44% and 10.9%±0.65% in the 6thweek after immunization,respectively. It was higher in both p-OmpK-CCL4 and p-OmpK-CCL19 groups than those in the p-OmpK group (P<0.05). In addition, the percentage of sIgM+B lymphocytes of the head kidney in p-OmpK-CCL4 group was higher than the p-OmpK-CCL19 group in the 6thand 7thweeks after immunization (P<0.05).
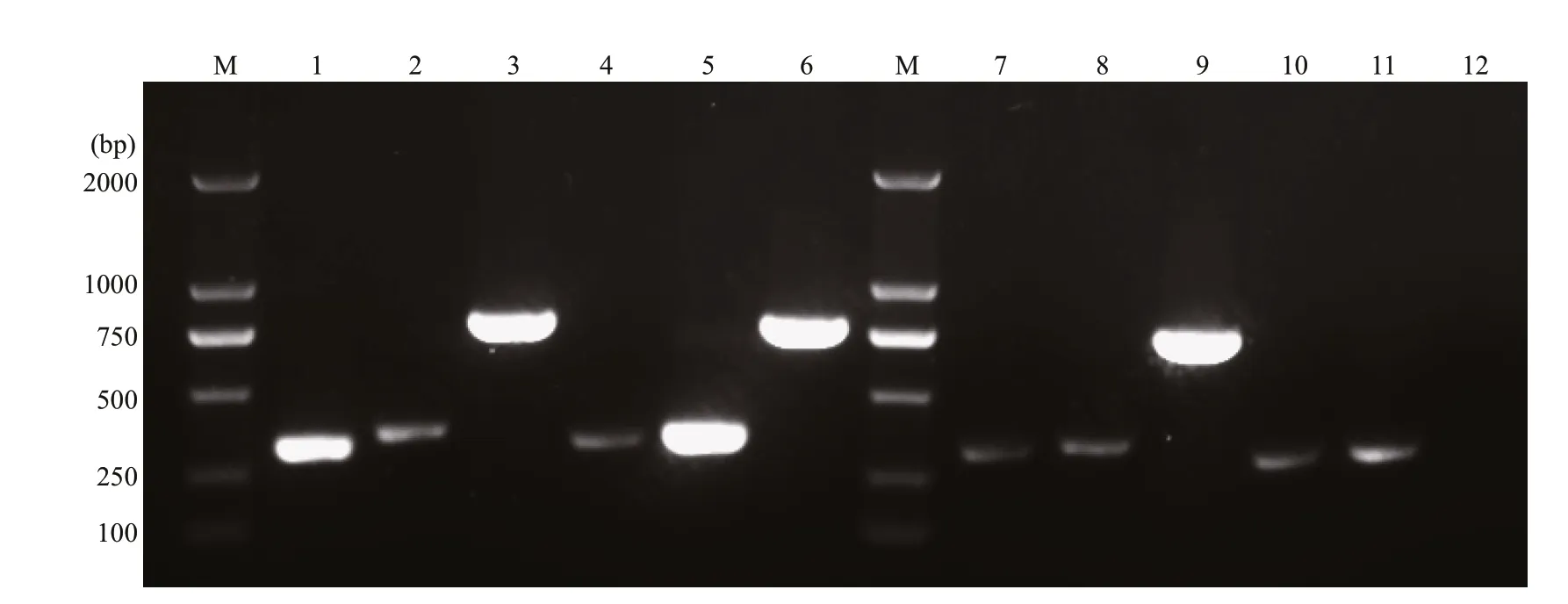
Fig.4 The transcription of p-OmpK-CCL4, p-OmpK-CCL19, p-OmpK, and pBudCE4.1 in f lounder detected by RT-PCR on the 3 rd day post-immunization
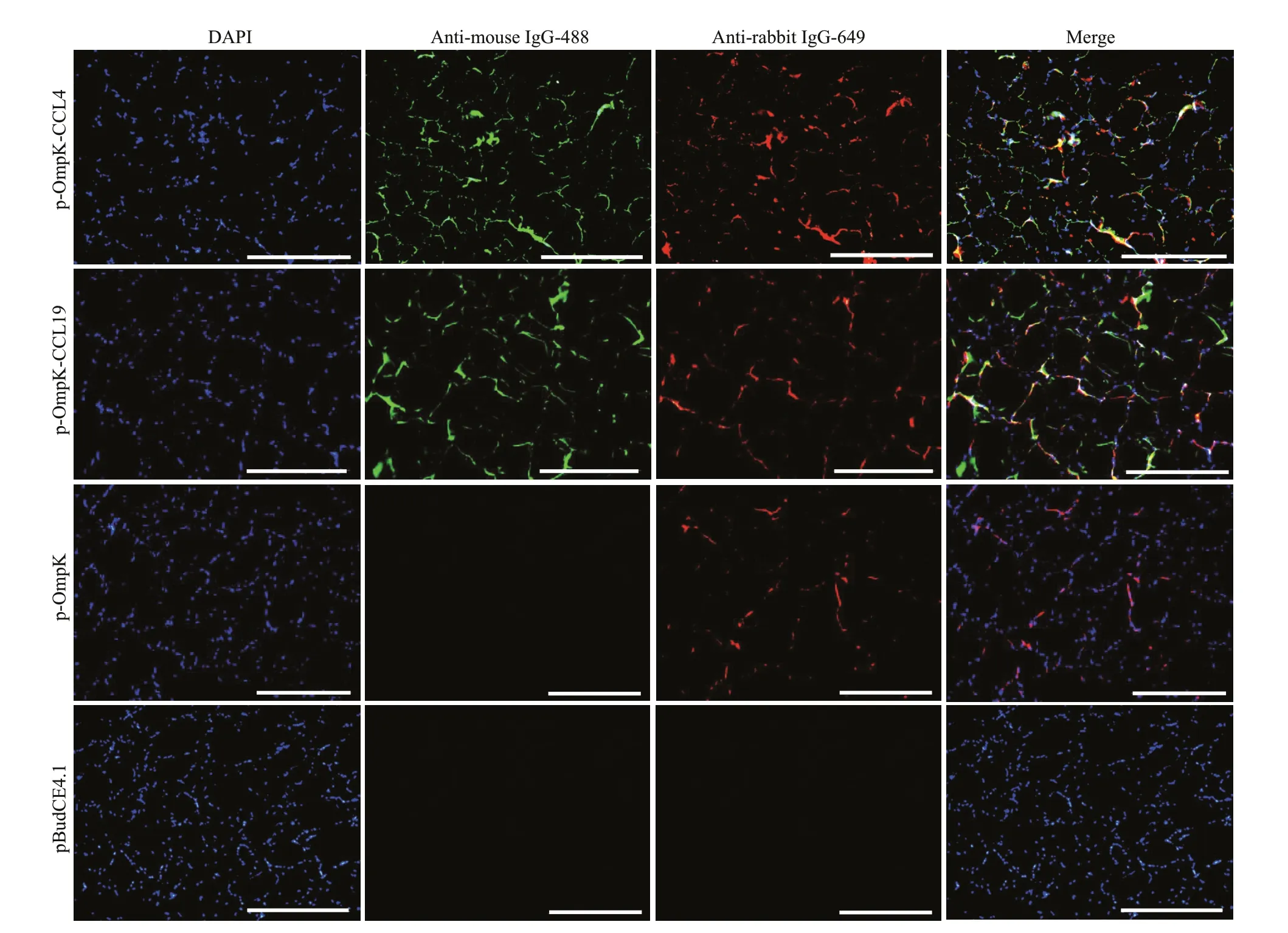
Fig.5 Detection of CCL4, CCL19, and OmpK expression in vaccinated f ish
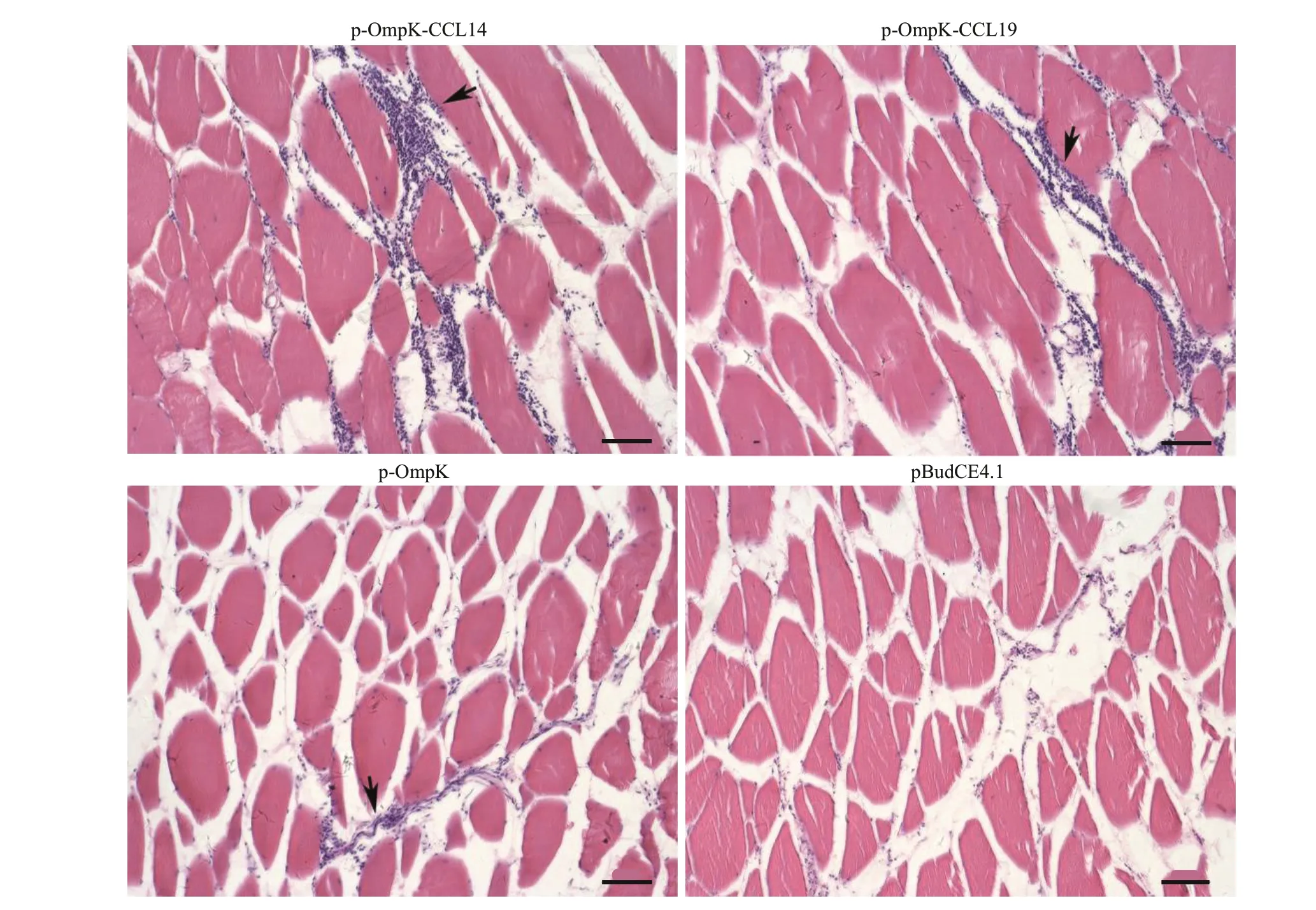
Fig.6 Injection of recombinant plasmids leads to local recruitment of leucocytes
The above results indicated that CCL4 and CCL19 can further increase the percentage of sIgM+B lymphocytes based on the OmpK DNA vaccine.
3.7 Expression of immune-related genes
In muscles of p-OmpK-CCL4 and p-OmpKCCL19 groups, the expression prof iles of IFN-γ,GATA-3, IL-2, CD83, MHC I, and MHC Ⅱ genes signif icantly increased after immunization and signif icantly higher than that of the pBudCE4.1 group.The expression prof iles of IFN-γ, IL-2, CD83, MHC I, and MHC Ⅱ genes were also signif icantly higher than that of the p-OmpK group (Fig.9a;P<0.05), but the expression prof iles of CD4-1, CD4-2, CD8β, and IL-6 were not signif icantly diff erent from that of the control groups (p-OmpK and pBudCE4.1) (data not shown).
In the spleen, there was no signif icant diff erence in the expression prof iles of IL-6 genes among the four groups, the expression prof iles of CD8β, IFN-γ, IgM,IL-2, CD4-1, CD4-2, MHC I, and MHC Ⅱ genes in p-OmpK-CCL4 and p-OmpK-CCL19 groups were signif icantly higher than that of pBudCE4.1 group,and the expression prof iles of IgM, IL-2, CD4-1,CD4-2, MHC I, and MHC Ⅱ genes were signif icantly higher than that of the p-OmpK group after immunization (Fig.9b,P<0.05).
In the head kidney, the relative expressions of IL-6,IFN-γ, IL-2, IgM, CD4-1, CD4-2, CD8β, MHC I, and MHC Ⅱ genes in p-OmpK-CCL4 and p-OmpKCCL19 groups were signif icantly higher than that of the pBudCE4.1 group after immunization, and the expression prof iles of IL-2, CD4-1, CD4-2, MHC I,and MHC Ⅱ genes were signif icantly higher than that of the p-OmpK group (Fig.9c;P<0.05).
3.8 Relative percentage survival
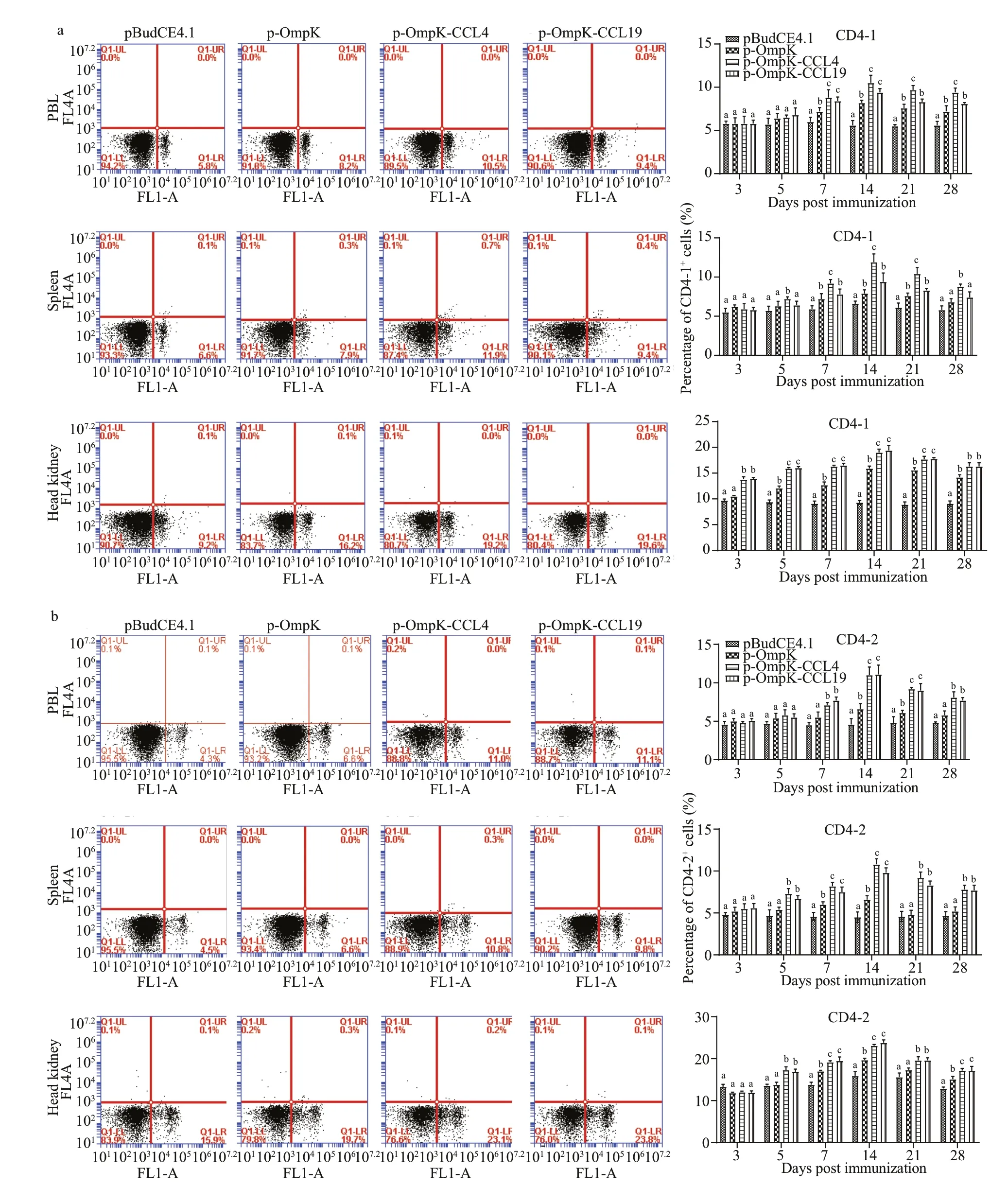
Fig.7 The T lymphocytes immune response induced by recombinant plasmids
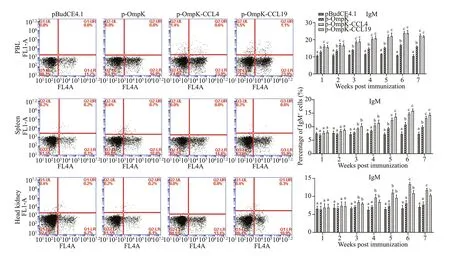
Fig.8 The sIgM + B lymphocytes immune response induced by recombinant plasmids
After the challenge, f lounder in p-OmpK-CCL4 and p-OmpK-CCL19 groups began to die on the 1st-2ndday and died quickly during the 3rd-10thdays, and then the mortalities remained stable till the end of the experiment. However, f lounder in p-OmpK and pBudCE4.1 groups died almost every day in 14 days after the challenge. The cumulative mortality rates of p-OmpK-CCL4, p-OmpK-CCL19, and p-OmpK groups were signif icantly lower than those in the pBudCE4.1 group, which were 23.3%, 33.3%, and 53.3%, respectively, conferred to RPS of 74.1%,63.3%, and 40.8% (Fig.10a). Additionally, the results of Log-Rank test revealed that survivals of p-Ompk-CCL4, p-OmpK-CCL19, and p-OmpK groups were signif icantly higher than the pBudCE4.1 group, and there was no diff erence in mortality rates among p-OmpK-CCL4, p-OmpK-CCL19, and p-OmpK groups (Fig.10b). For all challenge trials, examination of moribund f ish indicated thatV.anguillarumwere the only types of bacterial strains isolated from the liver, spleen, and the head-kidney tissue of challenged f ish, respectively, suggesting that mortalities were caused by the challenging bacteria.
It should be emphasized that the RPS was calculated on the 14thday after the challenge, and the results might have been diff erent if the challenge had been extended to 14 days or more.
4 DISCUSSION
Outer membrane protein is an important pathogenic factor of gram-negative bacteria and plays a key role in bacterial infection and colonization (Abdelhamed et al., 2017). Outer membrane protein exists in a variety of pathogenic bacteria, and because it can be easily recognized by the immune system of f ish, it is considered to be an important factor that stimulates f ish to produce immune responses (Li et al., 2014).Researchers have shown that the outer membrane protein of bacteria is an antigenic protein that locates on the surface of bacteria, and it has the potential to become a candidate vaccine for bacteria, such asV.anguillarum,Vibrioharveyi,Edwardsiellatarda(Hamod et al., 2012; Liu et al., 2016a; Zhu et al.,2019). Moreover, some studies have shown that the outer membrane of pathogenic bacteria can be used as a DNA vaccine or subunit vaccine to induced specif ic antibodies and provided protection against pathogenic bacteria (Vazquez-Juarez et al., 2005; Kumar et al.,2007; Liu et al., 2017a). In 1991, it was f irst reported that antigens could be expressed by recombinant plasmids in f ish muscles (Hansen et al., 1991), and DNA vaccines of f ish are still widely studied today.DNA vaccines have many advantages, such as being easy to produce and store, and relatively inexpensive,moreover, DNA vaccines can also induce strong and durable humoral and cellular immune responses(Adel et al., 2016; Zahm et al., 2017; Lee et al., 2018).However, in terms of animal safety and environmental protection, DNA vaccines have some potential and unknown risks.
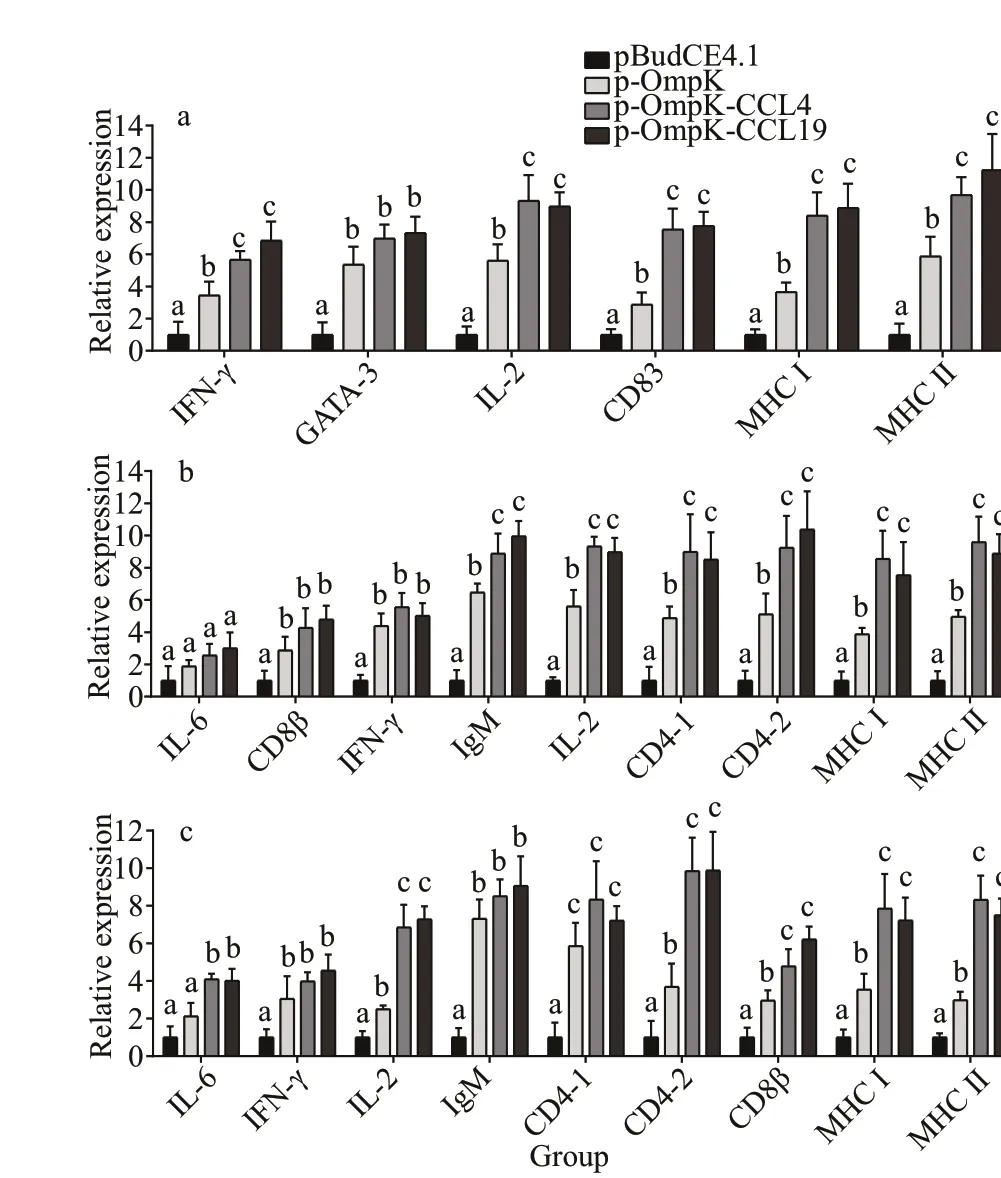
Fig.9 Detection of the expression of immune-related genes induced by recombinant plasmids
Our previous study has demonstrated that the OmpK gene ofV.anguillarumin the form of recombinant plasmids and injected it into the f lounder,resulting in an RPS of 50% in f lounder (Xu et al.,2019a). Although the OmpK-based DNA vaccine can induce humoral and cellular immune responses in f lounder, and higher levels of antibodies can be detected after immunization, but it did not provide the f lounder with ideal protection. Many factors aff ect the protection of DNA vaccines, such as the type of expression vector, use of adjuvants, diff erent inoculation methods, inoculation routes, and environmental factors (Cheng et al., 1993; Fynan et al., 1993). The use of adjuvants is a simple, convenient,and eff ective method to improve the protection of vaccines. Common adjuvants include Freund’s adjuvant, aluminum salt adjuvant. Previous research has demonstrated that the OmpW subunit vaccine ofE.tardaformulated with Freund’s complete adjuvant can result in an RPS of 60% in f lounder (Liu et al.,2017b). However, compared with the most common adjuvants in f ish farming (aluminum salt adjuvants and oil adjuvants), cytokines as adjuvants have advantages in initiating the expression of costimulatory molecules and the polarization of antigen-presenting cells (Tovey and Lallemand, 2010). Xu et al. (2020)pointed out that CCL3/CCL4/CCL19/CCL21 as adjuvants for VAA-based DNA vaccine could eff ectively protect f lounder againstV.anguillarumafter immunization.
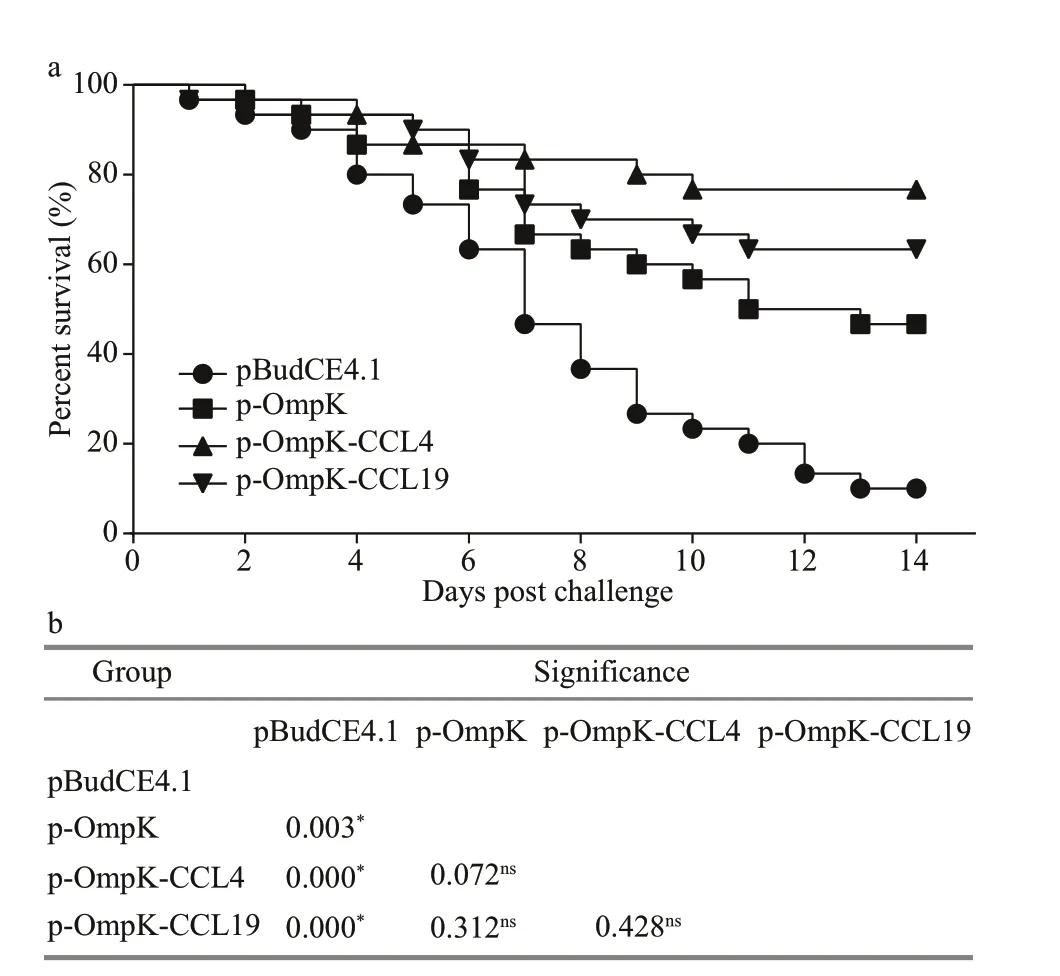
Fig.10 Survival percentage of immunized f ish after challenged with V. anguillarum
The pBudCE4.1 plasmid is a eukaryotic dual expression vector with CMV and EF-1α promoters,enabling the simultaneous expression of two foreign genes inserted into the plasmid. Research on mice showed that the genes of mycolyl-transferase Ag85A and phosphate transport receptor PstS-3 fromMycobacterium tuberculosiswere inserted into the pBudCE4.1 plasmid, and the antigens expressed by recombinant plasmid were proved to have good immunogenicity (Romano et al., 2006). pBudCE4.1 plasmid as a vector, intramuscular injection of naked DNA plasmids encoding proinsulin and pancreatic regeneration III protein can reduce the incidence of hyperglycemia and diabetes in mice (Hou et al.,2011). In this research, the OmpK gene ofV.anguillarumand the CCL4/CCL19 genes of f lounder were inserted into the pBudCE4.1 vector under the control of EF-1α and CMV promoters,respectively, to generate two bicistronic plasmids that co-expressed OmpK and CCL4/CCL19. To prove that the foreign genes were inserted into the pBudCE4.1 plasmid successfully, double digestion and agarose gel electrophoresis assay were performed. The results proved that these foreign genes were successfully inserted into the plasmids. In addition, DNA sequencing results showed that the foreign genes were accurate. Bright green and red f luorescence can be observed by IFA in HINAE cells transfected with recombinant plasmids, which revealed that these recombinant plasmids can be successfully expressed in vivo and in vitro.
Chemokines are small secreted proteins and usually range between 8-12 kDa, they belong to the subfamily of chemokine cytokines, which play a key role in mediating leukocyte migration, regulating immune responses and diff erentiation of the recruited cells(Moser et al., 2004). To date, a variety of chemokines have been reported in mammals and teleost. The chemokine CC-motif ligand 4 (CCL4) is a member of the CC chemokine subfamily, it is also known as MIP-1β because it was f irst discovered from the macrophages stimulated by lipopolysaccharide (LPS)(Chensue et al., 1996). CCL4 in mammals is usually secreted by antigen-presenting cells (APC), such as B cells, T cells, macrophages, and DC, and it binds to its specif ic receptor CCR5 to induce monocytes,migration, and recruitment of phages and Th1 cells(Bystry et al., 2001). Although CCL4 has been well studied in mammals, it has been identif ied in only a few species of f ish, and few kinds of research on CCL4 as an adjuvant for f ish vaccines (Xu et al.,2020). Mice as an immune model and the pcDNA3.1 encoding the CCL4 gene as an adjuvant for rabies virus glycoprotein DNA vaccine, the results found that CCL4 can improve the immune response against rabies virus in mice after vaccination and CCL4 had the potential to be an adjuvant for rabies virus DNA vaccine (Pinto et al., 2003). p-CCL4 was successfully constructed and inoculated into f lounder by intramuscular injection in the experiment. After immunization, the percentage of T and B lymphocyte subsets, the expression diff erences of immune-related genes, and the RPS were detected and the results showed that there was no signif icant diff erence between the p-CCL4 group and the pBudCE4.1 group(data not shown). In this paper, p-OmpK-CCL4 was injected into f lounder and results showed that the percentage of CD4-1+and CD4-2+T lymphocytes,and sIgM+B lymphocytes were signif icantly higher than those in pBudCE4.1 and p-OmpK groups after immunization, which proved that CCL4 as an adjuvant for OmpK-based DNA vaccine can signif icantly improve the cellular immune response of f lounder.And it can recruit a lot of leucocytes at the injection site post-immunization and conferred an RPS of 74.1% againstV.anguillarum, which revealed that CCL4 can further enhance the recruitment of leucocytes based on the OmpK DNA vaccine and can provide better protection. Moreover, the expression prof iles of all immune-related genes except IFN-γ in p-OmpK-CCL4 groups were signif icantly higher than that of control groups.
The chemokine CC-motif ligand 19 (CCL19) also belongs to the CC chemokine subfamily. In mammals,CCL19 is called MIP-3β or ELC, it can be synthesized and secreted by many immune cells, such as macrophages, neutrophils, NK cells, and DC (Yoshida et al., 1997; Katou et al., 2003). CCL19 is abundantly expressed in thymus and lymph nodes and secreted by DC during activation and migration (Radstake et al., 2005). CCR7 is the specif ic receptor for CCL19,and it is induced upon activation of DC, that is, by external infectious stimuli via TLR (Stewart and Smyth, 2008). CCL19 increases the chance of interaction between DC, T cells, and B cells in secondary lymphatic tissue, thus regulates the primary or secondary adaptive immune response(Nguyen-Hoai et al., 2012). CCL19 has only been studied in a few f ish species, including f lounder,turbot (Scophthalmusmaximus), striped murrel(Channastriatus), and ayu (Plecoglossusaltivelis)(Chen et al., 2013; Arockiaraj et al., 2015; Zhang et al., 2015; Fu et al., 2017). CCL19 has been abundantly studied in mammals, but there is not much investigation of CCL19 as a vaccine adjuvant in f ish.Nguyen-Hoai et al. (2012) showed that recombinant plasmid encoding the human CCL19 gene and immunized mouse tumor models with the anti-tumor DNA vaccine pVax/E2A by gene gun, the results showed that CCL19 can enhance protection of vaccine and B cells function as APC. Yan et al. (2016)pointed that a nasal adjuvant composed of CCL17 and CCL19 is used together with an anti-caries DNA vaccine, they can recruit more mature DC to secondary lymphoid tissues, induce the body to produce high titer antibodies, and reduce S mutants infection in rodents after vaccination. The results of T and B lymphocyte subsets percent, the expression diff erences of immune-related genes, and the RPS in p-CCL19-injected f ish also showed that there was no signif icant diff erence between the p-CCL19 group and the pBudCE4.1 group after immunization (data not shown). In this study, p-OmpK-CCL19 was injected into the f lounder intramuscularly, and the results of FCM were similar to those of the p-OmpKCCL4 group, the percentage of T, B lymphocytes subpopulations peaked in the 6thweeks after immunization, respectively. The percentage of CD4-1+and CD4-2+T lymphocytes in PBL and spleen was lower than in the p-OmpK-CCL4 group but higher than in head kidney of the p-OmpK-CCL4 group.Moreover, p-OmpK-CCL19 also recruited a lot of leucocytes at the injection site compared with p-OmpK and pBudCE4.1 plasmids, the results were similar to the p-OmpK-CCL4 group as described above. Both p-OmpK-CCL4 and p-OmpK-CCL19 can induce leucocytes recruitment in local muscle tissue, various cells including neutrophils,mononuclear macrophages, lymphocytes, etc. They can be recruited at the injection site, but the mechanism of leucocytes recruitment and the types of cells recruited by p-OmpK-CCL4 and p-OmpKCCL19 will be further studied. The relative expression of immune-related genes was also close to that of the p-OmpK-CCL4 group and p-OmpK-CCL19 provided an RPS of 63.3% againstV.anguillarum.
It is worth noting that the percentage of CD4-1+and CD4-2+T lymphocytes of f lounder in p-OmpKCCL4 and p-OmpK-CCL19 groups were almost the same after immunization, but there is a diff erence in the number of CD4-1+and CD4-2+lymphocytes. In fact, in contrast with the situation of tetrapod, which possesses a single CD4 gene, f lounder contain two CD4 genes, CD4-1 and CD4-2. Research has shown that the CD4-1+ and CD4-2+T lymphocytes of teleost are functionally similar to those of tetrapod CD4+T lymphocytes, but the functional diff erences between the CD4-1+and CD4-2+T lymphocytes of teleost are still unclear (Takizawa et al., 2016). Mouse-anti f lounder CD4-1/CD4-2 monoclonal antibodies were produced in our lab previously and can be used to identify the CD4-1+and CD4-2+T lymphocytes of f lounder (Tian et al., 2019). CD4-1/CD4-2, CD4-1,CD4-2 molecules can be expressed by immune cells according to the transcript analysis of sorted CD4-1+cells in Ginbuna crucian carp and fugu (Kono and Korenaga, 2013; Somamoto et al., 2014). Thus CD4-1 and CD4-2 were not fully co-expressed, which may explain the diff erent percentages of CD4-1+and CD4-2+T lymphocytes after immunization in this paper.Both CD4-1 and CD4-2 may function as Th cells suggested by a study, therefore it might show the diff erent immune role of the two subsets (Edholm et al., 2007).
5 CONCLUSION
In this research, two bicistronic plasmids (p-OmpKCCL4 and p-OmpK-CCL19) were constructed successfully, and p-OmpK and pBudCE4.1 as control,their immune eff ects were detected after immunization.The results showed that the plasmids could be transcribed and expressed in cell lines and muscles of f lounder. After immunization, p-OmpK-CCL4 and p-OmpK-CCL19 induced higher expressions of CD4-1+and CD4-2+T lymphocytes, and sIgM+B lymphocytes compared with control groups (P<0.05),and recruited more leucocytes to the injection site. In addition, p-OmpK-CCL4 and p-OmpK-CCL19 upregulated the expressions of all immune-related genes except IL-6 in muscle, spleen, and head kidney of f lounder, which were signif icantly higher than those in the pBudCE4.1 group (P<0.05), and conferred an RPS of 74.1% and 63.3%, respectively, higher than p-OmpK alone (40.8%). In summary, this study indicated that CCL4 and CCL19 have signif icant adjuvant eff ects on OmpK-based DNA vaccine, and could be employed as effi cient adjuvants in aquaculture.
6 DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
7 DECLARATION OF INTEREST STATEMENT
The authors declare that they have no known competing f inancial interests or personal relationships that could have appeared to inf luence the work reported in this paper.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
