Screening and characterization of proteases produced by deep-sea cold seep bacteria*
Chenchen GUO , Chaomin SUN , Shimei WU ,**
1 College of Life Sciences, Qingdao University, Qingdao 266071, China
2 CAS Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071,China
Abstract Fifty protease-producing strains were screened from sediment of deep-sea cold seep, and divided into four diff erent categories: Bacillus, Pseudoalteromonas, Vibrio, and Alteromonas according to the sequences of 16s rRNA. Their abilities to produce protease, amylase, and lipase were determined,and a Bacillus strain gcc-1 displayed very strong alkaline protease activity and stability under diff erent thermal and acidic conditions. The purif ication of the protease produced by strain gcc-1 was carried out by precipitation with ammonium sulfate, and sequentially chromatographed by anion exchange column and gel f iltration. The purif ied protease showed a single band at the molecular weight of 28 kDa by SDS-PAGE.The characterization results show that the purif ied protease exhibited a considerable activity and stability in a wide thermal range of 10-80 ℃ and a wide acidic range of pH 6.5-11.5, and displayed highest activity at 40 °C and pH 8.5. Notably, the protease still maintained high activity even at low to 10 ℃. Furthermore,the protease exhibited good stability in presence of diff erent surfactants, organic solvents, oxidizing agent H 2 O 2, and commercial detergents. Therefore, the protease produced by gcc-1 is a cold active and high stable enzyme, and has a promising potential in laundry detergent as an additive.
Keyword: deep-sea; Bacillus; alkaline protease; purif ication; characterization
1 INTRODUCTION
Proteases dominate the enzyme manufacturing commerce in the washing industry applications and leather processing (Fathi et al., 2005). Proteases have contributed around 60% of the total enzyme market around the world (Ali et al., 2006; Joshi and Satyanarayana, 2013a). Alkaline proteases are of particular interest due to large-scale use in a great many industrial applications such as laundry detergents, fur processes, food additives, household organic, diagnostic reagents, and waste management processes (Dube et al., 2000; Ichida et al., 2001; Lee et al., 2009; Del Rosso, 2013; Niyonzima and More,2015b).
A considerable part of alkaline proteases is used as detergent additives, which account for two-thirds of the total market of alkaline protease (Haki and Rakshit, 2003). As for being applicable additives of laundry detergent, proteases need to exhibit a high activity and stability under a wide range of pH,temperature, as well as the condition of surfactants,chelating agents, oxidizing and bleaching agents(Oberoi et al., 2001; Ogino and Ishikawa, 2001; Jain et al., 2012). Since the f irst alkaline protease fromBacilluslicheniformiswas used as an additive in laundry detergent in 1960s, many commercialized proteases were tested, but seldom alkaline proteases exhibited high activity at low temperature (Joshi and Satyanarayana, 2013b; Niyonzima and More, 2015a).Therefore, alkaline proteases with superior performance for commercial exploitation, especially for detergents, are still under exploration.
Marine occupies more than 70% of the Earth’s surface, and marine environment is a unique habitat,especially the deep-sea cold seep, which endows marine microorganisms with unique metabolic and physiological capabilities to adapt to the lowtemperature environment (Ting et al., 2010; Wu et al.,2015a). Various cold-adapted enzymes were isolated from marine microorganisms, and exhibited promising potential applications in diff erent aspects (Marx et al.,2007; Martin et al., 2014; Hassan et al., 2018). In this study, to obtain cold active protease with excellent performance as detergent additive, the proteaseproducing strains isolated from the deep-sea cold seep were screened, and their abilities to produce amylase and lipase were detected. Among them, a strain designated as gcc-1 exhibited the highest protease activity and stability even at low temperature,which indicates that the isolated strain gcc-1 has a promising potential as detergent additive. In order to provide basis for its future application, purif ication and characterization of the protease produced by gcc-1 was further carried out.
2 MATERIAL AND METHOD
2.1 Isolation and cultivation of protease producing strain
Microorganisms were f irstly isolated from the sediment of deep-sea cold seep of in South China Sea on 2216E medium (5-g/L tryptone, 1-g/L yeast extract and seawater), then their abilities to produce proteases were screened by inoculating the isolated strains on plates containing 2216E medium supplemented with 10- g/L casein. The plates were incubated at 28 ℃ for 48 h, and strains, which produced a clear zone on the plate, were evaluated as protease-producing strain.The isolated strains were streaked three times on the same medium to ensure their purity.
2.2 Detection of other hydrolytic enzymes of screened strains
The isolated protease-producing strains were further screened for their ability to produce amylase and lipase. Amylase activity was screened on plates containing 2216E medium supplemented with 1%(w/v) starch and 1% (v/v) Lugol’s iodine solution(0.05-mg/mL iodine, 0.01-mg/mL potassium iodide).The lipase activity was screened on plates containing 2216E medium supplemented with 1% (w/v) Tween 80 in 2216E. After 3 days of incubation at 28 °C, clear zones were detected around the positive strains directly.
2.3 DNA extraction and amplif ication of 16S rRNA
Bacterial genomic DNA was extracted from pure culture and amplif ied by PCR to analyze the 16S rRNA (Mamiatis et al., 1985). The primers to amplify 16S rRNA were 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). PCR was performed using a 20-μL reaction mixture as following (per reaction): 10-μL 2X Rapid Taq Master Mix (Vazyme, China), 9 μL of sterilized water, 0.5 μL of each primer (10 mmol/L), and 1 μL of template DNA. The following PCR conditions were used: 95 °C for 10 min followed by 30 cycles of 95 °C for 15 s, 53 °C for 15 s, and 72 °C for 15 s, and f inally an extension step of 3 min at 72 °C.
2.4 Nucleotide sequence accession numbers and phylogenetic analysis
The 16S rRNA sequences of protease-producing strains were submitted to GenBank under the accession number from MT815472 to MT815493,MT815495 to MT815521, and MW674640. The 16S rRNA sequences were further compared with sequences available in NCBI database by Nucleotide BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/, NCBI, Bethesda, MD). Phylogenetic tree was constructed based on neighbor-joining method by using MEGA 6.0 software (Tamura et al., 2013).
2.5 Protease purif ication and SDS-PAGE
Strain gcc-1 was cultured in Luria-Bertani (LB)medium (10-g/L tryptone, 5-g/L yeast extract, and 10-g/L NaCl) with 10-g/L casein at 28 °C. After incubation of 48 h, the protease of the strain gcc-1 was purif ied as described previously with minor modif ication (Wu et al., 2015b). Cell-free supernatant was obtained after centrifugation at 10 000×gfor 20 min, then precipitated by 80% saturation with(NH4)2SO4at 4 °C overnight. The precipitate was collected after centrifugation, and dissolved in 50-mmol/L NaCl with 10-mmol/L Tris-HCl (pH 8.0),then purif ied by a 5-mL HiTrapTMQ HP column (GE Healthcare) with gradient elution buff er from 50 to 500-mmol/L NaCl in 10-mmol/L Tris-HCl (pH 8.0)on AKTA purif ier system (Amersham Biosciences,Piscataway, NJ, USA). Then active fractions, which performed hydrolytic activity, were concentrated by ultra-f iltration (3-kDa MW interception membrane,Millipore), and subjected to gel f iltration on a HiloadTM16/600 SuperdexTM200 column (GE Healthcare) after pre-equilibrated by 150-mmol/L NaCl with 20-mmol/L Tris-HCl (pH 8.0). The purif ied active fractions were eluted by the same buff er at a f low rate of 1 mL/min, and the active fractions were used for further analysis. All purif ication processes were performed at 4 °C. The fraction with highest activity was executed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE),then stained with Coomassie Brilliant Blue R250(Sigma) and decolorized with destaining solution(10% acetic acid, 5% ethanol). Zymography analysis was performed on 10% polyacrylamide gel according to the method described by Garciacarreno et al. (1993)with trif ling alteration. The fraction was not heated before electrophoresis. After electrophoresis, the gel was soaked in sterile water three times to wash away the bacteria, and then placed on a solid plate containing casein in 28-°C incubator. The clear area of the gel indicates the presence of protease activity.
2.6 Protease activity assay
The protease activity was measured with minor modif ication according to previous method (Oh et al.,1999). The reaction mixture (1 mL) consisted of 100 μL of casein solution (2 mg/mL), 100 μL of diluted purif ied protease at the concentration of 50 μg/mL, and 800 μL of 10 mmol/L Tris-HCl (pH 8.0). The reaction mixture without any protease was used as control. The mixture was incubated at 37 °C for 30 min before an equal volume of 10%trichloroacetic acid (TCA) solution was added to stop the reaction, and then centrifuged at 10 000×gfor 15 min. Absorbance of supernatant was estimated at 278 nm, which indicates the hydrolysis ability of the protease. The standard curve was generated with tyrosine solution of 0-50 μg/mL. One unit (U) of protease activity was def ined as the amount of enzyme to release 1 μg of tyrosine per minute under the test conditions.
2.7 Eff ect of pH on protease activity and stability
The pH eff ect on enzyme activity was determined by incubating the reaction mixture at diff erent pH value of 6.5-11.5 at 37 °C for 30 min with following buff er systems: 0.1-mol/L potassium phosphate buff er(pH 6.5-7.5), 0.1-mol/L Tris-HCl (pH 8.5), 0.1-mol/L glycine-NaOH (pH 9.5-11.5). The reaction mixture(1 mL) contained 100 μL of 2-mg/mL casein solution,100 μL of 50-μg/mL protease, and 800 μL of buff er at diff erent pH value of 6.5-11.5. The pH eff ect on enzyme stability was determined by detecting the residual activity after the protease was incubated with diff erent buff ers at room temperature for 1 h. And the highest enzyme activity was considered as the control(100%). Presented values are averages of three independent experiments.
2.8 Eff ect of temperature on protease activity and stability
The eff ect of temperature on enzyme activity was determined by incubating the reaction mixture at diff erent temperatures (10, 20, 30, 40, 50, 60, 70, and 80 °C) for 30 min. The protease stability in diff erent temperature was performed by detecting the residual activity after the protease was incubated for 1 h at diff erent temperatures. The highest enzyme activity was considered as control (100%). Presented values are averages of three independent experiments.
2.9 Eff ect of various metal ions and inhibitors on protease activity
The eff ect of diff erent metal ions (Ba2+, Fe3+,Mg2+, Mn2+, Cu2+, Ca2+, Hg2+, and Zn2+) on enzyme activity was determined by measuring the residual activity after the protease was incubated with 5-mmol/L metal ions for 1 h at 37 °C, individually.The eff ect of inhibitors ethylene diamine tetraacetic acid (EDTA), phenylmethylsulfonyl f luoride(PMSF), or dithiothreitol (DTT) on protease activity was determined by measuring the residual activity after the protease was incubated with 5-mmol/L inhibitor for 1 h at 37 °C, individually. The enzyme without any treatment was considered as control(100%). Presented values are averages of three independent experiments.
2.10 Eff ect of detergent additives and organic solvents on protease activity
The eff ect of detergent additives on enzyme stability was monitored by measuring the residual activity after the protease was incubated with diff erent detergent additives for 1 h at 37 °C, separately. The detergent additives include H2O2(1%, v/v), SDS (1%,v/v), Tween 20, 80 (10%, v/v) or Triton X-100 (10%,v/v). The eff ect of organic solvents on protease stability was determined by measuring the residual activity after the protease was incubated with 50%(v/v) diff erent organic solvents for 1 h at 37 °C,individually. The organic solvents included methanol,ethanol, isopropanol, dimethylbenzene, glycerin,Dimethyl sulfoxide (DMSO), hexyl hydride, and trichloromethane. The enzyme without any treatment was considered as control (100%). Presented values are averages of three independent experiments.
In order to evaluate the compatibility of the protease produced by strain gcc-1 with commercial detergents (Ariel and Tide, Procter & Gamble,America and Chaoneng, Nice, China) were bought from local supermarkets, then dissolved to a f inal concentration of 7 mg/mL separately to simulate the domestic laundry environment (Espósito et al., 2009).The compatibility was indicated by measuring the residual activity after protease was incubated with diff erent detergent solution for 1 h at 37 °C individually. The enzyme without any treatment was considered as control (100%). Presented values are averages of three independent experiments.
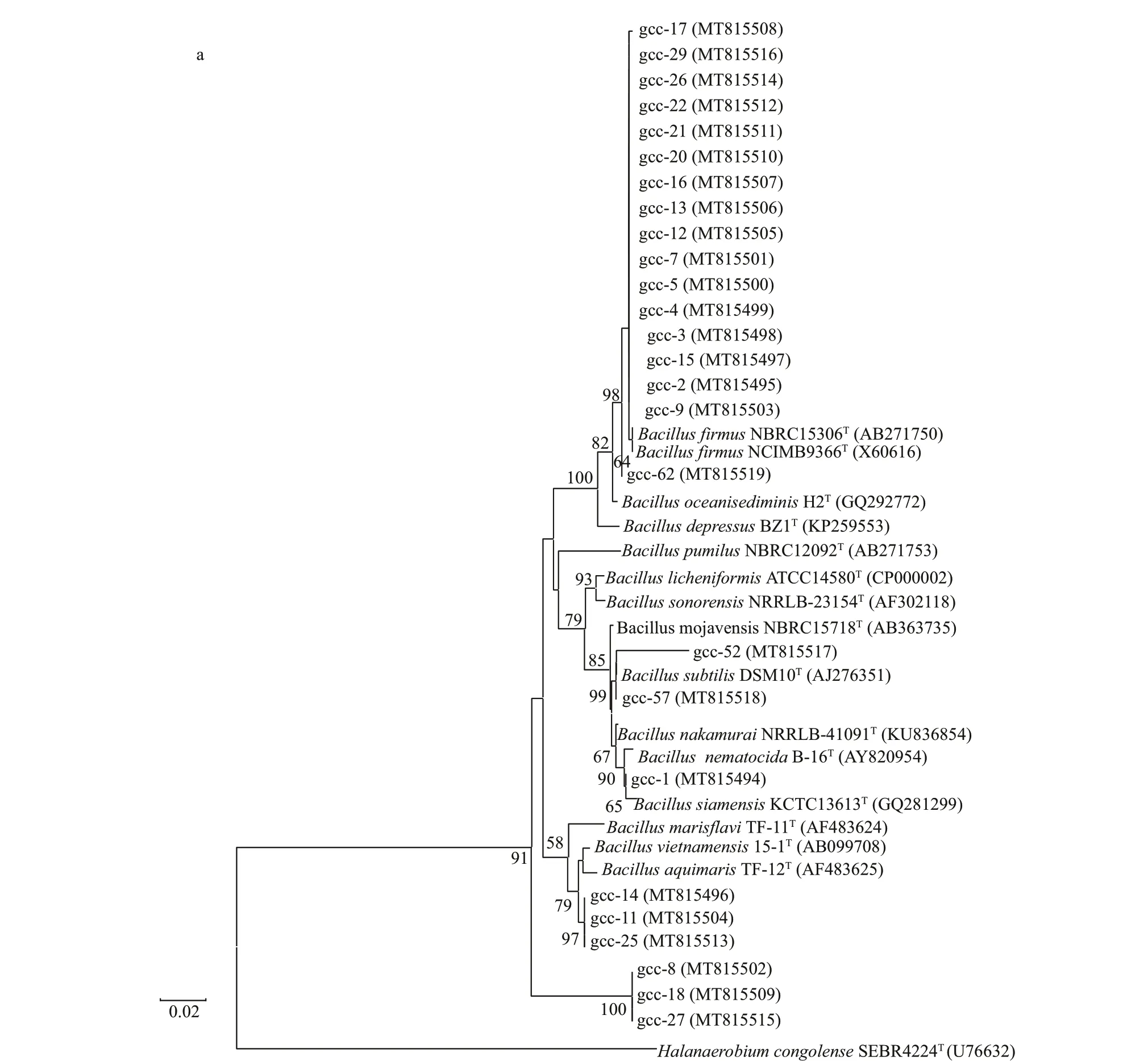
Fig.1 Phylogenetic tree of isolates belonging to four categories
3 RESULT
3.1 Identif ication of protease-producing strains
To screen the protease-producing strains, 250 strains were isolated from the sediment of the deepsea cold seep in South China Sea, and 50 proteaseproducing strains were screened out. 16S rRNA of the 50 protease-producing strains were sequenced and analyzed by phylogenetic tree. As shown in Fig.1, the50 strains were divided into four categories. Twentysix strains belong toBacillus(Fig.1a), 17 strains belong toPseudoalteromonas(Fig.1b), f ive strains belong toVibrio(Fig.1c), and two strains belong toAlteromonas(Fig.1d), which indicates that the main protease-producing strains belong toBacillusandPseudoalteromonas.
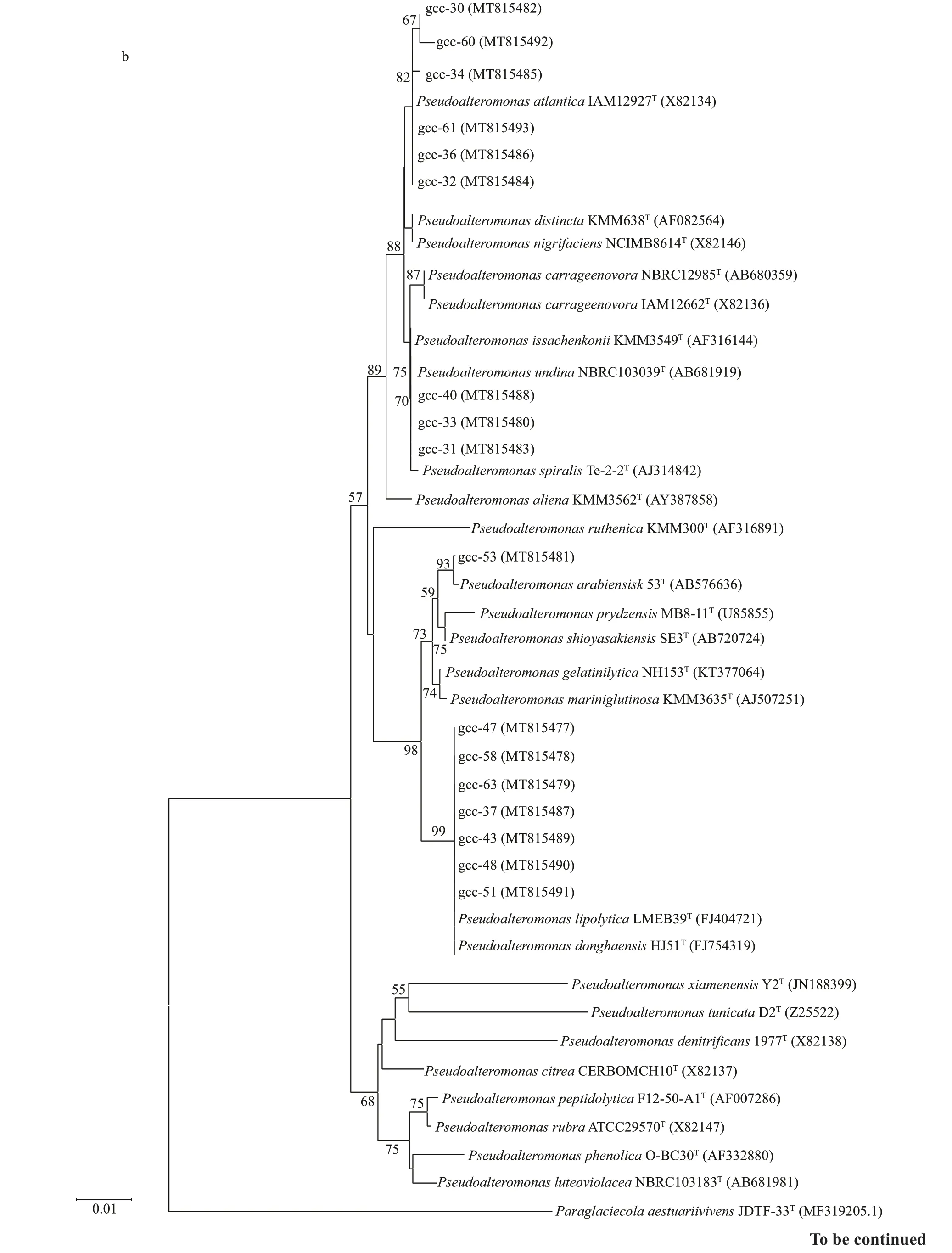
Fig.1 Continued
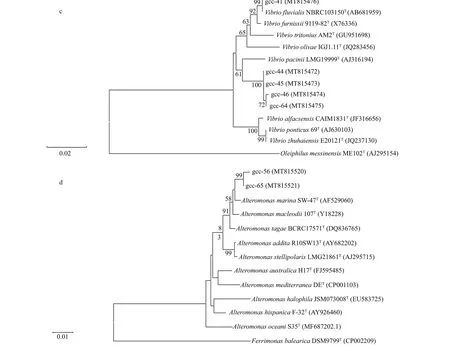
Fig.1 Continued
3.2 Determination of hydrolytic enzymes of protease-producing strains
Among the 50 protease producing strains, 9 strains(gcc-1, gcc-14, gcc-25, gcc-33, gcc-41, gcc-47, gcc-53, gcc-58, and gcc-63) exhibited strong protease activity (Table 1), which contained 3Bacillusstrains,5Pseudoalteromonasstrains and 1Vibriostrain.Furthermore, the strain gcc-1, which belongs toBacillus, displayed strongest protease activity and stability under diff erent temperature and pH conditions. Therefore, strain gcc-1 was selected for further protease analysis.
In order to detect whether the 50 proteaseproducing strains could produce other hydrolytic enzymes except protease, the activity of amylase and lipase was also detected among the screened 50 strains (Table 1). 39 amylase-producing strains were also detected among the 50 strains, which contained 21Bacillusstrains, 11Pseudoalteromonasstrains, 5Vibriostrains, and 2Alteromonasstrains.And 29 strains displayed good lipase activity,which contained 14Bacillusstrains, 12Pseudoalteromonasstrains and 3Vibriostrains.The results indicate that the microorganism isolated from the deep-sea cold seep is a rich source to screen hydrolytic enzymes.
3.3 Purif ication of protease produced by strain gcc-1
Since strain gcc-1 exhibited strongest protease activity and stability under diff erent conditions, thestrain gcc-1 was cultured in LB broth for 48 h to further investigate its protease activity. The protease was purif ied by sequentially ammonium sulfate precipitation, anion exchange chromatography and gel f iltration, and a main peak with protease activity was obtained at the last purif ication step (Fig.2a). The specif ic activity of the f inal purif ied protease is 176.9 U/mg with the recovery of 16.3% (Table 2).The occurrence of a single band with a molecular weight of 28 kDa in SDS-PAGE illustrated the homogeneity of the purif ied protease, and a clearance zone at the position of 28 kDa was observed by zymography assay, which further conf irmed the activity of the purif ied protease (Fig.2b).
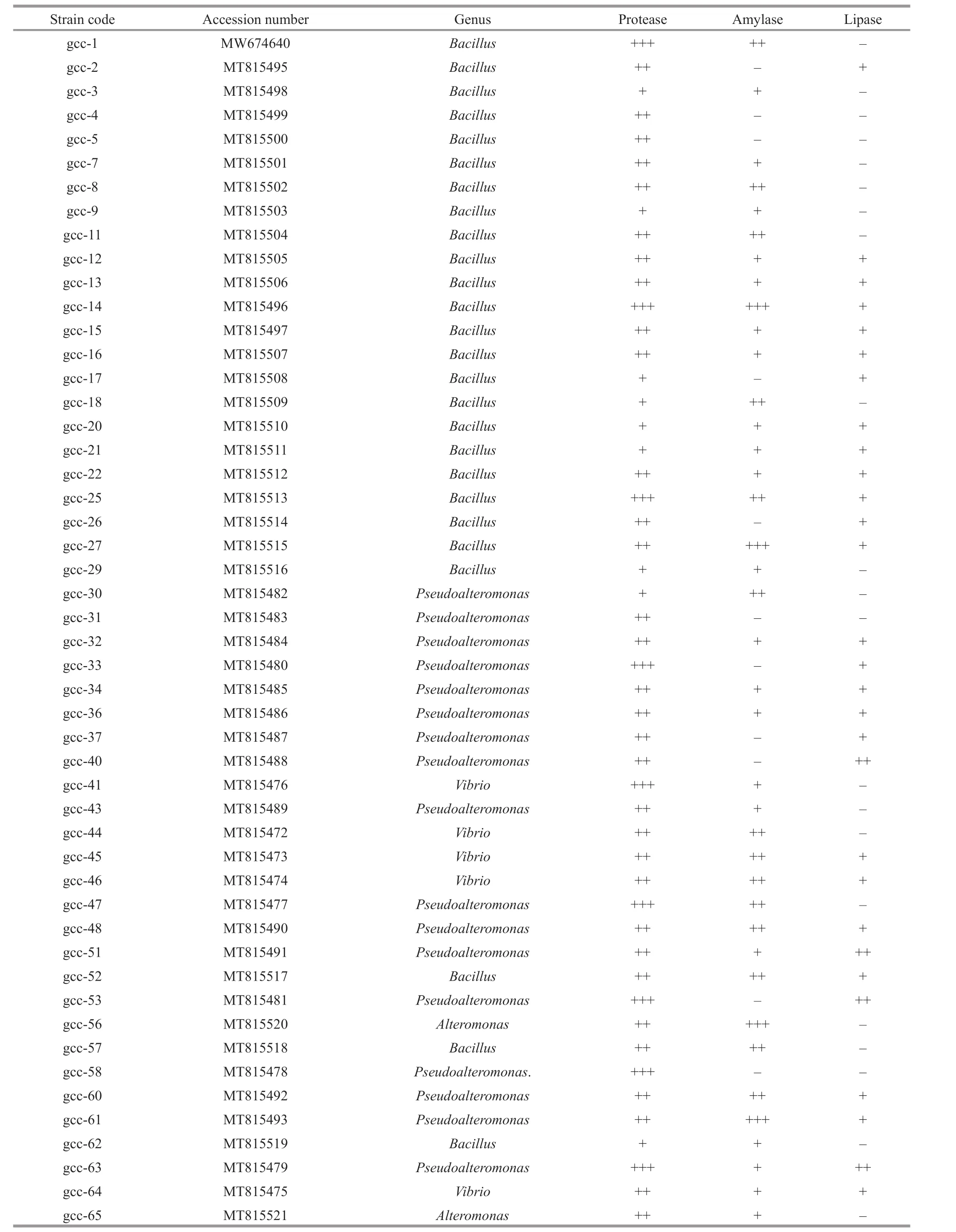
Table 1 Determination of hydrolytic enzymes of protease-producing strains isolated from deep-sea cold seep
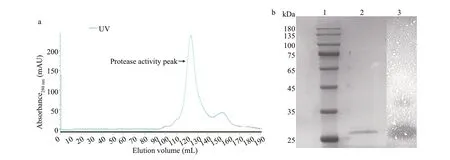
Fig.2 Chromatograms map of the purif ication, SDS-PAGE and zymography analyses of the protease produced by gcc-1
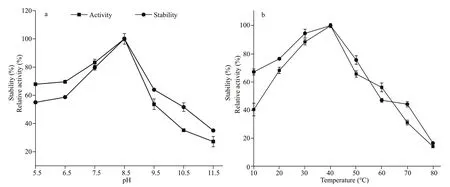
Fig.3 Eff ect of pH (a) and temperature (b) on the activity and stability of protease produced by gcc-1

Table 2 Purification of protease produced by strain gcc-1
3.4 Eff ect of pH and temperature on protease activity and stability
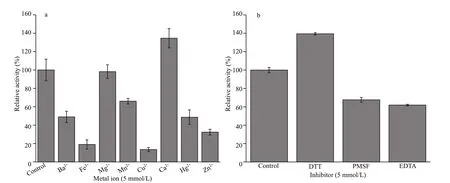
Fig.4 Eff ect of 5-mmol/L diff erent metal ions (a) and 5-mmol/L inhibitors (b) on the activity of protease produced by gcc-1
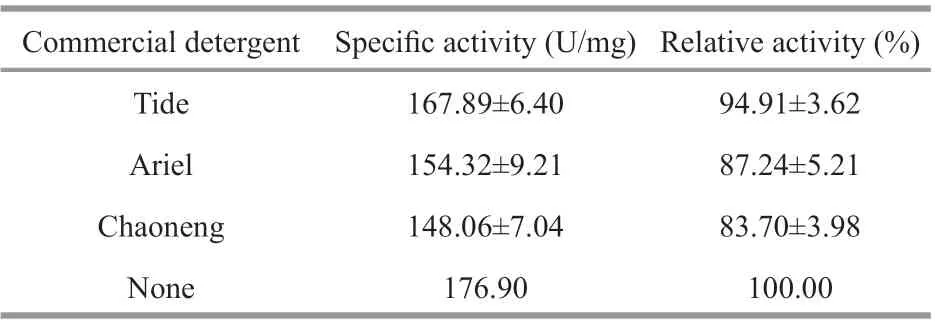
Table 3 Stability assay of the purif ied protease in presence of commercial detergents
In order to determine the activity and stability of the purif ied protease, the eff ect of pH and temperature on protease activity and stability was detected. As shown in Fig.3a, the purif ied protease exhibited high activity in a wide range of pH 6.5-11.5, and protease activity under the pH of 7.5-9.5 was over 50%. The optimum activity occurred at pH 8.5, which is similar to the stability assay under the range of pH 6.5-11.5.The eff ect of temperature on gcc-1 protease activity was also measured under diff erent temperature ranging from 10 to 80 °C. As shown in Fig.3b,although the optimum temperature of the protease activity was 40 °C, the protease exhibited 40%activity at 10 °C and almost 70% at 20 °C, and the stability assay showed the protease remained high activity in the range of 10 °C to 50 °C, indicating that the protease produce by gcc-1 is a cold active and high stable protease.
3.5 Eff ect of metal ions and protease inhibitors on protease activity
Eff ect of diff erent ions on protease stability was also detected and shown in Fig.4a. The protease activity changed greatly when 5-mmol/L diff erent metal ions were added. Ca2+could promote protease activity up to 134.5%, and Mg2+showed little eff ect on the protease activity with a residual activity of 98.2%, while other metal ions reduced the protease activity to diff erent extent. Eff ect of diff erent protease inhibitors on protease stability was also detected and shown in Fig.4b. The activity of protease was promoted to 139.4% by DTT, but inhibited by serine protease inhibitor PMSF and metallo-protease inhibitor EDTA, indicating that the protease produced by gcc-1 belongs to serine protease and metallo-type protease.
3.6 Eff ect of detergent additives and organic solvents on protease activity
Since the ability to coexist with many diff erent detergent additives is vital for proteases in the application market, and the stability of protease produced by strain gcc-1 against diff erent detergents was detected. As shown in Fig.5a, the protease activity in Tween 20, Tween 80, and Triton X-100 was increased to 113.0%, 100.8%, and 119.5%,respectively, while SDS and H2O2had little eff ect on its activity. In addition, the protease showed good compatibility under diff erent organic solvents. As shown in Fig.5b, the purif ied protease remained about 80% activity under treatment with DMSO,dimethylbenzene, methanol, and glycerin, and remained more than 50% activity with other organic solvents.
To determine the compatibility of the purif ied protease with commercial detergents, the protease activity was measured after incubated with common detergents bought from local supermarket. As shown in Table 3, the purif ied protease retained more than 80% activity after incubated with Ariel and Chaoneng,and retained about 94% activity after incubated with Tide, indicating that the protease produced by gcc-1 has a very good compatibility with commercial detergents.
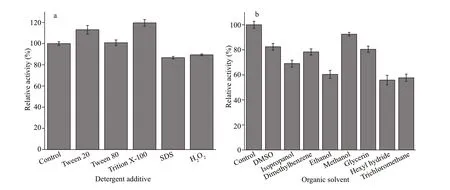
Fig.5 Eff ect of diff erent detergents (a) and organic solvents (b) on the activity of protease produced by gcc-1
4 DISCUSSION
Proteases are widely used in industry, most of which come from microbial metabolites, and are the main source of global enzyme market (Outtrup and Boyce, 1990). The ocean covers more than 70% of the Earth’s surface, and contained countless marine microorganisms, which provides rich resources to isolate biological enzymes (Makino et al., 1981; Ma et al., 2007; Zhou et al., 2009; Cristóbal et al., 2011).In this study, 50 protease-producing strains were screened from cold seep of the South China Sea,among them 52% of the strains belong toBacillusand 34% of the strains belong toPseudoalteromonas.Furthermore, the abilities of these 50 strains to produce amylase and lipase were also tested. As for the amylase detection, 39 strains exhibited good amylase activity, in which 53.8% of the strains belong toBacillusand 28.2% of the strains belong toPseudoalteromonas. As for the lipase detection, 29 strains displayed good lipase activity, and 48.2% of the strains belong toBacillusand 41.3% of the strains belong toPseudoalteromonas. Therefore,BacillusandPseudoalteromonasare the main groups producing hydrolytic enzymes, which may be due to their easy cultivation in marine microorganisms.
Alkaline proteases are widely used in laundry industry, while trends in energy effi ciency and demands of low-temperature washing raise the awareness of exploring cold-active proteases (Sun et al., 2000; Vázquez et al., 2008; Wang et al., 2010). The ocean is a cold environment, especially the deep-sea cold seep, which forces the microorganisms living in it to evolve corresponding cold adaptation mechanisms,such as the production of cold active enzymes (Zhou et al., 2019; Li et al., 2020a). Normally, this kind enzyme have evolved a range of structural features that confer a high level of f lexibility, and the high f lexibility, particularly around the active site, is translated into low-substrate affi nity and high specif ic activity at low temperatures (Marx et al., 2007). In our study, the protease producing strain gcc-1 was isolated from deep-sea cold seep, and the produced protease exhibited good activity at low temperature with 40%residual activity at 10 °C and almost 70% residual activity at 20 °C. Therefore, the protease produced by gcc-1 has a very good characteristic to be used in coldwashing laundry industry. Our results also conf irmed that the deep-sea cold seep is a rich source to isolate cold active hydrolytic enzymes.
Generally, enzymes compatible with detergents must withstand alkaline conditions. Because the pH value of detergent is alkaline, it is important for protease to keep high activity under alkaline condition as detergent additives (Li et al., 2013). The protease produced by gcc-1 maintained high activity within a wide pH range of 6.5-9.5. Furthermore, the activity of protease produced by gcc-1 is improved by Ca2+,which is consistent with previous report (Xu et al.,2013), and could hardly be aff ected by Mg2+. The stability of the protease in present of Ca2+and Mg2+,which are common ions in water (Li et al., 2020b),makes it has more superiority as a household detergent additive. Notably, most signif icant commercial detergent protease additives such as Savinase and Esperase (Novo Nordisk, Denmark), and Maxinase(Gist-Brocades, The Netherlands) are stable with laundry surfactants, yet unresisting to oxidizing agents (Gupta et al., 2002). In our study, the protease produced by gcc-1 not only exhibited high activity in presence of most detergent additives and organic solvents, but also maintained high stability when treated with oxidizing agent H2O2, which endow it more advantages compared with other proteases.Therefore, the protease produced by gcc-1 has a promising prospect applied in laundry industry as a good additive in the future.
5 CONCLUSION
In this study, we screened f ifty protease-producing strains from sediment of deep-sea cold seep, which belong to four diff erent categories:Bacillus,Pseudoalteromonas,Vibrio, andAlteromonas. Among them, strain gcc-1, which displayed strongest protease activity, was further purif ied and investigated. The purif ied protease produced by gcc-1 exhibited high activity at a wide temperature range and a wide pH range, and maintained high stability in present of diff erent surfactants, oxidizing agent, organic solvents and commercial detergents. Therefore, the protease produced by gcc-1 is a cold active and high stable enzyme, and has a high potential in laundry detergent as an additive.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年2期
Journal of Oceanology and Limnology2022年2期
- Journal of Oceanology and Limnology的其它文章
- Identif ication of Antarctic minke and killer whales with passive acoustic monitoring in Prydz Bay, Antarctica*
- Eff ects of dissolved oxygen and nutrients from the Kuroshio on hypoxia off the Changjiang River estuary*
- Methane in the Yellow Sea and East China Sea: dynamics,distribution, and production*
- Longitudinal genetic analysis of growth-related traits in red swamp crayf ish Procambarus clarkii (Girard)*
- Early life migration and population discrimination of the small yellow croaker Larimichthys polyactis from the Yellow Sea: inferences from otolith Sr/Ca ratios*
- A new oil spill detection algorithm based on Dempster-Shafer evidence theory*
