Transcriptome of hepatopancreas in kuruma shrimp Marsupenaeus japonicus under low-salinity stress*
Tingjun CHEN , Zhimin LI , , Jianyong LIU , , Caifeng LIANG , , Le YUAN
1 Guangdong Ocean University, Zhanjiang 524088, China
2 Guangdong Provincial Shrimp Breeding and Culture Laboratory, Guangdong Ocean University, Zhanjiang 524088, China
Abstract The kuruma shrimp Marsupenaeus japonicus is one of the most commercially important shrimp species in the world. Low salinity would aff ect the penetration and immunity, and even led to its death of the shrimp. However, little is known about the molecular mechanism of the eff ect. Therefore, hepatopancreas of M. japonicus reared under low-salinity stress for 6, 12, 24, 48, and 96 h was analyzed and the results were compared with that of the control group using transcriptomics. After removing reads containing adapters,88 890 960-1 051 300 444 clean reads were generated from 10 libraries in the control group and experimental group. Compared with the control group, 811, 589, 1 095, 745, and 875 diff erentially expressed genes were obtained in the f ive treatment groups. The N50 and N90 lengths of the transcripts were 1 746 bp and 436 bp,respectively. The top 20 gene ontology terms and Kyoto Encyclopedia of Genes and Genomes pathways associated with the diff erentially expressed genes were related mainly to osmotic regulation (ion exchange,lipid metabolism and carbohydrate metabolism), immune regulation (cellular and humoral immunity), chitin metabolism, and related functions. The diff erential expression patterns of nine randomly selected genes were conf irmed by quantitative real-time PCR. This is the f irst report of osmotic regulation-related genes that are diff erentially expressed under low-salinity stress in the hepatopancreas of M. japonicus. Furthermore, we found that M. japonicus initiated its own immune regulation under low-salinity stress. These results will help elucidating the mechanism of osmotic regulation and immune responses in this shrimp species.
Keyword: Marsupenaeus japonicus; transcriptome; osmotic regulation; immune responses
1 INTRODUCTION
The kuruma shrimpMarsupenaeusjaponicusis one of the most commercially important shrimp species worldwide, distributed in Japan, the South China Sea, and the Indo-West Pacif ic (Hamasaki and Kitada, 2008; Tsoi et al., 2014). The adaptability ofM.japonicusto salinity is relatively poor, the suitable salinity range is 15-30, the survival salinity is 7-35,and it is diffi cult to survive below 7 (Weng et al.,2012). Due to its advantages of rapid reproduction,tolerance to low temperature and long-distance transport capacity, this species has become one of the most important shrimp species in China’s coastal waters (Paterson, 1993; Duan et al., 2016). In 2016,the production ofM.japonicusin China was 55 885 t(http://www.fao.org/home/en/). In recent decades,there have been many studies onM.japonicus, and mostly concerned about the life activities (Chen and Lai, 1993; Hewitt and Duncan, 2001), cultivation mode (Cheng and Chen, 2001; Li et al., 2014b),environmental stress (Coman et al., 2002; Lee and Chen, 2003), and molecular biology (Lin et al., 2008;Leu et al., 2011). Recently, production ofM.japonicushas declined; in particular, production was approximately 2 000 t less in 2017 than in 2016(http://www.fao.org/home/en/). This decrease may be related to the broodstock ofM.japonicuscollected from wild populations, which exhibit poor stress resistance (Liu et al., 2019). The another, it may be the environmental stress, which combined with a bottom environment rich in ammonia nitrogen and nitrite nitrogen in farms undergoing a series of environmental changes (Cheng et al., 2004, 2013),such as salinity (Setiarto et al., 2004), temperature(Lu et al., 2016), and dissolved oxygen (Chien and Shiau, 2005).
Salinity is one of the most important environmental factors aff ecting the survival of aquatic organisms(Heugens et al., 2001; Hop et al., 2002; Laing, 2002;Chong-Robles et al., 2014). With the rapid change of the global marine environment, ocean warming(Johnson et al., 2011), typhoon activity (Balaguru et al., 2016), tide movement (Kourafalou et al., 1996)and other natural and human factors can cause the decrease of the salinity of the natural sea water, which has a great impact on aquatic organisms. Low salinity stress has been reported in many shrimps, such asLitopenaeusvannamei(McGraw et al., 2002),Fenneropenaeuschinensis(Liu et al., 2006),Penaeusmonodon(Ye et al., 2009), andM.japonicus(Via,1986). Low salinity aff ected the growth and survival of prawn, and the survival rate decreased signif icantly with the decrease of salinity (Via, 1986; McGraw et al., 2002; Ye et al., 2009).In addition, low salinity aff ected osmotic regulation (McNamara et al., 2015;Fregoso-López et al., 2017) and immune regulation(Lin et al., 2012). For example,M.japonicusandL.vannameiare susceptible to white spot virus andVibrioalginolyticusunder low-salinity conditions (Yu et al., 2003; Wang and Chen, 2005; Li et al., 2010),which may be due to shrimp consuming a large amount of stored energy to regulate osmotic pressure in vivo,leading to an increase in metabolic rate and decreases in growth rate and stress tolerance upon salinity change (Ingram et al., 2002; Setiarto et al., 2004). To clarify the osmotic regulation mechanism used by shrimp under low-salinity conditions, molecular research has intensif ied, including studies using gene cloning (Tiu et al., 2007; Meng et al., 2011; Rajesh et al., 2012), suppression subtractive hybridization (Gao et al., 2012; Shekhar et al., 2013), transcriptome sequencing (Hu et al., 2015), proteomics (Fan et al.,2019), and digital gene expression techniques (Zhao et al., 2015). Among these methods, transcriptome sequencing has been used most widely. Unfortunately,there is still a lack of research on low salt molecules ofM.japonicus, the specif ic mechanism of osmotic and immune regulation remains unknown.
Transcriptome sequencing can be used for accurate quantif ication of the expression of various RNA molecules, such as mRNAs, lncRNAs, and microRNAs,in diff erent tissues, developmental stages, and disease states, to characterize complex molecular pathways and diff erential gene expression patterns (Brawand et al., 2011; He et al., 2012; Zhao et al., 2012; Park et al.,2013; Kornienko et al., 2016; Ludwig et al., 2016). To date, the transcriptomes of several prawn species,includingLitopenaeusvannamei(Li et al., 2012),Fenneropenaeuschinensis(Li et al., 2013),Fenneropenaeusmerguiensis(Powell et al., 2015), andMacrobrachiumrosenbergii(Jung et al., 2011), have been sequenced. Those studies focused mainly on development, immunity, and osmotic regulation in shrimp. Previous studies have shown that the main osmotic regulator of shrimp is the gill (Shekhar et al.,2013; Hu et al., 2015). However, recent studies have found that the hepatopancreas is also involved in osmotic regulation (Chen et al., 2015). The hepatopancreas is the main organ responsible for not only the digestion, metabolism, absorption, and storage of nutrients in decapod crustaceans but also for energy metabolism under low-salinity stress (Wang et al.,2014b; Zhang et al., 2016). The purpose of this study is to evaluate transcript levels in the hepatopancreas ofM.japonicusunder low-salinity stress for diff erent periods using transcriptome sequencing, identifying the pathways related to osmotic regulation, immune response, detoxif ication, and apoptosis, and revealing diff erentially expressed genes (DEGs) involved in lowsalinity stress. The results not only enrich the information of shrimp cDNA database, but also provide important molecular evidence for breeding improved strains ofM.japonicusin the future.
2 MATERIAL AND METHOD
2.1 Salinity challenge and sample preparation
A 120-day-old mixed family ofM.japonicuswith an average body length of 49.28±4.79 mm and average body weight of 1.39±0.38 g was obtained from Guolian Aquatic Limited Company, Guangdong Province, China. Before the experiment, the shrimp were acclimated in a cement pond in 28±0.2 °C,salinity 29.8±0.2, and dissolved oxygen 6.0 mg/L for 7 days to reduce any stress response. In the preliminary experiment, the adaptability ofM.japonicasto low salinity was studied. The results show that the survival rate ofM.japonicuswas 80% for 96 h in salinity 8.Thus, we determined that the salinity of low salt stress was 8 in this study. The shrimp were divided into the control group (CG) and low-salinity group (LS).Salinity was regulated by mixing seawater with disinfected tap water, and the low-salinity and control groups were exposed to salinities of 8 and 29.8±0.2,respectively. Three experimental replicates were performed per group. A 1 500-L plastic bucket was used for the experiment, and all other experimental conditions were consistent with the domestication conditions. No food was given during the experiment,which lasted 96 h, with salinity determined every 6 h to maintain a constant level. The hepatopancreas was collected from 9 shrimp of each group at the 6th, 12th,24th, 48th, and 96thh and stored in an enzymatic centrifuge tube containing 1-mL RNA stabilization solution. The samples were stored overnight at 4 °C and then held at 20 °C until RNA extraction.
2.2 RNA extraction and Illumina sequencing
Total RNA from the hepatopancreases was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA degradation and contamination were monitored by 1% agarose gel electrophoresis. RNA purity was checked using the NanoPhotometer®spectrophotometer (Implen, CA,USA). The RNA concentration was measured using the Qubit®RNA Assay Kit and Qubit®2.0 f luorometer(Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, CA,USA) (Hu et al., 2015). Ten libraries were constructed,f ive from the low-salinity group at each time point(LS6, LS12, LS24, LS48, and LS96) and f ive from the control group at each time point (CG6, CG12,CG24, CG48, and CG96). These 10 libraries were generated using the NEBNext®UltraTMRNA Library Prep Kit for Illumina®(NEB, USA) following the manufacturer’s recommendations, and index codes were added to identify the sequences expressed in each sample. The total RNA content of each sample was 3 μg. Finally, PCR products were purif ied (The Agencourt AMPure XP system, Beckman Coulter,Brea, CA, USA), and the library quality was assessed on the Agilent Bioanalyzer 2100 system. Clustering of the index-coded samples was performed on the cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation,library preparation was carried out on the Illumina Hiseq platform, and 125-150 bp paired-end reads were generated (Fu and Zou, 2018).
2.3 Bioinformatics analysis of the transcriptome data
2.3.1 Sequencing data f iltering and assembly
Raw data (raw reads) in the fastq format were f irst processed using in-house Perl scripts. In this step,clean data (clean reads) were obtained by removing reads containing adapter sequences or poly-N sequences and low-quality reads (The base number of the qphred ≤ 20 accounts for more than 50% of the total reads) from the raw data. At the same time, the Q20, Q30, and GC content of the clean reads were calculated, respectively. All downstream analyses were based on high-quality clean reads (Li et al.,2020). Clean reads were assembled with Trinity(Grabherr et al., 2011). Brief ly, Trinity is an effi cient and stable transcriptome splicing software developed by Broad Institute and Hebrew University of Jerusalem for RNA-seq data. It combines three independent software modules (Inchworm, Chrysalis,and Butterf ly) to process and splice a large number of RNA-seq. The main process was as follows: By software Inchworm, f irst, all the fq f iles of the read were read and converted into the fa format read f iles,and then merged the 3′-end reads and 5′-end reads to obtain both.fa. The reads were decomposed bykmers (short fragment ofk-bp length), and the types ofk-mers and the number ofk-mers of each type were counted, and then sorted from high to low by the frequency ofk-mers. Thek-mer with the highest frequency, as a seed, was extended to 3′-end. The frequency of eachk-mer after the extension was counted. Thek-mer with the highest frequency was selected as the extension path. Finally, following the greedy algorithm, thek-mers were extended by the overlap relationship to form the contig sequence.Using software Chrysalis, all contigs with similar regions more thank-1-mers were clustered to form components. The de Bruijn graph was constructed according to diff erent components, and meantime,the reads and components were compared and verif ied. With the software Butterf ly, the transcripts were listed, and the graph was split into linear sequence. The error sequence was eliminated by the read and pairs relationship, and the de Bruijn graph of each component was simplif ied. The full-length transcripts of the variable splicing subtypes were output, and the transcripts corresponding to the paralogous genes were sorted out. Finally, the splicing result f ile is obtained and named as TRINITY.fasta.
2.3.2 Annotation of gene functions and diff erential expression analysis
Gene functions were annotated based on the following databases: National Center for Biotechnology Information non-redundant protein(NR) and nucleotide sequences (NT), Pfam (protein family database), Clusters of Orthologous Groups of proteins (KOG/COG), Swiss-Prot (a manually annotated and reviewed protein sequence database),Kyoto Encyclopedia of Genes and Genomes (KEGG)(Kanehisa et al., 2007), KEGG Orthology database(KO) (Mao et al., 2005), and gene ontology (GO)databases (Young et al., 2010).
Clean reads were aligned to transcriptome sequences by bowtie2 (Langmead et al., 2009), and the alignment results of bowtie were counted by RSEM (http://deweylab.biostat.wisc.edu/rsem/) (Li and Dewey, 2011). The read counts of each sample aligned to each gene were obtained, and the FPKM conversion were carried out (Trapnell et al., 2010).Read counts for each sequenced library were adjusted using the edgeR v.3.0.8 package of R software and a normalization scaling factor, TMM was used to standardize the read count data, and the DEGs between LS groups and CG groups were analyzed with DEGseq R package. Diff erential genes were determined by combiningqvalue (qvalue≤0.05) and fold change (|log2FC(sample2/sample1)|≥1).Pvalues were adjusted usingqvalues. Aqvalue of 1 was set as the threshold for def ining signif icant diff erential expression (Wang et al., 2010).
2.4 Validation of mRNA-seq data using quantitative real-time PCR (qPCR)
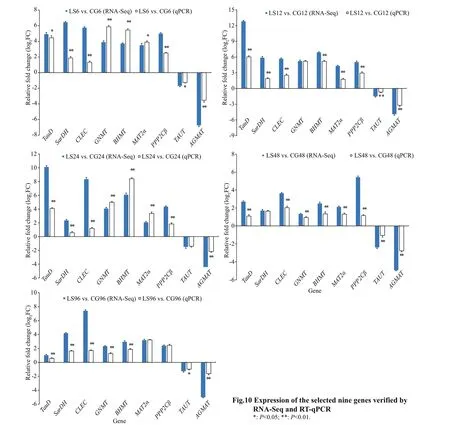
Nine DEGs from f ive libraries were randomly selected, including taurine catabolism dioxygenase(TauD), sarcosine dehydrogenase mitochondrial isoform X1 (SarDH), C-type lectin (CLEC), glycine N-methyltransferase (GNMT), betaine-homocysteine S-methyltransferase (BHMT), methionine adenosyltransferase II alpha (MAT2α), serine/threonine-protein phosphatase 2A catalytic subunit beta isoform (PPP2Cβ), taurine transporter (TAUT),and agmatinase mitochondrial-like (AGMAT) were conf irmed by qPCR. Using TransStart Tip Green SuperMix (Beijing TransGen Biotech Co., Ltd.) and performed on the Light Cycler 480II (Roche) thermal cycler according to the manufacturer’s instructions.Elongation factor 1α (EF1α) was used as the reference gene, and each gene amplif ication was performed inthree wells. Gene-specif ic primers were designed using Premier 5 (Table 1). Amplif ication was performed in a 384-well plate in a 10-μL reaction volume containing 1-μL cDNA, 0.2 μL each of the gene-specif ic forward and reverse primers, 5-μL TransStart Tip Green qPCR SuperMix and 3.6-μL RNase-free water. The thermal prof ile for the PCR was 94 °C for 30 s followed by 45 cycles of 94 °C for 5 s, 60 °C for 15 s and 72 °C for 10 s. The results are presented as the change in relative expression normalized to the reference gene (EF1α) using the 2-ΔΔCtmethod. The data was mean±SD, and the diff erence was statistically signif icant (P<0.05) by one-way ANOVA in SPSS19.0.
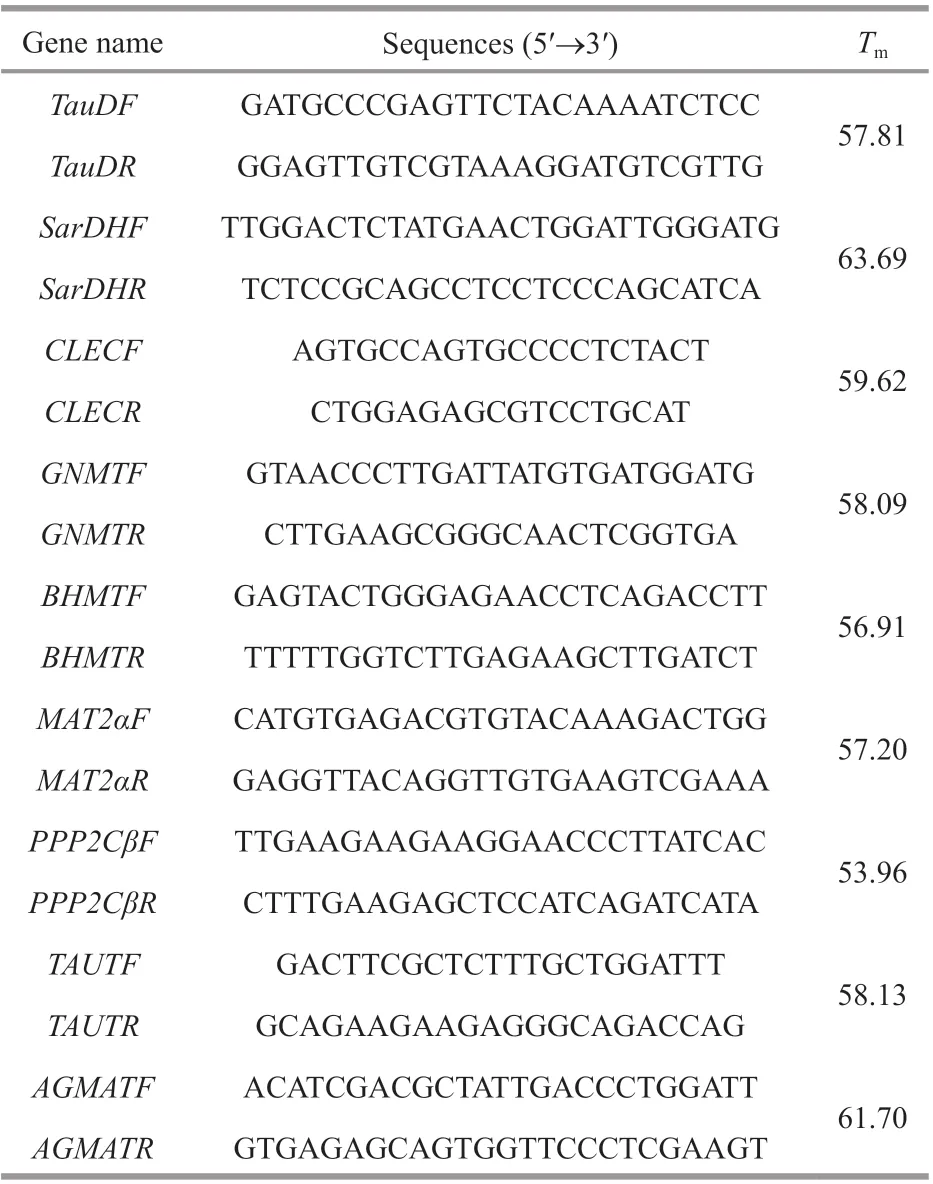
Table 1 Primer sequences for qPCR validation
3 RESULT
3.1 Sequencing and de novo assembly of the transcriptome
After transcriptome sequencing, 92 184 260-108 448 152 raw reads were generated from the 10 libraries prepared from the control and low-salinity groups. After removing the reads containing adapter sequences or poly-N sequences and low-quality reads from the raw reads, 88 890 960-1 051 300 444 clean reads were obtained, the data volume is 6.67G-7.88G,the GC contents of the raw data were 50.29%-52.48%,the Q20 percentages were 97.62%-98.11%, and theQ30 percentages were 93.34%-94.53% (Table 2).Using the Trinity to assemble the obtained clean reads, and after removing the redundancy, 48 807 unigenes were obtained from 52 826 925 nucleotides.N50 and N90 were 1 746 bp and 436 bp, respectively,with an average length of 1 082 bp (Table 3).According to the length distribution statistics of the assembled unigenes, the minimum length is 301 bp;there are 19 581 unigenes distributed between 300-500 bp, accounting for 40.12% of the total, with the largest number; there are only 6 383 unigenes more than 2 000 bp, accounting for 13.08% of the total(Table 4; Fig.1).
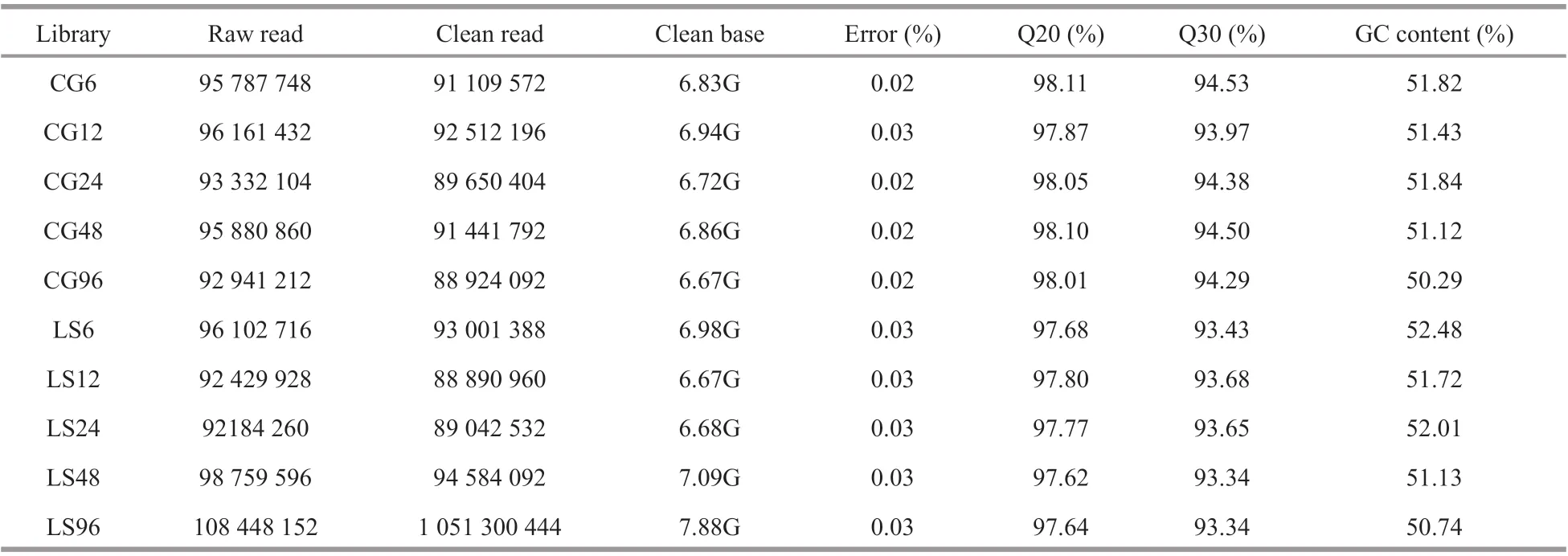
Table 2 Sequencing and assembly statistics of the transcriptome data
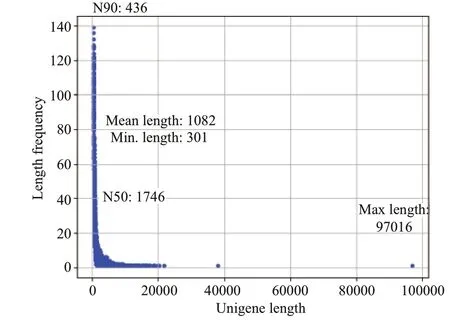
Fig.1 Unigenes length distribution of spliced transcripts
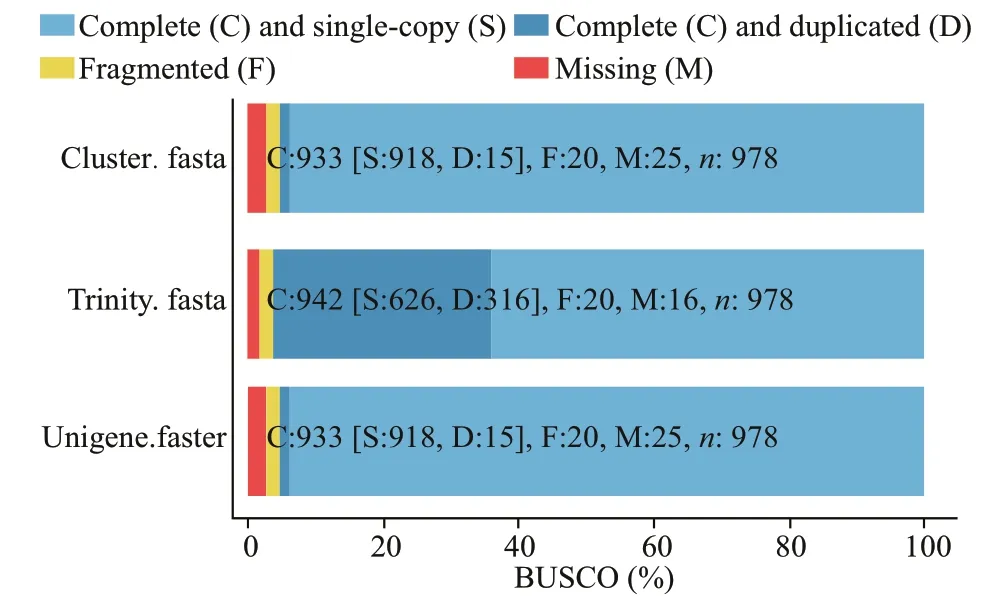
Fig.2 BUSCO evaluation results of splicing transcript
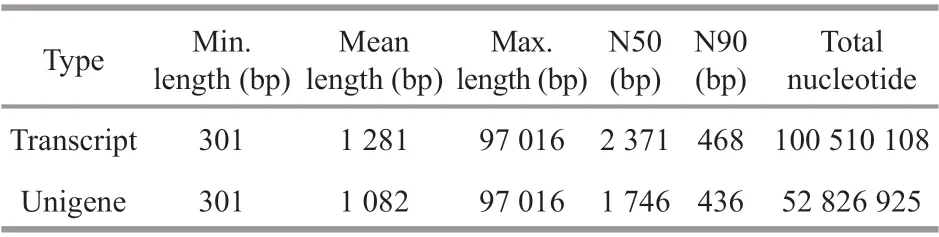
Table 3 Splicing length distribution
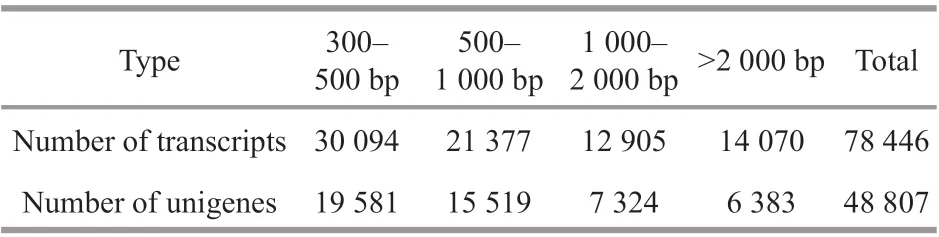
Table 4 Splicing length and frequency distribution
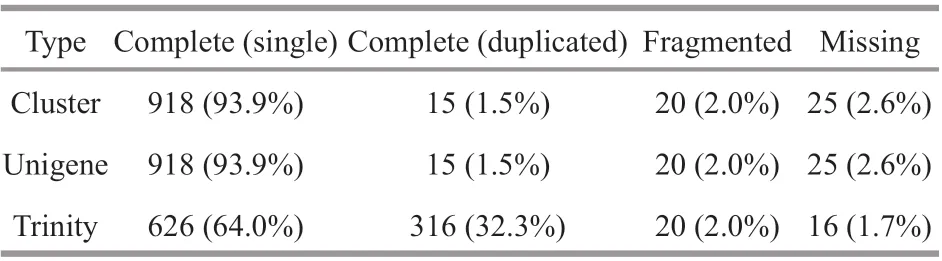
Table 5 BUSCO evaluation of splicing transcript
We used BUSCO to evaluate the splicing quality of unigenes, and compared with the conservative genes to evaluate the accuracy and integrity of the splicing results. The results showed that 978 BUSCO were completely covered, and 918 (93.9%), 15 (1.5%), 20(2.0%), and 25 (2.6%) unigenes were completely matched, including complete and single copy,complete and duplicated, partial fragment, and missing, respectively (Table 5; Fig.2). These data greatly enrich the genetic resources available forM.japonicus, which may facilitate further molecular studies ofM.japonicusunder low-salinity conditions for various periods.
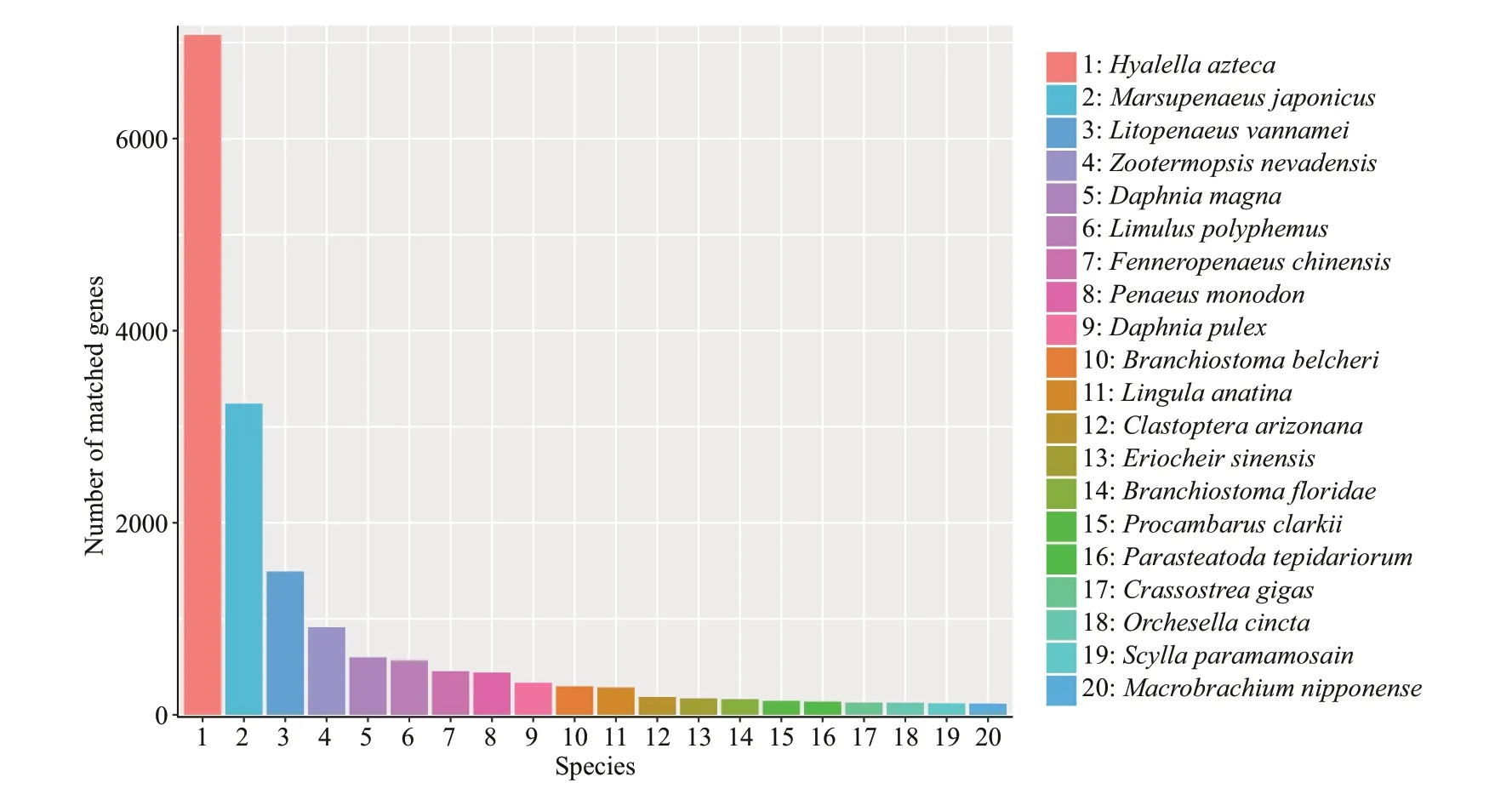
Fig.3 NR database species annotation
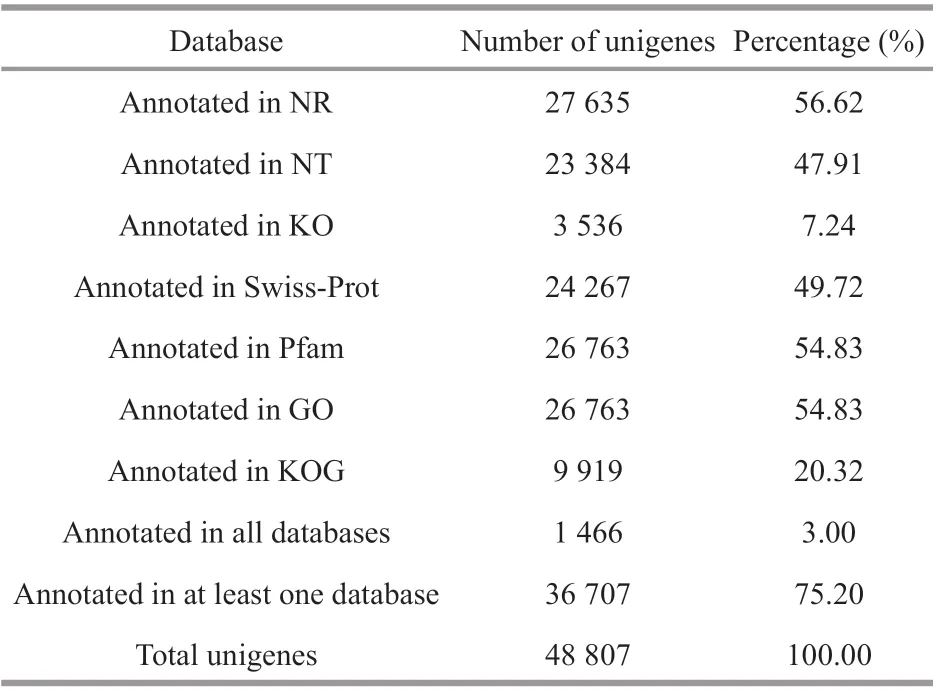
Table 6 Gene annotation rate
3.2 functional annotation of genes
A total of 48 807 unigenes were annotated by NR,NT, KO, Swiss-Prot, Pfam, GO, and KOG. The number of unigenes annotated to at least one database in seven databases is 36 707, accounting for 75.20%of the total, while the number of unigenes annotated to all seven databases is 1 466, only accounting for 3.00% of the total (Table 6). Among them, in the light of the analysis of species sources, 31.6%, 14.4%, and 6.6% of the transcript data compared to the NR database were annotated to the top 3 species,Hyalellaazteca,M.japonicus, andLitopenaeusvannamei(Fig.3).
According to the GO annotation of unigenes,26 763 unigenes obtained GO annotation, accounting for 54.83% of the total. The annotation can be divided into three categories: biological process (BP),molecular function (MF), and cell component (CC).In the biological process, the most involved are cell process (56.80%), metabolism process (52.31%), and single cell tissue process (49.19%). In the cell component, cell part, cell, and membrane account for 26.49%, 26.49%, and 20.20% of the total, respectively.In molecular function, it is mainly related to binding and catalytic activity, accounting for 47.56% and 46.31% of the total, respectively (Fig.4).
A total of 9 919 (20.32%) unigenes were successfully annotated in the KOG database and classif ied according to 26 KOG, among which the most unigenes annotated to R (general function prediction) accounted for 14.30% of the total,followed by O (post translation modif ication, protein transformation, and molecular chaperone), T (signal transduction mechanism), E (amino acid transport and metabolism), accounting for 9.87%, 9.17%, and 8.56% of the total, respectively. However, X (unnamed protein) accounts for only 0.02% of the total, with the least annotated unigenes (Fig.5).
After KO annotation of 3 536 (7.24%) unigenes,according to the KEGG metabolic pathway involved by unigenes, there were 485 (13.72%), 427 (12.08%),553 (15.64%), 2 006 (56.73%), and 795 (22.48%)unigenes involved in the A, B, C, D, and E (cellular processes, environmental information processing,genetic information processing, metabolism, and organismal systems) of KEGG metabolic pathway,respectively Middle branch. Among all pathways, the most abundant unigenes were 395 (11.17%) in carbohydrate metabolism. Secondly, there are 351 and 337 unigenes in amino acid metabolism and signal transduction, respectively, accounting for 9.93% and 9.53% of the total (Fig.6).
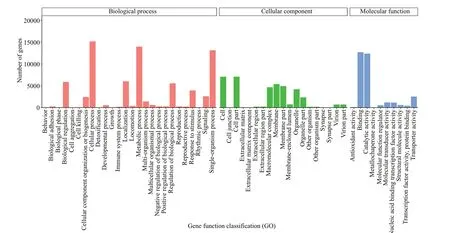
Fig.4 GO annotation classif ication statistics
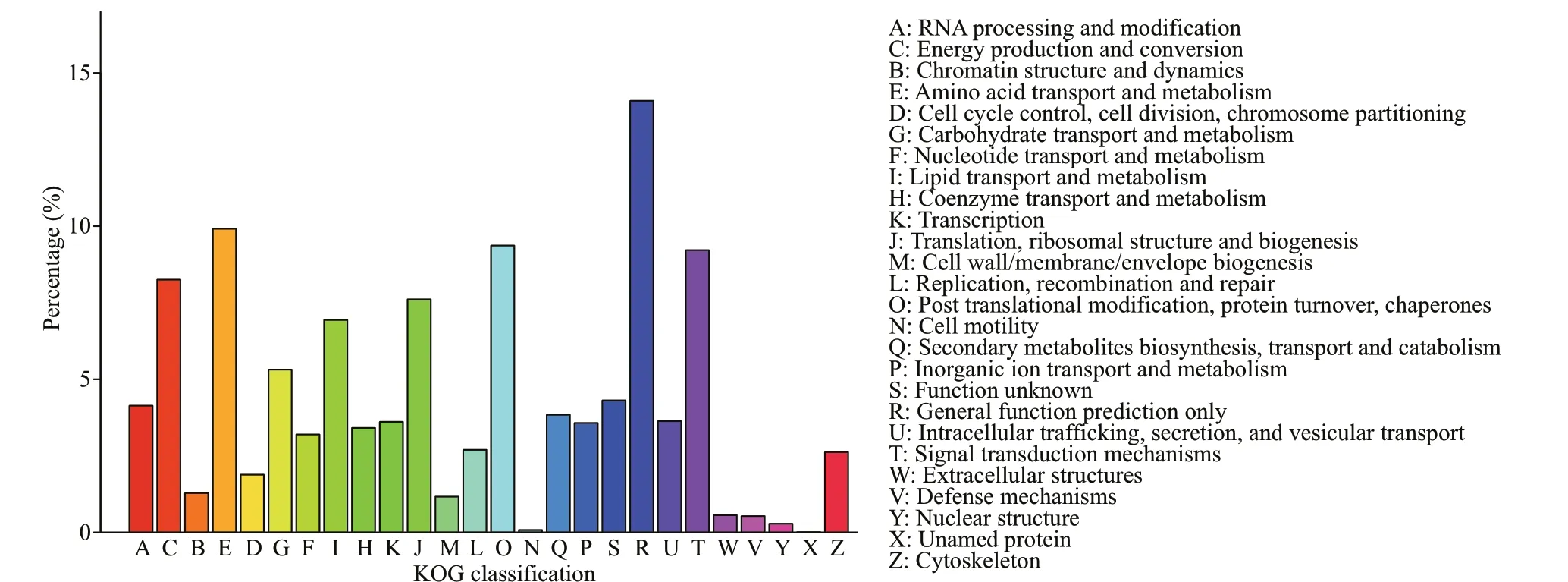
Fig.5 Classif ication statistics of KOG notes
3.3 Diff erential gene expression analysis
The annotated genes in the control and low-salinity groups were screened to obtain DEGs. A total of 811,589, 1 095, 745, and 875 DEGs were identif ied in the LS6, LS12, LS24, LS48, and LS96 libraries compared with the CG6, CG12, CG24, CG48, and CG96 libraries, respectively (Fig.7). More genes were upregulated than down-regulated among all of the DEGs in 6 h, with approximately double the number of upregulated than down-regulated genes. However, the number of down-regulated genes exceeded the number of up-regulated genes in 12 h, 48 h, and 96 h.
3.4 Functional annotation of DEGs
For DEG annotation, a total of 811, 589, 1 095,745, and 875 genes in the LS6, LS12, LS24, LS48,and LS96 groups, respectively, were identif ied using the NR, NT, KO, Swiss-Prot, Pfam, and KOG databases (Table 7). The percentage is ranked as NR> Pfam > Swiss-Prot > KOG > NT > KO, of which the percentages of NR were all greater than 80%,while those of the KO database were 9.34%-11.89%.
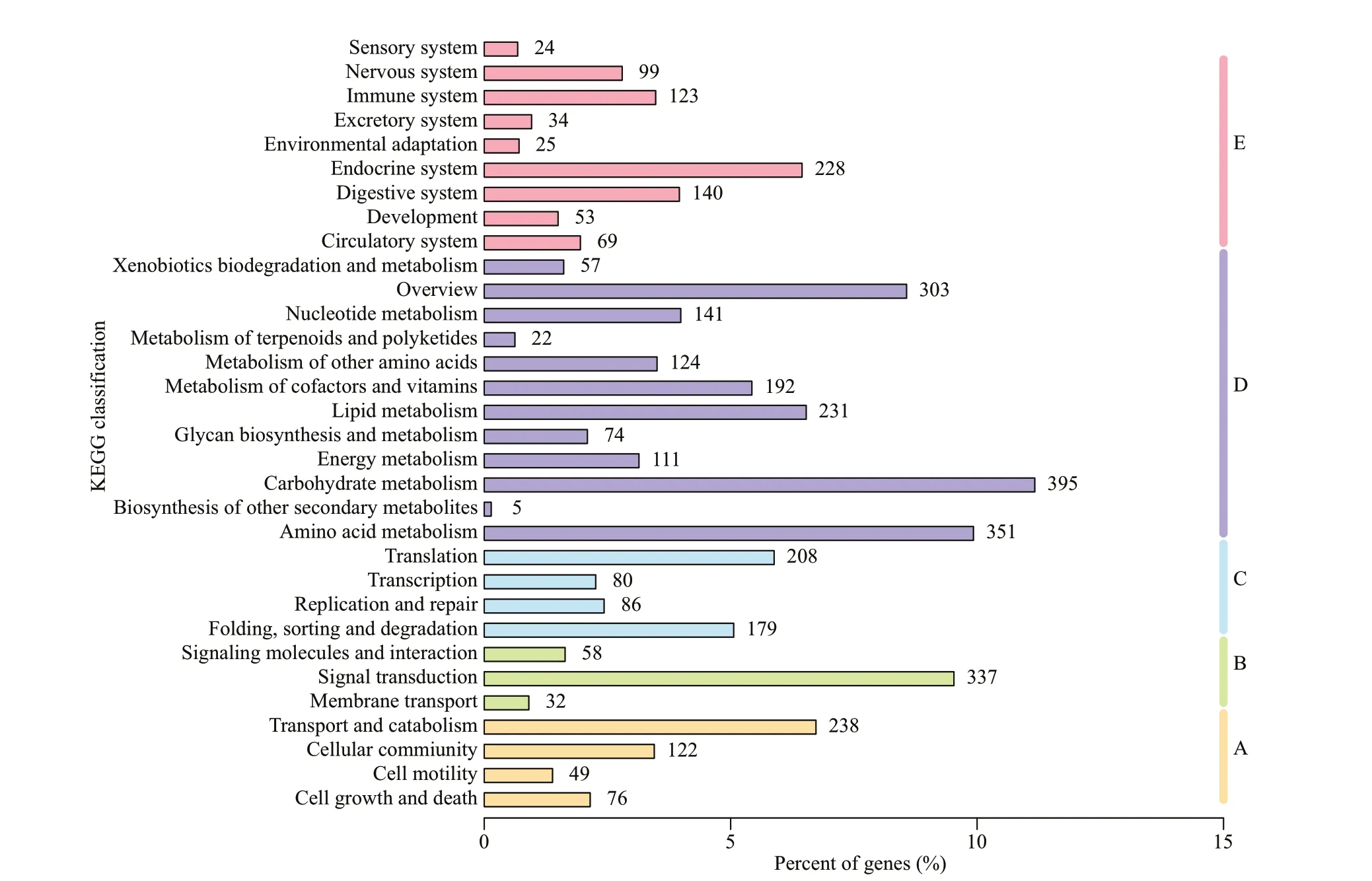
Fig.6 Classif ication statistics of KEGG metabolic pathway
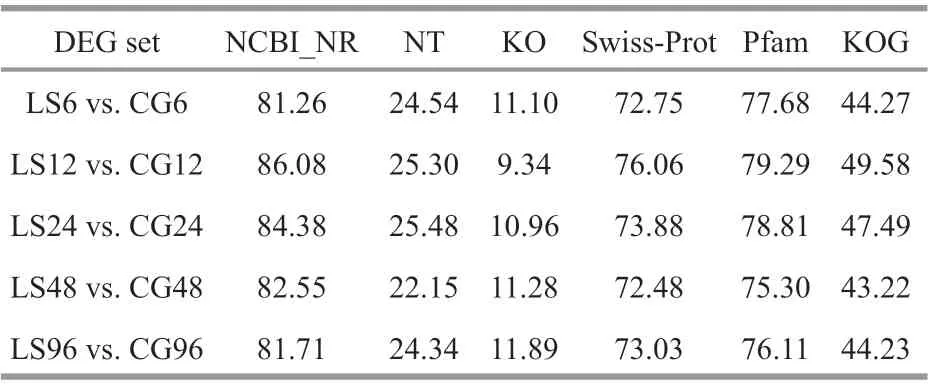
Table 7 Annotation of DEG functions (in % for all)
3.5 GO and KEGG enrichment analysis of DEGs
The DEGs from the comparisons of LS6 vs. CG6,LS12 vs. CG12, LS24 vs. CG24, LS48 vs. CG48, and LS96 vs. CG96 were analyzed based on GO enrichment and classif ied according to the three major GO categories: biological processes, cellular components, and molecular functions. There are 1 822-2 388 GO terms types. DEGs are the most abundant in biological processes, accounting for 56.97%-60.29% of the total, followed by molecular function (Table 8). Among the numerous biological processes represented, metabolic processes were the best represented. Chitin metabolic process (GO:0006030), glucosamine-containing compound metabolic process (GO: 1901071), and amino sugar metabolic process (GO: 0006040) were the most commonly enriched among the groups. Only the LS6 vs. CG6, LS24 vs. CG24, and LS48 vs. CG48 comparisons showed signif icant enrichment of cellular component-related DEGs, with most related to extracellular components and the nucleosome. In addition, genes related to chitin and iron binding were signif icantly enriched in all groups among molecular functions (Fig.8).
DEGs were mapped to KEGG pathways to conf irm the biological pathways activated or repressed during the response ofM.japonicusto low salinity. A total of 151, 85, 149, 97, and 111 KEGG pathways were enriched among the DEGs identif ied in the LS6 vs.CG6, LS12 vs. CG12, LS24 vs. CG24, LS48 vs.CG48, and LS96 vs. CG96 comparisons, respectively,among which the f irst 20 signif icantly enriched pathways are shown (Fig.9). The results show that metabolic pathway and immune pathway were signif icantly enriched, which may play important roles related to salinity stress include the Linoleic acid metabolism (ko00591), Arachidonic acid metabolism (ko00590), Sphingolipid metabolism(ko00600), AMPK signaling pathway (ko04152),Sphingolipid signaling pathway (ko04071), Lysosome(ko04142), and TGF-beta signaling pathway(ko04350).
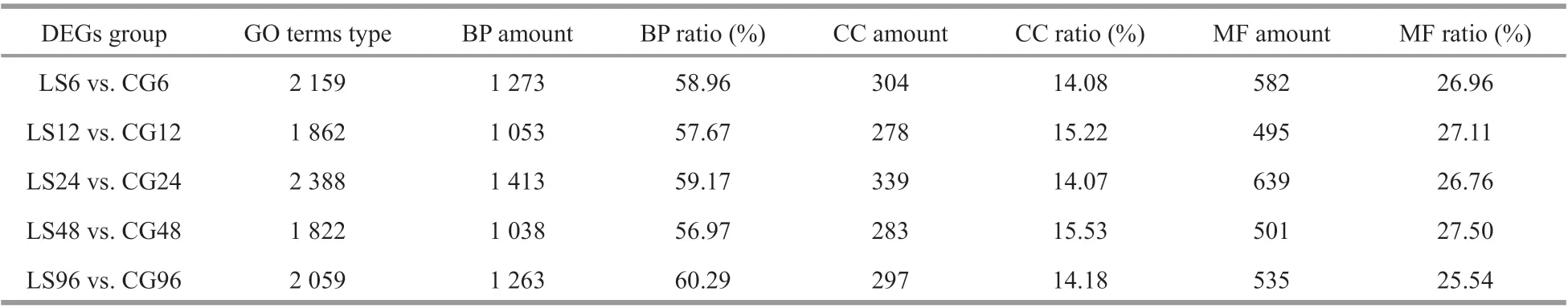
Table 8 GO enrichment analysis of diff erential genes
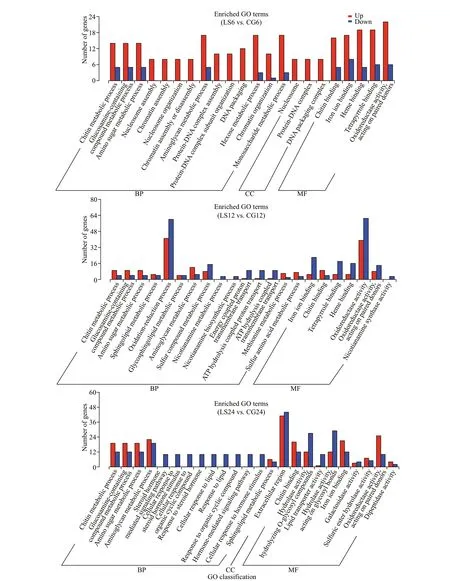
Fig.8 Go enrichment of diff erential genes at f ive diff erent time points
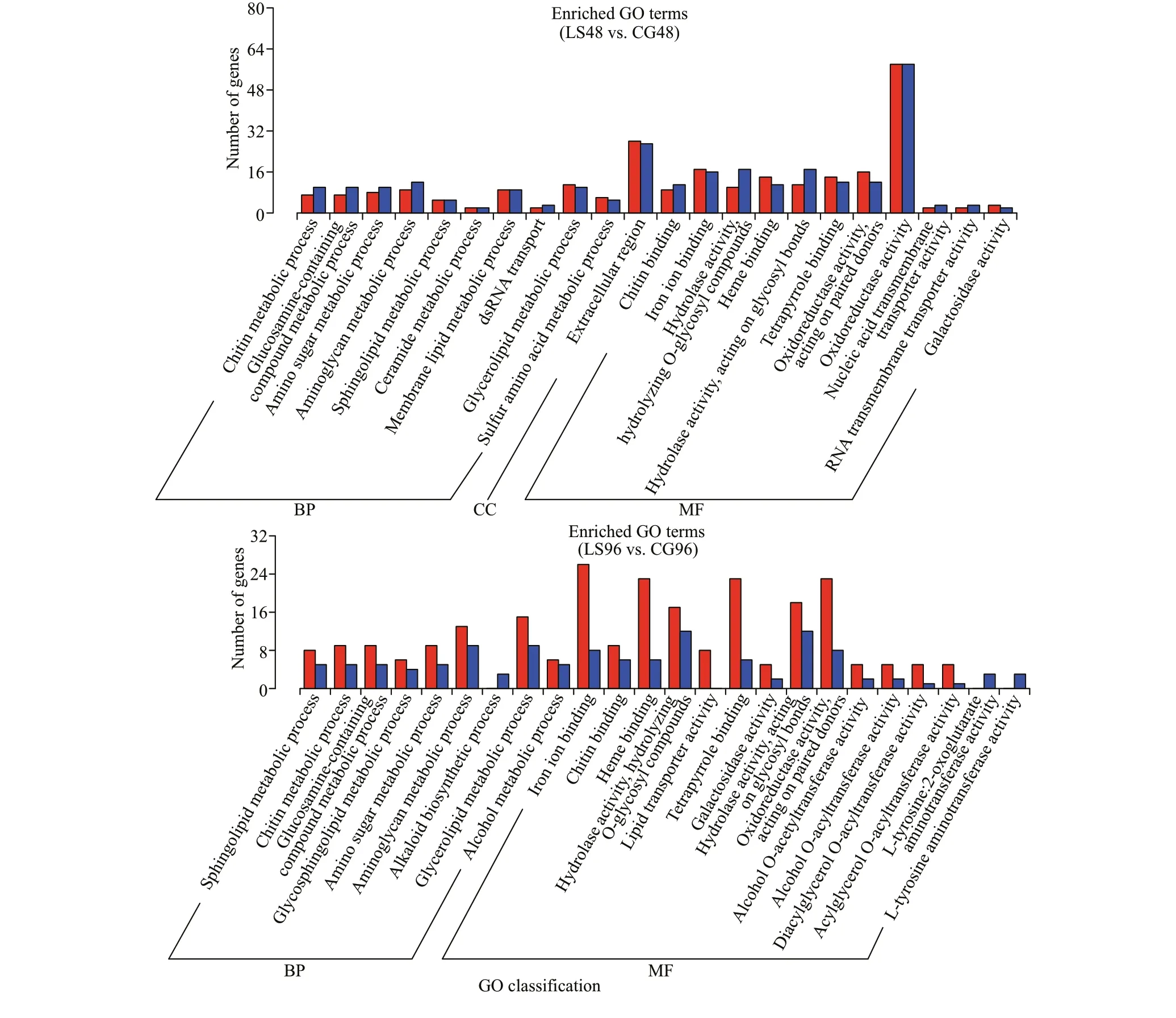
Fig.8 Continued
3.6 Verif ication of the signif icant DEGs by qPCR
Nine DEGs were selected to verify the RNA-seq results in the comparisons of the experimental (LS6,LS12, LS24, LS48, and LS96) and control (CG6,CG12, CG24, CG48, and CG96) groups using qPCR.The qPCR results showed that the trends in DEG variations were consistent with the RNA-seq results,verifying the accuracy of RNA-seq (Fig.10).According to qPCR,TauD,SarDH,CLEC,GNMT,BHMT,MAT2α, andPPP2Cβshowed up-regulation in the control group, whereasTAUTandAGMATshowed down-regulation in the control group.
4 DISCUSSION
4.1 Ion exchange-related processes under salt stress
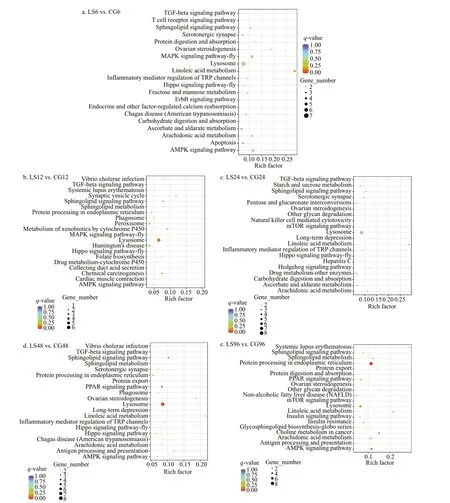
Fig.9 KEGG pathway enrichment of the diff erentially expressed genes
Decapod crustaceans exhibit a wide range of osmoregulatory patterns and capabilities (McNamara and Faria, 2012). In the low ionic freshwater environment, decapods face four major osmoregulation challenges simultaneously: absorbing ions from food and the surrounding water, minimizing diff usive ion loss via semi-permeable membranes,restricting incoming water f lux and regulating cell volume decreases to maintain cellular stability. These osmotic and ionic regulation processes are conducted mainly in the gills (Freire et al., 2008; Furriel et al.,2010; Rahi et al., 2017). Numerous enzymes and transporters are involved in ion transport in crustacean gills, including Na+/K+-ATPase, V (H+)-ATPase,carbonic anhydrase and Na+/K+/2Cl cotransporter(Weihrauch et al., 2001; Tsai and Lin, 2007; Furriel et al., 2010; Havird et al., 2013). Interestingly, we found three biological processes related to osmotic regulation in the LS12 vs. CG12 comparison ofM.japonicusunder low-salinity stress: energycoupled proton transmembrane transport (GO:0015988), ATP hydrolysis-coupled transmembrane transport (GO: 0090662) and ATP hydrolysis-coupled transport proton (GO: 0015991). These three processes were enriched among the DEGs identif ied at the 12thh only, and almost all of these DEGs were down-regulated. This result shows that the hepatopancreas ofM.japonicuswas involved in osmotic regulation, via inhibition of the ion transport process, under low-salinity stress for 12 h. We found signif icant diff erences in two DEGs: V (H+)-ATPase and V-H-ATPase subunits. V (H+)-ATPases are a family of membrane-related ATP-dependent proton pump enzymes. When located in the plasma membrane, these proteins couple the energy released during ATP hydrolysis with active transport of protons from the cytoplasm to intracellular or extracellular spaces (Forgac, 2007). The V1 region of V (H+)-ATPases contains eight diff erent subunits (A-H), with catalytic sites located at the interface between the A and B subunits participating in ATP hydrolysis(Jeff eries et al., 2008). WhenL.vannameiwas moved from seawater with a salinity of 20 to water with a salinity of 5, the expression of V-H-ATPase subunit A in the gills was increased at approximately 12 h after the change in salinity, whereas that of V-H-ATPase subunit A in the hepatopancreas was decreased (Wang et al., 2012). In this study, we found that the expression levels of V(H+)-ATPase and V-H-ATPase subunits were down-regulated in 12 h, similar to previous f indings inL.vannamei. We speculate that the hepatopancreas ofM.japonicusinhibits H+transport from the intracellular to extracellular space as an adaptation to hypoosmotic stress. The specif ic reason for this response remains to be explored.
The ion transporter Na+/K+-ATPase alpha subunit was also diff erentially expressed in the LS24 vs.CG24 comparison. Ion absorption by crustaceans is achieved mainly via the Na+/K+-ATPase (Lucu and Towle, 2003). The Na+/K+-ATPase is located in the basolateral membrane of gill ion-transport epithelial cells. The establishment of an electrochemical gradient shows that the Na+/K+-ATPase is the main force driving ion transport (Towle and Kays, 1986).The Na+/K+-ATPase maintains a gradient of excess Na+outside and excess K+inside the cell, and this gradient is used as an energy source to create a membrane potential for regulating cytoplasmic composition and transepithelial transport (Skou and Esmann, 1992; Suhail, 2010). Expression of the Na+/K+-ATPase alpha subunit gene in the hepatopancreas was increased under hypoosmotic stress, but the transcript level of this gene was down-regulated in 24 h, which was not consistent with previous research(Li et al., 2015). This pattern may result from the diff erences of species, and the specif ic reasons require further exploration.
4.2 Lipid metabolism and glucose metabolism related to osmotic regulation under salt stress
Under low-salinity stress, shrimp require additional saturated and polyunsaturated fatty acids to maintain their osmotic regulation and ion exchange, and this process involves high-energy consumption (Chen et al., 2014, 2019). A number of lipid metabolic pathways in the hepatopancreas ofL.vannameiare aff ected by salinity stress, including fatty-acid biosynthesis, arachidonic acid metabolism,adipocytokine signaling, and glycerophospholipid metabolism (Chen et al., 2015). Fatty acid metabolism and arachidonic acid metabolism are also related to osmotic regulation inEriocheirsinensis(Li et al.,2014a). In this study, enrichment of DEGs associated with linoleic acid metabolism (ko00591) and arachidonic acid metabolism (ko00590) was found in the comparisons of LS6 vs. CG6, LS24 vs. CG24,LS48 vs. CG48, and LS96 vs. CG96, and most of these DEGs were up-regulated. We speculate that lipid metabolism in the hepatopancreas (the main organ of fat production) and lacrimal secretion are increased inM.japonicus. Acceleration of synthetic renewal can provide enough energy for osmotic regulation in shrimp at low salinity. Previous studies have revealed two major strategies for osmotic regulation in crustaceans, the “limiting process” and“compensatory process”, which are carried out mainly by the gills. The limiting process is a strategy to maintain hemolymph osmotic pressure and ions in branchial membranes by adjusting the permeability of boundary structures, which can eff ectively reduce ion diff usion and water inf low (Péqueux, 1995; Rainbow and Black, 2001). Our results suggest that the strategy of the limiting the process may play an important role in osmotic regulation inM.japonicus, which is similar to that inPortunustrituberculatus(Lv et al., 2013)andL.vannamei(Chen et al., 2019).
Energy metabolism plays an important role in survival and normal body functions, as well as in stress adaptation and tolerance (Sokolova et al.,2012). In this study, we found that glycometabolism was signif icantly aff ected by low-salinity stress inM.japonicus, with changes in fructose and mannose metabolism (ko00051), sphingolipid metabolism(ko00600), and other glycan degradation (ko00511)pathways and signif icant up-regulation of the glucosamine-containing compound metabolic process(GO: 1901071), amino sugar metabolic process (GO:0006040), and aminoglycan metabolic process (GO:0006022) terms in all groups. DEGs related to glycometabolism in the hepatopancreas ofL.vannameiwere more represented at a salinity of 3 than in the control in terms of gluconeogenesis, amino sugar and nucleotide sugar metabolism,glycosphingolipid biosynthesis, glycosaminoglycan degradation, and other glycan degradation, suggesting a requirement for extra energy byL.vannameiunder chronic low-salinity stress, which is usually derived from the metabolism of nutrient substances (Xu et al.,2017). Therefore, we speculate that the active glycometabolism ofM.japonicusunder low-salinity stress is an adaptation to maintain osmotic regulation.Fructose-1,6-bisphosphate aldolase is a key enzyme involved in glycolysis and gluconeogenesis. It is ubiquitously expressed in animals, plants and microorganisms (De-Simone et al., 2006; Lu et al.,2012; Dawson et al., 2013). In this study, fructose-1,6-bisphosphate aldolase was up-regulated at the 6thh and then returned to a normal level, ref lecting an increase in glycometabolism during the early stage of low-salinity stress inM.japonicus. Previous studies have shown that supplementation with appropriate carbohydrates can improve the stress resistance ofL.vannameito low salinity (Wang et al., 2014c).Carbohydrate metabolism appears to play a major role in the energy supply for iono- and osmoregulation, and the liver is the main source of carbohydrate metabolites for osmoregulatory organs(Tseng and Hwang, 2008). Up-regulation of related genes indicated that carbohydrate metabolism was increased to provide more energy during the early stage of low-salinity stress inM.japonicus. We identif ied a DEG, alpha-amylase (alpha-1,4-glucanglucanohydrolase), that was signif icantly associated with carbohydrate metabolism. Alpha-amylase belongs to the glycosyl hydrolase family 13, and it is a major carbohydrate hydrolase involved in the digestive functions of the liver and pancreas in shrimp(Kuriki and Imanaka, 1999; Glenn et al., 2005). In this study, alpha-amylase expression returned to normal after 6 h of up-regulation and then was downregulated after 24 h. Therefore, we speculate that this gene is related to low-salinity stress inM.japonicus,and that the hydrolysis of carbohydrates is increased as an adaptation to osmotic regulation during the early stages of stress. In addition, we found that phosphoenolpyruvate carboxykinase expression was increased signif icantly at 6, 12, and 24 h, after which it returned to normal, indicating that phosphoenolpyruvate carboxykinase is involved in the early stage of low-salinity stress inM.japonicus.
4.3 Immunity under low-salinity stress
Low-salinity stress can reduce the immunity ofM.japonicusand increase its sensitivity to viral infection (Yu et al., 2003). KEGG analysis showed that the DEGs identif ied at 48 h were enriched in the Chagas disease (American trypanosomiasis)(ko05142) andVibriocholeraeinfection (ko05110)pathways, and most of these DEGs were downregulated, suggesting thatM.japonicusis more resistant to pathogens under low-salinity stress.Shrimp lack an acquired immune system, and its innate immune system is divided into humoral immunity and cellular immunity. Shrimp are thought to be totally dependent on a congenital and nonadaptive mechanism to resist pathogen invasion (Li and Xiang, 2013; Tassanakajon et al., 2013; Wang et al., 2014a). KEGG analysis showed that the DEGs were enriched in lysosome (ko04142), peroxisome(ko04146), phagosome (ko04145), and apoptosis(ko04210) pathways related to cellular immunity,consistent with the changes in pathways identif ied previously in theL.vannameihepatopancreas under low-salinity stress (Xu et al., 2017). Immune-related genes such as calnexin involved in the phagosome(ko04145) and cathepsin A involved in the lysosome(ko04142) showed signif icantly down-regulated expression in response to low-salinity stress,suggesting that low-salinity stress reduced phagocytosis and oxygen-independent reactivity in phagocytes ofL.vannamei(Zhao et al., 2015). In this study, cathepsin C and lysosomal alpha-mannosidase in the lysosome (ko04142) and calreticulin in the phagosome (ko04145) were signif icantly downregulated. Of these genes, cathepsin C returned to normal after 12 h of down-regulation, lysosomal alpha-mannosidase returned to normal after 6 hours of down-regulation, and calreticulin was down-regulated only during the late stage (after 48 h) of stress,indicating that these genes are involved in diff erent stages of cellular immunity and reduce the phagocytic capacity of shrimp. Apoptosis is a genetically programmed cell suicide process that eliminates unwanted or diseased cells and plays important roles in embryogenesis, homeostasis, insect metamorphosis and immunity (Steller and Grether, 1994; Opferman and Korsmeyer, 2003). Cytochrome C is a proapoptotic factor that is released from the outer surface of the inner mitochondrial membrane during the early stage of apoptosis. In combination with certain cytosolic proteins, it induces conversion of the latent apoptosis-promoting protease pro-caspase 9 into its active form (Skulachev, 1998; Marsden et al., 2002).In this study, we found that cytochrome C, involved in the apoptosis (ko04210) signaling pathway, was upregulated at 6 h; this is consistent with its previously reported up-regulation inL.vannameiinfected with WSSV, indicating that cytochrome C may be induced by low-salinity stress to participate in apoptosis (Hu and Yao, 2016). Interestingly, we found that caspase 3 was down-regulated at 6 h, suggesting thatM.japonicusresponds to low-salinity stress by inhibiting caspase 3 and thus preventing apoptosis.
The humoral response includes the prophenoloxidase (proPO) system, hemolymph coagulation, and a variety of antimicrobial peptides(Li and Xiang, 2013; Tassanakajon et al., 2013; Wang et al., 2014a). Melanization is regulated by phenoloxidase (PO), which is activated by the proPO cascade control and plays an important role in the immune system of invertebrates, responding quickly to pathogen infection (Amparyup et al., 2013).Prophenoloxidase b has been shown to contribute to PO activity in crustacean hemolymph plasma (Masuda et al., 2012). We found that prophenoloxidase b was increased signif icantly at 12 and 24 h in oxidoreductase activity (GO: 0016491), suggesting that prophenoloxidase b promotes humoral immune regulation inM.japonicusunder low-salinity stress.proPO activation and Toll pathway initiation in arthropods are mediated by serine proteinase cascades and regulated by serpins in the hemolymph (Liu et al.,2009). Injecting rPmSERPIN3 along withVibrioharveyiinto shrimp decreased the clearance rate of bacteria from the hemolymph, suggesting that PmSERPIN3 functions as a regulator of the proPO activation system (Wetsaphan et al., 2013). We found that the expression of serpin 3 was up-regulated after 6 hours of stress. We speculate that serpin 3 promotes activation of proPO and the Toll pathway during the early stage of low-salinity stress. In addition, antilipopolysaccharide factor 2, an antimicrobial peptide,was down-regulated in 6 and 12 h, indicating that anti-lipopolysaccharide factor 2 is also involved in immune regulation.
4.4 Chitin metabolic process under salt stress
Notably, the level of GO enrichment of DEGs related to chitin metabolism (qvalue: 6.4593E-10)was the highest among all metabolic processes. This indicates that chitin metabolism may be involved in osmotic regulation or aff ected by salinity stress,supporting the results of a salinity stress-based transcription analysis conducted inPortunustrituberculatus(Lv et al., 2013). In addition, we found DEGS in the control and low-salinity groups about the chitinase 1, peritrophin and peritrophin-like protein genes. In total, seven, f ive, and three chitinases were identif ied inL.vannamei,PenaeusmonodonandM.japonicus, respectively. This study showed agreement that chitinase 1 is mainly expressed in the hepatopancreas (Watanabe et al., 1998; Huang et al.,2010; Proespraiwong et al., 2010; Rocha et al., 2012),and consistent with the data from this experiment. The growth, molting and development of crustaceans require chitinase, which functions in digesting chitinous food, modifying the parenteral nutrition membrane and degrading the chitin exoskeleton (Zou and Bonvillain, 2004; Zhang et al., 2014). In this study, chitinase 1 was initially down-regulated. We speculate that chitinase 1 is involved in the response to low-salinity stress and improves the ability ofM.japonicusto resist low-salinity stress. The specif ic regulatory mechanism of this process remains to be explored. We presume that expression of chitinase elicits an immune response quickly under low-salinity stress, which promotes ecdysis, and then allows gradual adaption to changes in salinity, which may be related to osmotic regulation. Its subsequent inhibition under low-salinity conditions may be a regulatory mechanism to prevent energy waste. Peritrophin is the most widely investigated soluble protein in the insect peritrophic membrane. It has strong interactions with chitin-binding domain 2 and the envelope protein A domain to form the peritrophic membrane, which promotes food digestion and protects insects from microbial invasion (Jasrapuria et al., 2010). In recent years, many peritrophin-like genes have been identif ied and cloned in crustaceans such asF.merguiensis(Loongyai et al., 2007),P.monodon(Chen et al.,2009),F.chinensis(Du et al., 2006), andExopalaemoncarinicauda(Wang et al., 2013). Du et al. (2006) and Wang et al. (2013) found that silencing peritrophinlike genes by dsRNA interference improved the survival rate of shrimp infected with WSSV, suggesting that peritrophin-like genes may have an immune defense function. In our transcriptome data, chitinase 1 was initially up-regulated, then returned to normal,and then was down-regulated, while the peritrophin gene was initially down-regulated and then returned to normal, under low salinity compared with the control group. Interestingly, peritrophin-like protein gene showed the opposite trend of the peritrophin gene. We speculate that these three genes are involved in the regulation of low-salinity adaptation. Salt stress promotes a rapid immune response in shrimp. Shrimp may adapt to the low-salinity environment by selfregulating osmotic pressure. Down-regulation of chitinase levels may be undertaken to prevent energy waste. The specif ic regulatory mechanism of this response remains to be explored.
5 CONCLUSION
The transcriptome libraries in hepatopancreas ofM.japonicusunder low salt stress were successfully constructed, and many diff erentially expressed genes identif ied. The DEGs were annotated to obtain the pathways and genes related to osmoregulation,metabolism, and immunity. These functional genes provide valuable information for the further understanding of molecular mechanism ofM.japonicusunder low salt stress.
6 DATA AVAILABILITY STATEMENT
All data supporting the results of this study are available upon reasonable request from the corresponding author.Reference s
Amparyup P, Charoensapsri W, Tassanakajon A. 2013.Prophenoloxidase system and its role in shrimp immune responses against major pathogens.Fish&Shellf ish Immunology, 34(4): 990-1001.
Balaguru K, Foltz G R, Leung L R, Emanuel K A. 2016. Global warming-induced upper-ocean freshening and the intensif ication of super typhoons.NatureCommunications,7(13670): 1-8.
Brawand D, Soumillon M, Necsulea A, Julien P, Csárdi G,Harrigan P, Weier M, Liechti A, Aximu-Petri A, Kircher M, Albert F W, Zeller U, Khaitovich P, Grützner F,Bergmann S, Nielsen R, Pääbo S, Kaessmann H. 2011.The evolution of gene expression levels in mammalian organs.Nature, 478(7369): 343-348.
Chen J C, Lai S H. 1993. Eff ects of temperature and salinity on oxygen consumption and ammonia-N excretion of juvenilePenaeusjaponicusBate.JournalofExperimental MarineBiologyandEcology, 165(2): 161-170.
Chen K Y, Hsu T C, Huang P Y, Kang S T, Lo C F, Huang W P,Chen L L. 2009.Penaeusmonodonchitin-binding protein(PmCBP) is involved in white spot syndrome virus(WSSV) infection.Fish&Shellf ishImmunology, 27(3):460-465.
Chen K, Li E C, Gan L, Wang X D. 2014. Growth and lipid metabolism of the pacif ic white shrimpLitopenaeus vannameiat diff erent salinities.JournalofShellf ish Research, 33(3): 825-833.
Chen K, Li E C, Li T Y, Xu C, Wang X D, Lin H Z, Qin J G,Chen L Q. 2015. Transcriptome and molecular pathway analysis of the hepatopancreas in the Pacif ci white shrimpLitopenaeusvannameiunder chronic low-salinity stress.PLoSOne, 10(7): e0131503.
Chen K, Li E C, Xu C, Wang X D, Li H F, Qin J G, Chen L Q.2019. Growth and metabolomic responses of Pacif ic white shrimp (Litopenaeusvannamei) to diff erent dietary fatty acid sources and salinity levels.Aquaculture, 499:329-340.
Cheng S Y, Chen J C. 2001. The time-course change of nitrogenous excretion in the Kuruma shrimpPenaeus japonicusfollowing nitrite exposure.AquaticToxicology,51(4): 443-454.
Cheng S Y, Lee W C, Shieh L W, Chen J C. 2004. Increased production and excretion of urea in the kuruma shrimp(Marsupenaeusjaponicus) exposed to combined environments of increased ammonia and nitrite.Archives ofEnvironmentalContaminationandToxicology, 47(3):352-362.
Cheng S Y, Shieh L W, Chen J C. 2013. Changes in hemolymph oxyhemocyanin, acid-base balance, and electrolytes inMarsupenaeusjaponicusunder combined ammonia and nitrite stress.AquaticToxicology, 130-131: 132-138.
Chien Y H, Shiau W C. 2005. The eff ects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn,Marsupenaeus japonicusBate.JournalofExperimentalMarineBiology andEcology, 318(2): 201-211.
Chong-Robles J, Charmantier G, Boulo V, Lizárraga-Valdéz J,Enríquez-Paredes L M, Giff ard-Mena I. 2014.Osmoregulation pattern and salinity tolerance of the white shrimpLitopenaeusvannamei(Boone, 1931) during postembryonic development.Aquaculture, 422-423: 261-267.
Coman G J, Crocos P J, Preston N P, Fielder D. 2002. The eff ects of temperature on the growth, survival and biomass of diff erent families of juvenilePenaeusjaponicusBate.Aquaculture, 214(1-4): 185-199.
Dawson N J, Biggar K K, Storey K B. 2013. Characterization of fructose-1,6-bisphosphate aldolase during anoxia in the tolerant turtle,Trachemysscriptaelegans: an assessment of enzyme activity, expression and structure.PLoSOne,8(7): e68830.
De-Simone S G, Salles C M C D, Silva C M B, Hassón-Voloch A. 2006. Purif ication and amino acid sequence of fructose-1,6-bisphosphate aldolase from the electric organ ofElectrophoruselectricus(L.).ZeitschriftfürNaturforschung CAJournalofBiosciences. 61(11-12): 884-888.
Du X J, Wang J X, Ning, L, Zhao X F, Li F H, Xiang J H. 2006.Identif ication and molecular characterization of a peritrophin-like protein from f leshy prawn (Fenneropenaeus chinensis).MolecularImmunology, 43(10): 1633-1644.
Duan Y F, Zhang J S, Dong H B, Wang Y, Liu Q S, Li H. 2016.Eff ect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimpMarsupenaeus japonicus.Fish&Shellf ishImmunology, 49: 91-99.
Fan L F, Wang L, Wang Z L. 2019. Proteomic characterization of the hepatopancreas in the Pacif ic white shrimpLitopenaeusvannameiunder cold stress: revealing the organism homeostasis mechanism.Fish&Shellf ish Immunology, 92: 438-449.
Forgac M. 2007. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology.NatureReviews MolecularCellBiology, 8(11): 917-929.
Fregoso-López M G, Morales-Covarrubias M S, Franco-Nava M A, Ramírez-Rochín J, Fierro-Sañudo J F, Ponce-Palafox J T, Páez-Osuna F. 2017. Histological alterations in gills of shrimpLitopenaeusvannameiin low-salinity waters under diff erent stocking densities: potential relationship with nitrogen compounds.Aquaculture Research, 48(12): 5854-5863.
Freire C A, Onken H, Mcnamara J C. 2008. A structurefunction analysis of ion transport in crustacean gills and excretory organs.ComparativeBiochemistryand PhysiologyPartA:Molecular&IntegrativePhysiology,151(3): 272-304.
Fu B Z, Zou L L. 2018. De novo transcriptome assembly and comparison of walnut (JuglansregiaL.) cv. Qingxiang organs: roots, stems and leaves.SwiftJournalof AgriculturalResearch, 4(1): 1-20.
Furriel R P M, Firmino K C S, Masui D C, Faleiros R O, Torres A H, McNamara J C. 2010. Structural and biochemical correlates of Na+, K+-ATPase driven ion uptake across the posterior gill epithelium of the true freshwater crab,Dilocarcinuspagei(Brachyura, Trichodactylidae).JournalofExperimentalZoology.PartA:Ecological GeneticsandPhysiology, 313(8): 508-523.
Gao W H, Tan B P, Mai K S, Chi S Y, Liu H Y, Dong X H, Yang Q H. 2012. Prof iling of diff erentially expressed genes in hepatopancreas of white shrimp (Litopenaeusvannamei)exposed to long-term low salinity stress.Aquaculture,364-365: 186-191.
Glenn K L, Grapes L, Suwanasopee T, Harris D L, Li Y, Wilson K, Rothschild M F. 2005. SNP analysis ofAMY2andCTSLgenes inLitopenaeusvannameiandPenaeusmonodonshrimp.AnimalGenetics, 36(3): 235-236.
GrabherrM G, HaasB J, Yassour M, Levin J Z, Thompson D A,Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q D,Chen Z H, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren B W, Nusbaum C, Lindblad-Toh K,Friedman N, Regev A. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome.NatureBiotechnology, 29(7): 644-652.
Hamasaki K, Kitada S. 2008. Potential of stock enhancement for decapod crustaceans.ReviewsinFisheriesScience,16(1-3): 164-174.
Havird J C, Henry R P, Wilson A E. 2013. Altered expression of Na+/K+-ATPase and other osmoregulatory genes in the gills of euryhaline animals in response to salinity transfer:ameta-analysis of 59 quantitative PCR studies over 10 years.ComparativeBiochemistryandPhysiologyPartD:GenomicsandProteomics, 8(2): 131-140.
He L, Wang Q, Jin X K, Wang Y, Chen L L, Liu L H, Wang Y,Liu Z J. 2012. Transcriptome prof iling of testis during sexual maturation stages inEriocheirsinensisusing Illumina sequencing.PLoSOne, 7(3): e33735.
Heugens E H W, Hendriks A J, Dekker T, van Straalen N M,Admiraal W. 2001. A review of the eff ects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment.CriticalReviewsin Toxicology, 31(3): 247-284.
Hewitt D R, Duncan P F. 2001. Eff ect of high water temperature on the survival, moulting and food consumption ofPenaeus(Marsupenaeus)japonicus(Bate, 1888).AquacultureResearch, 32(4): 305-313.
Hop H, Pearson T, Hegseth E N, Kovacs K M, Wiencke C,Kwasniewski S, Eiane K, Mehlum F,Gulliksen B,Wlodarska-Kowalczuk M, Lydersen C, Weslawski J M,Cochrane S, Gabrielsen G W, Leakey R J G, Lønne O J,Zajaczkowski M, Falk-Petersen S, Kendall M, Wängberg S Å, Bischof K, Voronkov A Y, Kovaltchouk N A, Wiktor J, Poltermann M, di Prisco G, Papucci C, Gerland S.2002. The marine ecosystem of Kongsfjorden, Svalbard.PolarResearch, 21(1): 167-208.
Hu D X, Pan L Q, Zhao Q, Ren Q. 2015. Transcriptomic response to low salinity stress in gills of the Pacif ic white shrimp,Litopenaeusvannamei.MarineGenomics, 24:297-304.
Hu W Y, Yao C L. 2016. Molecular and immune response characterizations of a novel AIF and cytochrome c inLitopenaeusvannameidefending against WSSV infection.Fish&Shellf ishImmunology, 56(3): 84-95.
Huang Q S, Yan J H, Tang J Y, Tao Y M, Xie X L, Wang Y, Wei X Q, Yan Q H, Chen Q X. 2010. Cloning and tissue expressions of seven chitinase family genes inLitopenaeus vannamei.Fish&Shellf ishImmunology, 29(1): 75-81.
Ingram B A, Mckinnon L J, Gooley G J. 2002. Growth and survival of selected aquatic animals in two saline groundwater evaporation basins: an Australian case study.AquacultureResearch, 33(6): 425-436.
Jasrapuria S, Arakane Y, Osman G, Kramer K J, Beeman R W,Muthukrishnan S. 2010. Genes encoding proteins with peritrophin A-type chitin-binding domains inTribolium castaneumare grouped into three distinct families based on phylogeny, expression and function.Insect BiochemistryandMolecularBiology, 40(3): 214-227.
Jeff eries K C, Cipriano D J, Forgac M. 2008. Function,structure and regulation of the vacuolar (H+)-ATPases.ArchivesofBiochemistryandBiophysics, 476(1): 33-42.
Johnson C R, Banks S C, Barrett N S, Cazassus F, Dunstan P K, Edgar G J, Frusher S D, Gardner C, Haddon M,Helidoniotis F, Hill K L, Holbrook N J, Hosie G W, Last P R, Ling S D, Melbourne-Thomas J, Miller K, Pecl G T,Richardson A J, Ridgway K R. 2011. Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania.JournalofExperimentalMarineBiologyandEcology,400(1-2): 17-32.
Jung H, Lyons R E, Dinh H, Hurwood D A, McWilliam S,Mather P B. 2011. Transcriptomics of a giant freshwater prawn (Macrobrachiumrosenbergii):denovoassembly,annotation and marker discovery.PLoSOne, 6(12):e27938.
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T,Yamanishi Y. 2007. KEGG for linking genomes to life and the environment.NucleicAcidsResearch, 36: D480-D484.
Kornienko A E, Dotter C P, Guenzl P M, Gisslinger H,Gisslinger B, Cleary C, Kralovics R, Pauler F M, Barlow D P. 2016. Long non-coding RNAs display higher natural expression variation than protein-coding genes in healthy humans.GenomeBiology, 17: 14.
Kourafalou V H, Lee T N, Oey L Y, Wang J D. 1996. The fate of river discharge on the continental shelf: 2. Transport of coastal low-salinity waters under realistic wind and tidal forcing.JournalofGeophysicalResearch:Oceans,101(C2): 3435-3455.
Kuriki T, Imanaka T. 1999. The concept of the α-amylas
e family: structural similarity and common catalytic mechanism.JournalofBioscienceandBioengineering,87(5): 557-565.
Laing I. 2002. Eff ect of salinity on growth and survival of king scallop spat (Pectenmaximus).Aquaculture, 205(1-2):171-181.
Langmead B, Trapnell C, Pop M, Salzberg S L. 2009. Ultrafast and memory-effi cient alignment of short DNA sequences to the human genome.GenomeBiology, 10(3): R25.
Lee W C, Chen J C. 2003. Hemolymph ammonia, urea and uric acid levels and nitrogenous excretion ofMarsupenaeus japonicusat diff erent salinity levels.Journalof ExperimentalMarineBiologyandEcology, 288(1): 39-49.
Leu J H, Chen S H, Wang Y B, Chen Y C, Su S Y, Lin C Y, Ho J M, Lo C F. 2011. A review of the major penaeid shrimp EST studies and the construction of a shrimp transcriptome database based on the ESTs from four penaeid shrimp.MarineBiotechnology, 13(4): 608-621.
Li B, Dewey C N. 2011. RSEM: accurate transcript quantif ication from RNA-Seq data with or without a reference genome.BMCBioinformatics, 12(1): 323.
Li B, He X L, Zhao Y P, Bai D Y, Du M, Song L J, Liu Z, Yin Z C, Manglai D. 2020. Transcriptome prof iling of developing testes and spermatogenesis in the Mongolian horse.BMCGenetics, 21(1): 46.
Li C C, Yeh S T, Chen J C. 2010. Innate immunity of the white shrimpLitopenaeusvannameiweakened by the combination of aVibrioalginolyticusinjection and lowsalinity stress.Fish&Shellf ishImmunology, 28(1): 121-127.
Li C Z, Weng S P, Chen Y G, Yu X Q, Lü L, Zhang H Q, He J G, Xu X P. 2012. Analysis ofLitopenaeusvannameitranscriptome using the next-generation DNA sequencing technique.PLoSOne, 7(10): e47442.
Li E C, Wang S L, Li C, Wang X D, Chen K, Chen L Q. 2014a.Transcriptome sequencing revealed the genes and pathways involved in salinity stress of Chinese mitten crab,Eriocheirsinensis.PhysiologicalGenomics, 46(5):177-190.
Li F H, Xiang J H. 2013. Recent advances in researches on the innate immunity of shrimp in China.Developmental&ComparativeImmunology, 39(1-2): 11-26.
Li J T, Ma P, Liu P, Chen P, Li J. 2015. The roles of Na+/K+-ATPase α-subunit gene from the ridgetail white prawnExopalaemoncarinicaudain response to salinity stresses.Fish&Shellf ishImmunology, 42(2): 264-271.
Li S H, Zhang X J, Sun Z, Li F H, Xiang J H. 2013. Transcriptome analysis on Chinese shrimpFenneropenaeuschinensisduring WSSV acute infection.PLoSOne, 8(3): e58627.
Li Y Q, Jiang L X, Wang R J. 2014b. Layered farming forMarsupenaeusjaponicusBate.ChineseJournalof OceanologyandLimnology, 32(3): 549-553.
Lin Y C, Chen J C, Li C C, Morni W Z W, Suhaili A S N A,Kuo Y H, Chang Y H, Chen L L, Tsui W C, Chen Y Y,Huang C L. 2012. Modulation of the innate immune system in white shrimpLitopenaeusvannameifollowing long-term low salinity exposure.Fish&Shellf ish Immunology, 33(2): 324-331.
Lin Y C, Vaseeharan B, Chen J C. 2008. Identif ication and phylogenetic analysis on lipopolysaccharide and β-1,3-glucan binding protein (LGBP) of kuruma shrimpMarsupenaeusjaponicus.Developmental&Comparative Immunology, 32(11): 1260-1269.
Liu B, Yu Z M, Song X X, Guan Y Q, Jian X F, He J G. 2006.The eff ect of acute salinity change on white spot syndrome(WSS) outbreaks inFenneropenaeuschinensis.Aquaculture, 253(1-4): 163-170.
Liu J H, Zheng J J, Liu J Y. 2019. Genetic parameters for growth-related traits and survival with age in the Kuruma shrimpMarsupenaeusjaponicus.AquacultureResearch,50(1): 42-48.
Liu Y C, Li F H, Wang B, Dong B, Zhang X J, Xiang J H.2009. A serpin from Chinese shrimpFenneropenaeus chinensisis responsive to bacteria and WSSV challenge.Fish&Shellf ishImmunology, 26(3): 345-351.
Loongyai W, Avarre J C, Cerutti M, Lubzens E, Chotigeat W. 2007. Isolation and functional characterization of a new shrimp ovarian peritrophin with antimicrobial activity fromFenneropenaeusmerguiensis.Marine Biotechnology, 9(5): 624-637.
Lu W, Tang X L, Huo Y Q, Xu R, Qi S D, Huang J G, Zheng C C, Wu C A. 2012. Identif ication and characterization of fructose 1,6-bisphosphate aldolase genes in Arabidopsis reveal a gene family with diverse responses to abiotic stresses.Gene, 503(1): 65-74.
Lu X, Luan S, Hu L Y, Mao Y, Tao Y, Zhong S P, Kong J. 2016.High-resolution genetic linkage mapping, high-temperature tolerance and growth-related quantitative trait locus (QTL)identif ication inMarsupenaeusjaponicus.Molecular GeneticsandGenomics, 291(3): 1391-1405.
Lucu Č, Towle D W. 2003. Na++K+-ATPase in gills of aquatic crustacea.ComparativeBiochemistryandPhysiology PartA:Molecular&IntegrativePhysiology, 135(2): 195-214.
Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T,Pallasch C, Rheinheimer S, Meder B, Stähler C, Meese E,Keller A. 2016. Distribution of miRNA expression across human tissues.NucleicAcidsResearch, 44(8): 3865-3877.
Lv J J, Liu P, Wang Y, Gao B Q, Chen P, Li J. 2013. Transcriptome analysis ofPortunustrituberculatusin response to salinity stress provides insights into the molecular basis of osmoregulation.PLoSOne, 8(12): e82155.
Mao X, Cai T, Olyarchuk J G, Wei L. 2005. Automated genome annotation and pathway identif ication using the KEGG Orthology (KO) as a controlled vocabulary.Bioinformatics, 21(19): 3787-3793.
Marsden V S, O’Connor L, O’Reilly L A, Silke J, Metcalf D,Ekert P G, Huang D C S, Cecconi F, Kuida K, Tomaselli K J, Roy S, Nicholson D W, Vaux D L, Bouillet P, Adams J M, Strasser A.2002. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.Nature,419(6907): 634-637.
Masuda T, Otomo R, Kuyama H, Momoji K, Tonomoto M,Sakai S, Nishimura O, Sugawara T, Hirata T. 2012. A novel type of prophenoloxidase from the kuruma prawnMarsupenaeusjaponicuscontributes to the melanization of plasma in crustaceans.Fish&Shellf ishImmunology,32(1): 61-68.
Mcgraw W J, Davis D A, Teichert-Coddington D, Rouse D B.2002. Acclimation ofLitopenaeusvannameipost larvae to low salinity: inf luence of age, salinity endpoint, and rate of salinity reduction.JournaloftheWorldAquaculture Society, 33(1): 78-84.
Mcnamara J C, Faria S C. 2012. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: a review.JournalofComparativePhysiology B, 182(8): 997-1014.
Mcnamara J C, Freire C A, Torres A H Jr, Faria S C. 2015. The conquest of fresh water by the palaemonid shrimps: an evolutionary history scripted in the osmoregulatory epithelia of the gills and antennal glands.Biological JournaloftheLinneanSociety, 114(3): 673-688.
Meng X L, Dong Y W, Dong S L, Yu S S, Zhou X. 2011.Mortality of the sea cucumber,ApostichopusjaponicusSelenka, exposed to acute salinity decrease and related physiological responses: osmoregulation and heat shock protein expression.Aquaculture, 316(1-4): 88-92.
Opferman J T, Korsmeyer S J. 2003. Apoptosis in the development and maintenance of the immune system.NatureImmunology, 4(5): 410-415.
Park S J, Komata M, Inoue F, Yamada K, Nakai K, Ohsugi M,Shirahige K. 2013. Inferring the choreography of parental genomes during fertilization from ultralarge-scale wholetranscriptome analysis.Genes&Development, 27(24):2736-2748.
Paterson B D.1993. Respiration rate of the kuruma prawn,PenaeusjaponicusBate, is not increased by handling at low temperature (12°C).Aquaculture, 114(3-4): 229-235.
Péqueux A. 1995. Osmotic regulation in crustaceans.Journal ofCrustaceanBiology, 15(1): 1-60.
Powell D, Knibb W, Remilton C, Elizur A. 2015.De-novotranscriptome analysis of the banana shrimp(Fenneropenaeusmerguiensis) and identif ication of genes associated with reproduction and development.Marine Genomics, 22: 71-78.
Proespraiwong P, Tassanakajon A, Rimphanitchayakit V. 2010.Chitinases from the black tiger shrimpPenaeusmonodon:phylogenetics, expression and activities.Comparative BiochemistryandPhysiologyPartB:Biochemistryand MolecularBiology, 156(2): 86-96.
Rahi M L, Amin S, Mather P B, Hurwood D A. 2017. Candidate genes that have facilitated freshwater adaptation by palaemonid prawns in the genusMacrobrachium:identif ication and expression validation in a model species(M.koombooloomba).PeerJ, 5(2): e2977.
Rainbow P S, Black W H.2001. Eff ects of changes in salinity on the apparent water permeability of three crab species:Carcinusmaenas,EriocheirsinensisandNecorapuber.JournalofExperimentalMarineBiologyandEcology,264(1): 1-13.
Rajesh S, Kiruthika J, Ponniah A G, Shekhar M S.2012.Identif ication, cloning and expression analysis of Catechol-O-methyltransferase (COMT) gene from shrimp,Penaeusmonodonand its relevance to salinity stress.Fish&Shellf ishImmunology, 32(5): 693-699.
Rocha J, Garcia-Carreño F L, Muhlia-AlmazánA, Peregrino-Uriarte A B, Yépiz-Plascencia G, Córdova-Murueta J H.2012. Cuticular chitin synthase and chitinase mRNA of whiteleg shrimpLitopenaeusvannameiduring the molting cycle.Aquaculture, 330-333: 111-115.
Setiarto A, Strüssmann C A, Takashima F, Watanabe S, Yokota M. 2004. Short-term responses of adult kuruma shrimpMarsupenaeusjaponicus(Bate) to environmental salinity:osmotic regulation, oxygen consumption and ammonia excretion.AquacultureResearch, 35(7): 669-677.
Shekhar M S, Kiruthika J, Ponniah A G. 2013. Identif ication and expression analysis of diff erentially expressed genes from shrimp (Penaeusmonodon) in response to low salinity stress.Fish&Shellf ishImmunology, 35(6): 1957-1968.
Skou J C, Esmann M. 1992. The Na, k-ATPase.Journalof BioenergeticsandBiomembranes, 24(3): 249-261.
Skulachev V P. 1998. Cytochromecin the apoptotic and antioxidant cascades.FEBSLetters, 423(3): 275-280.
Sokolova I M, Frederich M, Bagwe R, Lannig G, Sukhotin A A. 2012. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates.MarineEnvironmentalResearch,79: 1-15.
Steller H, Grether M E. 1994. Programmed cell death in Drosophila.Neuron, 13(6): 1269-1274.
Suhail M. 2010. Na+, K+-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions.JournalofClinical MedicineResearch, 2(1): 1-17.
Tassanakajon A, Somboonwiwat K, Supungul P, Tang S. 2013.Discovery of immune molecules and their crucial functions in shrimp immunity.Fish&Shellf ish Immunology, 34(3): 954-967.
Tiu S H K, He J G, Chan S M. 2007. The LvCHH-ITP gene of the shrimp (Litopenaeusvannamei) produces a widely expressed putative ion transport peptide (LvITP) for osmo-regulation.Gene, 396(2): 226-235.
Towle D W, Kays W T. 1986. Basolateral localization of Na++K+-ATPase in gill epithelium of two osmoregulating crabs,CallinectessapidusandCarcinusmaenas.Journal ofExperimentalZoology, 239(3): 311-318.
Trapnell C, Williams B A, Pertea G, Mortazavi A, Kwan G,van Baren M J, Salzberg S L, Wold B J, Pachter L. 2010.Transcript assembly and quantif ication by RNA-Seq reveals unannotated transcripts and isoform switching during cell diff erentiation.NatureBiotechnology, 28(5):511-515.
Tsai J R, Lin H C. 2007. V-type H+-ATPase and Na+, K+-ATPase in the gills of 13 euryhaline crabs during salinity acclimation.JournalofExperimentalBiology, 210(4):620-627.
Tseng Y C, Hwang P P. 2008. Some insights into energy metabolism for osmoregulation in f ish.Comparative BiochemistryandPhysiologyPartC:Toxicology&Pharmacology, 148(4): 419-429.
Tsoi K H, Ma K Y, Wu T H, Fennessy S T, Chu K H, Chan T Y.2014. Verif ication of the cryptic speciesPenaeus pulchricaudatusin the commercially important kuruma shrimpP.japonicus(Decapoda: Penaeidae) using molecular taxonomy.InvertebrateSystematics, 28(5):476-490.
Via G J D. 1986. Salinity responses of the juvenile penaeid shrimpPenaeusjaponicus: I. Oxygen consumption and estimations of productivity.Aquaculture, 55(4): 297-306.
Wang L K, Feng Z X, Wang X, Wang X W, Zhang X G. 2010.DEGseq: an R package for identifying diff erentially expressed genes from RNA-seq data.Bioinformatics,26(1): 136-138.
Wang L U, Chen J C. 2005. The immune response of white shrimpLitopenaeusvannameiand its susceptibility toVibrioalginolyticusat diff erent salinity levels.Fish&Shellf ishImmunology, 18(4): 269-278.
Wang L Y, Li F H, Wang B, Xiang J H. 2013. A new shrimp peritrophin-like gene fromExopalaemoncarinicaudainvolved in white spot syndrome virus (WSSV) infection.Fish&Shellf ishImmunology, 35(3): 840-846.
Wang L, Wang W N, Liu Y, Cai D X, Li J Z, Wang A L. 2012.Two types of ATPases from the Pacif ic white shrimp,Litopenaeusvannameiin response to environmental stress.MolecularBiologyReports, 39(6): 6427-6438.
Wang P H, Huang T Z, Zhang X B, He J G. 2014a. Antiviral defense in shrimp: from innate immunity to viral infection.AntiviralResearch, 108: 129-141.
Wang W, Wu X G, Liu Z J, Zheng H J, Cheng Y X, Buratti E.2014b. Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crabPortunustrituberculatus: gene discovery in the comparative transcriptome of diff erent hepatopancreas stages.PLoSOne, 9(1): e84921.
Wang X D, Li E C, Qin J G, Wang S F, Chen X F, Cai Y, Chen K, Hou Y M, Yu N, Zhang M L, Du Z Y, Chen L Q. 2014c.Growth, body composition, and ammonia tolerance of juvenile white shrimpLitopenaeusvannameifed diets containing diff erent carbohydrate levels at low salinity.JournalofShellf ishResearch, 33(2): 511-517.
Watanabe T, Kono M, Aida K, Nagasawa H. 1998. Purif ication and molecular cloning of a chitinase expressed in the hepatopancreas of the penaeid prawnPenaeusjaponicus.BiochimicaetBiophysicaActa(BBA)-ProteinStructure andMolecularEnzymology, 1382(2): 181-185.
Weihrauch D, Ziegler A, Siebers D, Towle D W. 2001.Molecular characterization of V-type H+-ATPase(B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake.Journal ofExperimentalBiology, 204: 25-37.
Weng X, Song S X, He J G, Li S D, Li Y J, Wang P. 2012. New technology of effi cient ecological culture ofMarsupenaeus japonicus. p.14. (in Chinese)
Wetsaphan N, Rimphanitchayakit V, Tassanakajon A,Somboonwiwat K. 2013. PmSERPIN3 from black tiger shrimpPenaeusmonodonis capable of controlling the proPO system.Developmental&Comparative Immunology, 41(2): 110-119.
Xu C, Li E C, Liu Y, Wang X D, Qin J G, Chen L Q. 2017.Comparative proteome analysis of the hepatopancreas from the Pacif ic white shrimpLitopenaeusvannameiunder long-term low salinity stress.JournalofProteomics,162: 1-10.
Ye L, Jiang S G, Zhu X M, Yang Q B, Wen W G, Wu K C.2009. Eff ects of salinity on growth and energy budget of juvenilePenaeusmonodon.Aquaculture, 290(1-2): 140-144.
Young M D, Wakef ield M J, Smyth G K, Oshlack A. 2010.Gene ontology analysis for RNA-seq: accounting for selection bias.GenomeBiology, 11(2): R14.
Yu Z M, Li C W, Guan Y Q. 2003. Eff ect of salinity on the immune responses and outbreak of white spot syndrome in the shrimpMarsupenaeusjaponicus.Ophelia, 57(2):99-106.
Zhang D, Wang F, Dong S L, Lu Y L. 2016.Denovoassembly and transcriptome analysis of osmoregulation inLitopenaeusvannameiunder three cultivated conditions with diff erent salinities.Gene, 578(2): 185-193.
Zhang S Y, Jiang S F, Xiong Y W, Fu H T, Sun S M, Qiao H,Zhang W Y, Jiang F W, Jin S B, Gong Y S. 2014. Six chitinases from oriental river prawnMacrobrachium nipponense: cDNA characterization, classif ication and mRNA expression during post-embryonic development and moulting cycle.ComparativeBiochemistryand PhysiologyPartB:BiochemistryandMolecularBiology,167: 30-40.
Zhao Q, Pan L Q, Ren Q, Hu D X. 2015. Digital gene expression analysis in hemocytes of the white shrimpLitopenaeusvannameiin response to low salinity stress.Fish&Shellf ishImmunology, 42(2): 400-407.
Zhao X L, Yu H, Kong L F, Li Q.2012. Transcriptomic responses to salinity stress in the Pacif ic oysterCrassostreagigas.PLoSOne, 7(9): e46244.
Zou E M, Bonvillain R. 2004. Chitinase activity in the epidermis of the f iddler crab,Ucapugilator, as an in vivo screen for molt-interfering xenobiotics.Comparative BiochemistryandPhysiologyPartC:Toxicology&Pharmacology, 139(4): 225-230.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
