Transcriptome of Eriocheir sinensis under air exposure*
Yi ZHANG , Mengqi NI , Jinbin ZHENG , Zhaoxia CUI ,2,**
1 School of Marine Sciences, Ningbo University, Ningbo 315020, China
2 Laboratory for Marine Biology and Biotechnology, Pilot Qingdao National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266071, China
Abstract Chinese mitten crab, Eriocheir sinensis, is an economically important crab in China. Air exposure is a common stress for E. sinensis, especially during the harvest and transportation. Several studies have investigated the eff ects of air exposure stress on E. sinensis at physiological and molecular levels. However, the common and distinct mechanisms adopted by male and female crabs to cope with air exposure stress remain unclear. In this study, we performed a comparative transcriptome analysis from hepatopancreatic tissue of female and male Chinese mitten crabs in response to air exposure stress. In total,428 and 1 322 diff erentially expressed genes (DEGs) were identif ied in female and male crabs under air exposure, respectively. Our results showed that the transcriptional levels of several glycolysis related genes and anti-apoptotic proteins were up-regulated in both female and male crabs in response to air exposure.Moreover, our f indings indicated that female E. sinensis might preferentially increase the expression of heat shock proteins (HSPs) to deal with air exposure stress, while male E. sinensis tend to resist air exposure stress via increasing antioxidant enzyme expression. Overall, this study provides novel insights into the molecular mechanisms underlying the air exposure stress response of E. sinensis.
Keyword: Eriocheir sinensis; air exposure; transcriptome; hepatopancreas
1 INTRODUCTION
The Chinese mitten crab,Eriocheirsinensis, is one of the most important commercial crustaceans in China.E.sinensishas the capability of being transported live without water, and the aquaculture industry ofE.sinensisin China has gone through rapid development in the recent decade. In recent years, the annual aquaculture production ofE.sinensisreached approximately 780 000 tons (MOA, 2020).Although the breeding industry of Chinese mitten crab has made great progress, the whole industrial chain of Chinese mitten crab still faces obstacles,especially during the transportation of Chinese mitten crab, and desiccation is an inevitable process. During this process, the ability ofE.sinensisto deal with air exposure stress ensures long-distance transportation survival.
Air exposure is a common stress for aquatic animals, especially during their harvest and transportation (Ivanina et al., 2010). Thus, basic research on the air exposure stress response of the Chinese mitten crab has received growing attention.Furthermore,E.sinensisexhibits a relatively stronger tolerance to desiccation (Wang et al., 2013), which makes it a suitable species for investigating the molecular mechanism of air exposure stress response in aquatic crustaceans. Previous studies showed thatE.sinensiscould adjust their energy utilization mode,respiratory metabolism and antioxidant enzymes activities to adapt to air exposure stress (Bao et al.,2019a). Moreover, transcriptome analysis of gill provides insight into the immune and metabolism response ofE.sinensisagainst air exposure stress(Bao et al., 2019b; Chen et al., 2019). However, the common and distinct mechanisms adopted by male and female crabs to cope with air exposure stress remain unclear.
With the development of high-throughput sequencing technologies, comparative transcriptome analysis has become a powerful method for investigating the molecular mechanisms of organisms under certain physiological status (Mutz et al., 2013).Recently, transcriptome analyses of gill have been performed to investigate the gene expression prof iles ofE.sinensisin response to air exposure (Bao et al.,2019b; Chen et al., 2019). However, diff erent sexes of crabs could respond diff erently to environmental stress (Vitorino et al., 2019), thus, we performed a comparative transcriptome analysis the hepatopancreas rather than gill of female and male Chinese mitten crabs in response to air exposure stress, focusing on the transcriptional changes of metabolism, apoptosis, antioxidant enzymes, and heat shock proteins related genes. This study provides novel insights into the molecular mechanisms underlying the air exposure stress response in diff erent sexes ofE.sinensis.
2 MATERIAL AND METHOD
2.1 Air exposure and sample collection
Crabs (99.87±13.01 g in weight) were purchased from Dongying, Shandong Province. All crabs were acclimated for seven days in f iltered aerated freshwater at a temperature of 22 °C and pH of 7.0. Crabs were fed once daily with clam until 24 h before beginning the experimental treatment. Preliminary experiments showed that crabs began to die at 30 h under air exposure, therefore, female/male crabs subjected to air exposure for 30 h were set as experimental groups(TR30FH/TR30MH) and crabs cultured in aerated freshwater served as control groups (TC30FH/TC30MH). For both the control and air exposure groups, the experiment was run under the acclimation conditions of 22 °C. The experiment was conducted in rectangular tanks (50 cm×35 cm×20 cm) and each group contained f ive individuals. After 30 h, the hepatopancreases of three individuals in each group of males and females were collected and were immediately frozen at -80 °C until the extraction of total RNA for analysis. No mortality was observed during the cultivation and analysis process.
2.2 Library construction and sequencing
Total RNA was extracted from hepatopancreas using TRIzol reagent (Invitrogen, Carlsbad, CA,USA) according to the manufacturer’s instructions.The quantity and purity of total RNA were detected using NanoDrop ND-1000 (NanoDrop, Wilmington,DE, USA). The RNA integrity was assessed by Bioanalyzer 2100 (Agilent, CA, USA) with RIN number >7.0. Poly (A) RNA is purif ied from 1-μg total RNA using Dynabeads Oligo (dT)25-61005(Thermo Fisher, CA, USA) using two rounds of purif ication. Then the poly(A) RNA was fragmented into small pieces using Magnesium RNA Fragmentation Module (NEB, cat.e6150, USA) under 94 °C for 5-7 min. Then the cleaved RNA fragments were reverse-transcribed to create the cDNA libraries using TruSeq Stranded mRNA Library Prep Kit(Illumina, San Diego, USA). Libraries were sequenced on an Illumina Hiseq 4000 (LC-Bio Technology Co.,Ltd., Hangzhou, China) platform and 2×150 bp paired-end reads were generated.
2.3 Quality control and reads mapping
Raw data were f irstly subjected to quality control before bioinformatics analysis. In this step, reads containing sequencing adaptor, reads containing sequencing primer, and low-quality reads were removed. The sequence quality was verif ied using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) including the Q20, Q30, and GCcontent of the clean data. All downstream analyses were based on clean data of high quality. The screened high-quality reads were mapped to theE.sinensisgenome (Cui et al., 2021) using HISAT package (Kim et al., 2015). Then, all transcriptomes from samples were merged to reconstruct a comprehensive transcriptome using Perl scripts.
2.4 Identif ication of diff erentially expressed genes
After the final transcriptome was generated,StringTie and Ballgown were used to estimate the expression levels of all transcripts. StringTie was used to perform expression level for mRNAs by calculating fragments per kilobase of transcript per million mapped reads (FPKM). Diff erential expression analysis was performed using the R package-Ballgown. Balldown was used for gene expression analysis (Frazee et al., 2015). Transcripts with |log2(fold change)|≥1 andP-values<0.05 were considered DEGs.
2.5 Functional annotation
For functional annotation, all transcripts were searched against databases including the NCBInonredundant protein sequences (NR) (http://www.ncbi.nlm.nih.gov/), Gene Ontology (GO) (http://www.geneontology.org/), and Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/) databases using BlastX to convert nucleotide sequences into protein sequences (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
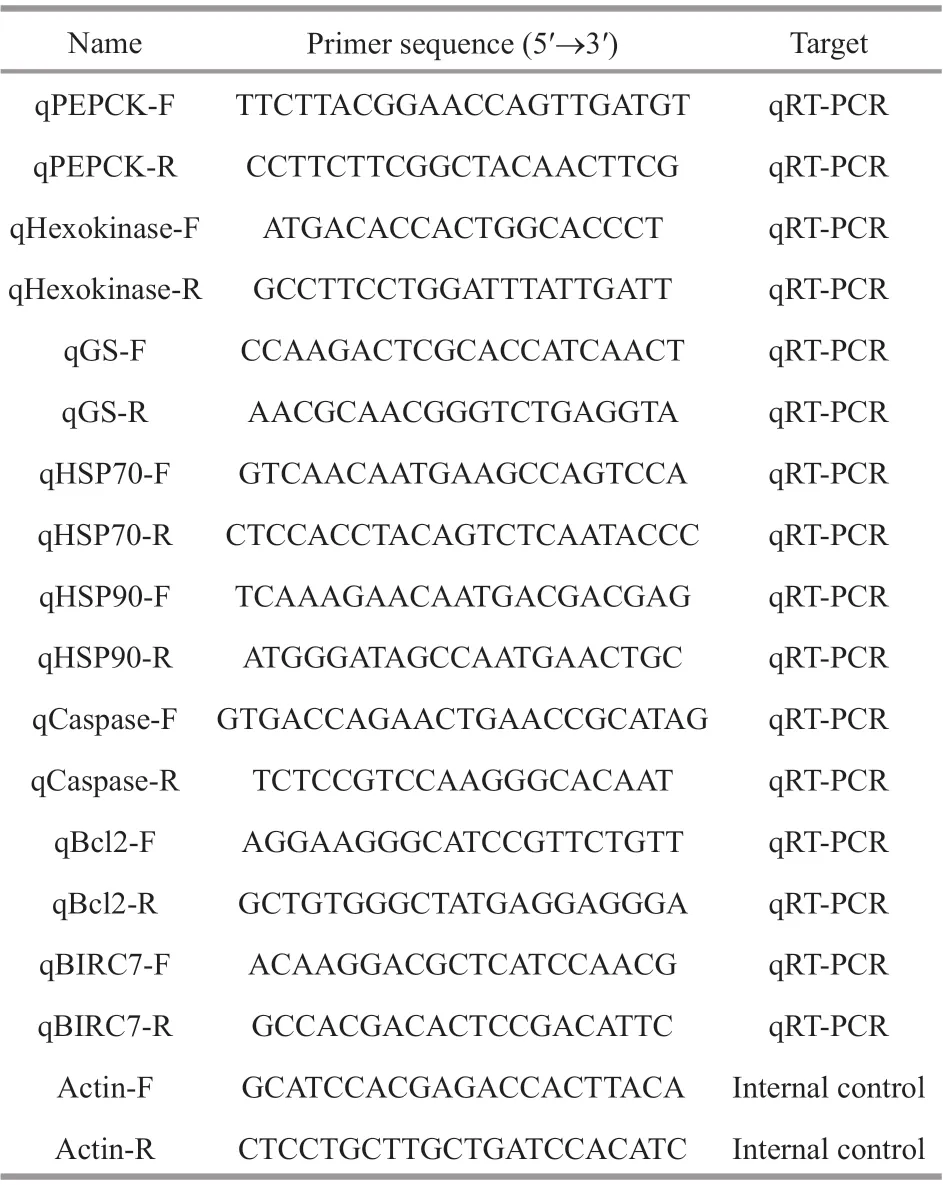
Table 1 PCR primers for the validation of RNA-Seq data by qRT-PCR
2.6 Validation of diff erentially expressed genes by quantitative real-time PCR
Several diff erentially expressed genes, including phosphoenolpyruvate-carboxykinase (PEPCK),hexokinase, glutamine synthetase (GS), heat shock protein 70 (HSP70), HSP90, caspase, B-cell lymphoma-2 (Bcl2), and baculoviral IAP repeatcontaining protein 7 (BIRC7), were selected for validation by quantitative real-time PCR (qRT-PCR).Total RNA of hepatopancreas was extracted using RNAiso plus (TaKaRa, Japan), and reverse transcription was conducted using PrimeScriptTMRT reagent Kit with gDNA Eraser (TaKaRa, Japan)following the manufacturer’s instructions. QRT-PCR was performed on an Applied Biosystems 7500 Realtime PCR System (Applied Biosystems, USA) using TB Green®Premix DimerEraserTM(TaKaRa, Japan)according to the manufacturer’s instructions. Fivebiological and three technical replicates were performed, and β-actin served as the reference for internal standardization according to previous study(Yu et al., 2013). The specif ic primers for qRT-PCR were designed based on the sequences obtained from the transcriptome sequencing using Primer 5.0 software and are listed in Table 1. The reaction was conducted under the following conditions: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 30 s and 72 °C for 30 s. Melting curve analysis was performed to evaluate the specif icity of amplif ication. The 2-ΔΔCtmethod was used to calculate the relative mRNA expression levels as previously described (Livak and Schmittgen, 2001). Statistical signif icance between groups was analyzed using a one-way analysis of variance (ANOVA) followed by multiple comparison testing with the LSD-ttest using the SPSS 17.0 software.
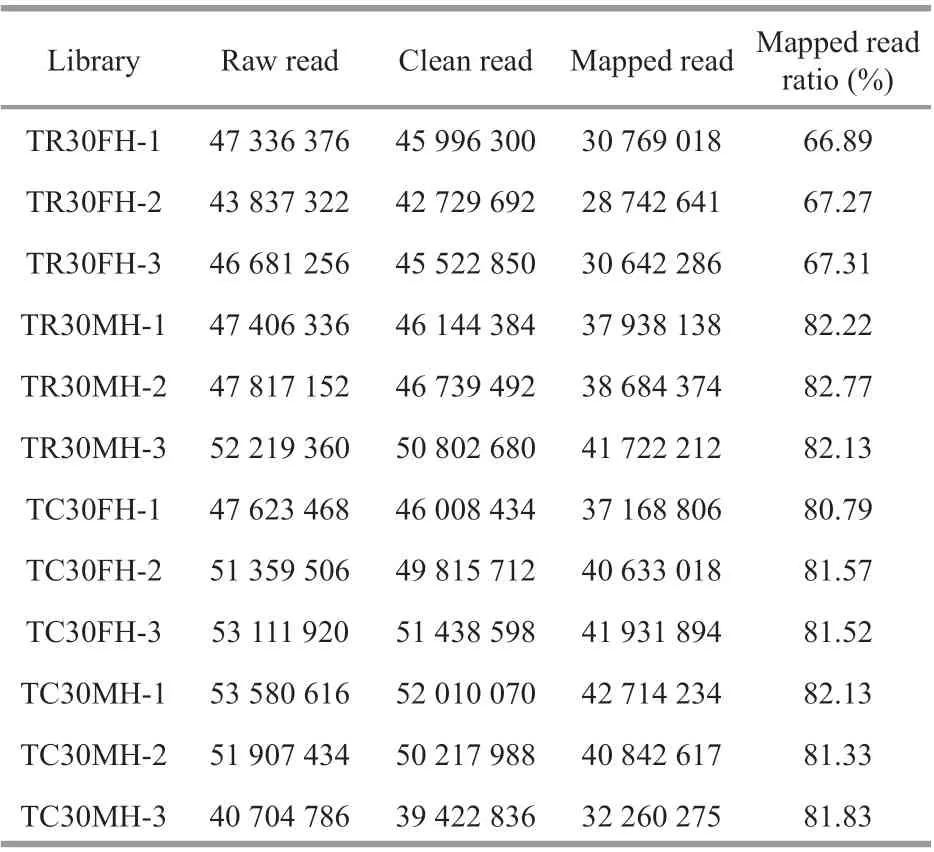
Table 2 Sequencing statistics of the transcriptome data
3 RESULT
3.1 Overview of the sequencing data
Based on the Illumina Hiseq 4000 sequencing platform, between 40 704 786-53 580 616 raw reads were generated from the 12 libraries (Table 2). After quality control, between 39 422 836-52 010 070 clean reads were obtained. The screened clean reads were mapped to theE.sinensisgenome, and 28 742 641-42 714 234 mapped reads were identif ied.The transcriptome sequencing data has been submitted to the SRA database and the accession number is PRJNA699917.
3.2 DEGs expression analysis
In order to identify genes potentially involved in the air exposure stress responses of Chinese mitten crabs, the relative gene expression abundance was compared to identify DEGs of diff erent treatments. A total of 2 186 and 4 813 DEGs were obtained in the hepatopancreas of female and male crabs under air exposure, respectively (Table 3). In detail, 956 upregulated genes and 1 230 down-regulated genes were identif ied in female crabs exposed to air. Meanwhile,433 up-regulated and 4 380 down-regulated genes were observed in male crabs under air exposure(Table 3).
3.3 GO enrichment and pathway analysis
To obtain comprehensive information on functions of DEGs, the transcripts were subjected to annotation analysis by searching against databases including NR, GO, and KEGG. In total, 428 and 1 322 transcripts were annotated. GO enrichment analysis showed that many DEGs identif ied in the TR30FH vs. TC30FH were enriched in categories of metabolism such as glycolytic process, glutathione metabolic process,and glutamine biosynthetic process (Fig.1a).Meanwhile, several DEGs observed in the TR30MH vs. TC30MH were enriched in arginine biosynthetic process and citrate metabolic process (Fig.1b). KEGG enrichment analysis showed that many DEGs identif ied in the TR30FH vs. TC30FH were enriched in metabolism processes including arachidonic acid metabolism, fatty acid biosynthesis, cholesterol metabolism, and steroid biosynthesis (Fig.2a). Some DEGs detected in the TR30MH vs. TC30MH were enriched in metabolism pathways such as pyrimidine metabolism, cholesterol metabolism, nicotinate and nicotinamide metabolism, and steroid hormone biosynthesis (Fig.2b). In the air exposure group of male and female crabs, several commonly expressed genes, associated with glycolysis, such as PEPCK,hexokinase, and lactate dehydrogenase (LDH), and associated with apoptosis, such as caspase, Bcl2 and BIRC7, showed the same expression trend (Fig.3).Interestingly, glutathione peroxidase (Gpx) and catalase (CAT) were up-regulated in the air exposure group of male crabs, while up-regulation of HSP60,HSP70, and HSP90 occurred in air exposure female crabs (Fig.3).

Table 3 Number of DEGs between libraries
3.4 QRT-PCR validation
To validate our sequencing results, several genes of interest were selected from the diff erentially expressed genes and verif ied by qRT-PCR. The results showed that PEPCK, GS, Hexokinase, Bcl2, BIRC7, HSP70,and HSP90 were up-regulated in the air exposure group of female crabs; PEPCK, GS, Hexokinase,Bcl2, and BIRC7 were up-regulated, and caspase were down-regulated in the air exposure group of male crabs. The diff erential expression tendencies of these genes detected by RNA-Seq and qRT-PCR is consistent (Fig.4), indicating that the transcriptome data were reliable.
4 DISCUSSION
In crustaceans, the hepatopancreas plays essential roles in digestion, metabolism, detoxif ication,immune, and antioxidant defense, and has served as a crucial target tissue for assessing eff ects of environmental stress on organisms (Steenvoorden and van Henegouwen, 1997; Li et al., 2013; Cheng et al., 2020a). Air exposure led to a progressive degradation of the hepatopancreas tissue inNorwaylobstersand caused the inf lation of the liver tubules,and disappearance of connective tissue in black tiger shrimp (Ridgway et al., 2006; Duan et al., 2016).However, no information on the transcriptome analysis of hepatopancreas inE.sinensisin response to air exposure stress is available. Herein, a comparative transcriptome analysis of hepatopancreas was conducted to elucidate the common and distinct molecular mechanisms adopted by male and femaleE.sinensisto deal with air exposure stress.
Based on transcriptome sequencing and bioinformatics analysis, a total of 2 186 and 4 813 genes in the hepatopancreas of female and male crabs changed their expression levels in response to air exposure, implying that male crabs might be more sensitive to air exposure. To understand the possible functions of the DEGs, genes were searched against databases including NR, GO, and KEGG. However,approximately only less than one-third of the genes were annotated (Table 3), which may be related to the limited information available on the genetic background of crustaceans and arthropods (Li et al.,2012). Functional annotation showed that various genes such as heat shock proteins, antioxidant enzymes, apoptosis and glycolysis related genes participated in the air exposure response ofE.sinensis.
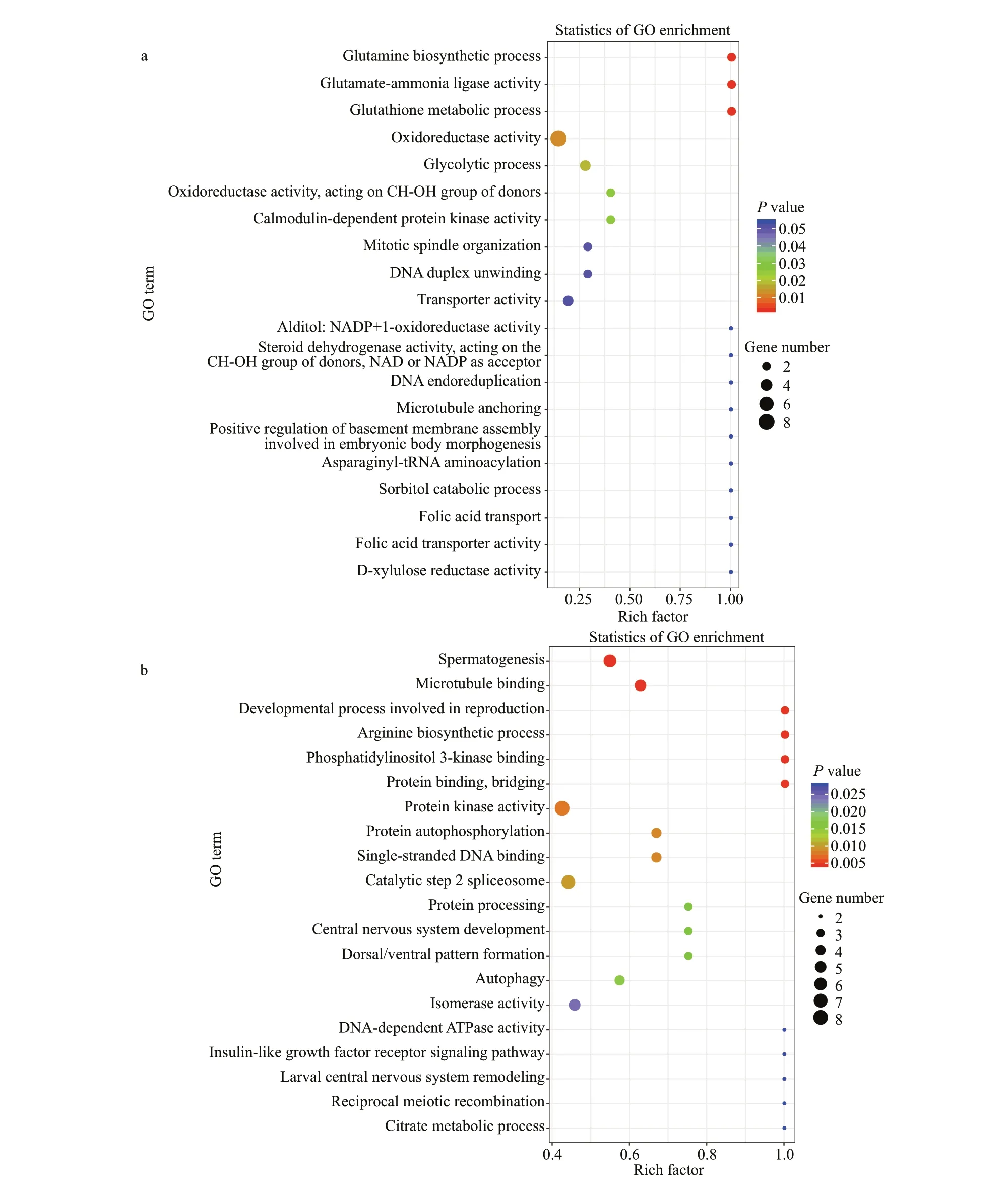
Fig.1 Scatter diagram of Gene ontology (GO) enrichment for diff erentially expressed genes (DEGs) in E. sinensis
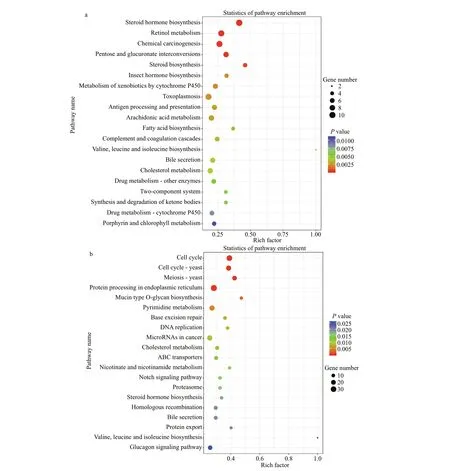
Fig.2 Scatter diagram of KEGG pathway enrichment for DEGs
Several common molecular mechanisms were found to be adopted by both male and female crabs to deal with air exposure stress (Fig.3). Previous studies have revealed that air exposure stress could enhance glycolysis, which is the primary process of carbohydrate metabolism under acute hypoxic stress(Li et al., 2018; Sun et al., 2018). In this study, the transcriptional level of several key enzymes involved in glycolysis, including hexokinase, PEPCK, and LDH were signif icantly increased in the hepatopancreas of male and femaleE.sinensisunder air exposure stress (Fig.3). The up-regulated expression level of these enzymes implies an enhancement of glycolysis in the hepatopancreas ofE.sinensis. Under the air exposure state, the crabs are unable to obtain enough oxygen, causing severe hypoxic stress and hindering aerobic respiration (Bao et al., 2019a). Consequently,anaerobic respiratory metabolism might be enhanced to provide suffi cient energy for maintaining basic physiological activities. According to research, dietary carotenoids supplementation also may be a useful way forE.sinensisagainst air exposure (Liu et al., 2020).
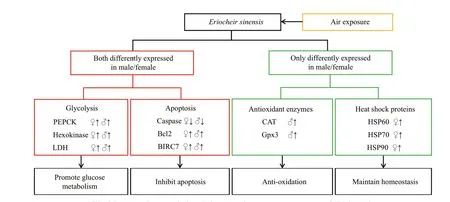
Fig.3 Proposed scenario involving the air exposure response of E. sinensis
Apoptosis is a normal biological process during the lifespan of organisms, and properly regulated apoptosis under stress is essential for maintaining homeostasis (Shan et al., 2021). Apoptotic pathways involve a set of proteins, such as caspases, tumor necrosis factor (TNF), p53 protein, Bcl2 family proteins and inhibitor of apoptosis proteins (IAPs)(Xie et al., 2020). In this study, the expression of caspase, a protein that promotes apoptosis, was decreased in the hepatopancreas of male and femaleE.sinensissubjected to air exposure stress (Fig.3).Meanwhile, the transcriptional levels of two antiapoptotic proteins, i.e. Baculoviral IAP repeatcontaining protein 7 (BIRC7) and Bcl2 were increased in the hepatopancreas ofE.sinensisregardless of gender (Fig.3). The transcriptional alterations of these genes implied an inhibition of apoptosis in the hepatopancreas ofE.sinensissuff ering from air exposure stress. Our f indings suggested that both male and femaleE.sinensismay inhibit cell apoptosis to resist air exposure stress.

Fig.4 Comparison of the relative fold changes of genes detected by RNA-seq and qRT-PCR in the hepatopancreas of female (a) and male (b) Chinese mitten crab
Interestingly, distinct molecular responses in diff erent sexes ofE.sinensiswere also observed. The expression levels of HSP60, HSP70, and HSP90 were increased in the hepatopancreas of femaleE.sinensis(Fig.3), which was consistent with previous studies(Frenkel et al., 2008; Zhang et al., 2009; Huang et al.,2013; Lou et al., 2014). No signif icant transcriptional changes of these genes were observed in maleE.sinensis. In this study, the expression levels of two important antioxidant enzymes, CAT and Gpx, were up-regulated in the hepatopancreas of maleE.sinensis(Fig.3), which was similar with f indings in previous studies (Bao et al., 2019b; Cheng et al., 2020a).Similarly, no signif icant transcriptional changes of the two genes were observed in femaleE.sinensis.
HSPs, the well-known anti-stress proteins,generally act as molecular chaperones to regulate protein homeostasis by assisting protein folding,degrading misfolded protein, and preventing proteins from aggregation (Picard, 2002; Pratt and Toft, 2003;Kondrikov et al., 2015). HSPs have been reported to play essential roles in helping organisms to cope with diverse stress (John, 2009).
It has been reported that air exposure stress could result in overproduction of reactive oxygen species(ROS) and leads to oxidative damage (Sun et al.,2020; Cheng et al., 2020b). To prevent the toxicity caused by ROS and maintain proper cellular functions,organisms have developed eff ective antioxidant mechanisms to eliminate excessive ROS and maintain a balance of ROS (Jacob, 1995; Limón-Pacheco and Gonsebatt, 2009). Antioxidant enzymes are critical components of the antioxidant system (Davis and Pennypacker, 2017; Trivedi and Lal, 2017).
The up-regulations of HSPs and antioxidant enzymes are two crucial adaptive processes by which organisms deal with environmental stress (Abeyrathne et al., 2018; Bolhassani and Agi, 2019). Our study indicated that femaleE.sinensismight preferentially increase the expression of HSPs to cope with air exposure stress, while maleE.sinensistend to rely more on the up-regulation of antioxidant enzymes expression under air exposure stress.
5 CONCLUSION
Based on comparative transcriptome analysis,several putative molecular mechanisms adopted by diff erent sexes ofE.sinensisto cope with air exposure stress were preliminarily identif ied. Both female and maleE.sinensiscan make adaptative responses to air exposure via increasing the expression of glycolysis and anti-apoptotic related genes. Furthermore, female and maleE.sinensisalso up-regulated the transcriptional levels of HSPs and antioxidant enzymes, respectively,to cope with air exposure stress. This study enriches our knowledge on the molecular mechanisms underlying the air exposure stress response ofE.sinensis.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to all the laboratory members.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
