Dinophysoid dinof lagellates from subphotic depths:Amphisolenia sp. aff . brevicauda, Dinofurcula tricornuta sp. nov., and Dinophysis profunda sp. nov. (Dinophysales,Dinophyceae)*
Fernando GÓMEZ
Department of Biological and Environmental Sciences and Technologies, University of Salento, Lecce 73100, Italy
Abstract Three species of dinophysoid planktonic dinof lagellates are described from the eastern Mediterranean Sea. Amphisolenia sp. aff . brevicauda was collected at 70-m depth in the Ionian Sea. This small species showed a more asymmetric midbody and a proportionally longer neck when compared to the closer relative A. brevicauda. Three individuals of Dinofurcula tricornuta sp. nov. collected at 154-m depth in the Marmara Sea are characterized by three posterior-oriented processes when compared to its congeneric species. This is the f irst record of the genus Dinofurcula beyond the eastern tropical Pacif ic Ocean. Dinophysis profunda sp. nov. collected at 500-m depth in the Ionian Sea is characterized by ovate hypotheca and a serrate crest-like left sulcal list when compared to its close relative D. alata. These f indings evidence an undescribed biodiversity in the under-sampled subphotic depths.
Keyword: deep ocean; Dinophyta; Dinof lagellata; Mediterranean Sea; mesopelagic; new species
1 INTRODUCTION
The microbial world represents the last truly unexplored frontier in the diversity of life on Earth.Microbial-community amplicon-based sequence abundance distributions have a long ‘tail’ of lowabundance organisms, referred to as the rare biosphere,which often comprises the large majority of species(Sogin et al., 2006). Microscopy-based plankton studies are focused in coastal waters and the surface of the ocean, while the sampling eff ort associated with subphotic depths is much lower. At the beginning of the 19thcentury, Kofoid described numerous species of dinophysoid dinof lagellates from the eastern tropical Pacif ic Ocean based on plankton net vertical hauls from 1 500-m depth to the surface (Kofoid,1907; Kofoid and Skogsberg, 1928). Kofoid described nearly all the known species ofTriposoleniaKof. and several species ofAmphisoleniaF. Stein, whose records in many cases remain restricted to the original descriptions. Kofoid also described the two species of genusDinofurculaKof. & Skogsb.; records that remain restricted to the eastern tropical Pacif ic Ocean.While dinof lagellate studies in the Mediterranean Sea,where about 1/3 of the described species has been reported (Gómez, 2003, 2012) have largely been focused on coastal and surface waters, sporadic samplings in mesopelagic zone (200-1 000 m-depth)revealed the presence of species of dinophysoid genera such asAmphisolenia, and especiallyTriposolenia(Gómez et al., 2011; Dolan et al., 2019). In this study,several samples were analysed from subphotic depths of the Ionian and Marmara Seas. The aim was the isolation of individuals to obtain molecular data following the method described in Gómez et al. (2011,2012). However, this study reports on three species that did not yield any molecular data, but whose distinctive morphologies deserve attention.
2 MATERIAL AND METHOD
The concentration of large volume of seawater is required for the observations of plankton at subphotic depths due to their very low abundances. Net sampling with vertical hauls inf licts mechanical damage to the individuals due to the drag from deep water, and even with closing plankton nets, there is the potential for contamination of the sample with individuals from upper depths. The use of oceanographic bottles (i.e.Niskin) allows sampling at discrete depths without contamination and reduces damage, but the sample volume is smaller. In this study, samples were collected with Niskin bottles during the research cruise MARM10_02 onboard R/VUraniain October 2010 (http://ricerca.ismar.cnr.it/CRUISE_REPORTS/2010-2019/MARM10_02_REP/MARM10_02_REP/node5.html). A 12-L sample was collected at 500-m depth (bottom at 3 226-m depth) in the Ionian Sea,site KM3 (36°28′58.3″N, 15°39′00.3″E) on October 1, 2010. Another 18-L sample was collected at 70-m depth (bottom at 3 633-m depth) in the Ionian Sea(36°36′28.8″N, 17°58′15.6″E) on October 2, 2010.On October 5, 2010, a third sample of 120 L was collected at 154-m depth (bottom at 161-m depth) in the Marmara Sea, site SN4 (40°43′43.3″N,29°23′11.9″E). Seawater from the Niskin bottles was gently poured through 200-μm pore size Nylon mesh to remove macrozooplankton, and then through 20-μm mesh to concentrate the sample. The material retained on the 20-μm mesh was washed and resuspended with 0.22-μm f iltered seawater. The plankton concentrate was divided into a sample preserved with Lugol’s solution at f inal concentration of 2% (volume/volume), and another sample f ixed with absolute ethanol at f inal concentration of 80%(volume/volume). Samples were kept in darkness and at a temperature of 4 °C until analyses. Lugol’s solution preserves the cell morphology, including most of the unarmoured forms, but PCR effi ciency is reduced compared to ethanol-f ixation. The ethanol lysed or distorted the unarmoured forms and discoloured the cells, and is sometimes associated with the formation of a mucilage or precipitation of salts that makes microscopy observation diffi cult.Lugol’s preserved samples were treated with small amounts (150-200 μL) of 10% (weight/volume)sodium thiosulfate to remove iodine. The Lugol’s samples were examined f irst, and the ethanol-f ixed samples were assessed in order to further identify individuals. Subsamples were examined in Utermöhl chambers with an inverted microscope (Eclipse TE2000-S, Nikon, Tokyo, Japan) and photographed with digital camera (Nikon DS-2M). Micrographs ofAmphisoleniabrevicaudaKof. are included for a comparison with theAmphisoleniaspecies. These individuals were collected in the surface waters of the Gulf of Lions at Marseilles and Banyuls-sur-Mer,France, following the method described in Gómez et al. (2011).
3 RESULT
3.1 Amphisolenia sp. aff . brevicauda
A single individual of a small amphisolenoid cell with a slight sigmoidal curvature of the midbody and antapical process was observed in the Ionian Sea at 70-m depth (36°36′28.8″N, 17°58′15.6″E). The total cell length was 133 μm, midbody of 50 μm, neck of 45 μm, and antapical process of 30 μm. The epitheca was tiny and dome-shaped, slightly inclined anteriorly.The cingulum was about ~7 μm in diameter. The cingular lists were 1.5 times as wide as the cingulum.The neck was straight, about 2.5 μm in diameter, with a triangular elongated left lateral sulcal list without ribs. The midbody was slightly laterally f lattened [12-μm depth (dorso-ventral distance) and 8-μm wide(transdiameter)], widened posteriorly, slightly convex in the dorsal side with a hump in the posterior ventral side, and an anterior ventral small hump associated with the f lagellar pore. The midbody tapered posteriorly to an antapical process (3 μm in diameter),unbranched, with an acute end devoid of spinules, and slightly oriented towards the ventral side. In lateral view, the outline of the midbody and antapical process was slightly sigmoidal. The theca was apparently structureless (Fig.1a-b).
The close relative,Amphisoleniabrevicauda, is shown in Fig.1e-h for comparison.Amphisoleniasp.aff .brevicaudawas 133-μm long, whileA.brevicaudawas 200-210-μm long. The epitheca ofAmphisoleniasp. aff .brevicaudawas dome-shaped and slightly protruded over the upper cingular list (Fig.1a-d),while the epitheca ofA.brevicaudawas f lat, and it did not protrude over the upper cingular list (Fig.1e-h).The cingulum ofA.brevicaudawas 16 μm in diameter,while 7 μm inAmphisoleniasp. aff .brevicauda. The neckAmphisoleniasp. aff .brevicaudawas straight and the length was almost similar to that of the midbody, while the neck ofA.brevicaudawas slightly dorsally def lected and one half of the midbody length.The ventral margin of the midbody ofA.brevicaudawas almost straight (Fig.1e-h), while more asymmetric with a dorsal hump inAmphisoleniasp.aff .brevicauda(Fig.1a-d). The midbody ofA.brevicaudagradually merged into the antapical process, while the antapical process ofAmphisoleniasp. aff .brevicaudawas well set off from the midbody.The individuals ofA.brevicaudawere collected in surface waters, and they harboured endosymbiont microalgae (Fig.1e-h). There was no evidence for the presence of endosymbiotic microalgae inAmphisoleniasp. aff .brevicaudadespite the fact that the collection depth of 70 m corresponded to the deep chlorophyll maximum. Nothing is known about the life cycle of the species ofAmphisolenia, so the potential thatAmphisoleniasp. aff .brevicauda(Fig.2a) is a part of the life cycle ofA.brevicauda(Fig.2b-d) cannot be discarded.
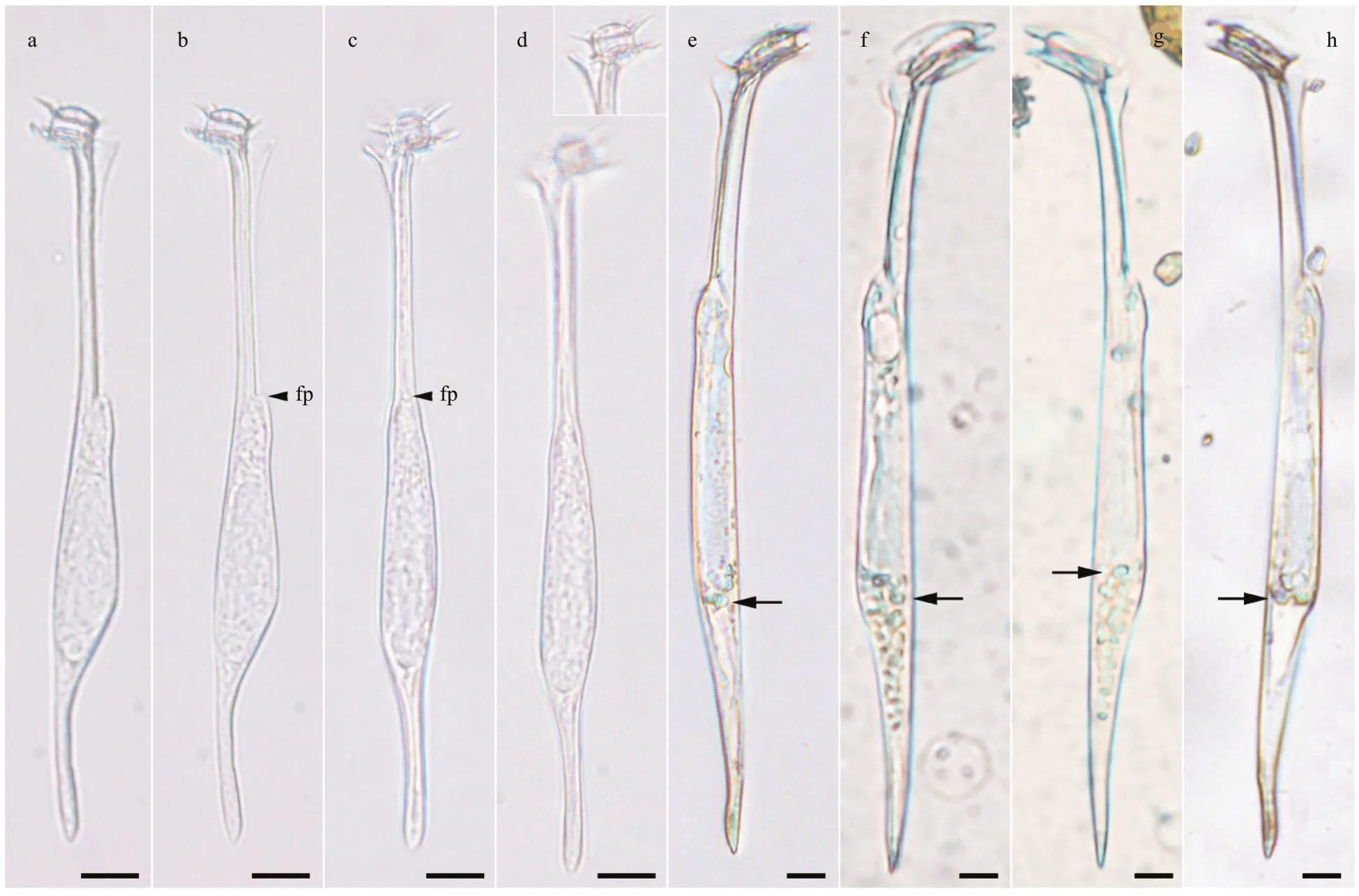
Fig.1 Light micrographs of Amphisolenia spp.
3.2 Dinophysis profunda F. Gómez sp. nov.(Figs.3a-h, 4a-b)
Diagnosis: A heterotrophic dinophysoid cell with ovoid hypotheca, narrower towards the antapex. The length of the cell body was 46-μm long, and it possessed a prominent antapical spine of 23-μm long.The greatest depth (dorso-ventral distance) was 27 μm. The epitheca was low, 18-μm depth and the cingulum was wide and excavated. The left sulcal list showed a serrate crest-like margin with a long third rib as a prominent spine. A scarcely developed sail emerged from the dorsal margin of the lower half of the hypotheca, with several posterior ribs. Theca with poroids.
Holotype: Fig.3e.
Iconotype: Fig.4a.
Isotype: Fig.3a-d, f-h.
Type locality: Ionian Sea (36°28′58.3″N,15°39′00.3″E) at 500-m depth.
Etymology:profundus, Latin from prō+fundus(“bottom”), meaning deep, profound. The individual was collected at 500-m depth.
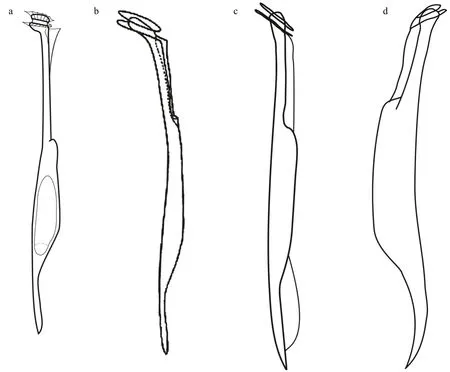
Fig.2 Line drawings of Amphisolenia spp.
Description: The most distinctive features of this dinophysoid cell are the cell shape, the prominent antapical spine, the dorsal sail, and especially the morphology of the left sulcal list (Fig.3). The straight antapical spine emerged from the ventral side of the antapex and extended slightly oriented towards the ventral side. The posterior half of the dorsal hypotheca showed a scarcely developed sail with a smooth margin and several ribs in the most posterior half(Fig.3a-b). The sulcal list and the antapical spine were placed in distinct focal planes, and the left sulcal list was a serrate crest with f ive peaks located between the second and third rib (Fig.3d-f). The f irst sulcal rib was short, partially hidden by the lower cingular list.The second rib was conspicuous and anteriorly curved. The third rib was a long spine of 15 μm that protruded over the sulcal list with an angle of 45°with respect to the longitudinal axis of the cell. The proximal half of the left sulcal list was reticulated,while the distal half was smooth (Fig.3d-f). The theca showed poroids (Fig.3e & h).
Closely related toD.profundasp. nov. (Fig.4a-b)areDinophysisalataJørg. (Fig.4c) andD.balechiiD. R. Norris & L. D. Berner (Fig.4d). The cingulum ofD.profundasp. nov. was wider than inD.alataandD.balechii. The shapes of the hypothecae ofD.alataandD.balechiiwere ellipsoidal and rotund,respectively, while the hypotheca of the new species was ovoid. The anterior cingular list ofD.balechiiwas narrow, while wider inD.profundasp. nov. The margin of the left sulcal list ofD.alataandD.balechiiwas smooth, while serrate in the new species. When compared to the other two species, the third rib ofD.profundasp. nov. largely protruded over the left sulcal list like an spine. The f irst rib of the left sulcal list ofD.alataandD.balechiiwas visible, while inconspicuous in the new species.Dinophysisalatais characterized by a large dorsal sail that extended from the cingulum to the antapex (Fig.4c), while absent inD.balechii(Fig.4d). The small dorsal sail ofD.profundasp. nov. extended along the posterior half of the hypotheca. The antapical spine of the new species was longer than in the two other species.Dinophysisprofundasp. nov. is described from mesopelagic depths, whileDinophysisalataandD.balechiiare only known from the euphotic zone.
3.3 Dinofurcula tricornuta F. Gómez sp. nov.(Figs.5a-p, 6a-b)
Diagnosis: Phalacromoid heterotrophic cell,bilaterally compressed of 53-57 μm in length. The epitheca was low and it did not protrude over the upper cingular list. The cingulum was wide. The hypotheca occupied most of the cell body and was characterized by three posterior-oriented process with round ends. The longer process emerged from the middle of the margin of the ventral hypotheca, and the two shorter processes emerged like a bifurcated antapex. The more dorsal antapical process was longer and oriented towards the right side, while the other one was shorter and oriented towards the left side.
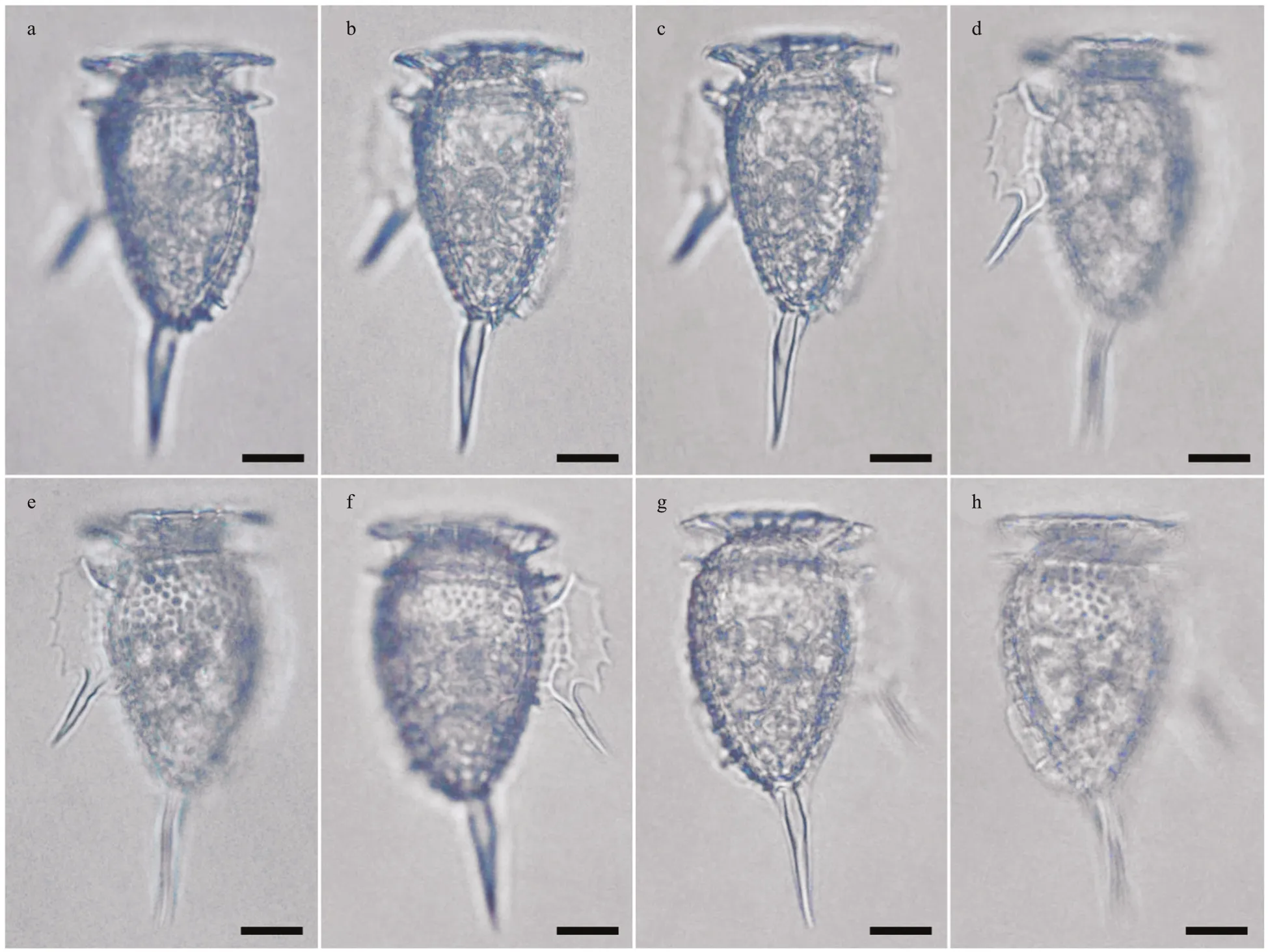
Fig.3 Light micrographs of Dinophysis profunda sp. nov. from the Ionian Sea
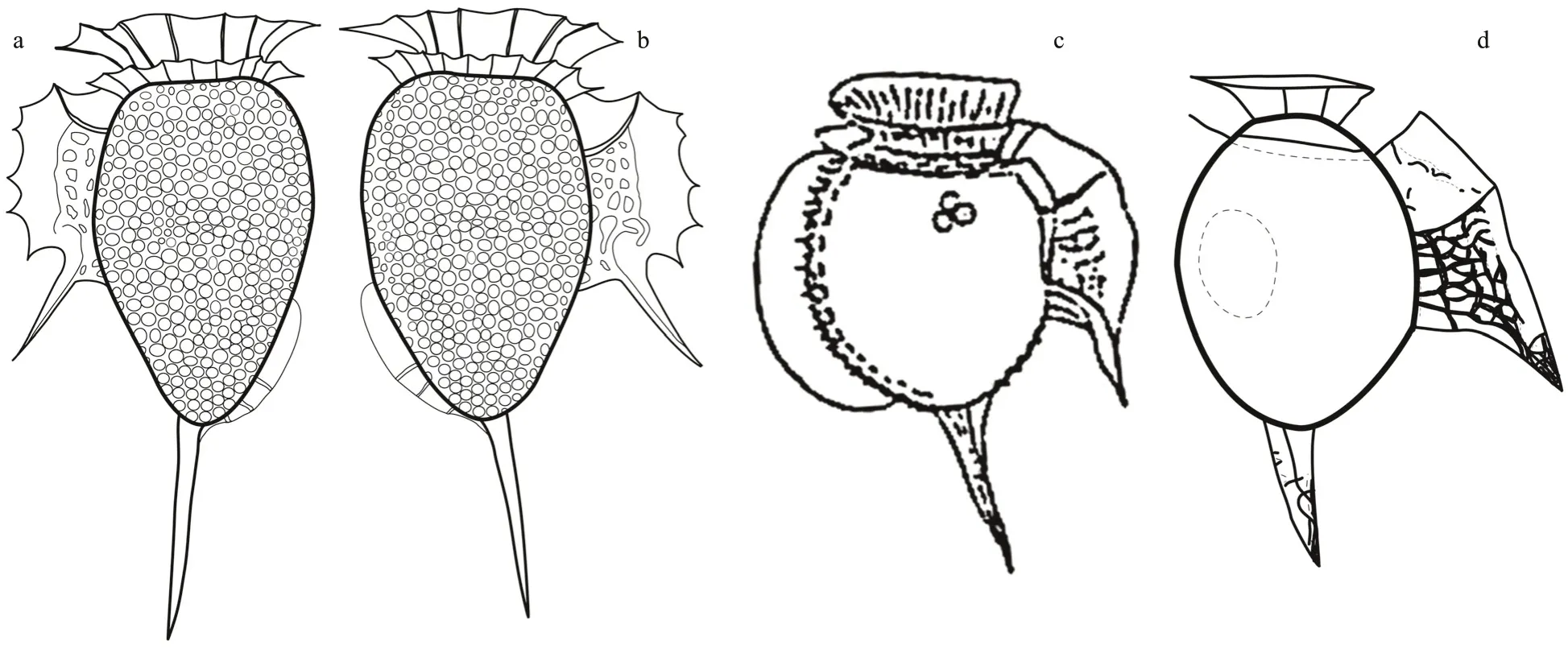
Fig.4 Line drawings of Dinophysis spp.
Holotype: Fig.5c.
Iconotype: Fig.6a.
Isotype: Fig.5a-b, d-h.
Type locality: Marmara Sea (40°43′43.3″N,29°23′11.9″E) at 154-m depth.
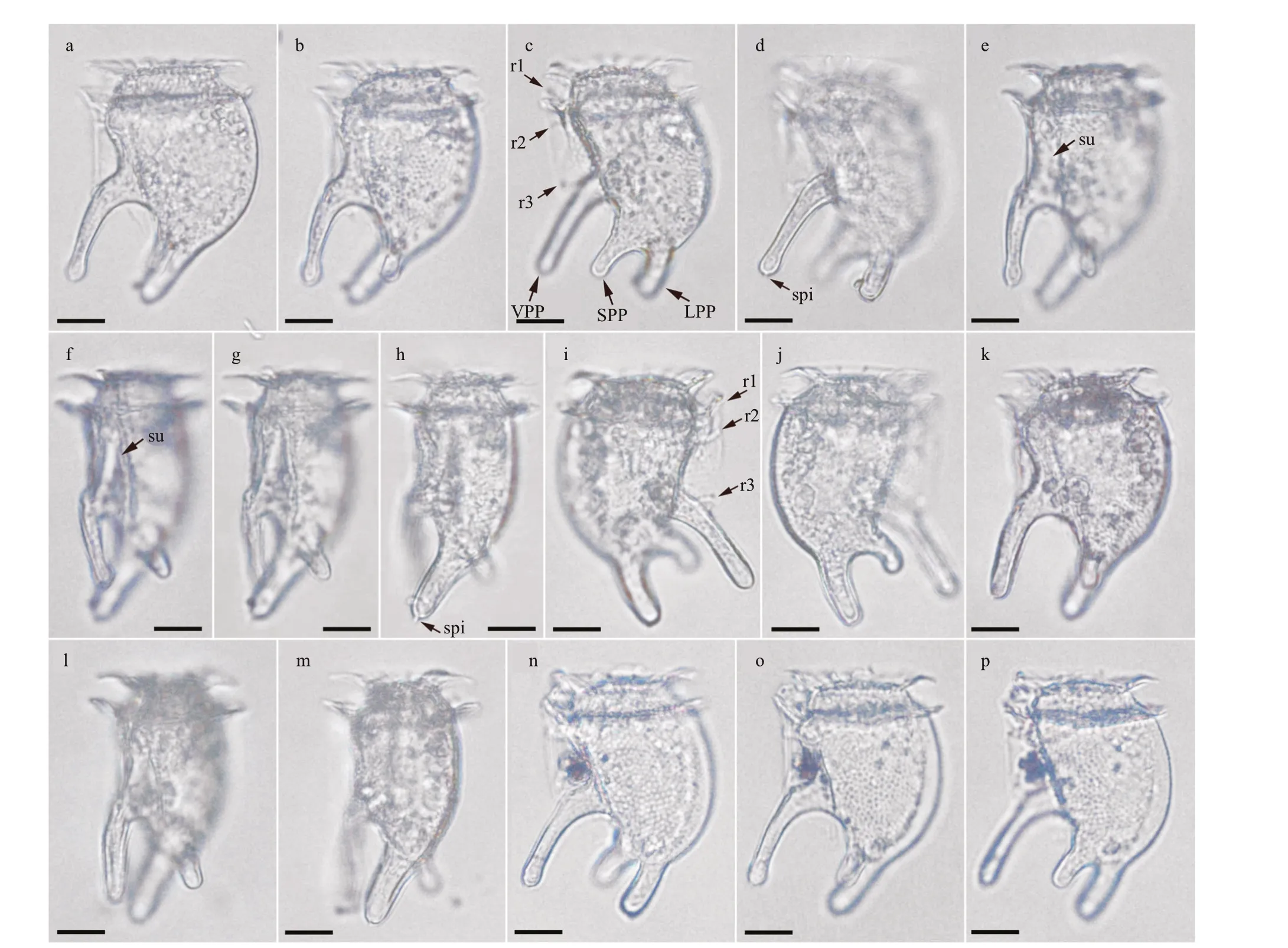
Fig.5 Light micrographs of three individuals of Dinofurcula tricornuta sp. nov. from the Marmara Sea
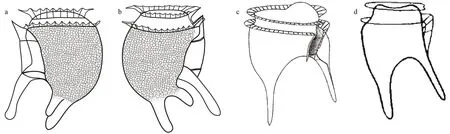
Fig.6 Line drawings of Dinofurcula spp.
Etymology:tri-, derived from both Latin and Greek roots, means three. Latin adjectivecornūtum,from cornū (“horn”), means horned, having horns.
Morphology: Three individuals were examined.They were similar in size and shape, with slight diff erences in the thickness and orientation of the ventro-posterior process, and the presence of corpuscles in the hyposome, probably food vacuoles(Fig.5). The length of the cells, including the antapical process ranged from 53-57 μm. The depth (dorsoventral distance) varied due to the irregular outline of the ventral margin of the hypotheca. The maximum depth excluding the ventral process was 30 μm. The epitheca was very low, dome-shaped, 17-20 μm in lateral view, and it did not protrude over the anterior cingular list. The cingulum was wide and excavated(5-μm wide), with a list at both the anterior and posterior edges and ribs almost perpendicular to the cell body. The left sulcal list was poorly developed,smooth with the margin parallel to the longitudinal axis of the cell. The f irst sulcal rib was straight and oriented anteriorly. The second rib was short and closer to the f irst rib than to the third rib. The third rib was straight and perpendicular to the hypotheca and emerged at the basis of the ventro-posterior process(Fig.5a, c, & i). The dorsal margin of the hypotheca was convex being wider at the middle. The ventral margin between the cingulum and the ventro-posterior process was sigmoidal, concave at the upper basis of the process. There were three posterior-oriented processes in the hypotheca. The longest process(25-μm long, named ventro-posterior process) was ventral and located in the right valve of the hypotheca,and it was slightly oriented towards the left side. This ventro-posterior process emerged with an angle of~45° with respect to the longitudinal axis of the cell,and decreased to ~20°. The distal end was rotund or spatulate depending on the individual, and it showed,at least, a spinule (Fig.5d). The other two posterior processes were shorter and located in the left hypotheca (Fig.5j & p). They can be interpreted as result of the bifurcation of the antapex. One process was shorter (named short posterior process), 10-11-μm long, emerged from a more ventral position and was oriented towards the left side of the cell (Fig.5f-h).The other process was longer (15-μm long, named long posterior process), located in the antapex and it was oriented towards the left side with more angle than the ventro-posterior process. The distal end of the long posterior process showed, at least, a spinule(Fig.5h). The cell theca was covered by poroids,apparently absent from the posterior processes(Fig.5b-c). The inner hypotheca showed corpuscles in distinct number and size, suggesting a heterotrophic nutrition (Fig.5k).
Currently, the genusDinofurculacontains two species:D.ultima(Kof.) Kof. & Skogsb. (Fig.6c) andD.ventralisKof. & Skogsb. (Fig.6d). The former showed a large, rounded hump on the ventral side of the epitheca and the sulcus in a lateral position(Fig.6c). These species possessed two processes, one is ventro-posterior and other one is antapical. In contrast,D.tricornutashowed two antapical process(Fig.6a-b). The records ofD.ultimaandD.ventralisare restricted to the eastern tropical Pacif ic. This is the f irst record of the genusDinofurculain another ocean region.
4 DISCUSSION
More than one century ago, plankton observations from open ocean research cruises were associated with the descriptions of new dinof lagellate species.For example, Kofoid and collaborators described more than 300 species of thecate dinof lagellates from several cruises in the eastern tropical Pacif ic Ocean(Kofoid, 1907; Kofoid and Michener, 1911; Kofoid and Skogsberg, 1928). In contrast, few new species of dinof lagellates have been described from open ocean research cruises in the last two decades (Gómez,2007; Hernández-Becerril et al., 2008; Parra-Toriz et al., 2014; Esqueda-Lara and Hernández-Becerril,2017). This should not be interpreted as all the existing dinof lagellates already having been described. In the last two decades, there has been an increase in the description of new species of neritic photosynthetic dinof lagellates collected near specialized laboratories and cultivated in standard culture media. The abundant material from the cultures allows detailed studies of the morphology and molecular phylogeny, but most of these new species lack distinctive features and cannot be easily recognized in routine plankton microscopy observations. In contrast, oceanic species inhabiting in the aphotic zone are heterotrophic, with unknown nutritional and environmental requirements,and are uncultivable with standard protocols. Due to low abundances, observations are often restricted to single or few individuals, making detailed morphological and molecular studies extremely diffi cult. Despite their distinctive morphologies, these species suff er a disadvantage compared to abundant and/or cultured organisms with respect to the process of new species descriptions. The consequence is that our knowledge of the biodiversity of dinof lagellates below the euphotic zone remains largely restricted to the observations by Kofoid and collaborators (Kofoid,1907; Kofoid and Michener, 1911; Kofoid and Skogsberg, 1928).
Dinof lagellates typical of subphotic depths are the dinophysoid generaTriposoleniaandDinofurcula,with highly distinctive generic diagnostic characters and easily recognizable in routine plankton observations. The records of most of the species ofTriposoleniaare Kofoid’s original descriptions, and there are only two records ofDinofurculaspecies after Kofoid. In this study, three distinctive species of dinophysoid dinof algellates are described after the observation of plankton concentrates from large volumes of seawater. This suggests a reservoir of undocumented biodiversity that remains in subphotic depths.
The dinophysoid dinof lagellates have received considerable attention in neritic waters because several chloroplast-containing species of the genusDinophysisEhrenb. are responsible of toxic events.Dinophysisprofundasp. nov. belongs to theD.hastata-group that contains heterotrophic species with an antapical spine, often with a high developed left sulcal list (Gómez et al., 2011; Esqueda-Lara et al., 2013). These species inhabit in the oligotrophic open ocean. The closest morphologic relatives ofDinophysisprofundasp. nov. areD.alata(Fig.4c;Jørgensen, 1923) andD.balechii(Fig.4d; Norris and Berner, 1970). The description ofDinophysis profundacollected at 500-m depth is the deepest recorded in the last century.
The records of the genusDinofurculawere restricted to the eastern tropical Pacif ic, especially the productive Peruvian Current (Kofoid and Skogsberg,1928). More recent records ofDinofurculafrom surface samples are associated with the upwelling of deep waters (Hernández-Becerril and Bravo-Sierra,2004; Ochoa and Baylón, 2005). Hernández-Becerril and Bravo-Sierra (2004) found several individuals in a single sample from a eutrophic site associated with deep water upwelling. In this study, three individuals were collected in a single sample collected at 154-m depth, near the bottom, in the eutrophic waters of the Marmara Sea. This suggests that the species ofDinofurculamay reach a relatively important local abundance below eutrophic waters. Taxonomic studies of dinof lagellates in the Marmara Sea are frequent, but mostly restricted to surface waters(Balkis, 2000).
The new species ofDinofurculashowed three posterior-oriented processes, while the other species of this genus have only two processes. The “tripartite”outline ofD.tricornutasp. nov. converges withTriposolenia, but the generaMetaphalacromaL. S. Tai and Skogsb. andPseudophalacromaJørg.are characterized by a crest-like structure in the epitheca. Scanning electron microscope observations ofDinofurculacf.ultimarevealed the presence of a crest on the epitheca, already cited in the original species description (Hernández-Becerril and Bravo-Sierra, 2004). This suggests thatDinofurculacould be related with the clade ofPseudophalacroma/Metaphalacromarather than to the clade ofPhalacromaF. Stein within the main clade of the Dinophysales. Species ofPseudophalacromaorMetaphalacromado not have processes or body extensions; however, the presence of processes are not always associated with genetic diff erences. For example, the speciesDinophysis caudataKent,D.triposGourret andD.milesCleve are characterized by distinct degrees of development of a dorsal process, while these species share identical SSU rRNA gene sequences.
The records ofAmphisoleniaare more common than those ofTriposoleniaandDinofurcula.Amphisoleniashows a large vertical distribution, with species containing endosymbiotic microalgae.Amphisoleniasp. aff .brevicaudawas collected at 70-m depth, but this single record does not allow comment on potential trends regarding the vertical distribution. The closely related speciesA.brevicaudais found in surface waters (Figs.1e-h & 2b-d; Wood 1963a, b). The most common species ofAmphisolenia(i.e.,A.bidentataSchröd.) are strongly anteroposteriorly elongated. These species have little diff erentiation between the outlines of midbody, neck and antapical process, but the end of the antapical process showed a distinct angle, named the foot, and the end had distal spinules or other ornamentations(Kofoid and Skogsberg, 1928). In contrast,Amphisoleniasp. aff .brevicaudabelongs to a less common group of species with a clearly diff erentiated midbody, and a short antapical process with a simple morphology, lacking bifurcations or spines. The thicker midbody ofA.brevicauda,A.inf lata, andAmphisoleniasp. aff .brevicaudasuggest that these species are basal in the clade ofAmphisoleniabecause they are closer to the morphology ofTriposolenia.Unfortunately, molecular data are not available to test that hypothesis.
5 CONCLUSION
The subphotic depths are the unexplored frontier in the diversity of life on Earth. Despite the low volume of sample, this study suggests a rich undocumented biodiversity. There are environmental sequencing surveys in subphotic depths, but they are rarely accompanied with microscope observations by qualif ied observers. The dinof lagellates inhabiting subphotic depths are at low abundance, but these species are not necessarily rare. What is rare is the opportunity to collect and examine samples where these species are located.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are included in this published article.
7 ACKNOWLEDGMENT
I thank P. López-García for sampling, and L.Gasperini and G. Bortoluzzi of the Istituto di Geologia Marina (ISMAR), CNR, Bolonia (Italy) for allowing to participate in the MARM10_02 R/VUraniacruise.I thank R. J. Gast for her improvements in the text.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
